Abstract
Introduction
Chronic use of cyclosporine A (CyA) induces nephrotoxicity primarily due to endothelial dysfunction. In our previous studies, potential mechanisms were identified in vitro and implicated NADPH oxidase and Interleukin-6 (IL-6) as key components in causing endothelial dysfunction. In this study, we tested the hypothesis that NADPH oxidase activity and IL-6 are key components in renal damage in an in vivo model.
Methods
Male mice C57B/6 mice from JAX Laboratories at 6–8 weeks were subjected to a low salt diet throughout the trial. After one week on a low salt diet, the mice were injected daily with treatments in 50µL vehicle composed of 75% cremaphor and Ethanol for five weeks. A vehicle alone group was also set aside. Mice were weighed and 25mg/kg/day cyclosporine was injected daily. Apocynin 20mg/kg were injected either alone or concomitantly with CyA. Another group of mice were administered IL-6 Antibody at 2µg/day along with CyA.
The kidneys were removed en bloc immediately and submitted in formalin for paraffin sections. Trichrome stains were performed.
Slides were blinded and ten photographs of cortical areas per treatment group were taken, which covered an estimate of 10% surface area in random fashion. Areas of renal damage, which were determined by tubular necrosis, were identified and quantified by amount of necrosis per photograph. Each photograph was divided into ten blocks, and the number of blocks that contained necrotic tubules per photo was recorded.
Results
The two control mice (low salt only) had no damage. The four vehicle mice had trace amounts of tubular necrosis. CyA treatment group demonstrated the highest amount of damage (29/70; 41%). CyA with apocynin, a specific NADPH oxidase inhibitor, was found to have 36% (22/60) damage, whereas the CyA with IL-6 antibody only was observed to have 15% (6/40) damage. Comparing imaging analysis, there was no difference between mice treated with CyA alone and with CyA with apocynin. However, the amount of damage in mice treated with CyA and IL-6 antibody was found to be significantly lower than both CyA and CyA with apocynin.
Conclusions
CyA action as a calcineurin inhibitor has allowed prolongation of kidney transplants, but its chronic use has led to devastating consequences such as allograft nephropathy. Previously, we have identified potential mechanisms of CyA induced endothelial dysfunction in vitro. The current study identifies increased IL-6 expression as a mechanism by which CyA induces renal damage and that the use of an IL-6 neutralizing antibody may be useful in reducing CyA induced renal damage.
Keywords: Cyclosporine A, Interleukin-6, Nephrotoxicity
Introduction
Maintaining the viability of transplanted tissues and organs is an ongoing clinical problem. Currently there is a shortage of viable tissues for transplantation thus increasing the survival time of transplants becomes a high clinical priority. Loss of graft function and transplant atherosclerosis are associated with endothelial cell dysfunction and loss of endothelial barrier integrity (1,5). Abnormalities in endothelial reactive oxygen species (ROS) generation has long been known to impact endothelial function both through degradation of nitric oxide and alterations in the endothelial barrier (2).
Calcineurin inhibitors (CNIs) are undoubtedly the most potent agents of immunosuppression. However, in pharmacological concentrations CNIs damage the endothelial barrier integrity without impairing the viability of the endothelial cells (4, 6, 7). We have observed that CyA, the prototypic CNI, disrupts endothelial in vitro capillaries and that this loss of endothelial integrity is due to a down regulation of VE-cadherins (12,13,14,15,16). More significant was our finding that the down-regulation of the VE-cadherins is consequent to dissociation of one of the key catenins, p120 ctn, from the VEcadherin/catenin complex (data communicated).
Based on the literature and our experiments we have identified in vitro that chronic use of cyclosporine A (CyA) induces nephrotoxicity primarily due to endothelial dysfunction by down regulation of VE-cadherins and implicated NADPH oxidase and Interleukin-6 (IL-6) as key components in regulation of VE-cadherin and causing endothelial dysfunction. Thus, we hypothesized that NADPH oxidase activity and IL-6 are key components in renal damage due to chronic use of CyA and inhibition of either would ameliorate the same. In this study, we tested the hypothesis that NADPH oxidase activity and IL-6 are key components in renal damage in an in vivo model.
