Abstract
The plant-derived acetylcholinesterase inhibitor physostigmine has previously been shown to act on the nicotinic acetylcholine receptor (nAChR) causing either direct activation or potentiation of currents elicited by low concentrations of nicotinic agonists, or, at higher concentrations, channel block. We examined mouse adult-type muscle nAChR activation by physostigmine and found that channel activation by physostigmine exhibits many characteristics common with channel activity elicited by nicotinic agonists. Single-channel conductance was indistinguishable, and mutants known to slow channel closing in the presence of nicotinic agonists had a similar effect in the presence of physostigmine. However, physostigmine is a very inefficacious agonist. The presence of physostigmine did not alter the effective opening rate for a subsaturating dosage of carbachol, suggesting that physostigmine does not interact with the nicotinic agonist binding site. Mutations to a residue (αK125) previously identified as part of the putative binding site for physostigmine reduced the duration of openings elicited by physostigmine but the effects were generally small and, in most cases, non-significant. At higher concentrations, physostigmine blocked channel activity. Block manifested as a reduction in the mean open time and the emergence of a closed state with a mean duration of 3-7 ms. The properties of block were consistent with two equivalent blocking sites per receptor with microscopic binding and unbinding rate constants for physostigmine of 20 μM-1s-1 and 450 s-1 (KD 23 μM). These observations indicate that physostigmine is able to activate muscle nAChR by interacting with a site other than the nicotinic ligand-binding site.
The muscle-type nicotinic acetylcholine receptor (nAChR) is a ligand-gated cation-permeable channel that initiates endplate depolarization of the neuromuscular junction. The endogenous ligand for the receptor is acetylcholine but the channel can be activated by numerous drugs including nicotine, choline and tetramethylammonium. While these drugs interact with the classic nicotinic agonist binding site, more recent work has demonstrated a novel class of agonists, called allosterically potentiating ligands (APLs), which appear to interact with a distinct binding site in the receptor. The plant-derived APLs, physostigmine and galantamine, were originally identified as acetylcholinesterase inhibitors, leading to their use in symptomatic treatment of neurological disorders involving memory deficits (Davis et al., 1978; van Dyck et al., 2000). Later work showed that the drugs also act on the nAChR where they potentiate currents elicited by low concentrations of nicotinic agonists, cause direct activation of the channel, and, at higher doses, block channel activity (Albuquerque et al., 1984; Okonjo et al., 1991; Zwart et al., 2000).
A direct activating effect of physostigmine has been observed in Locusta migratoria neurons (van den Beukel et al., 1998). In contrast, no whole-cell currents in response to up to 1 mM physostigmine were detected in oocytes expressing rat neuronal α4β2 or α4β4 receptors (Zwart et al., 2000), or COS cells expressing mouse muscle embryonic (αβγδ) receptors (Svobodova et al., 2006). Single-channel patch clamp has shown that channel openings in the presence of physostigmine have a conductance identical to that for ACh, but, in contrast to channel activity elicited by high concentrations of ACh, physostigmine-induced openings are typically not condensed into single-channel clusters (Shaw et al., 1985; Wachtel, 1993; Cooper et al., 1996). Together, the whole-cell and single-channel findings are suggestive of low potency and/or low efficacy of physostigmine on the nAChR. Physostigmine-mediated potentiation of currents elicited by low doses of ACh has been shown for neuronal (Zwart et al., 2000) as well as muscle-type nicotinic receptors (Svobodova et al., 2006).
Exposure to high concentrations of physostigmine leads to channel block. In macroscopic recordings, coapplication of physostigmine with ACh results in accelerated apparent desensitization and a reduction of peak response (Storch et al., 1995; Zwart et al., 2000; Svobodova et al., 2006). In single-channel recordings, high concentrations of physostigmine produce a reduction in the mean open duration and an emergence of a novel 7-8 ms closed time component (Shaw et al., 1985; Wachtel, 1993). In BC3H-1 cells that express the embryonic type nAChR, the properties of block were consistent with an open channel blocking scheme with a single blocking site per receptor (Wachtel, 1993).
Little is known about the location of the sites for physostigmine mediating activation or, for neuronal nicotinic receptors, potentiation. Photoafffinity labeling has shown that [phenyl-(n)-3H](-)physostigmine reacts with the K125 residue in the Torpedo receptor α subunit (Schrattenholz et al., 1993). Receptor activation by physostigmine is blocked by monoclonal antibody FK1 whose epitope is formed by segments 118-145 and 181-216 in the N-terminal region of the α subunit (Pereira et al., 1993; Schroder et al., 1994). These segments form strands β6-7 and β9-10 of the extracellular domain of the receptor thus placing the putative physostigmine activation site near, but not at, the ACh binding site (Brejc et al., 2001). Previous studies of galantamine have found that this APL can activate muscle nicotinic receptors without binding to the ACh-binding site (Akk & Steinbach, 2005), but did not locate the site. Clearly, further examination of the actions of physostigmine would help elucidate the actions of APLs.
In the present work, we have examined the adult mouse muscle-type nAChR activation by physostigmine. We find that physostigmine is a low efficacy agonist of the receptor. Channel activation by carbachol, a nicotinic agonist, was unaffected in the presence of physostigmine, consistent with previous data indicating different binding sites for nicotinic agonists and APLs. Mutations (αS269I, εT264P) previously shown to modify channel gating properties for nicotinic agonists act similarly on channel activation by physostigmine, while the single-channel conductance is indistinguishable for carbachol and physostigmine suggesting that the channel gating mechanisms are generally similar for APLs and nicotinic ligands. But in contrast to channel activation by nicotinic agonists, openings elicited by physostigmine show little voltage-dependence. Finally, mutations to the αK125 residue, previously associated with the physostigmine binding site, resulted in briefer channel openings from receptors activated by physostigmine but not carbachol.
MATERIALS AND METHODS
The mouse muscle adult-type receptor subunits (αβδε) were subcloned into a CMV promoter based expression vector, pcDNA3 (Invitrogen, Carlsbad, CA), and expressed in HEK 293 cells using a standard calcium phosphate precipitation-based transient transfection technique (Akk, 2002). In brief, a total of 3.5 μg of cDNA per 35 mm culture dish in the ratio of 2:1:1:1 (α:β:δ:ε) was mixed with 12.5 μl of 2.5 M CaCl2 and dH2O to a final volume of 125 μl. The mixture was then added slowly, without mixing to an equal volume of 2x BES-buffered solution. The combined solution was incubated at room temperature for 10 min followed by mixing the contents and another incubation of 15 min. The precipitate was then added to the cells. The cells were incubated at 37 °C with 5% CO2 for 16-20 h at which time the medium was replaced. The electrophysiological experiments were performed 40-72 h after the start of transfection.
Single-channel activity was recorded in the cell-attached configuration. The bath solution contained (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, 10 HEPES; pH 7.4. The pipette solution contained (in mM): 142 KCl, 1.8 CaCl2, 1.7 MgCl2, 5.4 NaCl, 10 HEPES; pH 7.4. The agonist (carbachol and/or physostigmine) was added to the pipette solution. In most experiments, the patch potential was held at -50 mV using a combination of cell membrane potential and applied pipette potential. The cell membrane potential was determined from the reversal potential of nicotinic receptor currents, several times during the course of a recording from a patch. Most HEK cells had a membrane potential of -30 to -20 mV in the bath solution used. All experiments were carried out at room temperature (18 - 21 °C).
Single-channel currents were amplified with an EPC7 (HEKA Instruments Inc, Port Washington, NY) or an Axopatch 200B amplifier (Molecular Devices Corp, Sunnyvale, CA), filtered at 10 kHz and digitized at 50 kHz using a Digidata 1322 Series interface (Molecular Devices). Channel event detection was carried out using program SKM (QuB suite, Qin et al., 1996, 1997). Typically, the currents were filtered at 5 kHz before idealization, and a minimal duration of 40 or 50 μs was imposed.
For currents recorded in the presence of carbachol, the analysis was restricted to clusters of single-channel currents, i.e., episodes of high open probability activity separated from other such episodes by prolonged silent intervals (Akk and Steinbach, 2003). In terms of kinetic mechanism, a cluster is defined as series of openings separated from each other by dwells in the un-, mono- and diliganded closed states. A cluster is initiated when a receptor returns from the long-lived desensitized state and terminated by receptor entry into the long-lived desensitized state. The advantage of employing single-channel cluster analysis is the near certainty that adjacent openings arise from the same receptor-channel. This allows to examine the durations of intracluster closed times and how they react to changes in agonist or modulator concentrations, and to correlate these parameters with channel activation properties.
