Abstract
Two strains of lymphocytic choriomeningitis virus (LCMV) differ in their ability to cause a lethal disease in outbred guinea pigs: the Armstrong (ARM) strain is not lethal at high doses (106 PFU), whereas the WE strain is lethal at less than 10 PFU inoculated intraperitoneally. The high pathogenic potential of LCMV WE has been mapped to the larger (L) of the two genomic RNA segments by genetic reassortment analysis (Riviere, Y., Ahmed, R., Southern, P.J., Buchmeier, M. J. and Oldstone, M. B. A., J. Virol. 55, 704–709, 1985). Here we describe the completed sequence of the LCMV WE L RNA, and its comparison to the L RNA of the non-virulent strain, LCMVARM. Similar to the L RNA of LCMVARM, the L RNA of WE is 7.2 kb long and contains two open reading frames (ORFs): the 5′ ORF encodes a small RING finger (zinc-binding) protein, p11 Z, and the 3′ ORF encodes the putative RNA-dependent RNA polymerase (RdRp or L protein). Comparison of nucleotide sequences for both viruses revealed 84% L RNA homology. At the amino acid level similarity between the two strains is 87% in the Z ORF, and 88% in the RdRp ORF. The most divergent regions are found in the N-terminal parts of the RdRp and Z proteins and are most likely to account for differences in pathogenic potential.
Keywords: Arenavirus, LCM virus, WE strain, Armstrong strain, Negative strand RNAvirus, RNA polymerase, RING-finger protein
Introduction
Lymphocytic choriomeningitis virus (LCMV), the prototype arenavirus, has been widely used to study virus-specific cell-mediated immunity and immunemediated pathogenesis in mice (1). Remarkable biological variations such as lethality, initiation of persistent infection and induction of liver injury are found among commonly used LCMV strains (2). These distinct properties also include the ability to produce disease in guinea pigs. Adult guinea pigs are extremely susceptible to the WE strain of the virus and at the same time are infected but not afflicted with the Armstrong (ARM) strain (2–4). In contrast to the murine LCMV model, immune reactions are not involved in the pathogenic mechanisms of LCMVinfected guinea pigs. Animals intraperitoneally inoculated with LCMV WE developed remarkable lymphopenia, polymorphonuclear neutrophil (PMN) interstitial infiltrates in lung and in the splenic red pulp, and bone marrow necrosis that may be the main cause of death (5,6). A similar PMN-mediated pneumonitis was reported for guinea pigs infected with another arenavirus, Junin virus, the etiological agent of Argentine hemorrhagic fever (7). Infection of guinea pigs with this virus as well as with Pichinde and Lassa viruses is a useful experimental model to study mechanisms of arenavirus virulence in humans (8–11).
The genome of LCMV consists of two singlestranded RNA molecules, a large RNA (L, 7.2 kb) and a small RNA (S, 3.4 kb). The nucleotide sequence was determined for both segments of LCMV ARM (GenBank acc. No. M20869, JO4331; 12–14) and for S RNA of LCMV WE (M22138; 15). Each of the RNA segments has an ambisense coding strategy. The S genome segment encodes nucleocapsid protein (NP), and a surface glycoprotein precursor (GPC) that undergoes cleavage maturation to become structural glycoproteins GP-1 and GP-2. The large (L) segment encodes the L protein and a zinc-binding (Z) protein (12,14). Reassortment analysis between the LCMV ARM and WE strains has been used to determine that the L and not the S RNA segment is responsible for the development of lethal disease in guinea pigs (5). It has been shown that only the WE/ARM reassortant (L RNA of WE and S RNA of ARM) was as virulent as the parental WE strain. Thus, a high level of viral replication in tissues, expression of viral antigens and histopathological lesions also correlated with the L RNA of LCMV WE. On the other hand, the parental and reassortant viruses replicate similarly in cultured guinea pig fibroblasts, suggesting that the fatal effects of LCMV WE must occur through mechanisms found only in vivo (5). In order to determine the role of L RNA in the lethality and high replication levels in guinea pigs we have completed the sequence of the WE L RNA and compared it to that of the avirulent strain, LCMV ARM.