Methods
Animals
Male mice C57B/6 mice aged 6–8 weeks were obtained from Jackson Laboratories (Bar Harbor, ME), harbored in an AALAC approved facility and had ad libitum access to food and water. All procedures were approved by the Care and Humane Use of Animals Committee at SUNY Upstate Medical University.
Experimental Protocol
At the start of the protocol mice were switched to a low salt diet (0.1 % NaCl, Purina Mills, MO) for the duration of the study. After one week on a low salt diet, the mice were injected daily with treatments in 50µL vehicle composed of 75% cremaphor (Sigma) and Ethanol for five weeks. Mice were weighed and 25mg/kg/day cyclosporine (Novartis Pharma) was injected intra peritoneally. Apocynin (Calbiochem) 20mg/kg was injected either alone or concomitantly with CyA. Another group of mice were administered IL-6 Antibody (R&D Systems, Cat # MAB406) at 2µg/day along with CyA. A vehicle alone group and a control group were also set aside.
After the treatment period, the mice were anesthetized with pentobarbital and approximately 1mL of blood was aspirated from the heart and assayed for CyA levels and IL-6 concentration.
The kidneys were removed en bloc immediately and submitted in formalin for paraffin sections. Trichrome stains were performed.
Histological Analysis
Slides were blinded and ten photographs of cortical areas per treatment group were taken, which covered an estimated 10% of the surface area in a random fashion. Areas of renal damage, which were determined by tubular necrosis, were identified and quantified by amount of necrosis per photograph. Each photograph was divided into ten blocks, and the number of blocks that contained necrotic tubules per photo was recorded. Histological analyses were blinded.
Quantification of serum IL-6
Serum IL-6 concentration of different treatment groups of mice was measured by ELISA using Quantikine Mouse IL-6 immunoassay kit (R&D systems) as per manufacturer’s protocol.
Statistical Analysis
Data were subjected to an ANOVA with significance set at p<0.05.
Results
The effect of a low-salt diet on renal architecture
The two control mice (low salt only) had no damage as demonstrated in figure 1 and table 1. In figure1 normal renal histology is observed with intact tubules and glomeruli.
Figure 1.
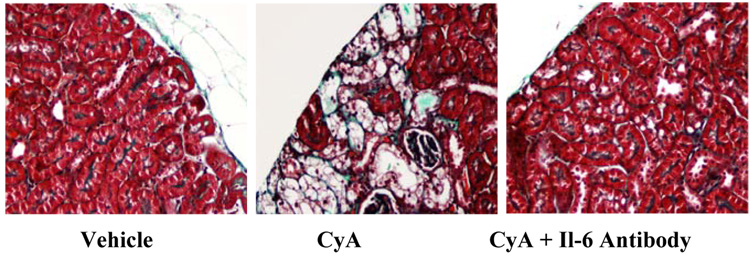
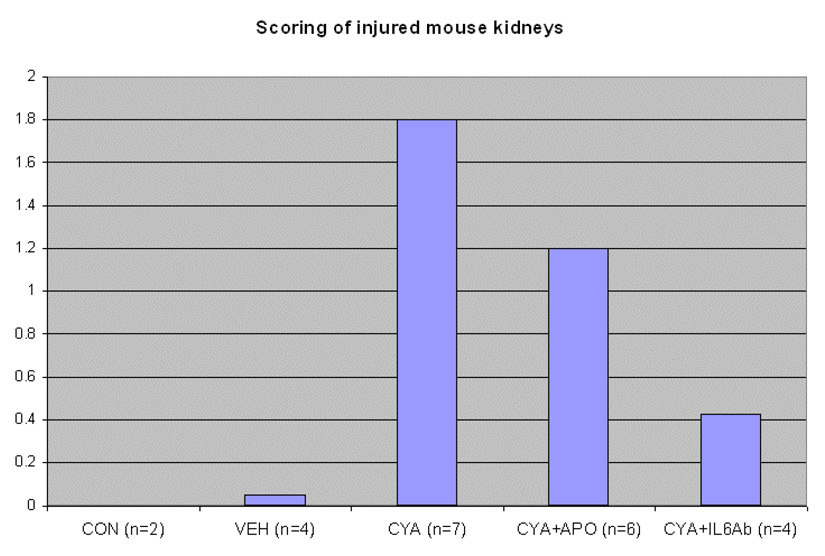
Figure 1a. Trichrome stained renal sections from mice maintained on a low salt diet for five weeks and treated with vehicle, CyA or CyA + an IL-6 neutralizing antibody.
