Abstract
The field of cancer vaccines is currently in an active state of preclinical and clinical investigations. While no therapeutic cancer vaccine has to date been approved by the FDA, several new paradigms are emerging from recent clinical findings in both the use of combination therapy approaches and, perhaps more importantly, in clinical trial design and endpoint analyses. This paper will review recent clinical trials involving several different cancer vaccines from which data are emerging contrasting classical “tumor response” (RECIST) criteria with “patient response” in the manifestation of increased patient survival post-vaccine therapy. Also described are several strategies in which cancer vaccines can be exploited in combination with other agents and therapeutic modalities that are quite unique when compared with “conventional” combination therapies. This is most likely due to the phenomena that (a) cancer vaccines initiate a dynamic immune process that can be exploited in subsequent therapies, and (b) both radiation and certain chemotherapeutic agents have been shown to alter the phenotype of tumor cells as to render them more susceptible to T-cell–mediated killing. Consequently, evidence is emerging from several studies in which patient cohorts who first receive a cancer vaccine (as contrasted with control cohorts) benefit clinically from subsequent therapies.
Keywords: cancer vaccines, immunotherapy, combination therapy, prostate cancer
The field of cancer vaccines is currently in a state of active preclinical and clinical investigations. While no therapeutic cancer vaccine has been approved to date by the FDA, recent preclinical and clinical findings have demonstrated that appropriate clinical trial design and endpoints, and the use of vaccines in new paradigms of combination therapies may well lead to cancer vaccines ultimately being employed for the therapy of several cancer types.
Cancer vaccines differ from other therapies in that they initiate a dynamic process of activating the host’s own immune system. This process could potentially influence both how patient responses are evaluated, and how responses to subsequent therapies post-vaccination are evaluated.
Evaluation of Cancer Vaccines: New Paradigms for Responses to Therapy
It is proposed that cancer vaccines are a therapeutic modality where one should evaluate “patient response” more so than “tumor response.” The two phenomena are not always mutually inclusive. Standardization of response criteria is of course critical for any given clinical trial, but one must be aware that the use of only one criterion for all therapeutics, cancer types, and disease stages can be classic “paradigm paralysis.” Response Criteria In Solid Tumors (RECIST) (1, 2) has served the oncology community well in the evaluation of passive therapeutic modalities such as chemotherapeutic agents and radiation therapy. With the advent of new targeted therapies, including cancer vaccines, however, the sole use of RECIST criteria as a clinical endpoint has now been called into question by, among others, several Cooperative Groups (2–5). An excellent example of this has been in the evaluation of sorafenib in clinical trials of patients with advanced renal cell carcinoma. In a randomized, placebo controlled Phase III trial involving 903 patients, progression-free survival doubled from 12 to 24 weeks (p<0.00001) for patients on sorafenib. At 3 months, the response rate (PR) was 10% with <1% CR (1 of 451) using RECIST criteria (6). Another example of this phenomenon is in the evaluation of imatinib in patients with gastrointestinal stromal tumor (GIST) (7). The converse of this phenomenon is also evident as seen, for example, in a trial in patients with metastatic renal cell carcinoma. A total of 306 patients were randomly assigned to high vs. low dose IL-2 therapy. There was a higher response rate using RECIST criteria (with more toxicity) in the high dose (21%) vs. the low dose (13%) IL-2–treated cohorts (p=0.048). However, there was no statistical difference in overall survival between the two groups (8). It is clear from these results and others that RECIST criteria do not always adequately assess patients’ clinical benefit, i.e., survival and quality of life.
A study of contrasts
The paradigm shift in analysis of clinical benefit from immunotherapy is exemplified in comparing results employing vaccines vs. adoptive transfer of T cells (9). Dramatic tumor size reductions satisfying RECIST criteria have been observed in the elegant studies with adoptive transfer of T cells (10–12). Unfortunately, however, in the 20+ years of reporting these types of responses, no randomized trial has demonstrated a statistical advantage in survival in patients receiving adoptive transfer of T cells over that seen with IL-2 alone (13–15). In contrast, while few responses satisfying RECIST were seen in vaccine trials, this article will review several recent trials where advantage in survival is being observed. Other contrasts are the reduced toxicities seen in vaccine therapy plus the potential for combination therapy, as will be discussed below.
Vaccine Clinical Trials
A prior review (16) listed 21 clinical trials in which a range of different cancer vaccines provided some evidence of clinical benefit in different patient populations. This article will review more recent clinical findings employing five different types of vaccines in the therapy of prostate cancer patients. Prostate cancer is a disease well suited for the efficacy of vaccines for several reasons: (a) it is a relatively slow growing tumor, (b) recurrence is often diagnosed early in the disease state, (c) there is a surrogate marker for disease prognosis and outcome, i.e., serum PSA doubling time (17, 18), and (d) after definitive primary therapy (surgery and/or radiation), there are few existing standard of care therapies that achieve long-lasting therapeutic effects.
