Abstract
Vasopressin (VP) secreted from parvocellular neurons of the hypothalamic paraventricular nucleus (PVN) stimulates pituitary ACTH secretion, through interaction with receptors of the V1b subtype (V1bR) in the pituitary corticotroph, mainly by potentiating the stimulatory effects of corticotrophin releasing hormone (CRH). Chronic stress paradigms associated with corticotroph hyperresponsiveness lead to preferential expression of hypothalamic VP over CRH and upregulation of pituitary V1bR, suggesting that VP has a primary role during adaptation of the hypothalamic pituitary adrenal (HPA) axis to long-term stimulation. However, studies using pharmacological of genetic ablation of V1b receptors have shown that VP is required for full ACTH responses to some stressors, but not for the sensitization of ACTH responses to a novel stress observed during chronic stress. Studies using minipump infusion of a peptide V1 antagonist in long-term adrenalectomized rats have revealed that VP mediates proliferative responses in the pituitary. Nevertheless, only a minor proportion of cells undergoing mitogenesis co-express markers for differentiated corticotrophs or precursors, suggesting that new corticotrophs are recruited from yet undifferentiated cells. The overall evidence supports a limited role of VP regulating acute ACTH responses to some acute stressors and points to cell proliferation and pituitary remodeling as alternative roles for the marked increases in parvocellular vasopressinergic activity during prolonged activation of the HPA axis.
Keywords: vasopressin, corticotrophin releasing hormone, ACTH secretion, hypothalamic paraventricular nucleus, stress, adrenalectomy, pituitary mitogenesis
INTRODUCTION
The nonapeptide vasopressin (VP) has been long recognized as a regulator of pituitary ACTH secretion (Baertschi et al., 1980; Buckingham, 1981; Chateau et al., 1979; McCann & Brobeck, 1954; Yasuda et al., 1978). Being a weak stimulator on its own, VP can act as an important modulator of ACTH responses to stress by potentiating the stimulatory effect of the major regulator, corticotrophin releasing hormone (CRH) (Antoni et al., 1983; Buckingham, 1982; Gillies et al., 1982). VP is produced by neurons of the hypothalamic paraventricular (PVN) and supraoptic nuclei (SON), which are organized into two major systems; a) the magnocellular system secreting VP into the peripheral circulation from axon terminals in the neural lobe of the pituitary, and b) the parvocellular system, with axons projecting to the external zone of the median eminence from where VP is secreted into the pituitary portal circulation (Antoni, 1993). VP of magnocellular origin is responsible for water conservation in the kidney, and regulation of its secretion depends upon osmotic stimulation (Leng et al., 1999; Stricker & Sved, 2002). On the other hand, parvocellular VP expression and secretion is independent of the osmotic status and increases during stress (Aguilera & Rabadan-Diehl, 2000; Antoni, 1993; de Goeij et al., 1991; Ma et al., 1997).
The actions of vasopressin are mediated by plasma membrane receptors belonging to the guanyl nucleotide binding protein (G-protein) family (Jard et al., 1987; Lolait et al., 1995a). The receptor present in pituitary corticotrophs is the V1b receptor subtype, which is coupled to Gq/11 and phospholipase C, leading to activation of protein kinase C and increases in cytosolic calcium (Jard et al., 1987; Thibonnier et al., 1997). Stress, and in particular chronic stress, increases not only VP expression in parvocellular neurons but also the content of V1b receptor mRNA and VP binding levels in the pituitary (Aguilera et al., 1994; Aguilera & Rabadan-Diehl, 2000; Ma et al., 1997). This contrast with the less pronounced or transient increases in CRH expression and the down-regulation of CRH receptor binding observed in repeatedly stressed rats (Aguilera, 1998). This shift in favor of a preferential activation of VP rather than CRH has lead to the proposal that VP becomes the major regulator of ACTH release during chronic stress.
This article will first present evidence supporting the prominence of the vasopressinergic system over CRH during chronic stress, and then discuss the current knowledge on the physiological importance of VP on the control of pituitary function during prolonged activation of the HPA axis.
Preferential vasopressin expression during chronic stress
Acute stress leads to rapid release of CRH and VP into the pituitary portal circulation from parvocellular neurons of the PVN (Berkenbosch et al., 1989; Kovacs & Sawchenko, 1996; Plotsky, 1988). Immunohistochemical studies have shown that in chronic somatosensory stress paradigms associated with hyperresponsiveness of the HPA axis to a novel stress, CRH stores remain unchanged but there is a progressive increase in VP stores as well as the number of CRH nerve endings containing VP (de Goeij et al., 1991). In contrast, during osmotic stimulation, a paradigm associated with an attenuated HPA axis response to stress, VP immunoreactivity in parvocellular terminals in the median eminence remains unchanged (Aguilera & Rabadan-Diehl, 2000; Grinevich et al., 2001). This correlation between CRH and VP content in parvocellular terminals and HPA axis responsiveness to stress can also be seen at the level of transcriptional regulation of the peptides.
