Summary
The annual percent change (APC) has been used as a measure to describe the trend in the age-adjusted cancer incidence or mortality rate over relatively short time intervals. The yearly data on these age-adjusted rates are available from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. The traditional methods to estimate the APC is to fit a linear regression of logarithm of age-adjusted rates on time using the least squares method or the weighted least squares method, and use the estimate of the slope parameter to define the APC as the percent change in the rates between two consecutive years. For comparing the APC for two regions, one uses a t-test which assumes that the two datasets on the logarithm of the age-adjusted rates are independent and normally distributed with a common variance. Two modifications of this test, when there is an overlap between the two regions or between the time intervals for the two datasets have been recently developed. The first modification relaxes the assumption of the independence of the two datasets but still assumes the common variance. The second modification relaxes the assumption of the common variance also, but assumes that the variances of the age-adjusted rates are obtained using Poisson distributions for the mortality or incidence counts. In this paper, a unified approach to the problem of estimating the APC is undertaken by modeling the counts to follow an age-stratified Poisson regression model, and by deriving a corrected Z-test for testing the equality of two APCs. A simulation study is carried out to assess the performance of the test and an application of the test to compare the trends, for a selected number of cancer sites, for two overlapping regions and with varied degree of overlapping time intervals is presented.
Keywords: Age, adjusted incidence/mortality rates, age, stratified Poisson Regression, annual percent change (APC), surveillance, trends, hypothesis testing
1 Introduction
The American Cancer Society (ACS) in its annual publication Cancer ACS 2007 (http://www.cancer.org/) reports that in 2007 about 1.5 million new cancer cases are expected to be diagnosed, and approximately 560,000 Americans are expected to die of cancer. Cancer is the most common cause of death in US, exceeded only by heart disease, and accounts for 1 of every 4 deaths. The same report also reveals that, for a number of cancer sites (such as breast, stomach, colon and rectum, lung and bronchus and leukemia), the age-adjusted cancer mortality rates have been steadly decreasing in recent years. In addition, the National Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI) periodically publishes similar reports on trends of cancer incidence at http://seer.cancer.gov/csr; see Ries et al. (2003) So much has been at stake in terms of human life and cost - for example, the government agencies such as the National Health of Institutes (NIH), and many private sectors spend billions of dollars every year on cancer research, health insurance and medical and other costs - that there is an urgent need for new methods that produce more accurate and reliable estimates of measures of cancer trends.
The annual percent change (APC) has been used as a measure of cancer trends over short time periods, and to compare the recent cancer trends by gender or by geographic regions, one compares their APC values using the two-sample pooled t-test ( Kleinbaum et al., 1988) that assumes that the datasets on age-adjusted rates under the study are independent. However, a fundamental statistical difficulty arises when such comparisons, largely for policy making purposes, have to be made for regions or time intervals that overlap, e.g. comparing the most recent changes in trends of cancer rates in a local area (e.g. the mortality rate of breast cancer in California) with a more global level (i.e. the national mortality rate) over two overlapping time periods. For example, as detailed in the data analysis section, it is of substantial interest to compare the changes in California cancer mortality rates with the national cancer mortality rates in the last 15 years.
Recently, Li and Tiwari (2007) and Li et al. (2007) developed Z-tests which adjust for the dependence between the two APCs, and are more efficient than the naive test which assumes independence. However, these tests are based on the logarithmic transformation of the age-adjusted rates, and fits a simple linear regression model of the transformed data on time using either the ordinary least squares (OLS) or the other weighted least squares (WLS) procedures. The proposed test procedure is based on the natural assumption that the age-specific mortality or incidence counts are results of underlying Poisson processes (Brillinger, 1986), and hence are realizations of independent Poisson random variables. The age-specific instantaneous hazards are modeled by a log-link function, thus leading to an age-stratified Poisson model. The estimation of the parameters is then carried out using a likelihood-based approach.
The rest of the paper is organized as follows. In Section 2, we briefly review the existing tests, and derive the new test in Section 3. To compare the performance of the proposed test with respect to the above mentioned tests, a simulation study is carried out in Section 4. In this section, we also give application to breast cancer mortality data from California (CA) and the US extracted from the SEER*STAT software of the SEER Program. Section 5 ends this paper with a short discussion.