Cell-based vaccines
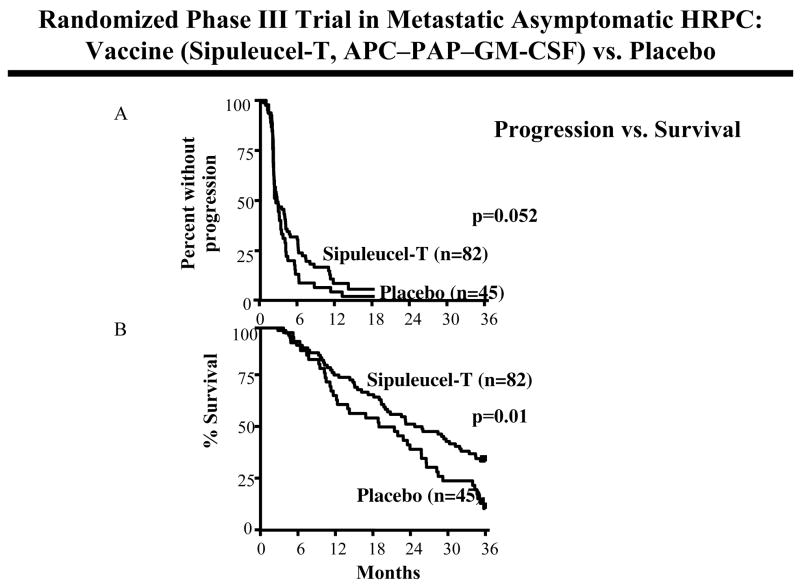
One of these prostate cancer vaccines is Sipuleucel-T (i.e., Provenge, Dendreon, Inc.), which consists of autologous antigen-presenting cells (APC) and a fusion protein composed of prostatic acid phosphatase (PAP) and GM-CSF (19). Early Phase I/II trials demonstrated increases in T-cell responses to the vaccine antigen, serum PSA declines in patients, and limited toxicity. A placebo controlled randomized Phase III trial in patients with metastatic asymptomatic androgen-independent prostate cancer (AIPC) using Sipuleucel-T has recently been reported (19). Patients were randomly assigned in a 2 to 1 ratio to receive vaccine (n = 82) or placebo (n = 45). The primary endpoint of this study, which was progression-free survival, did not achieve statistical significance (0.052) (Fig. 1A). Overall survival, however, was statistically significant (p=0.01, HR 1.70) between vaccine (25.9 months) vs. placebo (21.4 months) (Fig. 1B). A second randomized trial with Sipuleucel-T in this patient population showed a trend toward increased survival (19 months for vaccine vs. 15.7 months for placebo) that did not reach statistical significance. Thirty-six months survival was 32% for vaccine vs. 21% for placebo treated patients. The integrated analysis of both of these randomized trials, vaccine (n = 147) vs. placebo (n = 78), showed a statistically significant increase in overall survival (p=0.011, HR = 1.5) in vaccine-treated patients. Thirty-six months survival was 15% for placebo and 33% for vaccine. The survival advantages seen in these trials, as well as those described below, were obtained with little or no evidence of “objective” responses using RECIST criteria. A Phase III clinical trial employing the Sipuleucel-T vaccine is currently ongoing using survival as a primary endpoint.
Fig. 1.
Randomized, placebo controlled, Phase III trial of Sipuleucel-T vaccine (antigen-presenting cells with PAP-GM-CSF fusion protein) vs. placebo in patients with metastatic asymptomatic hormone refractory prostate cancer. (A) Time to progression, i.e., percent without progression. (B) Overall survival. Ref. (19).
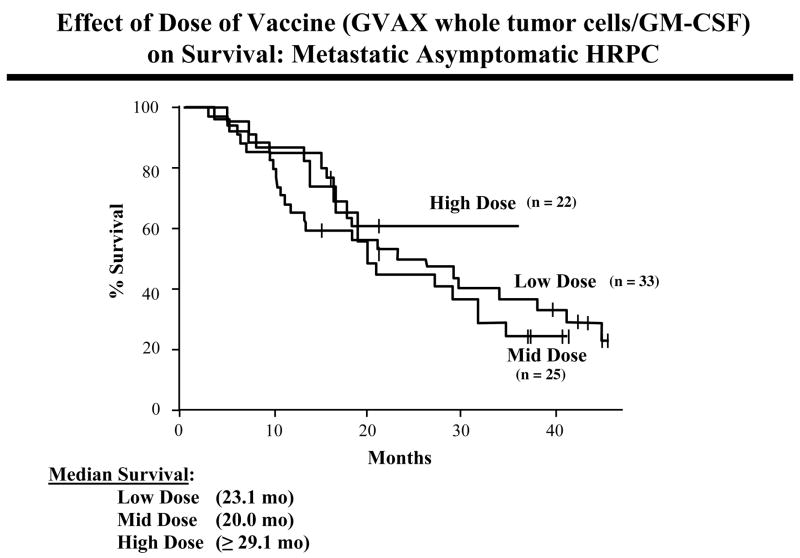
GVAX is another prostate cancer vaccine in advanced clinical trial testing. GVAX (Cell Genesys, Inc.) consists of two irradiated allogeneic prostate cancer cell lines engineered to secrete GM-CSF (20, 21). Two Phase II clinical studies have now been completed in patients with asymptomatic metastatic AIPC. In the first trial (n = 34), GVAX was given at two dose levels. Decreases in PSA velocity were seen in 67% of patients given low dose vaccine (n = 24) and in 90% of patients given high dose vaccine (n = 10). This correlated with survival results, with a median survival of 24.0 months in the low dose vaccine group and 34.9 months in the high dose vaccine group. A second Phase II trial in the same patient population (n = 80) employed five vaccine dose levels: two low dose (n = 33), one intermediate dose (n = 25), and two high dose cohorts (n = 22). The median survival of patients in the low dose and middle dose cohorts was 23.1 and 20.0 months, respectively. The median survival of the high dose cohorts has not yet been reached, but will be = 29.1 months (Fig. 2). Predicted survival was estimated for each patient using a nomogram; the median measured survival was greater than 6 months longer than the predicted survival for the high-dose cohorts. There were no dose limiting toxicities in either trial. These results have formed the basis for two ongoing Phase III trials, with overall survival as the primary endpoints.