The clusters were identified by eye and isolated for further analysis. Clusters containing overlapping currents, which are an indication of two or more simultaneously active receptors, were discarded. In the absence of clear clusters (e.g., for receptors activated by physostigmine) stable sections of the record were selected so as to include enough channel events for dwell time analysis, but also to avoid overlapping currents. Physostigmine-elicited activity from the εT264P receptors contained, in addition to single isolated openings, bursts or groups of activity. We focused our analysis on bursts of activity, but we note that the kinetic origin of bursts is unknown to us.
Open and closed interval durations were estimated using program MIL (QuB suite). The records were initially analyzed by fitting a simple C ↔ O model. The number of open states (or closed states, if estimating closed time durations) was then increased as long as the increase in the log-likelihood justified the addition of the extra free parameters (Horn, 1987). In general, an increase of >25 units was considered significant. The newly-added states were assumed to be unconnected to each other. In some cases, the presence of an additional state led to a significant increase in the log-likelihood, but the new state had a relative frequency of <0.1 %. In this case, data for that state were omitted. In some cases, parameters were estimated by simultaneous fitting of pooled data from several patches. Mean single channel current amplitudes were determined from the mean reported by the IDL module in QuB. Because some records were dominated by very brief events (e.g. 100 μM physostigmine), all amplitudes were estimated for events lasting longer than 200 μs, to allow full settling. Data are presented as mean ± SD, except when error estimates are calculated for fit parameters, in which case the data are best fitting value ± estimated SE of the value.
Voltage sensitivity of open or closed time durations was estimated from fitting the following equation:
where τ0 is the interval duration at 0 mV membrane potential, H is the change in membrane potential that produces an e-fold change in duration, and V is voltage. In cases where measurements were made at only two voltages, the voltage-sensitivity (H) was estimated as (V2 - V1) / (ln τ(V2) - ln τ(V1)).
All chemicals were purchased from Sigma-Aldrich (St Louis, MO). Physostigmine was stored in 1 mM aliquots at -20°C and diluted immediately before use.
RESULTS
Direct activation of wild type and mutant receptors by physostigmine
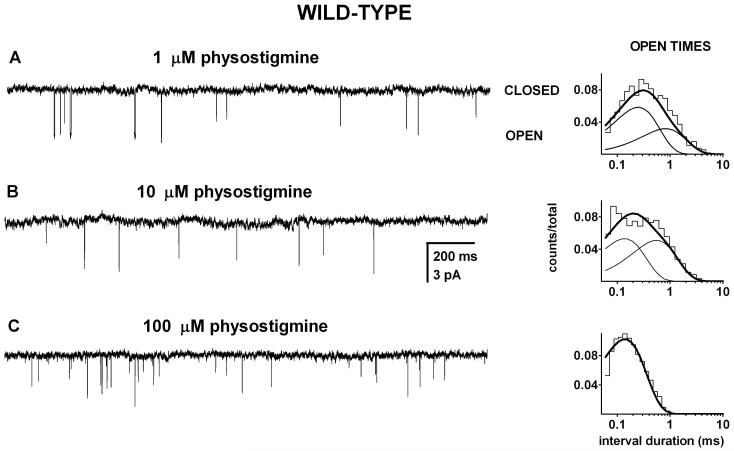
Physostigmine directly activates the mouse adult-type muscle nicotinic receptor. We recorded single-channel currents in the presence of 1-100 μM physostigmine and found that physostigmine elicits single, isolated openings which are not condensed into high open probability episodes (clusters) of activity. We saw no indication of agonist-dependent changes in the durations of long closed times suggesting that physostigmine is a low affinity or low efficacy (or both) agonist of the muscle-type receptor. Sample currents are shown in Figure 2.
Figure 2. Physostigmine directly activates the mouse adult wild type nAChR.
Single-channel currents and the respective open time histograms from patches exposed to 1 μM (A), 10 μM (B), or 100 μM physostigmine (C). The activity consisted of single, isolated openings without the characteristic clustering behavior observed with ACh or many other nicotinic agonists. The open time histograms were fitted to sums of two exponentials (1 and 10 μM physostigmine) or a single exponential (100 μM physostigmine). In the presence of 1 μM physostigmine, the open times for the data in this histogram were 0.22 ms (65%) and 0.74 ms. In the presence of 10 μM physostigmine, the open times were 0.12 ms (51%) and 0.49 ms. In the presence of 100 μM physostigmine, the mean open duration was 0.13 ms. Due to lack of clusters and the uncertainty in the number of active receptors in the patch, the closed times were not analyzed. The data are consistent with physostigmine being a low-potency or a low efficacy agonist on the wild type receptor.
At 1 and 10 μM physostigmine, the open time histograms were best-fitted by the sum of two exponentials. The brief openings (OT1) had mean durations of 0.30 and 0.22 ms at 1 μM physostigmine, and made up 78% and 65 % of all openings (data for 2 patches). The long duration openings (OT2) had durations of 0.72 and 0.74 ms. At 10 μM, the parameters were 0.35 and 0.12 ms (74% and 51 %) and .067 and 0.49 ms. At 100 μM physostigmine only a single component was see in the open times, with a duration of 0.17 ± 0.04 ms (mean for 3 patches).
In the absence of single-channel clusters, the durations of the closed times between adjacent openings depend, in addition to receptor activation properties, on the number of active receptors in the patch. The latter parameter is typically unknown and thus the durations of the closed times cannot be easily ascribed to a specific activation-related process. Accordingly, the closed intervals for physostigmine-activated wild type receptors were not analyzed. The lack of clusters suggests that physostigmine is a low affinity and/or low efficacy agonist for wild type muscle nicotinic receptors.
At -50 mV, the amplitude of single-channel events was similar at 1 and 10 μM (-3.6 ± 0.08 pA, 4 patches) and 100 μM physostigmine (-3.4 ± 0.2 pA, 3 patches). To obtain amplitudes of settled openings, only open durations longer than 200 μs were included in the analysis. Currents elicited by 1 mM carbachol exhibited a similar amplitude (-3.2 ± 0.4 pA, 7 patches) suggesting that the ion conduction pathway is unchanged when physostigmine, instead of the nicotinic agonist carbachol, is used to activate the receptor.
Previous work on nicotinic channels has shown that specific mutations to the M2 transmembrane domain (e.g., εT264P) or the linker region between the M2 and M3 domains (e.g., αS269I) enhance open probability (Po) of channels exposed to nicotinic agonists. The increase in the Po is mediated by an increase in the channel opening rate constant and/or a decrease in the channel closing rate constant (Chen and Auerbach, 1998; Zhou et al., 1999). These two mutations were useful in further studying physostigmine-elicited activation of the nicotinic receptor for two reasons. First, we could test whether the mutations similarly affect channel activation by physostigmine. Similarities in the effects of the mutations on channel activation by ACh and physostigmine would be an indication that channel gating proceeds similarly in the presence of these two classes of agonists. Second, prolonged open events and, consequently, higher open probability may allow isolation of channel activity originating from a single ion channel, i.e., clusters of activity. This would allow us to attempt to associate the channel closed time components with specific components of receptor activation, and give insight into physostigmine binding and gating properties.
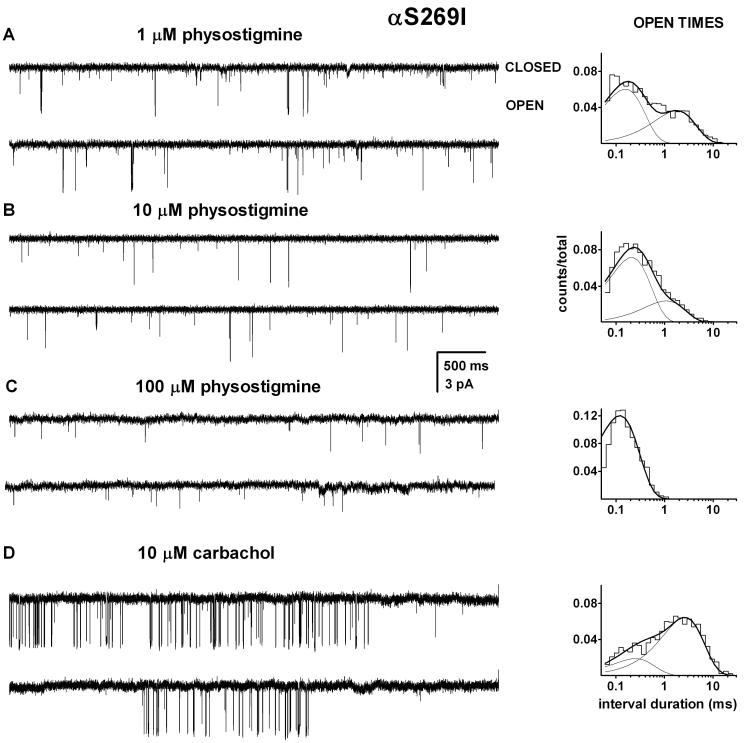
The activation of the αS269I mutant receptor was studied in the presence of 1 to 100 μM physostigmine (Figure 3). Two classes of open events were observed with the mutant receptor. In the presence of 1 μM physostigmine, OT1 had a mean duration of 0.17 ± 0.03 ms (4 patches) forming 75 ± 11 % of all open events. The mean duration of OT2 at 1 μM physostigmine was 2.3 ± 0.6 ms. Similar to what we saw with the wild type channel, the lifetime of OT2 was reduced at higher physostigmine concentrations. In the presence of 10 μM physostigmine, the mean duration of OT2 was 0.9 ± 0.3 ms (4 patches), and when activated by 100 μM physostigmine the channels opened to a single class of open events with the mean duration of 0.14 ± 0.03 ms (5 patches). When compared at a low physostigmine concentration (1 μM), the αS269I mutation prolonged the mean OT2 duration by ∼3-fold. This is similar to the effect observed for channel openings elicited by the nicotinic agonist choline (Zhou et al., 1999).