2 A Brief Review of Existing Tests
Consider two regions, and let dkji denote the number of counts (deaths or new cancer cases) from the population at risk nkji observed in Region k (k = 1, 2) in age-group j (j = 1, …, J) and at times T1, …, Tm for Region 1 and Ts+1, …, Ts+n for Region 2, where T1 ≤ Ts+1 < Tm ≤ Ts+n, with 0 ≤ s < m leading to overlapping time intervals. Note that this formulation is general and allows one region to have fewer time points than the other. In the SEER program, it is common to choose nkji (at year Ti) to be the mid-year population representing the total person-years in one year, with the assumption of “drop-outs” being uniform over the unit-intervals. The age-adjusted rates are defined as
where wj > 0, j = 1, …, J, are the known standards for the age group j so that . For the SEER analysis, there are J = 19 standard age-groups consisting of 0–1, 1–4, 5–9, …, 85+, and wj are chosen to be the year 2000 population standards (Fay et al. 2006).
Let yki = log(rki), be the logarithmic transformations of the age-adjusted rates. Consider the linear regression models
| (1) |
for k = 1, 2, flagging Regions 1 and 2, respectively. Here eki are random errors with mean 0, and tki corresponds to the calendar times of data collection in region k with I1 = m and I2 = n. More specifically, (t11, …, t1I1) = (T1, …, Tm), while (t21, …, t2I2) = (Ts+1, …, Ts+n). For the two regions, the annual percent change (APC) are defined as APCk = 100(eβ1k − 1)= 100β1k, for a small β1k, e.g. in the order of 10−2 (Kim et al., 2000; Fay et al., 2006; Tiwari et al., 2006).
Then under the assumptions that eki are independent and have common variance σ2, the two-sample pooled t-test (Kleinbaum et al., 1988) for testing the null hypothesis H0: APC1 = APC2 versus the alternative Ha: APC1 ≠ APC2 is given by
| (2) |
where for k = 1, 2, and σ̂2 is the “pooled” unbiased estimate of σ2 given by
where ŷki = β̂0k + β̂1ktki are the predictions for k = 1, 2. Here, β̂0k and β̂1k are obtained from the least squares estimation. That is,
where
The above test is not appropriate, however, when there is an overlap between the two regions or the two time periods. For this case, Li and Tiwari (2007) proposed the following corrected Z-test
| (3) |
where , , for k = 1, 2, and and are the numbers of at-risk population in the overlapping region. Note that there is no suffix k in . The sign of σ12 determines, whether the covariance between β̂11 and β̂12 is positive or negative, and when there is no overlap in time intervals, σ12 = 0. Under the log-normal model, the corrected ZCT test was shown to follow a standard normal distribution under the null hypothesis, and to be more efficient than the pooled t-test; see Li and Tiwari (2007).
However, one assumption in Li and Tiwari (2007) is the equal variance in both regression models, which may not be realistic, especially for rare cancers. A further refinement has been made to derive the variance of yki by using the Poisson assumptions on the first two moments of the counts dkji, i.e. E(dkji) = var(dkji). Under these assumptions, the consistent estimate of the error variance of eki is given by , leading to the following weighted least squares test proposed by Li et al. (2007), referred to as ZWLS:
| (4) |
with and ,
where
and are the counts in the overlapping region and during the overlapping period. Here,
and β̃11, β̃12 are weighted least square estimates of β11, β12.
Under the null hypothesis, because of the normal approximation, ZWLS approximately follows a standard normal distribution. The ZWLS has been shown to be more conservative than ZCT in retaining the size of the test, but is more powerful for the common cancer sites; see Li et al. (2007). However, there are several disadvantages of the existing methods. First, one key step of Li et al. (2007) is the normal approximation of the age-adjusted rates. Secondly, both Li et al. (2007) and Li and Tiwari (2007) need adjustments for zero counts.
3 Age-stratified Poisson Regression Model
As the existing approaches to dealing with age-adjusted cancer rates were all based on the normal approximation, we take a more natural route in the sequel by considering the Poisson nature of the underlying count data and propose an age-stratified Poisson regression to describe the change trend of incident (or death) counts on time. Based on this model, a proper test that accounts for overlapping is proposed.
