Abstract
We hypothesized that, in esophageal squamous epithelial cells, there are differences among individuals in the signal transduction pathways activated by acid reflux that might underlie the development of Barrett's esophagus. To explore that hypothesis, we immortalized nonneoplastic, esophageal squamous cells from patients with gastroesophageal reflux disease (GERD) with (NES-B3T) and without (NES-G2T) Barrett's esophagus and used those cells to study acid effects on MAPK proteins. During endoscopy in patients with GERD with and without Barrett's esophagus, we took biopsy specimens from the distal squamous esophagus to study MAPK proteins before and after esophageal perfusion with 0.1 N HCl. We used immunoblotting and Western blotting to study MEK1/2 phosphorylation at two activating sites (serines 217/221), MEK1 phosphorylation at an inhibitory site (threonine 286), and MEK1/2 activity. After acid exposure, both cell lines exhibited increased MEK1/2 phosphorylation at the activating sites; the NES-B3T cells had higher levels of MEK1 phosphorylation at the inhibitory site, however, and only the NES-G2T cells showed an acid-induced increase in MEK1/2 activity. Similarly, in the squamous epithelium of patients with GERD with and without Barrett's esophagus, acid perfusion increased MEK1/2 phosphorylation at the activating sites in both patient groups; the Barrett's patients had higher levels of MEK1 phosphorylation at the inhibitory site, however, and only the patients without Barrett's demonstrated an acid-induced increase in ERK1/2 phosphorylation. In esophageal squamous cell lines and biopsies from patients with GERD with and without Barrett's esophagus, we have found differences in MAPK pathways activated by acid exposure. We speculate that these differences might underlie the development of Barrett's metaplasia.
Keywords: gastroesophageal reflux disease, mitogen-activated protein kinase
gastroesophageal reflux disease (GERD) has been established as a strong risk factor for adenocarcinoma of the esophagus, and surveys suggest that up to 40% of adult Americans have GERD (12). GERD often results in inflammation in the esophageal squamous epithelium (reflux esophagitis), which, in most individuals, heals through the process of squamous cell regeneration. In some individuals, however, the reflux-damaged squamous epithelium heals through a metaplastic process in which squamous cells are replaced by intestinal-type columnar cells (26). This condition, called Barrett's esophagus, predisposes to esophageal adenocarcinoma, a deadly tumor whose incidence has increased profoundly in the United States over the past several decades (16, 26).
It is not known why only a minority of individuals with GERD develop Barrett's esophagus. Recent reports suggest that differences in the molecular mechanisms triggered when esophageal squamous cells are exposed to refluxed gastric material might play a role in determining whether reflux esophagitis heals through metaplasia or through squamous cell regeneration. In the esophageal squamous epithelium of patients with GERD with and without Barrett's esophagus, for example, differences have been found in baseline expression levels of Dickkopf-1 and -4 genes, which are known to regulate proliferation and apoptosis and which can be induced by acid exposure (2). When exposed to acid and bile salts, esophageal squamous cells from patients with GERD generate reactive oxygen species but through different intracellular generator pathways in patients with Barrett's esophagus compared with those without (4). In addition to causing cellular injury, reactive oxygen species molecules can regulate proliferation, differentiation, and apoptosis. Conceivably, differences in reflux-induced activation of molecular signaling pathways in esophageal squamous cells could underlie the development of Barrett's metaplasia.
The MAPK signaling cascade comprises three principal components including the ERK, JNK, and p38 kinase (9, 30). In general, ERK-mediated signaling influences cell proliferation, survival, and differentiation, whereas signals transmitted by JNK and p38 affect apoptosis (9). The best characterized MAPK signaling pathway is the Ras-Raf-MEK-ERK cascade (9, 30). ERK1/2 is activated when its tyrosine and threonine residues are phosphorylated by MEK, an upstream, dual-specificity MEK (24). This results in translocation of ERK1/2 to the nucleus, where the protein initiates the transcription of numerous growth regulatory genes (24). The activity of MEK is regulated by site-specific phosphorylation. For example, the phosphorylation of MEK1/2 at serines 217/221 enables the kinase activity that is required for activation of ERK1/2. In contrast, MEK1 phosphorylation at threonine 286 inhibits the kinase activity, thereby preventing ERK1/2 activation (19, 23, 24). Finally, ERK1/2 activity also can be regulated by dephosphorylation. For example, dephosphorylation of ERK1/2 by MAPK phosphatases (MKPs) inhibits ERK1/2 activity (11).