The main determinant controlling ACTH secretion is the secretion of the stimulants CRH and VP from parvocellular terminals into the pituitary-portal circulation (Antoni, 1993; Whitnall, 1993). The procedures to collect portal blood for analysis of CRH and VP present some technical challenges, especially in rodents, in which the use of anesthetics and problems with possible contamination of portal blood with peptides in the pituitary stalk make data on the effects of stress difficult to interpret (Antoni et al., 1990; Plotsky, 1988; Tannahill et al., 1991). Studies in sheep and horse have shown rapid and equal elevations in CRH and VP in the pituitary portal circulation following stress (Alexander et al., 1994; Alexander et al., 1997; Battaglia et al., 1998; Engler et al., 1989). The same studies in sheep show that some stressors, such as ketamine anesthesia, cause selective increases in VP (Engler et al., 1989). However, no information is available on changes in immunoreactive peptides in the pituitary portal circulation during chronic stress. Studies in rats have shown that changes in CRH and VP transcription in paraventricular parvocellular neurons are rapid and follow closely the activation of CRH neurons during stress (Kovacs & Sawchenko, 1996; Ma et al., 1999; Ma & Aguilera, 1999). The use of intronic in situ hybridization techniques to measure the nascent transcript or heteronuclear RNA (hnRNA) has facilitated studies on the effects of acute and chronic stress on CRH and VP transcription (Aguilera, 1998; Herman et al., 1991). These studies have revealed concomitant activation of CRH and VP transcription but with different temporal patterns; while the increases in CRH expression, assessed as changes in primary transcript or heteronuclear RNA (hnRNA), are transient and correlate with the stimulation of ACTH secretion, stimulation of VP expression is delayed and more prolonged (Aguilera, 1998). While in stress paradigms with sustained ACTH responses to the repeated stimulus (ip hypertonic saline injection, foot shock) activation of CRH transcription is sustained during repeated stimulation, in paradigms with habituation of ACTH responses (repeated restraint, cold, repeated immune challenge) CRH transcription responses decrease progressively with repeated exposure to the homotypic stressor. As shown in Fig 1, CRH hnRNA levels are almost undetectable in both naïve rats and in rats subjected to repeated restraint (1h for 14 days). In naïve rats 1 h restraint causes a marked increase in hnRNA levels but responses are absent in repeatedly restrained rats. This parallel pattern of activation of CRH transcription (and possibly secretion) and ACTH responsiveness supports a major role for CRH on acute stress responses. In contrast, VP hnRNA and mRNA responses to a repeated homotypic stress are preserved or increased, even when there is attenuation of CRH responses, such during repeated restraint (Fig 2) (Aguilera, 1998). The mechanism of the differential transcription responses of CRH and VP within the same cell remains to be elucidated but it is likely to involve selective interaction of transcription factors with responding elements in the CRH and VP promoters.
Figure 1.
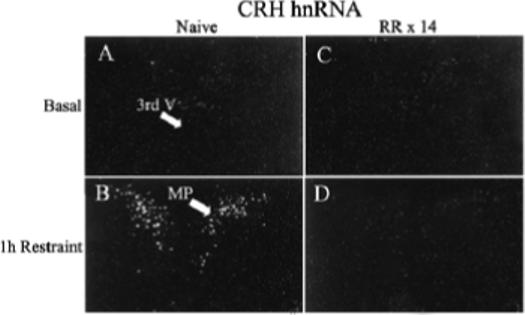
Effect of acute restraint stress for 1 h on CRH hnRNA levels in the PVN of naive rats (B) or 24 h after the last restraint in rats subjected to 1h repeated restraint for 14 days (D). Basal CRH hnRNA were almost undetectable in both naïve rats (A) and repeatedly restrained rats 24 h after the last episode (C). Darkfield photographs were taken through the medial parvocellular subdivision of the PVN (MP). 3rd V, Third ventricle; RR × 14, repeated restraint, 1h daily for 14 days.
Figure 2.
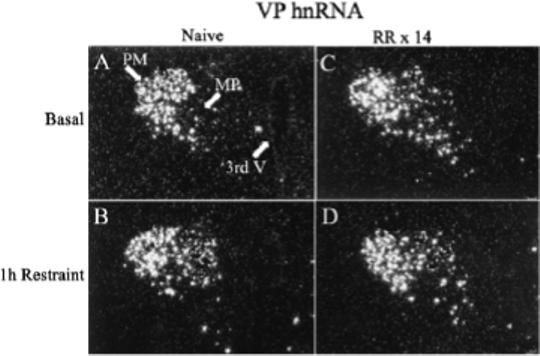
Effect of acute restraint stress for 1 h on VP hnRNA levels in the PVN of naive rats (B) or 24 h after the last restraint in rats subjected to 1h repeated restraint for 14 days (D). Basal VP hnRNA levels were undetectable in the parvocellular region of naïve rats (A), while they were markedly elevated 24 hr after the last stress in repeatedly restrained rats (C). Darkfield photographs of emulsion dipped slides were taken through the medial parvocellular subdivision of the PVN (MP). 3rd V, Third ventricle; PM, posterior magnocellular; MP, medial parvocellular; RR × 14, repeated restraint, 1h daily for 14 days.
Differential regulation of pituitary CRH and VP V1b receptors during stress
Studies in the rat have shown a good correlation between changes in pituitary VP binding levels and pituitary ACTH responsiveness (Aguilera et al., 1994). For example, reduced ACTH responses during chronic osmotic stimulation are associated with pituitary VP receptor downregulation, whereas somatosensory stressors leading to ACTH hyperresponsiveness to a novel stress (repeated immobilization or repeated i.p. hypertonic saline injections, a painful stress with an osmotic component) are associated with VP receptor up-regulation (Aguilera, 1994). In general, changes in VP receptors reflect changes in the number of binding sites with no significant alteration in binding affinity (Aguilera et al., 1994).