Specifically, since dkji, the number counts (deaths or new cancer cases) observed in Region k (k = 1, 2) in age-group j, is a count, we assume that
with
| (5) |
which is referred to as the Age-stratified Poisson Regression Model as the age-specific intercept β0kj is assumed for age-group j. The common slope β1k is of particular importance as it transcribes the trends of mortality or incidence and, in particular, determines the APC value.
Again let APC1 and APC2 be the corresponding APC values for these two Poisson regressions. A natural test for the null hypothesis H0: APC1 = APC2 versus the alternative hypothesis H1: APC1 ≠ APC2 would be
where β̂11 and β̂12 are the maximum likelihood estimates of β11 and β12 derived in the Appendix.
Because of the possible overlapping of Regions 1 and 2, β̂11 and β̂12 may be correlated. Thus the key to the derivation of the test lies in a correct evaluation of Cov(β̂11, β̂12).
3.1 Derivation of the Test
To proceed, we let βk = (β 1k, β 0k1, …, β 0kJ)′, k = 1, 2, whose estimates β̂k can be btained by solving the score equations [based on (5)]
for k = 1, 2, where Uk = (U1k,1, U0k,1, …, U0k,J)′ and
for j = 1, …, J.
As Uk(β̂k) = 0, expanding it around the true value βk, and ignoring the higher order terms yields
where are (J+1) × (J+1) matrices with its 1st row as and the (j + 1)th row (j = 1, …, J) as , for k = 1, 2. Here
and δjj′= 1 if j = j′ and 0 otherwise.
Denote by for k=1, 2, where plim denotes the limit in probability. Then for large I1(≡m) and I2(≡n), standard probabilistic arguments yield
Here, denotes approximate equivalence in joint distribution functions. Hence,
Let , , , and V̂, Ŵ, Σ̂ be the corresponding estimates, whose derivations are given in the Appendix.
Then,
These are three (J +1) × (J +1) matrices, the (1, 1) entries of which are , and σ̂12 = Ĉov(β̂11, β̂12), respectively. From this we compute V̂ar (β̂11 − β̂12) = V̂ar (β̂11)+V̂ar (β̂12) − 2Ĉov(β̂11, β̂12). Hence, the Z-test for comparing APC values is given by
| (6) |
which follows the standard normal distribution under H0: β11 = β12. The computation of β̂11, β̂12 is given in the Appendix.
3.2 ARE Comparison with the WLS test
It is of substantial interest to evaluate the gains in efficiency of the proposed test compared with the WLS test. First note (5) implies that . This in turn implies that
| (7) |
when Var(rki) is small, which is often the case for the cancer incidence and mortality data (Kim et al., 2000).
A comparison between (1) and (7) reveals that models (1) and (5) approximately specify the same first moment of the age-adjusted cancer rates, making it possible to compare the efficiency of the tests based on these two models via the measure of the Pitman Asymptotic Relative Efficiency. Specifically, standard asymptotic analysis will yield
while, for the WLS estimates,
Hence, the Pitman Asymptotic Relative Efficiency (ARE) comparing tests (6) and (4), which is the ratio of the noncentralities of the above two normal distributions, is given by
| (8) |
The evaluation of (8) typically involves numerical computations.
4 SEER mortality data analysis and Simulations
Li et al. (2007) demonstrated that ZWLS performs better than ZCT, via the calculation of ARE, as ZCT relies on the common variance assumption, which may not be realistic. Hence, we focus this paper on comparing ZPOIS with ZWLS. To comprehensively evaluate these tests, we consider several scenarios of overlap in two regions and in two different time intervals. Specifically, we assume that Region 1 consists of Georgia (GA), South Carolina (SC), and North Carolina (NC), and that Region 2 consists of NC, Virginia (VA) and Maryland (MD); with NC as the overlapping state between the two regions. The three different time intervals, with varying degree of overlap in the intervals, are taken to be: (a) [1980,1989] for Region 1, and [1990,1999] for Region 2 so that there is no overlap between the two time intervals and, hence, σ12 = 0; (b) [1980,1989] for Region 1, and [1983,1992] for Region 2 so that there a considerable overlap of six years between the two intervals and σ12 = 12.25; (c) [1980,1989] for Region 1, and [1987, 1996] for Region 2 so that there is a little overlap of three years’ between the two intervals and σ12 = −34.75.