Fig. 2.
Survival of patients with metastatic asymptomatic hormone refractory prostate cancer receiving low-, mid-, or high-dose GVAX whole tumor cell vaccine. Ref. (20, 21).
Another whole tumor cell vaccine for prostate cancer is also showing promising clinical results. Onyvax-P vaccine (Onyvax Limited) consists of three irradiated allogeneic prostate cell lines, the first two vaccinations given with BCG. A Phase II Onyvax-P trial has now been completed in AIPC patients (22). Eleven of 26 patients showed statistically significant prolonged decrease in their PSA velocity with no patient having a statistically significant increase in PSA velocity post-vaccination. Mean time to tumor progression was 58 weeks compared with recent studies of other agents and historical control values of approximately 28 weeks. Immunologic profiles by artificial neural network analysis of cytokines correlated with PSA velocity responses. A multi-center Phase IIb trial with Onyvax-P is currently under way.
Vector-based vaccines
There have also been several trials with poxviral vector–based vaccines with evidence of clinical benefit. Pox viruses (vaccinia (rV-), Modified Vaccinia Ankara (MVA), fowlpox (rF-)) have the ability to accept and express multiple transgenes and can thus be engineered to not only express tumor-associated antigens but also various immunostimulatory molecules. TG4010 (Transgene) is a recombinant MVA expressing both MUC-1 tumor antigen and IL-2. MVA is a replication incompetent vaccinia virus. A randomized Phase II study has been completed with MVA–MUC-1–IL-2 in prostate cancer patients with biochemical progression and no evidence of metastatic disease after local therapy (23). Patients were vaccinated every week for 6 weeks and then every 3 weeks in Arm I, and every 3 weeks in Arm II. Twenty-seven of 38 (71%) patients had lengthened PSA doubling time after vaccination. A statistically significant increase (p<0.001) in PSA doubling time (mean increase 3.8-fold) was observed in Arm I, providing evidence that vaccine dose scheduling can be an important variable.
In a Phase I study with recombinant vaccinia in prostate cancer patients, rV-PSA was administered to 33 patients with biochemical progression after local therapy or with metastatic disease (24). PSA levels in 13/33 men became stable for at least 6 months post-vaccination. Nine patients remained stable for 11–25 months and six remained progression free with stable PSA. At the time of publication, several patients remained without evidence of clinical progression for up to 21 months. An NCI-sponsored ECOG randomized Phase II trial was then carried out using two different PSA pox vectors in different prime/boost regimens: rV-PSA(V) and/or rF-PSA(F) in patients (n = 64) with biochemical progression after local therapy for prostate cancer (25). At the 2-year follow-up (26), median time to PSA and/or clinical progression was 9.2 months in the FFFF cohort, 9.0 months in the FFFV cohort, and 18 months in the VFFF cohort. The NCI has now developed rV- and rF- vectors containing the transgenes for PSA and three human costimulatory molecules (B7.1, ICAM-1 and LFA-3, designated TRICOM) (27). Recent Phase I/II trials in patients with metastatic and locally advanced prostate cancer have shown clinical responses and drops in serum PSA (28). A company-sponsored multi-center randomized Phase II study in 125 patients with metastatic androgen-independent asymptomatic prostate cancer did not meet its primary endpoint of progression-free survival (29). Patients’ overall survival data are currently being accumulated, with provocative results. Median overall survival thus far is 16.3 months for the control cohort (wild-type vector) (n = 41) vs. 24.4 months for those patients receiving PSA-TRICOM vaccines (n = 84) (Fig. 3).
Fig. 3.