In contrast to VP receptors, there is a poor correlation between pituitary responsiveness and the number of CRH receptors in the anterior pituitary (Aguilera, 1998). In this regard, following adrenalectomy and chronic stress there is marked down-regulation and desensitization of pituitary CRH receptors, with decreases in both receptor number and CRH-stimulated adenylate cyclase (Aguilera, 1998; Hauger et al., 1988). It is interesting to note that VP plays an important role on the loss of CRH receptors. In the regard, CRH receptor downregulation following adrenalectomy is markedly attenuated in the VP deficient, Brattleboro rat, and minipump infusion of VP and CRH, accentuate the downregulation induced by infusion of CRH alone (Hauger & Aguilera, 1993; Holmes et al., 1987). It is unlikely that decreases in pituitary CRH receptors during chronic stress account for the desensitization of the ACTH responses to the heterotypic stimulus, since responses to a novel stress are enhanced in these conditions (Aguilera, 1994) and CRH receptor downregulation also occurs in paradigms associated with sustained ACTH responses to the homotypic stressor. The molecular mechanisms involved in the regulation of both CRH and V1b receptors include transcriptional, translational and post-translational events controlled by the interactive effect of glucocorticoids and CRH and VP themselves. This has been extensively reviewed elsewhere (Aguilera, 1994; Volpi et al., 2004).
The positive correlation between the content of pituitary VP receptors but not CRH receptors and pituitary ACTH responsiveness to a novel stimulus, suggests that VP receptor regulation is part of the mechanism controlling corticotroph responses and supports the concept that during chronic stress regulation of HPA axis activity switches from CRH to VP (Aguilera & Rabadan-Diehl, 2000; Ma et al., 1997) .
Does VP mediate ACTH responsiveness during chronic stress?
As discussed above, the parallel changes in VP expression in parvocellular neurons and pituitary V1b receptors and ACTH responsiveness during chronic stress have suggested that the increase in vasopressinergic activity is a major determinant of ACTH responsiveness to a novel stress. However, the hypothesis that VP becomes the primary regulator of ACTH responses during chronic stress has been difficult to demonstrate in studies using genetic models of VP or V1b R deficiency or pharmacological blockade of VP receptors. For example, the VP deficient Brattleboro rat shows normal responses to most acute stressors and only a transient reduction in ACTH responses during repeated restraint (Baertschi et al., 1984; Zelena et al., 2004). On the other hand, studies in V1b receptor knockout mice show clear compromise of HPA axis responses to some stressors. Studies using a mouse carrying a deletion of the 3’ end of the coding region of the V1b receptor show reduced ACTH responses to acute hypoglycemia, lipopolysaccharide and ethanol administration but normal basal and acute restraint-stimulated ACTH (Lolait et al., 2007a; Lolait et al., 2007b; Tanoue et al., 2004; Wersinger et al., 2002). It is noteworthy that in contrast to rats (Aguilera, 1998; Maet al., 1999), wild type mice shoved no habituation of ACTH responses to the repeated homotypic stress of restraint. However, V1b receptor knock out mice showed no responses to restraint stress on day 14, suggesting that V1b receptors are required for sustained responses to repeated stress (Lolait et al., 2007a). Although ACTH responses are reduced, these mice are able to display sustained corticosterone responses to the repeated stimuli. Other investigators find severely deficient HPA axis responses to forced swim stress in a mouse model with a full deletion of the V1b receptor coding region (Tanoue et al., 2004). However, it is important to consider that the development of compensatory mechanisms due to the functional disruption of the gene since embryonic life could obscure the interpretation of the findings in models of non-inducible gene ablation.
An alternative approach is the use of VP receptor antagonists but only recently a selective V1b receptor antagonist, SSR149415, became available (Serradeil-Le Gal et al., 2005). This non-peptide, orally active antagonist binds to the V1b receptor with nM affinity, totally blocks radiolabeled VP binding to the V1b receptor in transfected cells, and inhibits VP-stimulated ACTH secretion in cultured pituitary cells (Serradeil-Le Gal et al., 2002). In vivo studies have shown that SSR149415 administered i.v., i.p. or orally, effectively blocked VP-induced ACTH secretion and also restraint stress-stimulated ACTH secretion (Serradeil-Le Gal et al., 2002; Serradeil-Le Gal et al., 2005). However, recent studies in repeatedly restrained rats show only minor effects of SSR149415 on ACTH responses to a novel stress, when given either as a single i.v. injection preceding a novel stress, or by repeated oral daily administration during the 14-day restraint stress (Chen et al., 2007). The same study suggests that lack of a significant effect of the selective V1b antagonist is due to a rather short biological half life of the antagonist in the experimental conditions used in the study, as shown by partial reduction of ACTH responses to exogenously injected VP (Chen et al., 2007). An additional issue to consider when interpreting the latter experiments is the fact that SSR149415 crosses the blood brain barrier and that blockade of central V1b receptors by the compound may influence the HPA axis responses.
In contrast, chronic osmotic minipump administration of the non-selective peptide V1 receptor antagonist, dGly[Phaa1,D-tyr(et), Lys, Arg]VP, has been shown to effectively block ACTH and corticosterone responses to exogenous vasopressin (Chen et al., 2007; Subburaju & Aguilera, 2007), indicating reasonable blockade of V1b receptors in the corticotroph. In contrast to the lack of effect of the orally active V1b receptor antagonist, chronic administration of the non-selective V1 antagonist caused a significant reduction of ACTH responses to i.p. hypertonic saline injection, suggesting that VP contributes to the response in this acute stress paradigm (Fig 3-A). The remarkable finding in the latter study was the total inability of the antagonist to inhibit ACTH responses to i.p. hypertonic saline injection in repeatedly stressed rats (Fig 3-B). The fact that partial (with SSR149415) or complete pituitary V1b receptor blockade (peptide non-selective V1 antagonist) failed to inhibit acute stress responses in repeatedly restrained rats, suggest that the VP receptor up-regulation observed following repeated restraint is not required for the sensitization of ACTH responses to a novel stress.
Figure 3.