The counts, dkji were generated based on model (5) with tki taking values in the intervals corresponding to the two regions stated above. More specifically, the t1i take values of {0, 1, …, 9}, while the t2i take values of {10, …, 19}, {3, …, 12}, and {7, …, 16}, respectively for cases (a)–(c).
In order to fully specify λkji in (5), we assume that β0kj = log(dkj1/nkj1) − β1ktk1, where dkj1 and nkj1 are respectively the observed number of deaths and the number of at-risk population at the beginning of the intervals considered, and take β1k = log(APCk/100 + 1), based on the specified values of APCk. The age-specific counts for the overlapping state, NC, are generated from Poisson distributions with means, , where denotes the at-risk population in the overlapping region. When λ1ji =λ2ji, this reduces to the situation specified by the null hypothesis. The number of at risk population and the observed number of deaths were obtained from the SEER database for all malignant male cancers and prostate cancer. The values of APC were assumed to range from −0.3% to 3.0%. For each parameter configuration, a total of 1000 simulated data were obtained. The results for the three time-overlapping cases are summarized in Tables 1–3.
Table 1.
Comparison of the power functions under various hypotheses between the Poisson-based test ZPOIS and the weighted-least-squares based test ZWLS for two overlapping regions over disjoint time intervals, Region 1 (1980–1989) vs Region 2 (1990–1999). APC1 and APC2 are the annual percent changes in Regions 1 and 2, respectively.
| Cancer Sites | APC1 | APC2 | ARE | ZWLS | ZPOIS |
|---|---|---|---|---|---|
| All Malignant | 0.100 | 0.100 | 1.1490 | 0.059 | 0.050 |
| −0.300 | −0.300 | 1.1491 | 0.049 | 0.045 | |
| 0.500 | 0.500 | 1.1492 | 0.060 | 0.049 | |
| 1.000 | 1.000 | 1.1493 | 0.048 | 0.053 | |
| 3.000 | 3.000 | 1.1504 | 0.047 | 0.057 | |
| 0.100 | 0.500 | 1.1507 | 0.877 | 0.907 | |
| −0.300 | 0.300 | 1.1509 | 0.998 | 1.000 | |
| 1.000 | 2.000 | 1.1515 | 1.000 | 1.000 | |
| 1.000 | 3.000 | 1.1518 | 1.000 | 1.000 | |
| Prostate | 0.100 | 0.100 | 1.1610 | 0.043 | 0.045 |
| −0.300 | −0.300 | 1.1611 | 0.041 | 0.047 | |
| 0.500 | 0.500 | 1.1612 | 0.051 | 0.041 | |
| 1.000 | 1.000 | 1.1613 | 0.046 | 0.051 | |
| 3.000 | 3.000 | 1.1614 | 0.045 | 0.051 | |
| 0.100 | 0.500 | 1.1617 | 0.182 | 0.212 | |
| −0.300 | 0.300 | 1.1619 | 0.357 | 0.40 | |
| 1.000 | 2.000 | 1.1622 | 0.773 | 0.830 | |
| 1.000 | 3.000 | 1.1634 | 1.000 | 1.000 |
Table 3.
Comparison of the power functions between the Poisson based ZPOIS and the weighted-least-squares based ZWLS for two overlapping regions over slightly overlapping time intervals, Region 1 (1980–1989) vs Region 2 (1987–1996). APC1 and APC2 are the annual percent changes in Regions 1 and 2, respectively.