We have hypothesized that, in esophageal squamous epithelial cells, there are differences among individuals in signal transduction pathways activated by acid reflux that might underlie the development of Barrett's esophagus. In an earlier report, we described the effects of acid perfusion on the ERK1/2 pathways in the esophageal squamous epithelium of patients with GERD with and without Barrett's esophagus (25). We found significant differences between those patient groups in baseline and acid-induced levels of ERK1/2 in esophageal squamous epithelium (25). The present study was designed to explore the mechanisms underlying those differences. To do so, we have immortalized nonneoplastic esophageal squamous epithelial cells from patients with GERD through the stable incorporation of human telomerase reverse transcriptase (hTERT). Using those cell lines and biopsies of esophageal squamous epithelium taken from patients with GERD with and without Barrett's esophagus during endoscopic examinations, we investigated the effects of acid exposure on the activity and phosphorylation of MEK1/2 and the expression levels of MKP-1 both in vitro and in vivo.
MATERIALS AND METHODS
Isolation and culture of esophageal squamous cells.
This study was approved by the institutional review board on human studies at the Dallas VA Medical Center. During endoscopic examinations, samples of the squamous epithelium of the distal esophagus were taken from patients with GERD with and without long-segment Barrett's esophagus (defined as ≥3 cm of specialized intestinal metaplasia in the distal esophagus) with the use of jumbo biopsy forceps (Olympus FB-50K-1). Biopsy tissues were placed in cold Hank's salt solution containing 1% antibiotic-antimycotic (both from Invitrogen, Carlsbad, CA) and processed within 2 h of collection. Six biopsies were placed into a conical tube containing trypsin (Invitrogen) at 37°C for 30 min. Following trypsinization, biopsies were dispersed into a single-cell suspension and cocultivated with mouse embryonic Albino Swiss 3T3 [American Type Culture Center (ATCC), CCL-92] fibroblast cells (1–3 × 106 cells/100-mm plate) pretreated with mitomycin C (10 μg/ml for 2 h), in a supplemented 3:1 mixture of DMEM/Ham's F12 medium (Invitrogen) as previously described (13, 18). For subculturing, fibroblast feeder cells were removed with EDTA washes (0.02% in 1× PBS, pH 8.0; Sigma, St. Louis, MO) before trypsinization of the epithelial cells. Squamous cells were resuspended in fresh medium containing 5% trypsin inhibitor (Sigma), reseeded at a 1:8 split ratio, and grown at 37°C in 5% CO2 with fresh media changes three times weekly. The number of population doublings (PD) per passage was calculated as log [(number of cells harvested/number of cells plated)/log2].
Retroviral vectors and transductions.
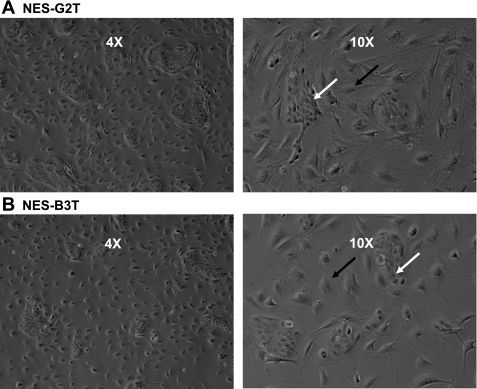
Esophageal squamous cells were retrovirally infected with hTERT using the Cre-lox recombination system as previously described by our laboratory (8). Cells at ∼30–50% confluence were infected twice with the retroviral vector containing hTERT flanked by lox-P sites (hTERTloxP) in the presence of 4 μg/ml of Polybrene (Sigma) at PDs 10–16. Esophageal squamous epithelial cell lines derived from patients who had GERD with Barrett's esophagus (NES-B3T) and without Barrett's esophagus (NES-G2T) containing the hTERTloxp vector were selected with 500 ng/ml puromycin for 7–9 days (Fig. 1).