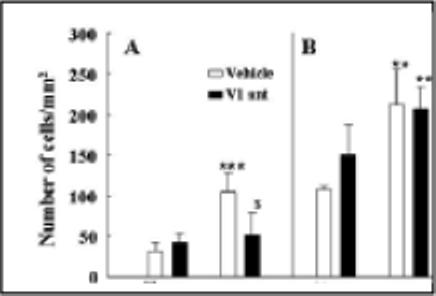
The effect of chronic osmotic minipump administration of the non-selective V1 receptor antagonist, dGly[Phaa1,D-tyr(et), Lys, Arg]VP (V1R Ant), or vehicle for 14 days on plasma ACTH responses to the novel stress of i.p. hypertonic saline injection (ipHS) in conscious naïve control rats (A), or rats subjected to restraint stress for 1h daily for 14 days (B). Data are the mean and SE of values obtained in 8 to 10 rats per group. Both groups showed a significant effect of ipHS on plasma ACTH levels (*, p<0.001). ACTH responses to ipHS in naïve rats were significantly reduced by the V1R Ant compared with the vehicle infused controls ($, p<0.04). ACTH responses to ipHS in repeatedly restrained rats were significantly higher than those in naïve rats (#, p<0.02), and these enhanced responses were unaffected by the V1R Ant.
Overall, the available evidence supports the view that VP contributes to the full ACTH response during some types of acute stress. Although the loss a sustained ACTH responses to repeated restraint in V1b receptor knock out mice suggest that VP is required for sustained responses, it appears that ACTH secretion in the absence of VP is sufficient to elicit full adrenal glucocorticoid responses. In addition, the ineffectiveness of the peptide V1 receptor antagonist to modify ACTH responses during chronic stress indicates that VP does not mediate the hyperresponsiveness of ACTH responses to a novel stress and suggests alternative roles for the peptide during stress adaptation.
Alternative actions of VP in the pituitary on mitogenesis
The disproportionality between the minor effects of genetic and pharmacologic VP blockade and the prominent increase in vasopressinergic activity (manifested as an increase in parvocellular VP and pituitary V1b receptor expression), raises the possibility that VP not only modulates ACTH secretion but has additional functions in the pituitary. It has been shown that VP stimulates mitogenesis in a number of systems, including mouse Swiss 3T3 cells (Rozengurt et al., 1979), rat bone marrow cells following hemorrhage (Hunt et al., 1977), rat liver cells (Russell & Bucher, 1983), mesangial cell (Ghosh et al., 2001), human osteoblast-like cells (Lagumdzija et al., 2004) and the murine corticotroph tumor cell line, AtT20 (van Wijk et al., 1995). VP has also been shown to increase the number of cells incorporating BrdU in primary cultures of rat anterior pituitary cells (McNicol et al., 1990). Since chronic stress and adrenalectomy induce an increase in the number of corticotrophs, it is likely that VP could mediate mitogenic responses in the pituitary. This question was examined in a recent study using long term infusion of a V1 antagonist in long-term adrenalectomized rats (Subburaju & Aguilera, 2007). As previously shown (Childs et al., 1989; Crane & Loomes, 1967; Nolan et al., 1998; Rappay & Makara, 1981) the latter study showed significant increases in the number of BrdU- and ACTH-labeled cells at 3 and 6 days, and a much larger increase at 28 days. Minipump infusion of the peptide V1 antagonist, dGly[Phaa1,D-tyr(et), Lys, Arg]VP (V1-ant) at doses blocking the increases in ACTH and corticosterone induced by exogenous VP, for the duration of the experiment starting at the time of adrenalectomy, prevented the increases in BrdU incorporation (Fig 4-A), but not irACTH cells induced by adrenalectomy (Fig.4-B)(Subburaju & Aguilera, 2007). This suggests that VP mediates mitogenic responses to adrenalectomy but that differentiation can occur in the absence of the peptide. However, in contrast to the findings in rats, in V1b receptor knockout mice, adrenalectomy for 6 or 14 days failed to increase either the number of cells incorporating BrdU or the number of irACTH cells, while inducing the expected increase in wild type mice. This suggests that life time deficient pituitary vasopressinergic activity has a more profound impact on the corticotroph population. Other studies showing a reduced number of corticotrophs in the VP-deficient Brattleboro rat compared with control Long Evans rats (Schmale & Richter, 1984; Tankosic et al., 1982), also support this view.
Figure 4.
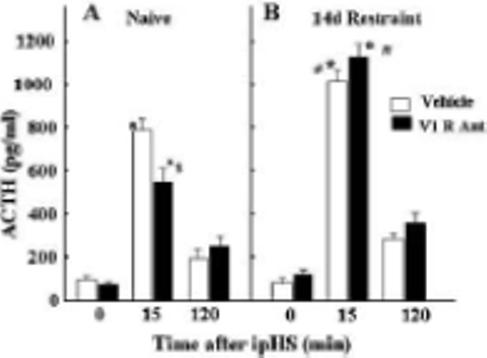
The effect of chronic administration of the non-selective V1 receptor antagonist, dGly[Phaa1,D-tyr(et), Lys, Arg]VP (V1R Ant), or vehicle on adrenalectomy (ADX)-induced changes in the number of cells immunostained for ACTH or deoxybromouridine (BrdU). Rats were subjected to adrenalectomy and implantation of osmotic minipumps (Alza, model 2004) containing the V1 antagonist (230 ng/h/28 days) or vehicle, and BrdU injections for 7 days before sacrifice at day 28. Rats were killed by decapitation and pituitaries fixed for BrdU and ACTH immunohistrochemistry. Bars represent the mean and SE of immunopositive cells counted in pituitary sections of 4 rats per experimental group. *p<0.05 vs. sham; ** p<0.002 vs. sham; *** p<0.001 vs. sham, $p<0.05 vs ADX
The effects of the V1 antagonist and V1b receptor ablation on the number of cells undergoing proliferation during long term adrenalectomy discussed above support the hypothesis that VP mediates the mitogenic activity in the pituitary following glucocorticoid withdrawal. In contrast, in another study, PVN lesions were unable to prevent the increase in pituitary cell proliferation induced by adrenalectomy (Nolan et al., 2004), suggesting that pituitary mitogenesis could be a direct consequence of glucocorticoid withdrawal in the pituitary. However, it is possible that VP of supraoptic origin with access to the pituitary portal circulation promotes mitogenesis (Antoni, 1993). Thus, it is conceivable that VP becomes a critical pituitary mitogenic agent during longer term adrenalectomy.