Figure 3. Activation of the αS269I mutant receptor by physostigmine.
Single-channel currents and the respective open time histograms from patches exposed to 1 μM (A), 10 μM (B), 100 μM physostigmine (C) or 10 μM carbachol (D). The open times were prolonged compared to the wild type receptor, but no clustering behavior was observed. The open time histograms were fitted to sums of two exponentials (1 and 10 μM physostigmine and 10 μM carbachol) or a single exponential (100 μM physostigmine). The open times for these patches were: 1 μM physostigmine, 0.15 ms (60%) and 1.43 ms; 10 μM physostigmine, 0.19 ms (75%) and 0.96 ms; 100 μM physostigmine, 0.11 ms (100%); 10 μM carbachol 0.23 ms (23%) and 2.34 ms. Carbachol produced activity grouped in clusters. Due to lack of clusters of activity elicited by physostigmine and the uncertainty in the number of active receptors in the patch, the closed times were not analyzed. The mutation enhances the channel opening rate constant for nicotinic agonists and the APL galantamine. The lack of clusters in the presence of physostigmine suggests that physostigmine is a low efficacy agonist of the adult-type muscle nAChR.
No clusters were observed at 1-100 μM physostigmine, and accordingly we did not attempt an analysis of closed time durations. Previous work has proposed that the αS269I mutation enhances the channel opening rate constant for nicotinic agonists by approximately 30-fold (Zhou et al., 1999; Grosman et al., 2000). Assuming a similar effect on channel opening by physostigmine, we conclude from the lack of clear-cut clusters that physostigmine is a low efficacy agonist of the mouse nicotinic receptor.
As a control, we examined the activation of the αS269I mutant receptor by 10 μM carbachol. Sample single-channel activity is shown in Figure 3D. The channel open time histograms were fitted to the sum of two exponentials. The mean open times were 0.33 ± 0.14 ms (23 ± 5 %) and 2.4 ± 0.2 ms (3 patches). It is likely that the shorter-lived component originates from monoliganded receptors while the longer-lived component can be associated with diliganded receptors. The mean duration of diliganded open events was ∼3-fold greater than in the wild-type receptor (see Figure 7A; also Akk et al., 2005). Thus, the mutation similarly affects channel open durations in the presence of physostigmine and the nicotinic agonist carbachol.
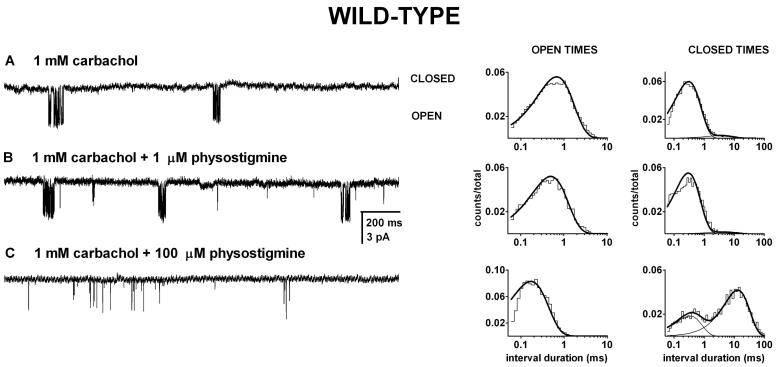
Figure 7. The presence of physostigmine does not interfere with channel activation by carbachol.
Single-channel clusters and the respective open and closed time histograms from wild type receptors exposed to 1 mM carbachol (A), carbachol + 1 μM physostigmine (B), or carbachol + 100 μM physostigmine (C). The activity consisted of easily identified clusters. In the presence of 1 mM carbachol, the mean intracluster open duration was 0.54 ms, and the closed times were 0.25 ms (96%) and 3.4 ms. In the presence of 1 mM carbachol + 1 μM physostigmine, the mean intracluster open duration was 0.39 ms, and the closed times were 0.24 ms (98%) and 9.6 ms. In the presence of 1 mM carbachol + 100 μM physostigmine, the mean intracluster open duration was 0.18 ms, and the mean closed times were 0.27 ms (40%) and 9.6 ms. The presence of 100 μM physostigmine led to a decrease in channel open probability through a decrease in the mean open duration and an increase in the prevalence and duration of the longerlived closed time component. The presence of physostigmine was ineffective at modifying the duration of the shorter-lived closed time component (inverse of the effective opening rate) which is a measure of agonist binding and channel opening. We conclude that physostigmine does not interact with the sites that mediate channel activation by carbachol.
The εT264P mutation produces an increase in Po due to a decrease in the channel closing rate constant and an increase in the channel opening rate constant (Chen and Auerbach, 1998; Zhou et al., 1999). In order to get further insight into the agonistic properties of physostigmine, we recorded single-channel currents from the mutant receptor exposed to 0.3-100 μM physostigmine.
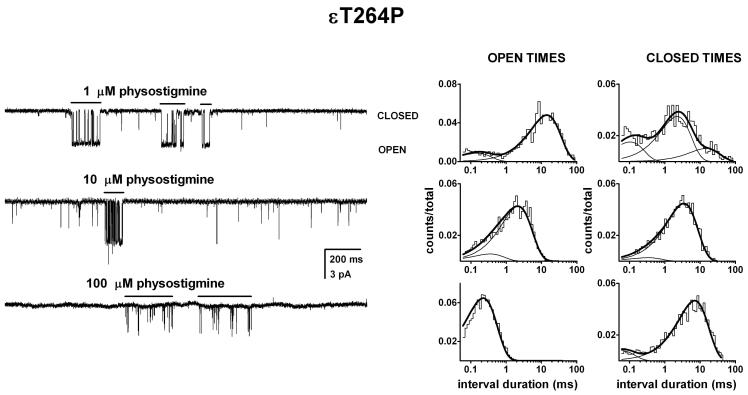
Single-channel activity from the εT264P mutant receptor activated by physostigmine exhibited complex behavior. In the majority of patches, physostigmine elicited 100-200 ms long groups of openings (bursts) interspersed with single isolated openings. However, we note that some patches contained only isolated openings. We do not have an explanation for this observation. Sample currents are shown in Figure 4.
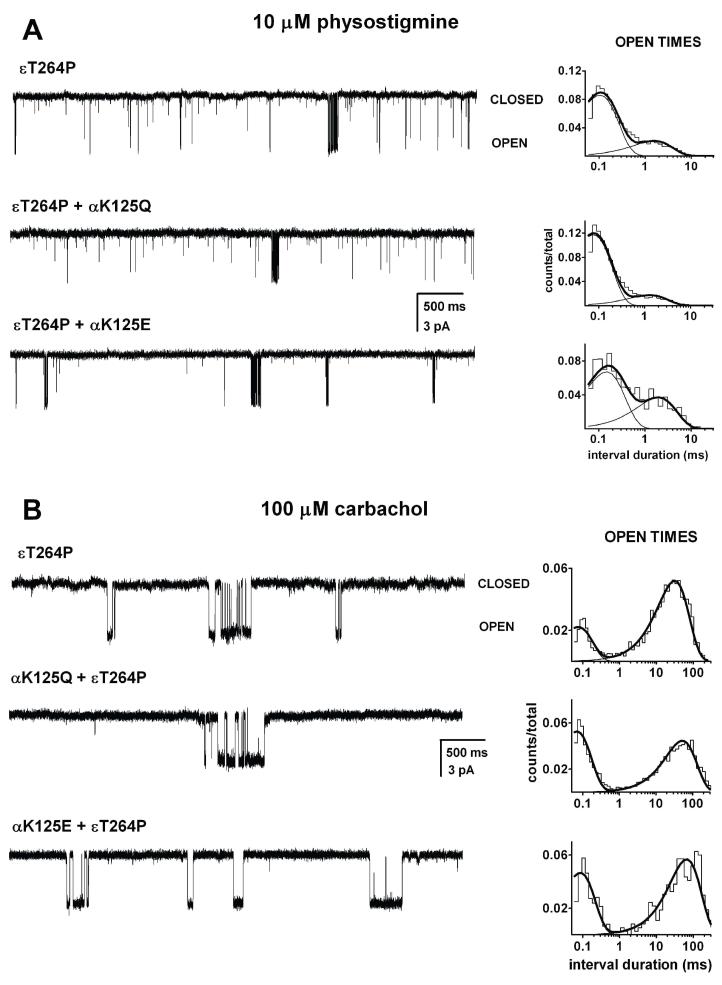
Figure 4. Activation of the εT264P mutant receptor by physostigmine.