Materials and Methods
The LCMV WE isolate 54 (kindly provided by Dr. D. H. L. Bishop) was grown in BHK-21 cells, inoculated at a multiplicity of one plaque-forming unit (PFU) per cell and then harvested at 48 and 72 h after infection. Viral RNA was purified from virus in TNE buffer (10mM Tris-HCl, pH 7.5, 100mM NaCl, 1mM EDTA) by phenol extraction, phenol chloroform extraction, and then ethanol precipitation. Sequence determination of viral RNA by primer extension, direct RNA sequencing, and cDNA cloning methods has been described previously (12,14,16,17). Sequence data were analyzed using the Genetics Computer Group (GCG) package from the University of Wisconsin, Madison. Nucleotide and amino acid sequence comparisons were performed using GAP software (UWGCG), with a gap size of 3.0. The sequence is available from the NCBI, GenBank database under the accession number AF004519.
Results and Discussion
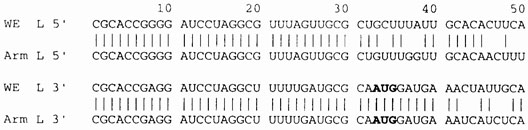
Reassortment studies between LCMV WE and LCMV ARM implicated the L RNA of WE in lethality for guinea pigs (5). A limited sequence, 1123 nucleotides at the 3′ end of the WE L RNA, had been published (18,19), and compared with the ARM L RNA sequence (13) revealing an 80% nucleotide homology and an 87% homology with predicted amino acid sequences. Now we have completed the WE L RNA and a comprehensive comparison has been done with the goal of finding those L RNA regions most likely to account for the different pathogenic potentials of the two strains. The L RNA of both viruses has the same genome structure encoding L (RdRp) and Z proteins in an ambisense manner. The LCMV WE L segment is 7219 nt long and has 30% U, 24% C, 28% A, and 18% G content. Nucleotide identity and homology (Bestfit option of GAP software) is 83% and 84%, respectively, between strains and transitions have occurred more frequently than transversions. As expected, the 3′ and 5′ inverted repeats that are probably involved in initiation of transcription, have been conserved and are identical for both viruses. The 5′ non-coding region of the LCMV WE L segment, which extends from the terminal nucleotide to the Z gene AUG initiation codon (nt 89–91), contains 16 nucleotide substitutions: 10 transitions and 6 transversions. The 3′ non-coding regions of the ARM and WE viruses are identical for the first 42 nucleotides (Fig. 1). Alignment of the 5′-and 3′-end sequences also shows an imperfect complementarity that may be involved in the circularization of genomic RNA thought to be necessary for replication/transcription of negative-stranded RNA viruses (20).
Fig. 1.
The 5′- and 3′- termini of L RNA of LCMV WE and LCMV ARM. The start codon for the Z protein is at position 89 (not shown) at the 5′ end. 3′ end sequences include the start codon of the large ORF encoding the viral polymerase at position 33 and indicated in bold.
After a short stretch of non-coding sequence at the 5′ end there is a small open reading frame (273 nt) encoding Z protein (90 aa), followed by almost 200 bases of non-coding sequence representing the intergenic region between the Z and ORFs. The intergenic region (nt 362–557) represents a highly structured RNA that was difficult to sequence by conventional methods and required some special techniques (17,21). This region has 78% identity between LCMV ARM and is presumably involved in termination of Z and RdRp mRNA synthesis.