An outstanding question is the origin of the cells undergoing mitogenesis during adrenalectomy. Since adrenalectomy increases the number of corticotrophs it would be expected that BrdU stained nuclei co-localize with ACTH immunoreactive cells. However, Subburaju and Aguilera (Subburaju & Aguilera, 2007) found that only a minor proportion of BrdU labeled nuclei corresponded to cells stained with ACTH or the corticotroph precursor marker, T-pit (Pulichino et al., 2004). Reports of co-localization of ACTH in pituitary cell types other than corticotrophs, have suggested that mature pituitary cells can cross-differentiate (Denef et al., 2005). However, the lack of co-localization of BrdU stained nuclei in lactotrophs, thyrotrophs, somatotrophs or gonadotrophs, shown in this study is against this possibility. These observations render unlikely that the increase in corticotrophs originates from the division of existing corticotrophs or already differentiated corticotroph precursors, but suggests that recruitment of corticotrophs during adrenalectomy occurs from undifferentiated cells. Other studies have also shown lack of co-localization of BrdU in corticotrophs following adrenalectomy (Gulyas et al., 1991; Nolan & Levy, 2001; Taniguchi et al., 1995). Nolan and Levy, (Nolan & Levy, 2006) also reported a minor incidence of mitogenesis in corticotrophs or gonadotrophs following 3- and 6-days adrenalectomy or gonadectomy in rats. In the latter study, there was no additivity of mitogenic responses to adrenalectomy and gonadectomy, supporting the view that mitogenesis in response to both stimuli occurs in an undifferentiated progenitor population (Nolan & Levy, 2006).
Of the two cell types examined as potential corticotroph progenitor cells, neither S100P-stained folliculo-stellate nor nestin-labeled stem cells have been found to colocalize BrdU. This suggest that folliculo-stellate cells do not act as precursors for the newly-formed corticotroph following adrenalectomy, and that non-nestin expressing stem cells are probably responsible for the mitogenic responses to long-term adrenalectomy.
The fact that the increase in cells undergoing mitogenesis is VP-dependent raises the question of whether pituitary cell types other than corticotrophs express VP receptors. While the main VP receptor subtype found in the pituitary is the V1b receptor, the major supporting evidence for VP-mediation of mitogenic responses was obtained using an antagonist equally effective for V1a and V1b receptors (Rabadan-Diehl et al., 1995). Thus, it is possible that blockade of V1a receptors located in pituitary cells other than corticotrophs or in the periphery could contribute to the effects of the antagonist. In addition, the published in situ hybridization image showing co-localization of V1b receptor mRNA and POMC mRNA (Lolait et al., 1995b), shows clusters of V1b receptor mRNA grains not overlaying POMC stained cells, suggesting that not only corticotrophs may express V1b receptors. Whether these cells correspond to pituitary progenitor cells remains to be elucidated.
CONCLUSIONS
It is clear that VP is a component of parvocellular hypothamic-pituitary corticotroph response to stress and glucocorticoid withdrawal, and that VP stimulates ACTH secretion, mainly by potentiating the stimulatory effect of CRH. The prominence of the increases in vasopressinergic activity during chronic stress, suggest that VP must have an important role in the adaptation of the HPA axis to chronic stress. Evidence from studies using genetic or pharmacological ablation of VP receptors supports the view that VP contributes to the full ACTH responses to some types of stress and sustained responses during chronic stress. However, the ineffectiveness of the peptide V1 antagonist to modify ACTH responses during chronic stress indicates that VP does not mediate the hypersensitivity of ACTH responses to a novel stress, and suggests alternative roles for the peptide during stress adaptation. Recent studies strongly suggest that one of these roles of VP is to mediate proliferative responses in the pituitary. On the other hand, the mechanisms mediating corticotroph differentiation appear to be more complex and at least in the rat are likely to involve factors other than VP. The minor co-localization of BrdU stained nuclei in ACTH or Tpit stained cells (or other pituitary cell types) suggests that newly produced corticotrophs following adrenalectomy and probably chronic stress originate from undifferentiated cells and not from division of existing corticotrophs. Taken as a whole, VP appears to play a minor role as a direct regulator of ACTH secretion, but one of the functions of the marked increases in parvocellular vasopressinergic activity during adrenalectomy (and probably chronic stress) is regulating cell proliferation and remodeling of the pituitary tissue.