| Cancer Sites | APC1 | APC2 | ARE | ZWLS | ZPOIS |
|---|---|---|---|---|---|
| All Malignant | 0.100 | 0.100 | 1.1350 | 0.044 | 0.044 |
| −0.300 | −0.300 | 1.1350 | 0.044 | 0.046 | |
| 0.500 | 0.500 | 1.1351 | 0.046 | 0.051 | |
| 1.000 | 1.000 | 1.1351 | 0.045 | 0.051 | |
| 3.000 | 3.000 | 1.1352 | 0.043 | 0.047 | |
| 0.100 | 0.500 | 1.1354 | 0.834 | 0.891 | |
| −0.300 | 0.300 | 1.1355 | 0.994 | 0.994 | |
| 1.000 | 2.000 | 1.1362 | 1.000 | 1.000 | |
| 1.000 | 3.000 | 1.1367 | 1.000 | 1.000 | |
| Prostate | 0.100 | 0.100 | 1.1410 | 0.045 | 0.051 |
| −0.300 | −0.300 | 1.1410 | 0.043 | 0.049 | |
| 0.500 | 0.500 | 1.1412 | 0.060 | 0.051 | |
| 1.000 | 1.000 | 1.1423 | 0.061 | 0.055 | |
| 3.000 | 3.000 | 1.1426 | 0.052 | 0.052 | |
| 0.100 | 0.500 | 1.1428 | 0.170 | 0.184 | |
| −0.300 | 0.300 | 1.1431 | 0.319 | 0.361 | |
| 1.000 | 2.000 | 1.1436 | 0.706 | 0.759 | |
| 1.000 | 3.000 | 1.1439 | 0.998 | 1.000 |
We remark that that, even though both ZWLS and ZPOIS are derived under different model assumptions, they are both valid tests for testing the equality of two APCs and hence the ARE defined in (8) is valid. The tables show that the ARE of ZPOIS with respect to ZWLS is greater than 1 for all the three cases, meaning that ZPOIS would be more powerful than ZWLS when the alternative hypothesis is true. The tables also show that in most situations ZPOIS outperforms the ZWLS in retaining the Type I error probabilities and, hence, yields a more valid test. Also the powers of both WLS and Poisson-based tests are sensitive to the delta values (the differences of APC values). The larger the delta values are, the more powerful the tests are. The larger delta values also lead to slightly larger AREs, though the differences are not so obvious.
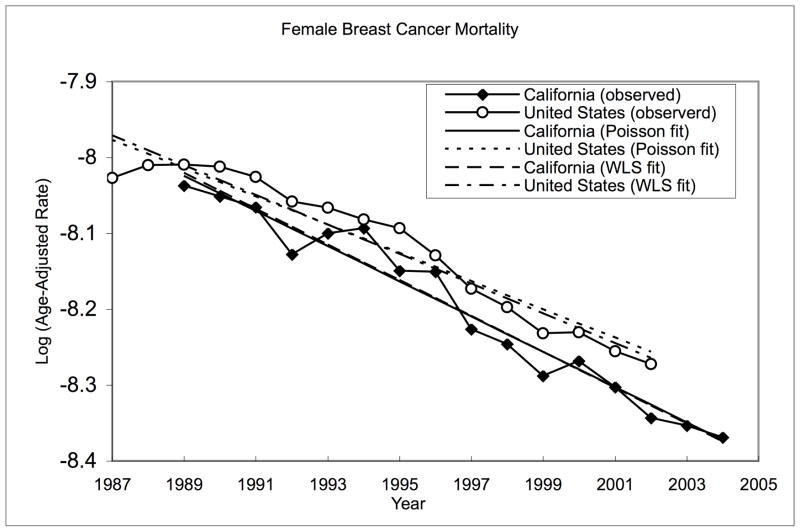
It is of substantial interest to compare the changes in cancer mortality rates in California with the national levels starting late 1980’s as a California law (Health and Safety Code, Section 103885) was passed then, which mandated the reporting of malignancies diagnosed throughout the state. For this purpose, we applied the proposed methodology to compare the annual percent change (APC) in the age-adjusted mortality rates for the United States (US) for the period from 1988–2002 to that of California (CA) for the period from 1990 to 2004. We fitted the weighted linear models as well as the age-stratified Poisson model, and applied both tests to compare the age-adjusted mortality rates of female breast cancer in CA for the 16-year period from 1989–2004 to that of US for the 16-year period from 1987–2002, for which the national mortality data were available. The observed values of the log-transformed annual age-adjusted rates and fitted regression lines from the ZPOIS test procedure are plotted in Figure 1. The parameter estimates and the values of the test statistics are summarized in Table 4. The results indicated the mortality rates of Breast cancer for California and the US have decreased. Both tests reject the null hypothesis of equality of the two APCs, indicating that the annual percent change (APC) of California, is significantly different from the national level. However, the p-value for ZPOIS is much smaller than that of ZWLS, rendering more evidence again the null hypothesis.