Fig. 1.
Telomerase-immortalized esophageal squamous epithelial cells in culture. Low (4×) and high (10×) power magnifications of esophageal squamous epithelial cells (white arrows) grown with ATCC Albino Swiss 3T3 fibroblast feeder cells (black arrows). A: NES-G2T squamous cells from a patient with gastroesophageal reflux disease (GERD) without Barrett's esophagus. B: NES-B3T squamous cells from a patient with GERD with Barrett's esophagus.
Telomerase activity.
Telomerase activity was measured in cultured cells before and after the introduction of hTERT with the TRAP-eze Telomerase Detection kit (Intergen, Burlington, MA) as previously described (7, 8). Telomerase-expressing cervical cancer-derived HeLa cells were used as positive controls. Lysis buffer was used as a negative control.
Contact inhibition and anchorage-dependent cell growth assay.
50,000 telomerase-immortalized NES-B3T and 120,000 telomerase-immortalized NES-G2T cells were plated in six-well plates and harvested at multiple time points ranging from 1 to 16 days. Cells were harvested with 0.05% trypsin and counted by Coulter counter. An in vitro marker for neoplastic cells is the ability to grow in soft agar. A 12-well soft agar plate was created with two layers: the bottom layer had 0.5% (wt/vol) of Noble agar (Sigma, A5431) in DMEM/F12 supplemented with 20% FBS (Atlanta Biologicals, Norcross, GA). The top layer consisted of cells with 0.3% agar in DMEM/F12 and 20% FBS. 5,000 telomerase-immortalized NES-B3T and NES-G2T cells were plated in triplicate. For positive controls, 1,000 SEG-1 cancer cells were also plated in triplicate as previously described (8). Cells were fed weekly with 200 μl of media containing 10% serum. Plates were examined daily for 3 wk. Cells were imaged using a Bio-Rad Molecular Imager (Bio-Rad, Hercules, CA). All experiments were performed in duplicate.
Western blotting and MEK activity assays.
Cells and tissues were lysed in 1× cell lysis buffer (Cell Signaling Technology, Beverly, MA). Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis; protein concentrations were determined using the BCA-200 Protein Assay kit (Pierce, Rockford, IL). After separation and transfer to nitrocellulose membranes, the membranes were incubated with primary antibodies to p53 and p21 (Oncogene, San Diego, CA), cytokeratins (CK) 13 and 4 (Novocastra, Newcastle upon Tyne, UK), phospho-MEK1/2 (serines 217/221), phospho-MEK1 (threonine 286), phospho-ERK1/2, or MKP-1 (Cell Signaling Technology). Horseradish peroxidase secondary antibodies were used, and chemiluminescence was determined using the Super Signal West Dura detection system (Pierce). Membranes were stripped using Restore Stripping Buffer (Pierce) and were reprobed with total ERK1/2 or total MEK1/2 (Cell Signaling Technology). Tubulin (Sigma) was used to confirm equal loading. Doxorubicin-treated MCF-7 cells were used as controls for p53 and p21. Neonatal human foreskin keratinocytes were used as a control for cytokeratins 4 and 13. MEK1/2 activity was determined using a commercially available immunoblot assay per the manufacturer's instructions (Sigma). In brief, equal amounts of protein lysate were immunoprecipitated with 2 μl of anti-MEK antibody and EZView Red Protein A affinity gel beads for 4 h at 4°C. The supernatant was then removed and the pellet washed three times with 1× wash buffer. Assay buffer and 1 μg of ERK2 substrate were added and incubated with the pellet for 30 min at 30°C. The reaction was terminated with 12 μl of 4× SDS sample buffer followed by Western blotting for phosphorylated ERK1/2. β-tubulin (Sigma) was used to confirm that equal amounts of protein lysates were added to the reaction. All Western blots were performed in duplicate.
UV irradiation.
Telomerase-immortalized NES-B3T and NES-G2T squamous epithelial cells were cultured overnight in 100-mm plates as described above. Cells were rinsed several times with 1× PBS and exposed to 200 J/m2 UV-B irradiation as previously described (8). Protein was harvested at 6, 24, and 48 h after irradiation. Nonirradiated cells served as controls.