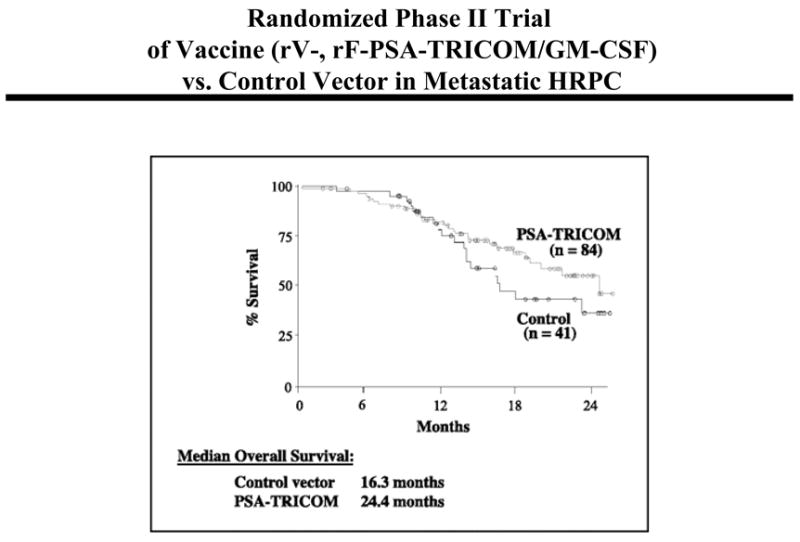
Randomized Phase II study in patients with metastatic androgen-independent asymptomatic prostate cancer receiving PSA-TRICOM vaccines (n = 84) versus control fowlpox vector (n = 41). Ref. (29).
This article has detailed vaccine trials in prostate cancer as one example of the progress being made in clinical vaccine therapy. Ongoing progress in pancreatic cancer, lymphoma, melanoma, lung cancer and other tumor types are also providing evidence in clinical trials of vaccine efficacy.
Separating Vaccine Efficacy from Poor Clinical Trial Design
There is clinical evidence that the ability to mount an immune response to vaccine can be altered by prior chemotherapy. Studies in patients with metastatic cancer demonstrated (30) that there was a negative correlation between the number of previous chemotherapy regimens and the magnitude of T-cell response to vaccine (p = 0.032). This same study also showed a positive correlation between the magnitude of a T-cell response to vaccine and time since last chemotherapy regimen (p = 0.005). Thus, patients who had received more cycles of prior chemotherapy, or who had received chemotherapy more recently, were shown to mount less effective immune responses to vaccine.
New Paradigms for Combination Therapies
There are five different strategies of combination therapies that can and are being used with cancer vaccines. All have been validated in preclinical models and several have provided preliminary evidence of clinical benefit. As the field matures, progress in this area will undoubtedly be accelerated.
(a) Conventional combination therapy
In many cases of combination therapies using two or more chemotherapeutic agents or a chemotherapeutic agent and a targeted therapy (e.g., herceptin and docetaxel), each agent works individually with the goal of additive anti-tumor effects. This too has been shown in numerous preclinical models using vaccines in combination with chemotherapeutic agents. Both preclinical and early clinical studies have highlighted the following important phenomena: while vaccines are less effective in patients heavily pre-treated with chemotherapy prior to vaccine, no detrimental effects in immune responses to vaccines have been seen in patients when vaccine is given in combination with certain chemotherapeutic agents such as 5FU and docetaxel (31, 32). For example, in preclinical studies, it has even been shown that the COX-2 inhibitor celecoxib, an established anti-inflammatory, had no adverse effect on immune responses to vaccine and worked well in combination with vaccine to enhance anti-tumor effects (33).
(b) Vaccine in combination with agents that affect the host immune system
There now exist a plethora of reagents that can be employed in combination with vaccines that either act as immune stimulants/adjuvants or inhibitors of immune regulatory cells or molecules. This phenomenon has been shown in multiple preclinical models. A major issue at this time, however, is that only a few of these agents have been approved by the FDA; sadly, there is still hesitancy on the part of many companies to employ proprietary agents of another company to enhance their agent’s efficacy.
Cytokines are well established for their ability to enhance immune response (see (16) for review). As examples: GM-CSF (FDA approved) has now been demonstrated in numerous clinical trials including several described above to enhance vaccine efficacy. IL-2 may not be as useful as thought due to its toxicity, its ability to induce apoptosis in activated T cells, and/or its ability to enhance regulatory T-cell activity. IL-15 and IL-7 both have the potential to be useful with vaccines in enhancing memory T-cell responses. Other immune stimulants, such as BCG, CpG motifs, and Aldara, are also currently being employed clinically with vaccines (see (16) for review).
One agent that is showing promise in patients with melanoma, ovarian cancer and prostate cancer is the monoclonal antibody anti-CTLA-4 (34–37). While the exact mechanism by which this agent works with vaccine has never been shown clinically, preclinical studies have clearly shown that anti-CTLA-4 renders higher avidity antigen-specific T cells when employed with vaccines (38).