Acknowledgements
This work was supported by the Intramural Research Program of the NIH/NICHD
REFERENCES
- Aguilera G. Corticotropin Releasing Hormone, Receptor Regulation and the Stress Response. Trends in Endocrinology and Metabolism. 1998;9:329–336. doi: 10.1016/s1043-2760(98)00079-4. [DOI] [PubMed] [Google Scholar]
- Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front. Neuroendocrinol. 1994;15:321–350. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Pham Q, Rabadan-Diehl C. Regulation of pituitary vasopressin receptors during chronic stress: relationship to corticotroph responsiveness. J.Neuroendocrinol. 1994;6:299–304. doi: 10.1111/j.1365-2826.1994.tb00586.x. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul.Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- Alexander SL, Irvine CH, Donald RA. Short-term secretion patterns of corticotropin-releasing hormone, arginine vasopressin and ACTH as shown by intensive sampling of pituitary venous blood from horses. Neuroendocrinology. 1994;60:225–236. doi: 10.1159/000126755. [DOI] [PubMed] [Google Scholar]
- Alexander SL, Roud HK, Irvine CH. Effect of insulin-induced hypoglycaemia on secretion patterns and rates of corticotrophin-releasing hormone, arginine vasopressin and adrenocorticotrophin in horses. Journal of Endocrinology. 1997;153:401–409. doi: 10.1677/joe.0.1530401. [DOI] [PubMed] [Google Scholar]
- Anton,i FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front. Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- Antoni FA, Fink G, Sheward WJ. Corticotrophin-releasing peptides in rat hypophysial portal blood after paraventricular lesions: a marked reduction in the concentration of corticotrophin-releasing factor-41, but no change in vasopressin. J.Endocrinol. 1990;125:175–183. doi: 10.1677/joe.0.1250175. [DOI] [PubMed] [Google Scholar]
- Antoni FA, Holmes MC, Jones MT. Oxytocin as well as vasopressin potentiate ovine CRF in vitro. Peptides. 1983;4:411–415. doi: 10.1016/0196-9781(83)90041-4. [DOI] [PubMed] [Google Scholar]
- Baertschi AJ, Gahwiler B, Antoni FA, et al. No role of vasopressin in stress-induced ACTH secretion? Nature. 1984;308:85–86. doi: 10.1038/308085c0. [DOI] [PubMed] [Google Scholar]
- Baertschi A,J, Vallet P, Baumann JB, Girard J. Neural lobe of pituitary modulates corticotropin release in the rat. Endocrinology. 1980;106:878–882. doi: 10.1210/endo-106-3-878. [DOI] [PubMed] [Google Scholar]
- Battaglia DF, Brown ME, Krasa HB, et al. Systemic Challenge with Endotoxin Stimulates Corticotropin-Releasing Hormone and Arginine Vasopressin Secretion into Hypophyseal Portal Blood: Coincidence with Gonadotropin-Releasing Hormone Suppression. Endocrinology. 1998;139:4175–4181. doi: 10.1210/endo.139.10.6226. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, de Goeij DC, Tilders FJ. Hypoglycemia enhances turnover of corticotropin-releasing factor and of vasopressin in the zona externa of the rat median eminence. Endocrinology. 1989;125:28–34. doi: 10.1210/endo-125-1-28. [DOI] [PubMed] [Google Scholar]
- Buckingham JC. The influence of vasopressin on hypothalamic corticotrophin releasing activity in rats with inherited diabetes insipidus. J. Physiol. 1981;312:9–16. doi: 10.1113/jphysiol.1981.sp013612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham JC. Potentiation of hypothalamic corticotropin releasing activity by vasopressin: studies in the Brattleboro rat. Ann. N. Y. Acad. Sci. 1982;394:580–586. doi: 10.1111/j.1749-6632.1982.tb37472.x. [DOI] [PubMed] [Google Scholar]
- Chateau M, Marchetti J, Burlet A, Boulange M. Evidence of vasopressin in adenohypophysis: research into its role in corticotrope activity. Neuroendocrinology. 1979;28:25–35. doi: 10.1159/000122841. [DOI] [PubMed] [Google Scholar]
- Chen J, Young S, Subburaju S, et al. Vasopressin does not mediate hypersensitivity of the hypothalamic pituitary adrenal axis during chronic stress. Ann. N. Y. Acad. Sci. 2007 doi: 10.1196/annals.1410.037. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs GV, Lloyd J, Unabia G, Rougeau D. Growth and secretory responses of enriched populations of corticotropes. Endocrinology. 1989;125:2540–2549. doi: 10.1210/endo-125-5-2540. [DOI] [PubMed] [Google Scholar]
- Crane WA, Loomes RS. Effect of age, sex, and hormonal state on tritiated thymidine uptake by rat pituitary. Br.J.Cancer. 1967;21:787–792. doi: 10.1038/bjc.1967.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goeij DC, Kvetnansky R, Whitnall MH, et al. Repeated stress-induced activation of corticotropin-releasing factor neurons enhances vasopressin stores and colocalization with corticotropin-releasing factor in the median eminence of rats. Neuroendocrinology. 1991;53:150–159. doi: 10.1159/000125712. [DOI] [PubMed] [Google Scholar]
- Denef C, Pals K, Hauspie A, et al. Combinatorial expression of phenotypes of different cell lineages in the rat and mouse pituitary. Ann.N.Y.Acad.Sci. 2005;1040:84–88. doi: 10.1196/annals.1327.010. [DOI] [PubMed] [Google Scholar]
- Engler D, Pham T, Fullerton MJ, et al. Studies of the secretion of corticotropin-releasing factor and arginine vasopressin into the hypophysial-portal circulation of the conscious sheep. I. Effect of an audiovisual stimulus and insulin-induced hypoglycemia. Neuroendocrinology. 1989;49:367–381. doi: 10.1159/000125141. [DOI] [PubMed] [Google Scholar]