The Z proteins of both LCMV strains encode a 90 amino acid protein with a high percentage of leucine + proline (25%), as well as abundant serine, lysine and cysteine. It has been shown that the 11 kDa Z protein of LCMV ARM binds zinc most likely through a RING finger motif at residues 32–68 (14). The RING structure binds two zinc ions and often mediates association with other proteins (22,23). Alignment of the deduced amino acid sequences of Z proteins encoded by LCMV strains (Fig. 2) indicates that the most conserved sequence is in the central part containing the RING-finger domain. Eleven conserved amino acid substitutions flanking the RING motif were found in the WE Z gene. Eight substitutions were located at the amino-terminal part of the protein and three conserved substitutions were located in the carboxy-terminal region. This region contains one non-conserved substitution (P > L) surrounding by 8 identical carboxy-terminal residues. Comparison of the predicted amino-acid sequences of WE and ARM Z proteins with the Z protein of LCMV TRAUB that has been partially sequenced (14) also revealed the greatest number of substitutions at the amino-terminal region of Z protein.
Fig. 2.
Alignment of the Z protein of LCMV (WE strain) to that of LCMV (ARM strain). Amino acid identity between the two viruses is indicated by lines (1). One or two dots signify increasing amino acid similarity, and the position of a single C3HC4 RING-finger motif is boxed. LCMV ARM sequence is from GenBank accession number M27693 (see ref. 14). The single letter amino acid code is A, alanine; C, cysteine; D, aspartic acid; E, glutamic acid; F, phenylalanine; G, glycine; H, histidine; I, isoleusine; K, lysine; L, leucine; M, methionine; N, asparagine; P, proline; Q, glutamine; R, arginine; S, serine; T, threonine; V, valine; W, tryptophan; T, tyrosine.
The 3′ portion of the L RNA encodes the viral RNA dependent RNA polymerase (RdRp. or L protein) with an ORF of 6630 nucleotides in the genome-complementary sense. The predicted gene product consists of 2209 amino acids with a calculated molecular mass of 254,328 and isoelecteric point of 6.12. Both LCMV WE and ARM L proteins are predicted to contain 13.8% acidic (D, E) and 15% basic (K, R, H) amino acids, and are rich in leucine, serine, valine and phenylalanine amino acid residues. The alignment analysis reveals a strong homology between the two LCMV L proteins with 1962 identical amino acids, 226 conserved and 30 non-conserved amino-acid substitutions (Fig. 3). The two most variable regions (356–458 and 874–918 residues) were located within the amino-terminal portion of the protein. These regions contained condensed clusters of conserved and non-conserved substitutions that affect the predicted secondary structure of the L protein (not shown). The conserved “A–E” domains (16,24) that are presumed to encode the RdRp activity of negative-strand viruses had no non-conserved substitutions. The carboxy-terminus of the WE L protein also differed from the ARM L protein in predominantly conserved amino-acid substitutions.
Fig. 3.
The predicted L proteins of LCMV WE (top) and LCMV ARM (bottom) were aligned from residue 1–2110. The differing amino acid residues are indicated by arrow. The most variable regions are underlined. Conserved regions of both LCMV L proteins belonging to RdRp module (A–E motifs) are boxed. A dash (−) indicates an amino acid deletion at position 1706 in the LCMV WE strain. Numbers indicate amino acid positions in the L proteins.
In summary, the Armstrong and WE strains of LCMV are very closely related, yet have remarkably different pathogenic potential for guinea pigs. This could be due to amino acid substitutions or to differences in the non-coding regions. We have noted numerous differences at the nucleotide level and have made a special note of the clusters of the most drastic changes in predicted protein sequence. These most divergent regions are likely to be important for the pathogenic potential of the LCMV WE strain.
Acknowledgments
This work was supported by NIH Grants AI-25522 and AI-32107 (MS). We are grateful for the initial technical support of Elaine Shimomaye.