Still another paradigm to be exploited in the therapy of prostate cancer is the phenomenon that androgen deprivation therapy (ADT) can enhance thymic regeneration. An elegant clinical study (39) has shown that biopsy samples of patients’ prostates, post- vs. pre-ADT, have a substantial increase in CD3 T cells infiltrating their prostate. This can also be exploited in future vaccine/ADT combination approaches.
It has become apparent from numerous preclinical studies and recent clinical studies that the control of immune inhibitory entities will play an important role in vaccine-mediated therapies. Preclinical and clinical studies have shown that the use of Ontak, a fusion protein consisting of diphtheria endotoxin and IL-2, can kill CD4/CD25/FOXP3 regulatory T cells (T regs) and enhance vaccine efficacy in inducing greater T-cell responses (40). The chemotherapeutic agent Cytoxan (cyclophosphamide) has been shown in preclinical studies to enhance vaccine efficacy. Cyclophosphamide not only reduces the number of T regs but also their functionality (41, 42). As the field matures, clinical application of agents that inhibit immunosuppressive molecules such as TGF-β and IL-10 will more likely also add to vaccine effectiveness.
(c) Multiple vaccine therapies
This approach may ultimately prove advantageous because (i) different types of vaccines can augment different arms of the immune system, (ii) each vaccine can carry different tumor-associated antigens, and (iii) limited toxicities have been associated with vaccine therapy. Efficacy of this approach in clinical trials has been observed in cancer patients who have received a recombinant (r) rV-prime (V) and multiple fowlpox (F) boosts (25, 26). As the field matures, it is anticipated that more diverse vaccine combinations will be employed.
(d) Dose scheduling of vaccine with other therapies
Perhaps the most unique feature of cancer vaccine therapy is the fact that a vaccine initiates a dynamic process of host immune responses that may be exploited in subsequent therapies. There are now several clinical studies that have provided evidence of this phenomenon.
In a Phase I study, 17 patients with advanced stage progressive cancer received a plasmid/microparticle vaccine directed against cytochrome P4501B1. Most patients who developed immunity to vaccine, but required salvage therapy upon progression, showed marked responses to their next treatment regimen, most of which lasted longer than 1 year (43). In another study (44), 29 patients with extensive stage small cell lung cancer received an adeno-p53 vaccine. A high rate (61.9%) of objective clinical responses was observed to chemotherapy that immediately followed vaccine therapy, and these clinical responses were also closely associated with induction or augmentation of immune response to vaccine.
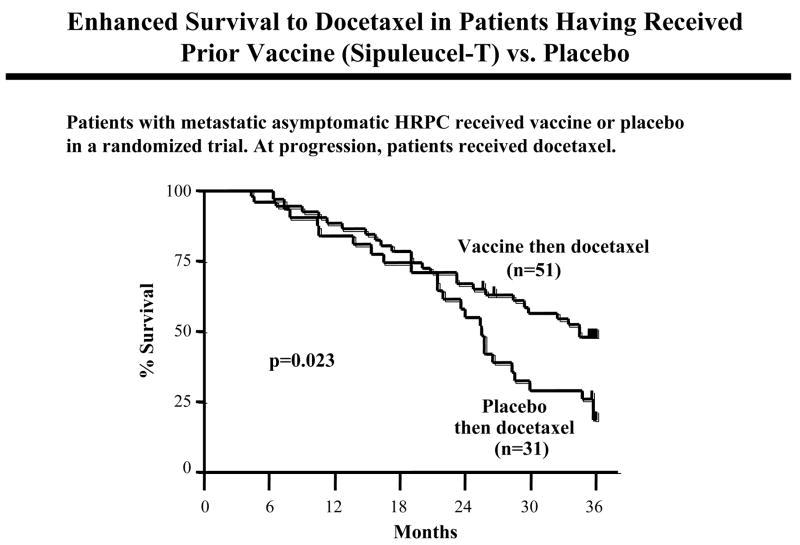
Three randomized clinical trials in prostate cancer also provided evidence of this phenomenon. The NCI has now completed studies with a diversified prime-boost strategy involving priming with rV-PSA + rV-B7.1 followed by rF-PSA booster vaccinations. In the first trial, 28 patients with metastatic AIPC were randomized to receive vaccine alone or vaccine plus weekly docetaxel (31). Patients on the vaccine arm alone were allowed to cross over to receive docetaxel at time of progression. After vaccine, median progression-free survival on docetaxel was 6.1 months compared with a progression-free survival of 3.7 months with the same docetaxel regimen and patient population at the same institution. Similar findings were observed employing the Sipuleucel vaccine (45). In the randomized multi-center Sipuleucel study described above, patients in both the vaccine arm (n = 51) or placebo arm (n = 31) went on to receive docetaxel at progression. There was a striking and statistically significant (p=0.023 HR=1.90) increase in overall survival with docetaxel treatment in patients having had prior vaccine vs. placebo (Fig. 4).
Fig. 4.