Single-channel currents and the respective open and closed time histograms from patches exposed to 1 μM (A), 10 μM (B), or 100 μM physostigmine (C). The channel activity consisted of episodes or bursts of activity (shown with lines above current traces) intermixed with brief, isolated openings. The bursts were isolated from the recording and analyzed for open and closed time durations. The open times were prolonged compared to the wild type receptor. In the presence of 1 μM physostigmine, the open times were 0.14 ms (15%) and 13.1 ms, and the closed times were 0.10 ms (25%), 2.1 ms (58%) and 13.6 ms. In the presence of 10 μM physostigmine, the open times were 0.33 ms (12 %) and 2.0 ms, and the closed times were 0.30 ms (6%) and 3.2 ms. In the presence of 100 μM physostigmine, the mean open duration was 0.21 ms, and the closed times were 0.05 ms (20%) and 4.9 ms. Due to the potent blocking action of physostigmine, we were unable to identify the activation-related closed time components. It is likely that the more prominent 3 to 5 ms closed time component arises from dwells in the blocked state.
We focused our attention on the bursts of activity. The bursts were visually identified, isolated from the remaining record, and analyzed to estimate the intraburst open time durations. At physostigmine concentrations up to 10 μM most bursts contained two open time components. In the presence of 1 μM physostigmine, the two components had mean durations of 0.16 ± 0.11 ms (14 ± 2 %) and 9.8 ± 2.3 ms (analysis of pooled data from 4 patches). As the physostigmine concentration was increased the mean duration of OT2 was reduced, and at concentrations above 10 μM, in most patches, only a single open time component could be resolved. At 10 μM physostigmine, the mean open times were 0.41 ± 0.26 ms (13 ± 1 %) and 3.2 ± 1.1 ms (3 patches), and in the presence of 100 μM physostigmine, the single open time component had the mean duration of 0.24 ± 0.03 ms (4 patches).
In sum, when measured at low (1-10 μM) physostigmine concentrations, the presence of the εT264P mutation increased the duration of the long openings by about 10-fold. This indicates that the mutation affects channel gating by cholinergic agonists and this APL in the same way. The reduction in open durations seen at higher physostigmine concentrations is indicative of the channel-blocking properties of physostigmine (see below).
Physostigmine-elicited openings are not modulated by voltage
The experiments described above indicate that despite its low efficacy, channel activation by physostigmine proceeds in principal similarly to that by nicotinic agonists. The single-channel conductance is comparable for openings elicited by physostigmine and carbachol, and mutations previously shown to affect channel gating by nicotinic agonists also modify channel gating by physostigmine. As an additional measure of conformity in the mechanisms of gating we examined the voltage-dependence of channel open durations. Previous work has demonstrated that, for a number of nicotinic agonists, the nAChR closing rate constant is reduced at more negative potentials with an e-fold change per 80-100 mV hyperpolarization (Auerbach et al., 1996; Akk and Steinbach, 2000).
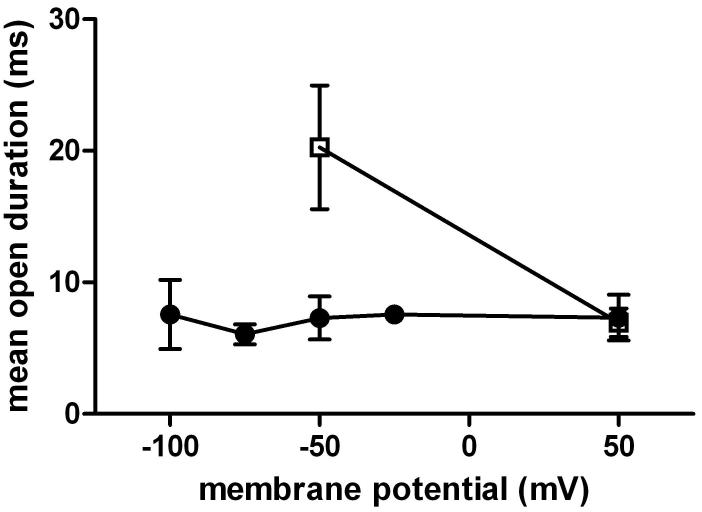
We investigated the effect of voltage on the durations of openings elicited from receptors containing the εT264P mutation. The mutant, instead of the wild type, receptor was used due to its longer open time durations increasing the fidelity of dwell time estimates. The experiments were conducted in the presence of a low concentration (1 μM) of physostigmine in order to avoid contribution from channel block to the apparent open time duration. We were particularly interested in the voltage-sensitivity of the longer-lived component, which presumably originates from fully-liganded receptors and should thus exhibit most voltage-sensitivity (Auerbach et al., 1996). Figure 5 shows the mean duration for the longer duration component estimated at -100, -75, -50, -25 and +50 mV membrane potentials. Overall, the data displayed minimal sensitivity to changes in membrane potential. At -100 mV, the mean lifetime of the longer-lived open time component was 7.6 ± 4.5 ms (3 patches) whereas at +50 mV, the mean duration was 7.3 ± 3.0 ms (3 patches). This gives a voltage-sensitivity of >3000 mV per e-fold change in open interval duration.
Figure 5. Voltage does not affect channel open durations in the presence of physostigmine.
The long open time component from the εT264P mutant receptor activated by 1 μM physostigmine (circles) was estimated at -100, -75, -50, -25 and +50 mV membrane potentials. No voltage-sensitivity was observed. For control, we estimated the open interval durations from the εT264P mutant receptor activated by 100 μM carbachol (squares) at -50 and +50 mV membrane potentials. Carbachol-elicited openings showed a voltage sensitivity of 93 mV per e-fold change. This value is similar to previous estimates for the wild type receptor and a number of mutant receptors activated by nicotinic agonists (Auerbach et al., 1996; Akk and Steinbach, 2000). Points show mean ± SD for data from 3 to 6 patches.
As a control, we estimated the voltage-sensitivity of durations of openings elicited by carbachol. At -50 mV, the εT264P receptors exposed to 100 μM carbachol showed two open time components. The brief openings had a mean duration of 70 ± 40 μs, and the long openings had a mean duration of 25 ± 10 ms (3 patches). When the membrane potential was changed to +50 mV, the mean duration of brief openings remained unchanged (70 ± 30 μs), but the mean duration of long-lived openings decreased by almost 3-fold (8.8 ± 1.4 ms). Based on previous work (Auerbach et al., 1996), we believe that the voltage-insensitive short-lived openings arise from unliganded or monoliganded receptors. The change in the mean duration of long openings (96 mV/e-fold change) is consistent with previously published data on voltage-sensitivity of doubly-liganded channels (e.g., Auerbach et al., 1996; Akk and Steinbach, 2000).
Effects of mutations to the αK125 residue
Photoaffinity labeling studies with [phenyl-(n)-3H](-)physostigmine have shown that the compound associates with the K125 residue in the Torpedo receptor α subunit (Schrattenholz et al., 1993). The αK125 residue is at a considerable distance from the acetylcholine binding pocket (Brejc et al., 2001) suggesting that the physostigmine binding site does not overlap with the ACh binding site. We examined the effects of mutations to the αK125 residue on adult mouse nAChR activation by physostigmine. The positively charged lysine residue was mutated to a neutral glutamine (Q) and a negatively charged glutamate (E). For enhanced opening frequency as well as longer open time durations, the experiments were conducted on the background of the εT264P mutation.
Figure 6A shows sample currents elicited by 10 μM physostigmine from receptors containing the εT264P, αK125Q+εT264P, or αK125E+εT264P mutant subunits. In five cells expressing the wild type α subunit (along with the εT264P mutant subunit), the open durations were 0.13 ± 0.02 ms (66 ± 19 %) and 2.7 ± 1.4 ms, and the weighted average open duration was 1.0 ms. When the wild type α subunit was replaced with one containing the αK125Q mutation, the mean duration of OT1 was 0.08 ± 0.02 ms (80 ± 19 %) and the mean duration of OT2 was 0.9 ± 0.5 ms (5 patches) resulting in a weighted mean open time of 0.25 ms. Finally, having a glutamate residue in the α125 position resulted in mean open durations of 0.13 ± 0.01 ms (79 ± 13 %) and 1.4 ± 0.4 ms (3 patches) with a weighted mean open time of 0.39 ms. Thus, a replacement of the positively charged lysine residue with a neutral glutamine or a negatively charged glutamate in the putative physostigmine binding site leads to shorter open durations, but only the effect of the αK125Q mutation on the OT2 duration was statistically significant (two-tailed t-test, p<0.05).