Figure 1b. Quantification of tubular necrosis from Trichrome stained slides from treated animals. Slides were blinded and ten photographs of cortical areas per treatment group were taken, which covered an estimated 10% of the surface area in a random fashion. Areas of renal damage, which were determined by tubular necrosis, were identified and quantified by amount of necrosis per photograph. Each photograph was divided into ten blocks, and the number of blocks that contained necrotic tubules per photo was recorded. Data is represented as average block damage per all images and was anlysed by performing an ANOVA, p= 0.027.
Table 1.
Quantification of tubular necrosis from Trichrome stained slides from treated animals. Slides were blinded and ten photographs of cortical areas per treatment group were taken, which covered an estimated 10% of the surface area in a random fashion. Areas of renal damage, which were determined by tubular necrosis, were identified and quantified by amount of necrosis per photograph. Each photograph was divided into ten blocks, and the number of blocks that contained necrotic tubules per photo was recorded. Data is represented as % damaged in each treatment group. # = significant difference for CyA treated.
| Blocks Damaged | Percent Damaged | |
|---|---|---|
| Control | 0 | 0 |
| Vehicle | Trace | Trace |
| CyA | 29/70 | 41% |
| CyA + Apocynin | 22/60 | 36% |
| CyA + IL-6 Ab | 6/40 # | 15% # |
The effect of Cremaphor combined with a low-salt diet on renal architecture
The vehicle treated mice (n=4) had trace amounts of tubular necrosis that was not significantly different from control. Further, the trace amount of tubular necrosis was only seen in 50% of mice in the group (table 2). However, there was no way to determine if the small amount of damage observed was simply artifact from the sample preparation or was due to the influence of the cremaphor vehicle.
Table 2.
Percentage of mice affected by CyA induced tubular necrosis in different treatment groups.
| Treatments | # Affected Mice | # Total Mice | % Affected |
|---|---|---|---|
| Control (n=2) | 0 | 2 | 0 |
| Vehicle (n=4) | 2 | 4 | 50 |
| CyA (n=7) | 6 | 7 | 86 |
| CyA + Apocynin (n=6) | 4 | 6 | 67 |
| CyA + IL-6ab (n=4) | 1 | 4 | 25 |
The effect of Cyclosporin combined with a low-salt diet on renal architecture
CyA treatment group demonstrated the highest amount of damage (29/70; 41%, n=7) which was expected. 86% of mice were affected in this group (6 out of 7, table 2). The renal tissue demonstrated widespread tubular destruction which is in accordance with the known renal toxicity of CyA. Further this damage was statistically significant when compared to the control data (p=0.027).
The effect of Apocynin and Cyclosporin combined with a low-salt diet on renal architecture
The CyA with apocynin, a specific NADPH oxidase inhibitor, was found to have 36% (22/60, n=6) renal damage which demonstrated a similar pattern as that seen with the cyclosporine treated group i.e. acute tubular necrosis. Cotreatment with apocynin also decreased the % of affected animals to 67, four out of six mice showed tubular necrosis.