Enhanced survival to docetaxel in patients having received prior vaccine. Overall survival in a randomized trial of patients with metastatic asymptomatic hormone refractory prostate cancer receiving Sipuleucel-T vaccine or placebo. At progression, patients went on to receive docetaxel. There was a statistically significant increase in survival (p=0.023, HR 1.9) when patients received prior vaccine. Ref. (45).
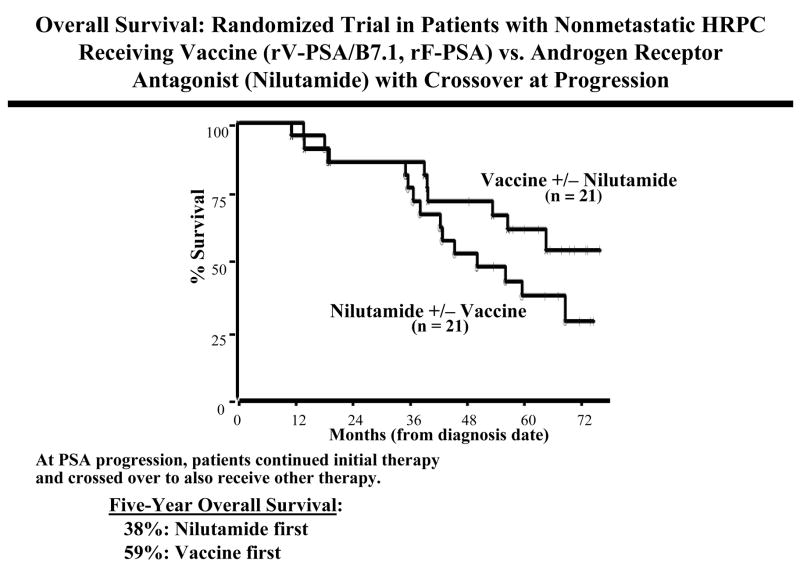
In another Phase II trial at the NCI (46), 42 patients with nonmetastatic AIPC and rising serum PSA were randomized to receive either vaccine (rV-PSA/B7.1 prime and rF-PSA boosts) or nilutamide, an androgen-receptor antagonist (ARA). After 6 months, patients with a rising PSA were allowed to “cross over” and receive a combination of both therapies. Median time to treatment failure was similar in the vaccine (9.9 months) and ARA (7.6 months) arms. However, for the patients who first received vaccine and then went on to receive vaccine plus ARA, time to treatment failure was 13.9 months from the time of initiation of ARA, and the time to treatment failure from the initiation of any therapy was 25.9 months. Of the initial randomized population (n = 21/cohort), for those patients who received nilutamide first (nilutamide alone or nilutamide then vaccine), 5-year overall survival was 38%, vs. a median overall survival of 59% for those patients who received vaccine first (vaccine alone or nilutamide plus vaccine) (47) (Fig. 5).
Fig. 5.
Overall survival in a randomized trial of patients with hormone refractory prostate cancer receiving vaccine (rV-PSA + rV-B7.1 prime, rF-PSA boosts, n = 21) vs. nilutamide therapy (n = 21) with patients “crossing over” to receive both therapies at progression. Ref. (46,47).
All of the above trials have provided evidence of the same phenomenon: patients who receive vaccine (and mount immune responses to vaccine if monitored) have enhanced outcome to subsequent therapies. This is unlikely due to patient population selection since three of these trials described were randomized. This phenomenon may be due to one or more factors: the subsequent therapy (a) may be reducing suppressor cell populations, allowing for enhancement of prior established T-cell responses, (b) may be lysing some tumor cells that are then, as a consequence of cross priming, activating relatively dormant T cells to elicit an anti-tumor response, (c) may enhance host T-cell activity, and/or (d) may alter the phenotype of tumor cells (see below).
(e) Phenotype alterations in tumor cells
Still another new paradigm to exploit in vaccine combination therapies is the phenomenon that certain standard of care therapeutics can actually alter the phenotype of tumor cells to render them more susceptible to T-cell–mediated lysis. This has been shown in a series of preclinical studies (48, 49). Sublethal doses of radiation delivered via external beam have been shown to upregulate tumor-associated antigens, fas, and/or adhesion molecules, and/or downregulate anti-apoptotic genes, subsequently rendering these phenotypically altered tumor cells more susceptible to antigen-specific T-cell–mediated lysis. Chemotherapeutic agents such as 5FU (50), cisplatnin and gemcitabine (48) have also been employed in sublethal doses inducing similar alterations of tumor-cell phenotype and subsequent susceptibility to T-cell–mediated lysis. This may ultimately lead to another paradigm shift in vaccine combination therapies, i.e., when a patient does not respond to a drug or radiation therapy due to its lack of ability to lyse tumor cells, it may continue to be used with vaccine therapy due to its ability to augment vaccine-induced T-cell lysis of tumor.