Figure 6. Mutations to the αK125 site reduce channel open durations in the presence of physostigmine but not carbachol.
Single-channel currents and the respective open time histograms for εT264P, αK125Q+εT264P, and αK125E+εT264P mutant receptors activated by 10 μM physostigmine (A), or 100 μM carbachol (B). In the presence of 10 μM physostigmine, the activity consisted of bursts of activity intermixed with brief, isolated openings. All the openings within long sections of the recording were analyzed for open time durations. The open times for the εT264P receptor activated by physostigmine were 0.10 ms (80%) and 1.4 ms, weighted average mean open duration of 0.28 ms. The open times for the αK125Q+ εT264P receptor activated by physostigmine were 0.07 ms (87%) and 1.2 ms, weighted average mean open duration of 0.22 ms. The open times for the εT264P+αK125E receptor activated by physostigmine were 0.13 ms (64%) and 1.7 ms, weighted average mean open duration of 0.54 ms. In the presence of 100 μM carbachol, the activity consisted of bursts of activity. The open times for the εT264P receptor activated by carbachol were 0.08 ms (25%) and 28.6 ms. The open times for the αK125Q+ εT264P receptor activated by carbachol were 0.07 ms (30%) and 48 ms. The open times for the εT264P+αK125E receptor activated by carbachol were 0.08 ms (45%) and 60 ms.
To rule out a more global effect of the αK125Q mutation that may amplify or offset the specific effects on physostigmine-mediated activation, we probed the effect of the mutations on channel activation by 100 μM carbachol. Activation of the εT264P receptor by this concentration of carbachol was characterized by clear-cut clusters (Figure 6B). The intracluster open time histograms contained two components. In the receptor containing the wild type α subunit, the open times were 90 ± 4 μs and 30 ± 0.4 ms (simultaneous fitting of data from 6 patches). In receptors containing the αK125Q, the mean open durations were 80 ± 2 μs and 30 ± 0.4 ms. Thus, the αK125Q mutation did not cause a reduction in the durations of openings elicited by carbachol, and we conclude that the effects of the mutation on open time durations are specific to physostigmine.
Physostigmine does not interact with the nicotinic agonist binding site
We also examined the possibility that physostigmine interacts with the nicotinic agonist binding site. To do that, we investigated receptor activation by a subsaturating concentration of carbachol in the absence and presence of physostigmine. We reasoned that if physostigmine binds to the carbachol site, then, due to its low efficacy, it will act as a competitive inhibitor of carbachol-elicited activity. This would manifest as a reduced effective opening rate because the receptor spends a fraction of time liganded with the low efficacy agonist physostigmine (Akk & Steinbach, 2003).
Single-channel currents were elicited by 1 mM carbachol from wild type receptors in the absence and presence of 1-100 μM physostigmine. For currents elicited by 1 mM carbachol alone, we observed the characteristic clustering behavior as described previously (Akk and Auerbach, 1999). The intracluster open time histograms were fitted to a single exponential with a mean open duration of 0.72 ± 0.22 ms (7 patches). The intracluster closed time histograms contained two components. The brief closed time component (CT1) had a mean duration of 0.33 ± 0.07 ms and the long-lived closed time component (CT2) had a mean duration of 4.4 ± 1.5 ms. It is likely that CT1 corresponds to sojourns in the unliganded and monoliganded closed states, i.e., reflects agonist re-binding and channel opening. The less-frequent CT2 component (7 ± 5 % of all intracluster closed events) corresponds to a short-lived desensitized state (Salamone et al., 1999). Sample single-channel currents are shown in Figure 7.
In order to test for physostigmine interactions with the carbachol binding site we examined the effect of physostigmine on the intracluster closed time distributions. We were particularly interested in changes in the duration of the CT1 component, as an increase in the duration of CT1 would be indicative of physostigmine-induced inhibition of channel activation. The intracluster closed time durations were estimated for 1 mM carbachol coapplied with 1, 10 or 100 μM physostigmine. Under all conditions the closed time histograms were best-fitted to the sum of two exponentials with one component similar in duration to CT1 and the other to CT2 for currents recorded in the presence of 1 mM carbachol alone. When 1 μM physostigmine was coapplied with carbachol, the closed times were 0.29 ± 0.01 ms and 3.9 ± 1.5 ms (3 patches). The relative contributions of the two components were similar to that in the absence of physostigmine - the CT2 component contributed 5 ± 4 % of intracluster closed events. Coapplication of 10 or 100 μM physostigmine with carbachol similarly failed to alter the duration of CT1. The mean duration of CT1 was 0.41 ± 0.16 ms (3 patches) or 0.35 ± 0.07 ms (6 patches) in the presence of 10 or 100 μM physostigmine, respectively. We conclude that physostigmine, at up to 100 μM, is ineffective at competing with carbachol for the nicotinic agonist binding site.
In contrast, physostigmine had significant effects on the longer duration closed time component, CT2. The mean duration of CT2 was 6.5 ± 0.7 ms at 10 μM physostigmine, and 10.6 ± 2.2 ms at 100 μM physostigmine. The increase in the concentration of physostigmine had a major effect on the prevalence of the CT2 component. The long-lived closed time component had a relative frequency of 22 ± 4 % or 67 ± 6 % in the presence of 10 or 100 μM physostigmine, respectively. These changes are likely associated with channel block that becomes the predominant contributor to the CT2 component at high physostigmine concentrations (see next section).
Channel block in the presence of physostigmine
The data presented above suggest that physostigmine can block the nicotinic channel. The open durations were reduced and, when such analysis was possible, the mean intracluster closed times were progressively longer when the receptors were exposed to high (100 μM) concentrations of physostigmine. In this section, we present a systematic characterization of physostigmine-induced channel block under a variety of conditions.
In the first set of experiments, we examined physostigmine-induced block of physostigmine-activated channels. Although comparison of the durations of wild type channel openings recorded at low vs. high concentrations of physostigmine demonstrated the blocking action of physostigmine, we chose to conduct a more thorough examination on the εT264P mutant receptor, for two reasons. First, physostigmine-elicited channel openings from the εT264P mutant channel are longer than those from the wild type receptor, allowing for less error and a greater dynamic range in studying the blocking actions of physostigmine. Second, we reasoned, the higher opening frequency and the presence of bursts of activity would allow us to isolate and study the closed time component that corresponds to sojourns in the blocked state.
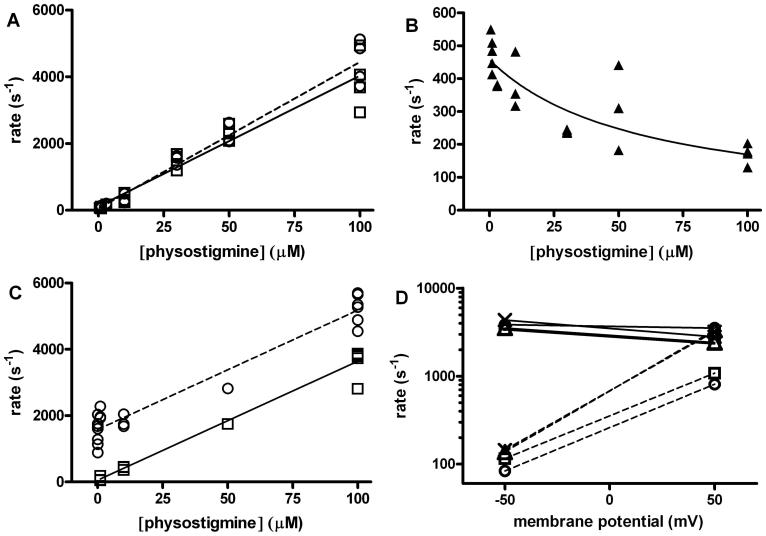
An increase in the concentration of physostigmine resulted in shorter open time durations. We estimated the apparent rate constant for development of block (k+B*) from the slope of the relationship between the inverse of the mean of the long duration open time component and physostigmine concentration according to 1/(mean duration) = (channel closing rate constant) + k+B* · [physostigmine] (see Figure 8A). This approach gave k+B* = 44 ± 2 μM-1s-1 for block of the εT264P receptor. An independent estimate for k+B* was obtained by examining the intraburst closed time distributions. The intraburst closed time histograms, which were fitted to the sums of three (at <10 μM physostigmine) or two exponentials, contained a closed interval component (nominally called CT2) that demonstrated a strong physostigmine concentration-dependency. The rate of entry into this closed state (k+CT2) was highly dependent on the concentration of physostigmine, suggesting that this closed duration component corresponds to dwells in the blocked state. Accordingly, we postulated that the rate of entry into this closed state can be used to estimate k+B* . The relationship between k+CT2 and physostigmine concentration is shown in Figure 8A, and a linear regression analysis of the data gave a k+B* = 39 ± 2 μM-1s-1. This value is essentially identical to the estimate from the open time analysis and confirms our identification of the blocked state in the closed time histograms.