The effect of an Interleukin-6 neutralizing antibody and Cyclosporin combined with a low-salt diet on renal architecture
The CyA with IL-6 antibody only was observed to have 15% (6/40, n=4) damage. Comparing imaging analysis, there was no difference between mice treated with CyA alone and with CyA with apocynin. However, the amount of damage in mice treated with CyA and IL-6 antibody was found to be significantly lower than both CyA and CyA with apocynin (p=0.027). The % of affected animals were decreased from 86 (CyA only group) to 25 when IL-6 antibody was used in combination with CyA.
The effect of CyA on serum IL-6 concentration
CyA administration increased the serum IL-6 levels from 3.4±0.6pg/ml in control mice to 9.25±1.4pg/ml in mice treated with CyA alone. IL-6 levels in mice that received IL-6 antibody treatment alongwith CyA was comparable to that of control at 3.96±2.05pg/ml as shown in figure 2 (p=0.05). The CyA levels in all the treatment groups were also measured and represented in table 2.
Figure 2.
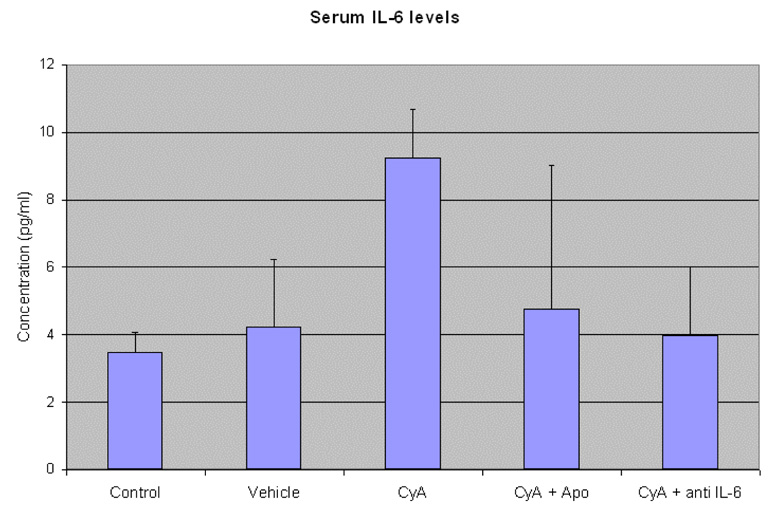
Serum IL-6 concentration in mice from the different treatment groups. Blood was collected from the mice of different experimental groups at the end of the experimental duration (6weeks) after the animals were euthanized. Data is represented as mean± SD and was analyzed by performing an ANOVA (p=0.05)
Discussion
CyA’s action as a calcineurin inhibitor has allowed prolongation of kidney transplants, but its chronic use has led to devastating consequences such as allograft nephropathy (3,8,9,10,11). Previously, we have identified potential mechanisms of CyA induced endothelial dysfunction in vitro. The current study identifies increased IL-6 expression as a mechanism by which CyA induces renal damage and that the use of an IL-6 neutralizing antibody may be useful in reducing CyA induced renal damage.
Increased IL-6 expression has been noted in chronically rejecting tissues and has been associated with graft dysfunction. CyA induces the secretion of IL-6 from endothelial cells secondary to activation of NADPH oxidase and we have shown in vitro that IL-6 antibodies prevent CyA mediated down regulation of VE cadherins and the disruption of in vitro capillaries (Data communicated). Therefore, we decided to determine if the protective effect of IL-6 neutralizing antibodies could be demonstrated in vivo. Our study demonstrates that co-treatment of animals with IL-6 neutralizing antibodies and CyA reduces the nephrotoxic effects of CyA.