Immune Responses
Several clinical studies have reported statistical correlations between antigen-specific immune responses to vaccine and patient benefit, while others have not. These findings may be confounded by several phenomena: (a) the vast majority of studies have examined only T-cell or antibody responses in blood, which may not always correlate with their presence in tumor, and this may vary with tumor size, vasculature, etc., (b) few studies, if any, have taken into consideration the presence of regulatory T cells and/or have analyzed multiple immune cell subsets, e.g., CD4, CD8, NK responses from a given patient population, (c) virtually all studies have measured the level of antigen-specific T cells, but few studies have monitored avidity of antigen-specific T-cell subsets (51), which is, perhaps, the most important parameter to measure, and (d) it has become apparent from preclinical studies that the more important antigen-specific T-cell subsets to monitor may not be those directed to the antigen in the vaccine. As a consequence of initial tumor-cell disruption by vaccine-induced cytolytic T cells, cross priming will lead to the generation of T cells directed against other tumor-associated antigens. Preclinical studies (49) have shown that these “antigen cascade” T cells can be of greater magnitude and greater avidity than those directed against the antigen in the vaccine, and are those that are principally responsible for tumor cure. Clinical studies (52–54) have also shown this phenomenon of “antigen cascade.”
New Vaccine Strategies, New Targets, New Paradigms
This article has reviewed only a few of the vaccine vehicles that are currently being employed with evidence of clinical benefit: allogeneic whole tumor cells, peptide- or protein-pulsed antigen-presenting cells (including dendritic cells), recombinant DNA and viral vectors, and recombinant Saccharomyces (yeast) are all currently in active clinical trial development. Moreover, there are a plethora of newly defined potential tumor-associated targets that are ripe for cancer vaccine development, including those involved in the neoplastic and/or tumor progression processes. As the field of cancer vaccine therapy matures, long-term safety profiles of several of these agents will most likely be realized. At that juncture, vaccines may well also be employed in neoadjuvant settings, and in certain preneoplastic conditions.
Skepticism is an important component of the scientific process and it should be an integral component in the development of any potential new therapy. Many are very much aware, for instance, of those skeptics and “naysayers” who, for a decade, dismissed monoclonal antibody-mediated cancer therapy. (There are now seven monoclonals approved for cancer management.) This too may well be the case for cancer vaccines. While skepticism is important, there are also those who realize the need for paradigm shifts in both exploiting vaccine combination therapy and analysis of patient benefit in terms of survival (with minimal toxicity) as the appropriate clinical trial endpoint.
Acknowledgments
The authors thank Debra Weingarten for her assistance in the preparation of this manuscript.
References
- 1.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 2.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–9. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Gore ME, Escudier B. Emerging efficacy endpoints for targeted therapies in advanced renal cell carcinoma. Oncology (Williston Park, NY. 2006;20:19–24. [PubMed] [Google Scholar]
- 4.Hoos A, Parmiani G, Hege K, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30:1–15. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- 5.Tuma RS. Sometimes size doesn’t matter: reevaluating RECIST and tumor response rate endpoints. J Natl Cancer Inst. 2006;98:1272–4. doi: 10.1093/jnci/djj403. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. The New England journal of medicine. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 7.Goodman VL, Rock EP, Dagher R, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13:1367–73. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 8.Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–32. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. The New England journal of medicine. 1987;316:889–97. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 13.Dillman RO. Lymphocyte therapy of renal cell carcinoma. Expert review of anticancer therapy. 2005;5:1041–51. doi: 10.1586/14737140.5.6.1041. [DOI] [PubMed] [Google Scholar]
- 14.Figlin RA, Thompson JA, Bukowski RM, et al. Multicenter, randomized, phase III trial of CD8(+) tumor-infiltrating lymphocytes in combination with recombinant interleukin-2 in metastatic renal cell carcinoma. J Clin Oncol. 1999;17:2521–9. doi: 10.1200/JCO.1999.17.8.2521. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg SA, Lotze MT, Yang JC, et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst. 1993;85:622–32. doi: 10.1093/jnci/85.8.622. [DOI] [PubMed] [Google Scholar]
- 16.Hodge JW, Schlom J, Abrams SI. Vaccines and Immunostimulants. In: Kufe DW, et al., editors. Holland-Frei Cancer Medicine 7. Hamilton, Ont.: BC Decker; 2006. pp. 786–801. [Google Scholar]
- 17.Arlen PMBF, Dahut WL, et al. Prostate-Specific Antigen Working Group’s guidelines on PSA doubling time. Manuscript submitted to J Clin Oncol. 2007 [Google Scholar]
- 18.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–90. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 19.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–94. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 20.Simons JHC, Smith D, Corman J, Steidle C, Gittelman M, et al. Clinical and immunologic findings in a phase 2 study of a GM-CSF-secreting prostate cancer cell line vaccine in patients with metastatic hormone-refractory prostate cancer (met HPRC). J Clin Oncol 2005; ASCO Annual Meeting Proceedings 2005; abstract 2517. [Google Scholar]