Figure 8. Properties of channel block by physostigmine.
The increase in the concentration of physostigmine was found to result in reduced open duration and an increase in the rate of entry into a ∼3-7 ms closed state. Both observations are consistent with physostigmine-induced channel block. (A) The inverse of the open duration (circles and dashed line) and the rate of entry into the putative blocked state (squares and solid line) for εT264P mutant receptors are plotted as a function of physostigmine concentration. The lines were fitted to rate = rate at no physostigmine + k+B* · [physostigmine], where k+B* is the apparent blocking rate. The estimates for k+B* were 44 ± 2 μM-1s-1 when fitting the reduction in the open duration, and 39 ± 2 μM-1s-1 when fitting the increase in the rate of entry into the putative blocked state. (B) The relationship between the inverse of the duration of the putative blocked state and physostigmine concentration in the εT264P mutant receptor. The increase in the duration of the putative blocked state at higher physostigmine concentrations is consistent with the presence of two (or more) blocking sites per receptor. The line was fitted to 1/τBlocked = k-B (2k-B / (2k-B + k+B* · [physostigmine])). This equation assumes the presence of two, equivalent blocking sites per receptor. The fitting results are: k+B = 15.6 ± 4.8 μM-1s-1, and k-B = 458 ± 28 s-1. (C) The inverse of the open duration (circles) and the rate of entry into the putative blocked state (squares) for the wild type receptor activated by 1 mM carbachol in the presence of physostigmine are plotted as a function of physostigmine concentration. The estimates for k+B* were 36 ± 2 μM-1s-1 when fitting the reduction in the open duration, and also 36 ± 2 μM-1s-1 when fitting the increase in the rate of entry into the putative blocked state. (D) Changes in membrane potential strongly affected the rate of return from the blocked state but were ineffective at altering the rate of entry into the blocked state. The values for k+B* (solid lines) and k-B* (dashed lines) in the presence of 100 μM physostigmine were estimated for wild type receptors activated by 1 mM carbachol (circles) or 200 μM ACh (squares), εT264P receptors activated by 100 μM carbachol (triangles), and εT264P receptors exposed solely to physostigmine (crosses) at -50 mV and +50 mV membrane potential. For the rate of development of block, the H value (change in membrane potential needed for an e-fold change in parameter) was 1141 mV (wild type + carbachol), 268 mV (wild type + ACh), 261 mV (εT264P + carbachol), or 227 mV (εT264P + physostigmine alone). For the rate of recovery from block, the H value was 44 mV (wild type + carbachol), 45 mV (wild type + ACh), 31 mV (εT264P + carbachol), or 32 mV (εT264P + physostigmine alone). For panels A through C, each point shows data from one patch, while for D each point shows results from the combined analysis of data from 2 or 3 patches.
In a model with a single blocking site per receptor, the lifetime of the blocked state is independent of blocker concentration. In contrast, the duration of the CT2 (putative blocked) state was longer at higher physostigmine concentrations suggesting that the receptor contains two or more blocking sites, while the occupancy of any one of the sites can produce block. If so, then the apparent blocking rate is in fact a composite rate reflecting the binding properties of all sites. For example, in a model with two equivalent sites the microscopic blocking rate constant (k+B) is equal to one-half of the composite blocking rate k+B*, and the relationship between the apparent blocked state lifetime (τBlocked) and the microscopic unblocking rate constant (k-B) can be expressed as 1/τBlocked = k-B · (2k-B / (2k-B + k+B · [physostigmine])). To test whether a model with two equivalent sites can adequately describe the data we fitted this equation to the experimental data. Figure 8B shows that physostigmine-induced block of the εT264P mutant receptor is well described by a model with two equivalent blocking sites per receptor yielding a k+B of 15.6 ± 4.8 μM-1s-1 and a k-B of 458 ± 28 s-1 for physostigmine-induced block of the εT264P mutant receptor. The predicted aggregate blocking rate constant (31 μM-1s-1) is gratifyingly similar to the values for k+B* (39-44 μM-1s-1).
To confirm these findings on the wild type receptor, we examined physostigmine-mediated block of currents elicited by 1 mM carbachol. The somewhat longer open times and the presence of single-channel clusters are more amenable to measuring changes in the open time duration and the identification of the closed time component associated with the blocked state(s). The receptors were additionally exposed to 1, 10 or 100 μM physostigmine, and the intracluster open and closed time durations determined. In the absence of physostigmine, 1 mM carbachol produced openings with a mean duration of 0.72 ± 0.22 ms (7 patches). The addition of physostigmine resulted in a reduction in the mean open duration. From the relationship between the inverse of the mean open duration and physostigmine concentration we estimated a k+B* of 36 ± 2 μM-1s-1 (Figure 8C). An independent estimate for the rate of development of block was obtained from the analysis of the rate of entry into the putative blocked state. Linear regression analysis of the relationship between the rate of entry into the blocked state and physostigmine concentration also gave a k+B* of 36 ± 2 μM-1s-1.
Thus, the analysis of block of the wild type and the εT264P mutant receptors in essence gave identical results. Accordingly, we conclude that the εT264P mutation does not interfere with the blocking actions of physostigmine. We estimate that the microscopic binding rate constant for physostigmine, k+B, is ∼20 μM-1 s-1, and the dissociation rate constant for physostigmine, k-B, is ∼450 s-1 producing a KD of 23 μM at -50 mV.
We next tested the voltage-dependence of physostigmine-induced block. These experiments were conducted under four principal experimental conditions. Block of wild type receptors activated by 1 mM carbachol or 200 μM ACh, and εT264P receptors activated by 100 μM carbachol was studied in the presence of 100 μM physostigmine. In addition, we examined voltage-dependence of block of εT264P receptors exposed to 100 μM physostigmine alone. In the latter case, physostigmine served as both agonist and blocker.
For each case, the apparent blocking (k+B*) and unblocking (k-B*) rates were determined at -50 mV and +50 mV. The closed time histograms were fitted to the sum of two exponentials and the closed time component corresponding to the blocked state was identified based on comparison with control data. The rate of entry into the blocked component (k+B*) was calculated from the inverse of the mean open duration while k-B* was calculated as the inverse of the duration of blocked state. The results are summarized in Figure 8D.
The data show that a change in membrane potential had little effect on the rate of development of block. The H value (change in membrane potential needed for an e-fold change in parameter) ranged from 227 to 1141 mV for k+B* for different receptor-agonist combinations. In contrast, k-B* was highly dependent on membrane potential. In the wild type receptor activated by 1 mM carbachol, the lifetime of the blocked state was 1.2 ± 0.04 ms at +50 mV, but 12.0 ± 0.3 ms at -50 mV giving an H of 44 mV. Comparable voltage-sensitivity was observed for the wild type receptor activated by 200 μM ACh as well as the εT264P mutant receptor.
DISCUSSION
In this study we have characterized the activation and block of the adult mouse muscle nAChR by physostigmine. Physostigmine is a low efficacy agonist, which produces single-channel openings of the same conductance as the nicotinic agonist carbachol. Physostigmine-elicited activity is affected by the mutations αS269I and εT264P in a manner similar to channel gating by nicotinic agonists. Mutations to the putative physostigmine binding site (αK125 residue) shorten the open dwell duration of channels in the presence of physostigmine but not carbachol. The channel effective opening rate in the presence of 1 mM carbachol was not affected by up to 100 μM physostigmine, indicating that physostigmine does not interact with the ACh-binding site. Finally, we demonstrate that the blocking actions of physostigmine can be accounted for by two equivalent binding sites per receptor.
Physostigmine elicits only a low level of activity, but previous studies have generally been noncommittal regarding the underlying cause for the weak activating properties of physostigmine (Pereira et al., 1993; Storch et al., 1995; Jackson et al., 2002). Our single-channel data from the wild type receptor activated by physostigmine showed low open probability activity that did not condense into recognizable clusters. The lack of clusters is typically attributed to low potency or low efficacy of the agonist on the particular receptor type. We therefore utilized a receptor containing a mutation previously shown to increase channel Po through effects on the channel opening and closing rate constants. The αS269I mutant increases the channel opening rate constant for receptors activated by a nicotinic agonist, choline, by ∼30-fold (Zhou et al., 1999; Grosman et al., 2000), and enhances the opening rate constant for another APL, galantamine, resulting in easily recognizable single-channel clusters (Akk and Steinbach, 2005). In contrast, no clusters were observed for the αS269I mutant receptor activated by physostigmine at concentrations up to 100 μM. While it is possible that the mutation selectively acts on channel gating by nicotinic agonists and galantamine, but not physostigmine, we note that the mutation had a similar effect on the channel closing rate (a ∼3-fold reduction) for nicotinic agonists and physostigmine. We conclude from this finding that physostigmine is a low efficacy agonist of the adult-type muscle nAChR, and estimate that the channel opening rate constant for wild-type receptors activated by physostigmine is less than 2 s-1. The studies were limited to 100 μM physostigmine, because potent block by physostigmine hindered studies at higher concentrations.