References
- 1.Salvato MS, Rai KS, Mahy B, Collier L, editors. Toply and Wilson's Microbiology and Microbial Infections 9th edition. Arnold Publishing; London: 1998. pp. 629–650. [Google Scholar]
- 2.Dutko FJ, Oldstone MBA. J Gen Virol. 1983;64:1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann-Grube F. Virol Monogr. 1971;10:78–81. [Google Scholar]
- 4.Buchmeier MJ, Welsh RM, Dutko FJ, Oldstone MBA. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 5.Riviere Y, Ahmed R, Southern P, Buchmeier MJ, Oldstone MB. J Virol. 1985;55:704–709. doi: 10.1128/jvi.55.3.704-709.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez Peralta LA, Laguens M, Ponzinibbio C, Laguens RP. Medicina. 1990;50:225–229. [PubMed] [Google Scholar]
- 7.Gonzalez PH, Ponzinibbio C, Laguens RP. J Med Virol. 1987;22:289–297. doi: 10.1002/jmv.1890220313. [DOI] [PubMed] [Google Scholar]
- 8.Peters CJ, Jahrling PB, Liu CT, Kenyon RH, McKee KT, Jr., Barrera Oro JG. Cur Top Microbiol Immunol. 1987;134:5–68. doi: 10.1007/978-3-642-71726-0_2. [DOI] [PubMed] [Google Scholar]
- 9.Qian C, Jahrling PB, Peters CJ, Liu CT. Lab An Sci. 1994;44:600–607. [PubMed] [Google Scholar]
- 10.Aronson JF, Herzog NK, Jerrells TR. Am J Pathol. 1994;145:228–235. [PMC free article] [PubMed] [Google Scholar]
- 11.Aronson JF, Herzog NK, Jerrells TR. Am J Trop Med Hyg. 1995;52:262–269. doi: 10.4269/ajtmh.52-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvato M, Shimomaye E, Southern P, Oldstone MB. Virol. 1988;164:517–522. doi: 10.1016/0042-6822(88)90566-1. [DOI] [PubMed] [Google Scholar]
- 13.Salvato M, Shimomaye E, Oldstone MB. Virol. 1989;169:377–384. doi: 10.1016/0042-6822(89)90163-3. [DOI] [PubMed] [Google Scholar]
- 14.Salvato M, Shimomaye E. Virol. 1989;173:1–10. doi: 10.1016/0042-6822(89)90216-x. [DOI] [PubMed] [Google Scholar]
- 15.Romanowski V, Matsuura Y, Bishop DH. Virus Res. 1985;3:101–114. doi: 10.1016/0168-1702(85)90001-2. [DOI] [PubMed] [Google Scholar]
- 16.Lukashevich IS, Djavani M, Shapiro K, Sanchez A, Ravkov E, Nichol ST, Salvato MS. J Gen Virol. 1997;78:547–551. doi: 10.1099/0022-1317-78-3-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djavani M, Lukashevich IS, Sanchez A, Salvato MS. Virol. 1997;235:414–418. doi: 10.1006/viro.1997.8722. [DOI] [PubMed] [Google Scholar]
- 18.Romanowski V, Bishop DH. Virus Res. 1985;2:35–51. doi: 10.1016/0168-1702(85)90058-9. [DOI] [PubMed] [Google Scholar]
- 19.Singh MK, Fuller-Pace FV, Buchmeier MJ, Southern PJ. Virol. 1987;161:448–456. doi: 10.1016/0042-6822(87)90138-3. [DOI] [PubMed] [Google Scholar]
- 20.Salvato MS, Salvato MS, editors. The Arenaviridae. Plenum Press; New York: 1993. p. 133.p. 156. [Google Scholar]
- 21.Shimomaye E, Salvato M. Gen Anal Tech. 1989;6:25–28. doi: 10.1016/0735-0651(89)90022-8. [DOI] [PubMed] [Google Scholar]
- 22.Lovering R, Hanson IM, Borden KL, Martin S, O'Reilly NJ, Evan GI, Rahman D, Pappin DJ, Trowsdale J, Freemont PS. Proc Natl Acad Sci USA. 1993;90:2112–2116. doi: 10.1073/pnas.90.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borden KL, Freemont PS. Cur Opin Structural Biol. 1996;6:395–401. doi: 10.1016/s0959-440x(96)80060-1. [DOI] [PubMed] [Google Scholar]
- 24.Poch O, Blumberg BM, Bougueleret L, Tordo N. J Gen Virol. 1990;71:1153–1162. doi: 10.1099/0022-1317-71-5-1153. [DOI] [PubMed] [Google Scholar]