- 21.Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urologic oncology. 2006;24:419–24. doi: 10.1016/j.urolonc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Michael A, Ball G, Quatan N, et al. Delayed disease progression after allogeneic cell vaccination in hormone-resistant prostate cancer and correlation with immunologic variables. Clin Cancer Res. 2005;11:4469–78. doi: 10.1158/1078-0432.CCR-04-2337. [DOI] [PubMed] [Google Scholar]
- 23.Dreicer RAR, Pantuck A, Stadler WM, Bizouarne N, Acres B, et al. ASCO Annual Meeting Proceedings 2005. Vaccine immunotherapy with MVA-Muc1-IL2 (TG4010) in prostate cancer patients with biochemical failure. J Clin Oncol. 2005:23. abstract 4518. [Google Scholar]
- 24.Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–8. [PubMed] [Google Scholar]
- 25.Kaufman HL, Wang W, Manola J, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–32. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman HLWW, Manola J, DiPaola RS, Ko YJ, Sweeny C, et al. Phase II prime/boost vaccination using poxviruses expressing PSA in hormone dependent prostate cancer: Follow-up clinical results from ECOG 7897. ASCO Annual Meeting Proceedings 2005, abstract 4501. J Clin Oncol. 2005;23 [Google Scholar]
- 27.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–7. [PubMed] [Google Scholar]
- 28.Todd NJ, Dahut W, Schlom J, Arlen P. A phase II study of PROSTVAC-VF vaccine, and the role of GM-CSF, in patients (pts) with metastatic androgen insensitive prostate cancer (AIPC) [abstract] J Clin Oncol. 2005;23(16S Pt 1):2504. [Google Scholar]
- 29.Kantoff PWGL, Tannenbaum SI, Bilhartz DL, Pittman WG, Schuetz TJ. Randomized, double-blind, vector-controlled study of targeted immunotherapy in patients (pts) with hormone-refractory prostate cancer (HRPC). 2006 ASCO Annual Meeting Proceedings, Part I, abstract 2501. J Clin Oncol. :24. [Google Scholar]
- 30.von Mehren M, Arlen P, Gulley J, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7. 1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7:1181–91. [PubMed] [Google Scholar]
- 31.Arlen PM, Gulley JL, Parker C, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–9. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foon KA, John WJ, Chakraborty M, et al. Clinical and immune responses in resected colon cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. J Clin Oncol. 1999;17:2889–5. doi: 10.1200/JCO.1999.17.9.2889. [DOI] [PubMed] [Google Scholar]
- 33.Zeytin HE, Patel AC, Rogers CJ, et al. Combination of a poxvirus-based vaccine with a cyclooxygenase-2 inhibitor (celecoxib) elicits antitumor immunity and long-term survival in CEA. Tg/MIN mice Cancer Res. 2004;64:3668–78. doi: 10.1158/0008-5472.CAN-03-3878. [DOI] [PubMed] [Google Scholar]
- 34.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–8. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 35.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson RH, Allison JP, Kwon ED. Anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) immunotherapy for the treatment of prostate cancer. Urologic oncology. 2006;24:442–7. doi: 10.1016/j.urolonc.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulley JL, Dahut WL. Future directions in tumor immunotherapy: CTLA4 blockade. Nature clinical practice. 2007;4:136–7. doi: 10.1038/ncponc0749. [DOI] [PubMed] [Google Scholar]
- 38.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174:5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–70. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 42.Machiels JP, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor- secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–97. [PubMed] [Google Scholar]
- 43.Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11:4430–6. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- 44.Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–87. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 45.Petrylak D. Defining the optimal role of immunotherapy and chemotherapy: Advanced prostate cancer patients who receive sipuleucel-T (PROVENGE) followed by docetaxel derive greatest survival benefit. 14th Annual Meeting of the Chemotherapy Foundation Symposium; New York, New York. November 8–11, 2006. [Google Scholar]
- 46.Arlen PM, Gulley JL, Todd N, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–46. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 47.Madan R, Gulley J, Dahut W, Steinberg S, Liewehr D, Schlom J. 5-year overall survival (OS) in non-metastatic androgen independent prostate cancer (AIPC) patients (pts) treated with nilutamide (N), vaccine (V), and combination therapy [abstract]. 2007 ASCO Prostate Cancer Symposium Program/Proceedings; 2007. p. 400. [Google Scholar]
- 48.Gelbard A, Garnett CT, Abrams SI, et al. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res. 2006;12:1897–905. doi: 10.1158/1078-0432.CCR-05-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 50.Aquino A, Prete SP, Guadagni F, et al. Effect of 5-fluorouracil on carcinoembryonic antigen expression and shedding at clonal level in colon cancer cells. Anticancer Res. 2000;20:3475–84. [PubMed] [Google Scholar]
- 51.Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 52.Cavacini LA, Duval M, Eder JP, Posner MR. Evidence of determinant spreading in the antibody responses to prostate cell surface antigens in patients immunized with prostate-specific antigen. Clin Cancer Res. 2002;8:368–73. [PubMed] [Google Scholar]
- 53.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–62. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 54.Butterfield LH, Ribas A, Dissette VB, et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res. 2003;9:998–1008. [PubMed] [Google Scholar]