Surprisingly, inhibition of NADPH oxidase had only a modest protective effect. In light of our previous studies that demonstrated that CyA increases IL-6 levels through activation of NADPH oxidase we had expected that the NADPH oxidase inhibitor apocynin would have been equally as effective as the IL-6 neutralizing antibody in preventing tubular sclerosis. However, our data clearly demonstrate that this is not the case. Given the fact that the IL-6 neutralizing antibody was so much more effective leads us to the conclusion that either apocynin is not nearly as effective in vivo as it is in vitro, or that CyA increases IL-6 both in a NADPH oxidase dependent and independent manner. In our study it is likely that the bioavailability of apocynin is reduced in vivo as compared to our in vitro studies. This is likely due to the fact that apocynin is hydrophobic and probably binds tightly to albumin and thus less is available to enter the cells.
Our observations of renal injury primarily demonstrated tubular necrosis rather than vascular or endothelial damage. While it is possible that CyA causes direct damage to tubular cells, we hypothesize that tubular damage is secondary to endothelial dysfunction possibly through the filtration of excessive IL-6 into the tubule or through loss of the glomerular endothelial barrier which would result in excessive protein being filtered into the tubule leading to osmotic stress. Further studies into the mechanism(s) of CyA induced tubular necrosis are needed to answer these questions.
The present study demonstrates that CyA administration induces an increase in serum IL-6 levels. And the use of Interleukin-6 neutralizing antibodies alongwith CyA immunosuppression regimen ameliorates the renal damage induced by the latter. However, increasing the duration of CyA administration in the experimental animals would certainly provide more insight into the role of IL-6 neutralizing antibody in ameliorating CyA induced renal damage. Further insights may also be obtained on the role of cotreatment of CyA and IL-6 neutralizing antibody on prevention of renal damage as well as chronic allograft vasculopathy in an allotransplantation model.
Table 3.
Cyclosporine A levels in the blood of mice from different treatment groups. Blood was collected from the heart of mice at the end of the experimental duration (6 weeks) after the animals were euthanized.
| Treatment Groups | CyA (ng/ml) |
|---|---|
| Control (n=2) | NA |
| Vehicle (n=4) | NA |
| CyA (n=7) | 336, 6010, 2095, 2040, 2455, 2680, 2320 |
| CyA + Apo (n=6) | 1038, 1780, 1605, 1370, 1715, 1580 |
| CyA + anti IL-6 (n=4) | 412, 795, 945, 319 |
Acknowledgements
This work was supported by a grant for the Rochester Finger Lakes Eye and Tissue Bank and a grant from the National Institutes of Health (5R21CA106515).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andriambeloson E, Pally C, Hengerer B, Cannet C, Nikolova Z, Bruns C, Zerwes HG, Bigaud M. Transplantation-induced endothelial dysfunction as studied in rat aorta allografts. Transplantation. 2001;72:1881–1889. doi: 10.1097/00007890-200112270-00004. [DOI] [PubMed] [Google Scholar]
- 2.Cailhier JF, Laplante P, Hebert MJ. Endothelial apoptosis and chronic transplant vasculopathy: recent results, novel mechanisms. Am J Transplant. 2006;6:247–253. doi: 10.1111/j.1600-6143.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 3.Hazzan M, Buob D, Labalette M, Provot F, Glowacki F, Hoffmann M, Copin MC, Noel C. Assessment of the risk of chronic allograft dysfunction after renal transplantation in a randomized cyclosporine withdrawal trial. Transplantation. 2006;82:657–662. doi: 10.1097/01.tp.0000229424.11872.a0. [DOI] [PubMed] [Google Scholar]