- Ghosh PM, Mikhailova M, Bedolla R, Kreisberg JI. Arginine vasopressin stimulates mesangial cell proliferation by activating the epidermal growth factor receptor. Am.J.Physiol Renal Physiol. 2001;280:F972–F979. doi: 10.1152/ajprenal.2001.280.6.F972. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Ma XM, Verbalis J, Aguilera G. Hypothalamic Pituitary Adrenal Axis and Hypothalamic-Neurohypophyseal Responsiveness in Water-Deprived Rats. Exp. Neurol. 2001;171:329–341. doi: 10.1006/exnr.2001.7784. [DOI] [PubMed] [Google Scholar]
- Gulyas M, Pusztai L, Rappay G, Makara GB. Pituitary corticotrophs proliferate temporarily after adrenalectomy. Histochemistry. 1991;96:185–189. doi: 10.1007/BF00315991. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Aguilera G. Regulation of pituitary corticotropin releasing hormone (CRH) receptors by CRH: interaction with vasopressin. Endocrinology. 1993;133:1708–1714. doi: 10.1210/endo.133.4.8404613. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Millan MA, Lorang M, et al. Corticotropin-releasing factor receptors and pituitary adrenal responses during immobilization stress. Endocrinology. 1988;123:396–405. doi: 10.1210/endo-123-1-396. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schäfer MK, Watson SJ, Sherman TG. In situ hybridization analysis of arginine vasopressin gene transcription using intron-specific probes. Mol. Endocrinol. 1991;5:1447–1456. doi: 10.1210/mend-5-10-1447. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Catt KJ, Aguilera G. Involvement of vasopressin in the down-regulation of pituitary corticotropin-releasing factor receptors after adrenalectomy. Endocrinology. 1987;121:2093–2098. doi: 10.1210/endo-121-6-2093. [DOI] [PubMed] [Google Scholar]
- Hunt NH, Perris AD, Sandford PA. Role of vasopressin in the mitotic response of rat bone marrow cells to haemorrhage. J.Endocrinol. 1977;72:5–16. doi: 10.1677/joe.0.0720005. [DOI] [PubMed] [Google Scholar]
- Jard S, Barberis C, Audigier S, Tribollet E. Neurohypophyseal hormone receptor systems in brain and periphery. Prog.Brain Res. 1987;72:173–187. doi: 10.1016/s0079-6123(08)60206-x. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J. Neuroscie. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagumdzija A, Bucht E, Stark A, et al. Arg-vasopressin increases proliferation of human osteoblast-like cells and decreases production of interleukin-6 and macrophage colony-stimulating factor. Regul.Pept. 2004;121:41–48. doi: 10.1016/j.regpep.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Leng G, Brown CH, Russell JA. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog. Neurobiol. 1999;57:625–655. doi: 10.1016/s0301-0082(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, O'Carroll AM, Brownstein MJ. Molecular biology of vasopressin receptors. Ann.N.Y.Acad.Sci. 1995a;771:273–292. doi: 10.1111/j.1749-6632.1995.tb44688.x. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, O'Carroll AM, Mahan LC, et al. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc.Natl.Acad.Sci.U.S.A. 1995b;92:6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolait SJ, Stewart LQ, Jessop DS, et al. The Hypothalamic-Pituitary-Adrenal Axis Response to Stress in Mice Lacking Functional Vasopressin V1b Receptors. Endocrinology. 2007a;148:849–856. doi: 10.1210/en.2006-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolait SJ, Stewart LQ, Roper JA, et al. Attenuated Stress Response to Acute Lipopolysaccharide Challenge and Ethanol Administration in Vasopressin V1b Receptor Knockout Mice. J. Neuroendocrinol. 2007b;19:543–551. doi: 10.1111/j.1365-2826.2007.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Aguilera G. Transcriptional responses of the vasopressin and corticotropin-releasing hormone genes to acute and repeated intraperitoneal hypertonic saline injection in rats. Brain Res. Mo.l Brain Res. 1999;68:129–40. doi: 10.1016/s0169-328x(99)00080-7. [DOI] [PubMed] [Google Scholar]
- Ma XM, Levy A, Lightman SL. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: a study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology. 1997;138:4351–4357. doi: 10.1210/endo.138.10.5446. [DOI] [PubMed] [Google Scholar]
- Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–32. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- McCann SM, Brobeck JR. Evidence for a role of the supraopticohypophyseal system in regulation of adrenocorticotrophin secretion. Proc.Soc.Exp.Biol.Med. 1954;87:318–324. doi: 10.3181/00379727-87-21368. [DOI] [PubMed] [Google Scholar]
- McNicol AM, Murray JE, McMeekin W. Vasopressin stimulation of cell proliferation in the rat pituitary gland in vitro. J.Endocrinol. 1990;126:255–259. doi: 10.1677/joe.0.1260255. [DOI] [PubMed] [Google Scholar]
- Nolan LA, Kavanagh E, Lightman SL, Levy A. Anterior pituitary cell population control: basal cell turnover and the effects of adrenalectomy and dexamethasone treatment. J.Neuroendocrinol. 1998;10:207–215. doi: 10.1046/j.1365-2826.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- Nolan LA, Levy A. Anterior pituitary trophic responses to dexamethasone withdrawal and repeated dexamethasone exposures. J.Endocrinol. 2001;169:263–270. doi: 10.1677/joe.0.1690263. [DOI] [PubMed] [Google Scholar]
- Nolan LA, Levy A. A population of non-luteinising hormone/non-adrenocorticotrophic hormone-positive cells in the male rat anterior pituitary responds mitotically to both gonadectomy and adrenalectomy. J.Neuroendocrinol. 2006;18:655–661. doi: 10.1111/j.1365-2826.2006.01459.x. [DOI] [PubMed] [Google Scholar]