Fig. 1.
Observed and fitted log-transformed age-adjusted breast cancer mortality rates in CA [1989–2004] and US [1987–2002]
Table 4.
Comparing APC of Breast Cancer Mortality Between CA and the US
| CA | US | |
|---|---|---|
| APCWLS | −2.33 | −1.94 |
| SEWLS | 0.084 | 0.027 |
| ZWLS | −4.95 | (p=0.000000757) |
|
| ||
| APCPOIS | −2.29 | −1.84 |
| SEPOIS | 0.083 | 0.026 |
| ZPOIS | −5.62 | (p= 0.000000019593) |
5 Discussion
In this paper, we have considered an important problem where comparisons have to be made for regions or time intervals that overlap. As opposed to the existing work, e.g Li and Tiwari (2007) and Li et al. (2007), this project advances this area in two distinct ways. First, the developed test does not rely on the normal approximation of the cancer rate, but directly model the counts to follow a Poisson regression model. The parameters are then estimated using their maximum likelihood estimates, and the Z-test is derived for testing the equality of two APCs. Secondly, the developed Poisson regression model can easily accommodate 0 count data (for the rare cancers), as opposed to the log normal model (Li and Tiwari, 2007; Li et al., 2007) which needs to involve extra zero-corrected adjustment. We have applied the developed methodology to the analysis of the major cancer sites from the SEER Program and have found that the corrected Z-test renders more power than the existing tests. A Bayesian Poisson regression would be a useful approach. However, choice of priors is always difficult and computation may not be so straightforward compared to this current work, wherein analytical solutions have been derived. Hence, we envision that the proposed method would be preferable because of simple interpretation of the model parameters, natural choice of the model and computational readiness.
In our technical development, we have modelled the logarithm transformation of the age-adjusted rates as a linear regression on time in (5) and have indeed explicitly assumed parallelism across age groups. That is, the growth curves of the cancer rates for various age groups share the same slope, which carries the information for the APC. Indeed, linearity parallelism for the cancer rates could be a debatable issue in cancer surveillance, which is likely to be violated for some cancers. One alternative, along the line of generalized mixed models, is to assume a random slope (as opposed to a constant slope) across age groups. This ongoing work will be reported in a subsequent communication.
Table 2.
Comparison of the power functions between the Poisson based ZPOIS and the weighted-least-squares based ZWLS for two overlapping regions over roughly the same time intervals, Region 1 (1980–1989) vs Region 2 (1983–1992). APC1 and APC2 are the annual percent changes in Regions 1 and 2, respectively.
| Cancer Sites | APC1 | APC2 | ARE | ZWLS | ZPOIS |
|---|---|---|---|---|---|
| All Malignant | 0.100 | 0.100 | 1.1710 | 0.055 | 0.057 |
| −0.300 | −0.300 | 1.1710 | 0.056 | 0.057 | |
| 0.500 | 0.500 | 1.1711 | 0.048 | 0.050 | |
| 1.000 | 1.000 | 1.1712 | 0.047 | 0.049 | |
| 3.000 | 3.000 | 1.1723 | 0.051 | 0.056 | |
| 0.100 | 0.500 | 1.1726 | 0.872 | 0.908 | |
| −0.300 | 0.300 | 1.1729 | 0.994 | 0.997 | |
| 1.000 | 2.000 | 1.1733 | 1.000 | 1.000 | |
| 1.000 | 3.000 | 1.1737 | 1.000 | 1.000 | |
| Prostate | 0.100 | 0.100 | 1.1820 | 0.043 | 0.047 |
| −0.300 | −0.300 | 1.1821 | 0.043 | 0.043 | |
| 0.500 | 0.500 | 1.1822 | 0.046 | 0.041 | |
| 1.000 | 1.000 | 1.1823 | 0.035 | 0.053 | |
| 3.000 | 3.000 | 1.1823 | 0.034 | 0.055 | |
| 0.100 | 0.500 | 1.1825 | 0.170 | 0.197 | |
| −0.300 | 0.300 | 1.1827 | 0.341 | 0.393 | |
| 1.000 | 2.000 | 1.1832 | 0.742 | 0.802 | |
| 1.000 | 3.000 | 1.1835 | 1.000 | 1.000 |
Acknowledgments
The authors thank the editor, an AE and two referees for their insightful suggestion, which led to a better version of this manuscript.