Results of several previous studies could be interpreted as demonstrating the involvement of the nicotinic agonist binding site in physostigmine-mediated channel activity. Cooper et al. (1996) found that in Xenopus oocytes expressing rat embryonic receptors, physostigmine-elicited single-channel currents were not observed following an incubation with α-bungarotoxin, while the presence of mecamylamine or methyllycaconitine strongly reduced the frequency of single-channel events. Methyllycaconitine, α-bungarotoxin and d-tubocurarine were also shown to inhibit receptors activated by physostigmine in Locusta migratoria neurons (van den Beukel et al., 1998; Jackson et al., 2002) while in oocytes expressing rat neuronal α4β4 nicotinic receptors physostigmine competitively displaced 125I-epibatidine, a high affinity ligand to the ACh site (Zwart et al., 2000). In contrast to these studies, methyllycaconitine inhibited channel activation by ACh but not by physostigmine in clonal rat pheochromocytoma cells (Storch et al., 1995), and saturating concentrations of d-tubocurarine and α-bungarotoxin were found to be ineffective at blocking the ability of physostigmine to activate the Torpedo receptor (Okonjo et al., 1991). Similarly, physostigmine had low potency to reduce binding of α-bungarotoxin to the Torpedo receptor (IC50 70 to 500 μM; Sherby et al, 1985). Accordingly, the existing data are not in agreement.
To re-examine this question, we tested the ability of physostigmine to alter the channel effective opening rate in the presence of a subsaturating concentration of carbachol. We reasoned that if physostigmine interacts with the nicotinic agonist binding site then its presence should lead to a reduction in the effective opening rate for carbachol because the binding site is occupied by the low efficacy agonist physostigmine a fraction of the time. We have previously shown that the presence of low efficacy agonists known to interact with the nicotinic agonist binding site, such as tetraethylammonium or choline, competitively reduces the effective opening rate for channels activated by carbachol (Akk and Steinbach, 2003; Akk et al., 2005). In contrast, we found that physostigmine, at concentrations up to 100 μM, was ineffective at modifying the effective opening rate for carbachol. These data are consistent with the idea that physostigmine activation is not mediated by interaction with the ACh-binding site. Previous studies had shown that another APL, galantamine, also activated nAChR but did not interact with the ACh-binding site (Akk & Steinbach, 2005).
We show that mutations to the αK125 site reduce channel open durations for physostigmine but not carbachol. This appears to be consistent with the proposal that the αK125 residue participates in the binding of physostigmine (Schrattenholz et al., 1993) insofar as changes in open interval durations in response to mutations can be used to judge an involvement of a residue in agonist binding. However, it seems unlikely that the αK125 residue forms a critical element of the binding pocket because of the limited effect that the αK125Q and αK125E mutations had on channel open durations.
Despite interacting with a distinct binding site, many basic features of channel activation by physostigmine were similar to activation by nicotinic agonists. The channel conductance was indistinguishable when physostigmine instead of the nicotinic agonist carbachol was employed for channel activation. Further, mutations to the M2-M3 linker (αS269I) and the M2 domain (εT264P) affected channel gating by physostigmine and nicotinic agonists in qualitatively similar ways. Previous work has shown that channel openings elicited by nicotinic agonists as well as the APL galantamine are prolonged at hyperpolarized potentials. But, surprisingly, changes in the membrane potential were ineffective at modifying the channel open durations in the presence of 1 μM physostigmine. It seems unlikely that block could produce the lack of voltage-sensitivity because the onset of block was largely independent of membrane potential, and, in any case, termination of the open event is dominated by channel closing at this low physostigmine concentration. We are unable to provide a definitive explanation for this lack of voltage-sensitivity.
Physostigmine is a strong channel blocker. We estimate that physostigmine-induced block develops with an apparent rate of ∼40 μM-1s-1. This is not drastically different from previous estimates for k+B* for fetal type muscle nAChR (6 μM-1s-1; Bufler et al., 1996; 16 μM-1s-1; Wachtel, 1993). However, our data on physostigmine-induced block of the adult muscle-type nAChR display several important distinctions from a study conducted on the embryonic-type nAChR in BC3H-1 cells (Wachtel, 1993). First, we observed voltage-dependence for recovery from block but not development of block, while Wachtel (1993) saw voltage-sensitivity of both reactions. We note that voltage-dependence has been reported for physostigmine block of neuronal nicotinic receptors in rat hippocampal neurons (Pereira et al., 1993), and endplate currents from frog muscle (Shaw et al., 1985). Second, our findings are best described by two equivalent physostigmine blocking sites per receptor while the study on embryonic receptors was consistent with a single blocking site per receptor (Wachtel, 1993). Previous studies on block by other APL have shown that tacrine-induced block is best described by two blocked states connected to the open state (Prince et al., 2002), but block by galantamine could be accounted for by interactions with a single site (Akk and Steinbach, 2005). The voltage-dependence for block by physostigmine corresponds to a movement of a single charge through approximately 60 to 80 % of the membrane field. This suggests that when physostigmine occupies the blocking site, the charge reaches a point near the cytoplasmic end of the channel. A binding site for the classic open channel blocker QX-222 has been described at the same distance (Neher and Steinbach, 1978).
Given the pKa value for physostigmine (8.1; Meloun and Cernohorsku, 2000), the majority of physostigmine is charged when dissolved in the pipette medium (pH 7.4) Voltage-dependence of block is a strong indication that ionized species of physostigmine produce channel block. It may be interesting to determine in future studies whether channel activation by physostigmine is accomplished by charged or neutral molecules, and whether more complete studies on the activation properties of physostigmine can be carried out at higher pH values where block is reduced.
In sum, physostigmine activates the muscle nicotinic receptor but does not interact with the nicotinic binding site. The single-channel conductance in the presence of physostigmine is indistinguishable from that in the presence of the nicotinic agonist carbachol. But unlike channel activity elicited by nicotinic agonists, the durations of openings elicited by physostigmine are not affected by voltage. Physostigmine is also a potent channel blocker, and the findings are well-described by two equivalent blocking sites per receptor.
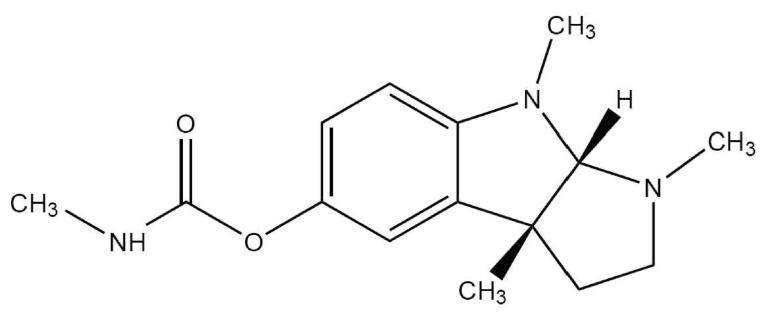
Figure 1. Structure of physostigmine.
CAS number 57-47-6.
Acknowledgments
This work was supported by NIH grant NS-22356 to JHS. JHS is the Russell and Mary Shelden Professor of Anesthesiology.