- Nolan LA, Thomas CK, Levy A. Pituitary mitosis and apoptotic responsiveness following adrenalectomy are independent of hypothalamic paraventricular nucleus CRH input. J.Endocrinol. 2004;181:521–529. doi: 10.1677/joe.0.1810521. [DOI] [PubMed] [Google Scholar]
- Plotsky PM. Hypophysiotropic regulation of stress-induced ACTH secretion. Adv.Exp.Med.Biol. 1988;245:65–81. doi: 10.1007/978-1-4899-2064-5_6. [DOI] [PubMed] [Google Scholar]
- Pulichino AM, Lamolet B, Vallette-Kasic S, et al. Tpit−/−NeuroD1−/− mice reveal novel aspects of corticotroph development. Endocr.Res. 2004;30:551–552. doi: 10.1081/erc-200043625. [DOI] [PubMed] [Google Scholar]
- Rabadan-Diehl C, Lolait SJ, Aguilera G. Regulation of pituitary vasopressin V1b receptor mRNA during stress in the rat. J.Neuroendocrinol. 1995;7:903–910. doi: 10.1111/j.1365-2826.1995.tb00734.x. [DOI] [PubMed] [Google Scholar]
- Rappay G, Makara GB. A quantitative approach to trace the corticotrophs in culture after adrenalectomy. Histochemistry. 1981;73:131–136. doi: 10.1007/BF00493139. [DOI] [PubMed] [Google Scholar]
- Rozengurt E, Legg A, Pettican P. Vasopressin stimulation of mouse 3T3 cell growth. Proc.Natl.Acad.Sci.U.S.A. 1979;76:1284–1287. doi: 10.1073/pnas.76.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell WE, Bucher NL. Vasopressin modulates liver regeneration in the Brattleboro rat. Am.J.Physiol. 1983;245:G321–G324. doi: 10.1152/ajpgi.1983.245.2.G321. [DOI] [PubMed] [Google Scholar]
- Schmale H, Richter D. Single base deletion in the vasopressin gene is the cause of diabetes insipidus in Brattleboro rats. Nature. 1984;308:705–709. doi: 10.1038/308705a0. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Wagnon J, Simiand J, et al. Characterization of (2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl) -2-oxo-2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. J.Pharmacol.Exp.Ther. 2002;300:1122–1130. doi: 10.1124/jpet.300.3.1122. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Wagnon J, Tonnerre B, et al. An overview of SSR149415, a selective nonpeptide vasopressin V(1b) receptor antagonist for the treatment of stress-related disorders. CNS Drug Reviews. 2005;11:53–68. doi: 10.1111/j.1527-3458.2005.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker EM, Sved AF. Controls of vasopressin secretion and thirst: similarities and dissimilarities in signals. Physiol. & Behavior. 2002;77:731–736. doi: 10.1016/s0031-9384(02)00926-5. [DOI] [PubMed] [Google Scholar]
- Subburaju S, Aguilera G. Vasopressin Mediates Mitogenic Responses to Adrenalectomy in the Rat Anterior Pituitary. Endocrinology. 2007;148:3102–3110. doi: 10.1210/en.2007-0103. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Tamatani R, Yasutaka S, Kawarai Y. Proliferation of pituitary corticotrophs following adrenalectomy as revealed by immunohistochemistry combined with bromodeoxyuridine-labeling. Histochem.Cell Biol. 1995;103:127–130. doi: 10.1007/BF01454009. [DOI] [PubMed] [Google Scholar]
- Tankosic P, Burlet A, Jegou S, et al. Fetal and postnatal maturation of corticotrope function in the vasopressin-deficient rat (Brattleboro strain): a radioimmunological, immunocytochemical, and morphometric study. Ann.N.Y.Acad.Sci. 1982;394:560–573. doi: 10.1111/j.1749-6632.1982.tb37470.x. [DOI] [PubMed] [Google Scholar]
- Tannahill LA, Sheward WJ, Robinson IC, Fink G. Corticotrophin-releasing factor-41, vasopressin and oxytocin release into hypophysial portal blood in the rat: effects of electrical stimulation of the hypothalamus, amygdala and hippocampus. J.Endocrinol. 1991;129:99–107. doi: 10.1677/joe.0.1290099. [DOI] [PubMed] [Google Scholar]
- Tanoue A, Ito S, Honda K, et al. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J.Clin.Invest. 2004;113:302–309. doi: 10.1172/JCI19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibonnier M, Preston JA, Dulin N, et al. The human V3 pituitary vasopressin receptor: ligand binding profile and density-dependent signaling pathways. Endocrinology. 1997;138:4109–4122. doi: 10.1210/endo.138.10.5432. [DOI] [PubMed] [Google Scholar]
- van Wijk PA, van Neck JW, Rijnberk A, et al. Proliferation of the murine corticotropic tumour cell line AtT20 is affected by hypophysiotrophic hormones, growth factors and glucocorticoids. Mol.Cell Endocrinol. 1995;111:13–19. doi: 10.1016/0303-7207(95)03541-e. [DOI] [PubMed] [Google Scholar]
- Volpi S, Rabadan-Diehl C, Aguilera G. Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress. 2004;7:75–83. doi: 10.1080/10253890410001733535. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O'Carroll AM, et al. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol.Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog.Neurobiol. 1993;40:573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- Yasuda N, Greer MA, Greer SE, Panton P. Studies on the site of action of vasopressin in inducing adrenocorticotropin secretion. Endocrinology. 1978;103:906–911. doi: 10.1210/endo-103-3-906. [DOI] [PubMed] [Google Scholar]
- Zelena D, Foldes A, Mergl Z, et al. Effects of repeated restraint stress on hypothalamo-pituitary-adrenocortical function in vasopressin deficient Brattleboro rats. Brain Res.Bull. 2004;63:521–530. doi: 10.1016/j.brainresbull.2004.04.007. [DOI] [PubMed] [Google Scholar]