Appendix A: Derivation of V̂, Ŵ, Σ̂
Write
where
Hence a consistent estimate is
Similarly,
where
Also, V22 = ((V22,jj′)) with V̂22,jj′= Ĉov (U01,j; U01,j′) = 0; j ≠ j′;
Next compute the estimate of W:
so that Similarly, Ŵ12 = (Ŵ12,1, …, Ŵ12,J) where . Also, Ŵ22 = ((Ŵ22,jj′)) with . Finally, the estimate of Σ is computed as follows:
where and the superscripts “NO” and “O” denote the non-overlapping and overlapping regions, respectively. Thus, . Let Σ̂12 = (Σ12,1, …, Σ̂12,J ) where . Also, Σ̂22 = ((Σ̂22,jj′)) with .
Appendix B: Computation of the MLEs
Note that the MLEs of β0jk and β1k satisfy:
where dkj· =Σi dkji, dk·i =Σj dkji, and for a = 0, 1, 2.
Since Ũ(β1k) is a monotonic function of β1k, there is a unique solution to this equation. Let β̂1k be the solution. This is the MLE of β1k. We can use a Newton-Raphson method to obtain β̂1k as follows. Let β̂1k(l) be the estimate at the lth iteration, then the estimate at the (l + 1)th iteration is given by β̂1k(l + 1) = β̂1k(l) − [Ũ(1)(β̂1k(l))]− 1Ũ(β̂1k(l)), where Ũ(1)(β) is the first (partial) derivative of Ũ(b) with respect to b evaluated at b = β. We stop iterating wh en |β̂1k(l + 1) − β̂1k(l)| < ε for some pre-specified value of ε. Note that
Substituting the MLE β̂1k in place for β1k in gives the MLE
References
- American Cancer Society. Cancer Facts & Figures. Atlanta: Georgia; 2007. [Google Scholar]
- Brillinger DR. The natural variability of vital rates and associated statistics (with discussion) Biometrics. 1986;42:693–734. [PubMed] [Google Scholar]
- Fay M, Tiwari R, Feuer E, Zou Z. Estimating Average Annual Percent Change for Disease Rates without Assuming Constant Change. Biometrics. 2006;62:847–854. doi: 10.1111/j.1541-0420.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Fay M, Feuer E, Midthune D. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in Medicine. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kleinbaum D, Kupper, Muller P. Applied Regression Analysis and Other Multivariable Methods. 2 PWS-Kent; Boston, Mass: 1988. [Google Scholar]
- Li Y, Tiwari R. Comparing Trends in Age-Adjusted Cancer Rates Across Overlapping Regions or Time Intervals for the NCI SEER Program. Technical Report. 2007 http://www.bepress.com/harvardbiostat/paper71.
- Li Y, Tiwari R, Walters K, Zou Z. A Weighted-Least-Squares Estimation Approach to Comparing Trends in Age-Adjusted Cancer Rates Across Overlapping Regions. Technical Report. 2007 http://biowww.dfci.harvard.edu/~yili/apc2.pdf. [PMC free article] [PubMed]
- Ries L, Eisner M, Kosary C, Hankey B, Miller B, Clegg L, Mariotto A, Feuer E, Edwards B. SEER Cancer Statistics Review, 1975–2002. National Cancer Institute; Bethesda, MD: 2003. http://seer.cancer.gov/csr/1975-2002/ [Google Scholar]
- Tiwari R, Clegg L, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Statistical Methods in Medical Research. 2006;15:547–569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]