- 4.Koskinen PK, Lemstrom KB, Hayry PJ. How cyclosporine modifies histological and molecular events in the vascular wall during chronic rejection of rat cardiac allografts. Am J Pathol. 1995;146:972–980. [PMC free article] [PubMed] [Google Scholar]
- 5.Menger MD, Vollmar B. Role of microcirculation in transplantation. Microcirculation. 2000;7:291–306. [PubMed] [Google Scholar]
- 6.Mercanoglu F, Turkmen A, Kocaman O, Pinarbasi B, Dursun M, Selcukbiricik F, Sever MS. Endothelial dysfunction in renal transplant patients is closely related to serum cyclosporine levels. Transplant Proc. 2004;36:1357–1360. doi: 10.1016/j.transproceed.2004.05.073. [DOI] [PubMed] [Google Scholar]
- 7.Morris ST, McMurray JJ, Rodger RS, Farmer R, Jardine AG. Endothelial dysfunction in renal transplant recipients maintained on cyclosporine. Kidney Int. 2000;57:1100–1106. doi: 10.1046/j.1523-1755.2000.00937.x. [DOI] [PubMed] [Google Scholar]
- 8.Nacar A, Kiyici H, Ogus E, Zagyapan R, Demirhan B, Ozdemir H, Haberal M. Ultrastructural examination of glomerular and tubular changes in renal allografts with cyclosporine toxicity. Ren Fail. 2006;28:543–547. doi: 10.1080/08860220600923086. [DOI] [PubMed] [Google Scholar]
- 9.Oflaz H, Turkmen A, Kazancioglu R, Kayacan SM, Bunyak B, Genchallac H, Erol B, Mercanoglu F, Umman S, Sever MS. The effect of calcineurin inhibitors on endothelial function in renal transplant recipients. Clin Transplant. 2003;17:212–216. doi: 10.1034/j.1399-0012.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- 10.Paul LC. Endothelial dysfunction, atherosclerosis and cyclosporine in renal transplantation. Neth J Med. 2001;59:1–3. doi: 10.1016/s0300-2977(01)00109-7. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz JC, Campistol JM, Grinyo JM, Mota A, Prats D, Gutierrez JA, Henriques AC, Pinto JR, Garcia J, Morales JM, Gomez JM, Arias M. Early cyclosporine a withdrawal in kidney-transplant recipients receiving sirolimus prevents progression of chronic pathologic allograft lesions. Transplantation. 2004;78:1312–1318. doi: 10.1097/01.tp.0000137322.65953.0a. [DOI] [PubMed] [Google Scholar]
- 12.Wilasrusmee C, Da Silva M, Singh B, Kittur S, Siddiqui J, Bruch D, Wilasrusmee S, Kittur DS. A new in vitro model to study endothelial injury. J Surg Res. 2002;104:131–136. doi: 10.1006/jsre.2002.6429. [DOI] [PubMed] [Google Scholar]
- 13.Wilasrusmee C, Da Silva M, Singh B, Siddiqui J, Bruch D, Kittur S, Wilasrusmee S, Kittur DS. Morphological and biochemical effects of immunosuppressive drugs in a capillary tube assay for endothelial dysfunction. Clin Transplant. 2003;17 Suppl 9:6–12. doi: 10.1034/j.1399-0012.17.s9.1.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilasrusmee C, Da Silva M, Siddiqui J, Bruch D, Kittur S, Wilasrusmee S, Kittur DS. Role of endothelin-1 in microvascular dysfunction caused by cyclosporin A. J Am Coll Surg. 2003;196:584–591. doi: 10.1016/S1072-7515(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 15.Wilasrusmee C, Ondocin P, Bruch D, Shah G, Kittur S, Wilasrusmee S, Kittur DS. Amelioration of cyclosporin A effect on microvasculature by endothelin inhibitor. Surgery. 2003;134:384–389. doi: 10.1067/msy.2003.233. [DOI] [PubMed] [Google Scholar]
- 16.Wilasrusmee C, Shah G, Kittur S, Halverson A, Bruch D, Kittur D. Signal transduction pathway in endothelial dysfunction. Surg Infect (Larchmt) 2004;5:9–14. doi: 10.1089/109629604773860255. [DOI] [PubMed] [Google Scholar]