List of abbreviations
- ACh
acetylcholine
- APL
allosterically potentiating ligand
- nAChR
nicotinic acetylcholine receptor
References
- Akk G. Contributions of the non-α subunit residues (loop D) to agonist binding and channel gating in the muscle nicotinic acetylcholine receptor. J Physiol. 2002;544:695–705. doi: 10.1113/jphysiol.2002.029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Auerbach A. Activation of muscle nicotinic acetylcholine receptor channels by nicotinic and muscarinic agonists. Br J Pharmacol. 1999;128:1467–1476. doi: 10.1038/sj.bjp.0702941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Milescu LS, Heckmann M. Activation of heteroliganded mouse muscle nicotinic receptors. J Physiol. 2005;564:359–376. doi: 10.1113/jphysiol.2004.078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Steinbach JH. Structural elements near the C-terminus are responsible for changes in nicotinic receptor gating kinetics following patch excision. J Physiol. 2000;527:405–417. doi: 10.1111/j.1469-7793.2000.t01-2-00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Steinbach JH. Activation and block of mouse muscle-type nicotinic receptors by tetraethylammonium. J Physiol. 2003;551:155–68. doi: 10.1113/jphysiol.2003.043885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Steinbach JH. Galantamine activates muscle-type nicotinic acetylcholine receptors without binding to the acetylcholine-binding site. J Neurosci. 2005;25:1992–2001. doi: 10.1523/JNEUROSCI.4985-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Akaike A, Shaw KP, Rickett DL. The interaction of anticholinesterase agents with the acetylcholine receptor-ionic channel complex. Fundam Appl Toxicol. 1984;4:S27–33. doi: 10.1016/0272-0590(84)90135-0. [DOI] [PubMed] [Google Scholar]
- Auerbach A, Sigurdson W, Chen J, Akk G. Voltage dependence of mouse acetylcholine receptor gating: different charge movements in di-, mono- and unliganded receptors. J Physiol. 1996;494:155–170. doi: 10.1113/jphysiol.1996.sp021482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Beukel I, van Kleef RG, Zwart R, Oortgiesen M. Physostigmine and acetylcholine differentially activate nicotinic receptor subpopulations in Locusta migratoria neurons. Brain Res. 1998;789:263–273. doi: 10.1016/s0006-8993(98)00007-9. [DOI] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Bufler J, Franke C, Parnas H, Dudel J. Open channel block by physostigmine and procaine in embryonic-like nicotinic receptors of mouse muscle. Eur J Neurosci. 1996;8:677–687. doi: 10.1111/j.1460-9568.1996.tb01253.x. [DOI] [PubMed] [Google Scholar]
- Chen J, h A. A distinct contribution of the delta subunit to acetylcholine receptor channel activation revealed by mutations of the M2 segment. Biophys J. 1998;75:218–225. doi: 10.1016/S0006-3495(98)77508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JC, Gutbrod O, Witzemann V, Methfessel C. Pharmacology of the nicotinic acetylcholine receptor from fetal rat muscle expressed in Xenopus oocytes. Eur J Pharmacol. 1996;309:287–298. doi: 10.1016/0014-2999(96)00294-4. [DOI] [PubMed] [Google Scholar]
- Davis KL, Mohs RC, Tinklenberg JR, Pfefferbaum A, Hollister LE, Kopell BS. Physostigmine: improvement of long-term memory processes in normal humans. Science. 1978;201:272–274. doi: 10.1126/science.351807. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Newhouse P, Falk WE, Mattes JA, Physostigmine Study Group Extended-release physostigmine in Alzheimer disease: a multicenter, double-blind, 12-week study with dose enrichment. Arch Gen Psychiatry. 2000;57:157–164. doi: 10.1001/archpsyc.57.2.157. [DOI] [PubMed] [Google Scholar]
- Grosman C, Salamone FN, Sine SM, Auerbach A. The extracellular linker of muscle acetylcholine receptor channels is a gating control element. J Gen Physiol. 2000;116:327–340. doi: 10.1085/jgp.116.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R. Statistical methods for model discrimination. Biophys J. 1987;51:255–263. doi: 10.1016/S0006-3495(87)83331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C, Bermudez I, Beadle DJ. Pharmacological properties of nicotinic acetylcholine receptors in isolated Locusta migratoria neurons. Microsc Res Tech. 2002;56:249–255. doi: 10.1002/jemt.10028. [DOI] [PubMed] [Google Scholar]
- Meloun P, Cernohorsky P. Thermodynamic dissociation constants of isocaine, physostigmine and pilocarpine by regression analysis of potentiometric data. Talanta. 2000;52:931–945. doi: 10.1016/s0039-9140(00)00448-3. [DOI] [PubMed] [Google Scholar]
- Neher E, Steinbach JH. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Hutchinson DO, Milone M, Brengman JM, Bouzat C, Sine SM, Engel AG. Congenital myasthenic syndrome caused by prolonged acetylcholine receptor channel openings due to a mutation in the M2 domain of the epsilon subunit. Proc Natl Acad Sci U S A. 1995;92:758–762. doi: 10.1073/pnas.92.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonjo KO, Kuhlmann J, Maelicke A. A second pathway of activation of the Torpedo acetylcholine receptor channel. Eur J Biochem. 1991;200:671–677. doi: 10.1111/j.1432-1033.1991.tb16231.x. [DOI] [PubMed] [Google Scholar]
- Pereira EF, Reinhardt-Maelicke S, Schrattenholz A, Maelicke A, Albuquerque EX. Identification and functional characterization of a new agonist site on nicotinic acetylcholine receptors of cultured hippocampal neurons. J Pharmacol Exp Ther. 1993;265:1474–1491. [PubMed] [Google Scholar]
- Prince RJ, Pennington RA, e S. Mechanism of tacrine block at adult human muscle nicotinic acetylcholine receptors. J Gen Physiol. 2002;120:369–393. doi: 10.1085/jgp.20028583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealid patch-clamp data containing missed events. Biophys J. 1996;70:264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, s F. Maximum likelihood estimation of aggregated Markov proceses. Proc R Soc London B Biol Sci. 1997;264:375–383. doi: 10.1098/rspb.1997.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone FN, Zhou M, Auerbach A. A re-examination of adult mouse nicotinic acetylcholine receptor channel activation kinetics. J Physiol. 1999;516:315–330. doi: 10.1111/j.1469-7793.1999.0315v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrattenholz A, Coban T, Schroder B, Okonjo KO, Kuhlmann J, Pereira EF, Albuquerque EX, Maelicke A. Biochemical characterization of a novel channel-activating site on nicotinic acetylcholine receptors. J Recept Res. 1993;13:393–412. doi: 10.3109/10799899309073669. [DOI] [PubMed] [Google Scholar]
- Schrattenholz A, Godovac-Zimmermann J, Schafer HJ, Albuquerque EX, Maelicke A. Photoaffinity labeling of Torpedo acetylcholine receptor by physostigmine. Eur J Biochem. 1993;216:671–677. doi: 10.1111/j.1432-1033.1993.tb18187.x. [DOI] [PubMed] [Google Scholar]
- Schroder B, Reinhardt-Maelicke S, Schrattenholz A, McLane KE, Kretschmer A, Conti-Tronconi BM, Maelicke A. Monoclonal antibodies FK1 and WF6 define two neighboring ligand binding sites on Torpedo acetylcholine receptor α-polypeptide. J Biol Chem. 1994;269:10407–10416. [PubMed] [Google Scholar]
- Sherby SM, Eldefrawi AT, Albuquerque EX, Eldefrawi ME. Comparison of the actions of carbamate anticholinesterases on the nicotinic acetylcholine receptor. Mol Pharmacol. 1985;27:343–8. [PubMed] [Google Scholar]
- Shaw KP, Aracava Y, Akaike A, Daly JW, Rickett DL, Albuquerque EX. The reversible cholinesterase inhibitor physostigmine has channel-blocking and agonist effects on the acetylcholine receptor-ion channel complex. Mol Pharmacol. 1985;28:527–538. [PubMed] [Google Scholar]
- Smulders CJ, Zwart R, Bermudez I, van Kleef RG, Groot-Kormelink PJ, Vijverberg HP. Cholinergic drugs potentiate human nicotinic alpha4beta2 acetylcholine receptors by a competitive mechanism. Eur J Pharmacol. 2005;509:97–108. doi: 10.1016/j.ejphar.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Storch A, Schrattenholz A, Cooper JC, Abdel Ghani EM, Gutbrod O, Weber KH, Reinhardt S, Lobron C, Hermsen B, Soskic V, Pereira EFR, Albuquerque EX, Methfessel C, Maelicke A. Physostigmine, galanthamine and codeine act as ‘noncompetitive nicotinic receptor agonists’ on clonal rat pheochromocytoma cells. Eur J Pharmacol. 1995;290:207–219. doi: 10.1016/0922-4106(95)00080-1. [DOI] [PubMed] [Google Scholar]
- Svobodova L, Krusek J, Hendrych T, Vyskocil F. Physostigmine modulation of acetylcholine currents in COS cells transfected with mouse muscle nicotinic receptor. Neurosci Lett. 2006;401:20–24. doi: 10.1016/j.neulet.2006.02.065. [DOI] [PubMed] [Google Scholar]
- Wachtel RE. Physostigmine block of ion channels activated by acetylcholine in BC3H1 cells. Mol Pharmacol. 1993;44:1051–1055. [PubMed] [Google Scholar]
- Zhou M, Engel AG, Auerbach A. Serum choline activates mutant acetylcholine receptors that cause slow channel congenital myasthenic syndromes. Proc Natl Acad Sci U S A. 1999;96:10466–10471. doi: 10.1073/pnas.96.18.10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, van Kleef RG, Gotti C, Smulders CJ, Vijverberg HP. Competitive potentiation of acetylcholine effects on neuronal nicotinic receptors by acetylcholinesterase-inhibiting drugs. J Neurochem. 2000;75:2492–2500. doi: 10.1046/j.1471-4159.2000.0752492.x. [DOI] [PubMed] [Google Scholar]