Cytogenetic analysis.
Cytogenetic preparations were made following conventional procedures. Briefly, metaphase NES-B3T (PD 53.9) and NES-G2T (PD 64) cells were obtained by Colcimid arrest followed by hypotonic treatment with prewarmed 0.075 M KCl. They were then fixed and washed in freshly made modified Carnoy's fixative (3:1 absolute methanol:glacial acetic acid), spread onto precleaned, wet microscope slides, and air dried. Trypsin G-banding was performed following a modified methodology (21).
Cell lines and acid exposure.
NES-B3T and NES-G2T cell lines were cultured in DMEM/F12 with supplements (growth media). Cells were cultured either in neutral full growth media (pH 7.2) or in acidic full growth media (brought to a pH of 5.0 with 1 M HCl) for 3, 5, or 10 min, after which cells were collected for analyses.
Patients and acid exposure.
Patients scheduled for elective endoscopy at the Dallas VA Medical Center who had GERD with and without long-segment Barrett's esophagus were invited to participate in the study. During endoscopy, three samples of the squamous mucosa were taken using a jumbo biopsy forceps (Olympus FB-50K-1) before and after perfusion of the distal esophagus with 20 ml of 0.1 N HCl for 3 min using the technique previously described by our laboratory (25). None of the patients with GERD had erosive esophagitis at the time of enrollment in the study.
Statistical analyses.
Quantitative data are expressed as the mean ± SE. Statistical analyses were performed using the paired Student's t-test with the Prism statistical software package (GraphPad Software, San Diego, CA). P values ≤0.05 were considered significant for all analyses.
RESULTS
Establishment of immortalized NES-B3T and NES-G2T esophageal squamous cell lines.
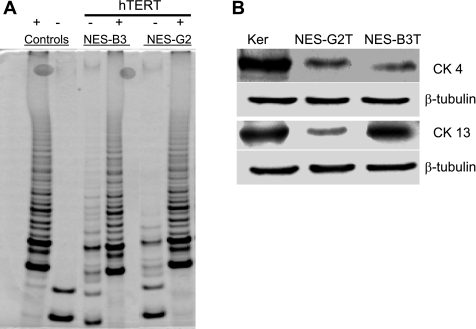
The log phase doubling times of hTERT-immortalized NES-B3T and NES-G2T esophageal squamous cells were 24–26 h and 36–40 h per PD, respectively. To date, NES-B3T cells have been cultured beyond 100 PDs, and NES-G2T cells have been cultured to 80 PDs, both without evidence of senescence. The TRAP-eze detection kit demonstrated substantial telomerase activity after the introduction of hTERT in both cell lines (Fig. 2A).
Fig. 2.
A: telomerase activity in parent and human telomerase reverse transcriptase (hTERT)-infected esophageal squamous cell lines. HeLa cells served as a positive (+) control; lysis buffer served as a negative (−) control. TRAP assay shows a characteristic, six-base-pair ladder in cells with telomerase activity, which include the positive control and the NES-B3 and NES-G2 cells infected with hTERT. B: both the NES-G2T and NES-B3T cell lines express the squamous cytokeratin (CK4 and CK13); β-tubulin served as a loading control. Skin derived keratinocytes (Ker) served as a positive control.
NES-B3T and NES-G2T cells express squamous cell cytokeratins and display minimal chromosomal abnormalities.
NES-B3T and NES-G2T cell lines showed immunoblot evidence for expression of both CK 4 and CK 13, which are markers of esophageal squamous cell differentiation (3, 5, 20) (Fig. 2B). To identify structural and numerical chromosomal abnormalities, we used conventional G-banding analysis. Our analysis of 20 metaphase cells (∼PD 64) for NES-G2T revealed a 47,XY,+i(8)(q10) in 19 cells with one cell containing a 47,XY,+5 karyotype. Our analysis of NES-B3T (∼PD 54) revealed a 47,XX, +20 karyotype as the sole abnormality. No other consistent numerical or structural changes were identified.
NES-B3T and NES-G2T cells are nontumorigenic.
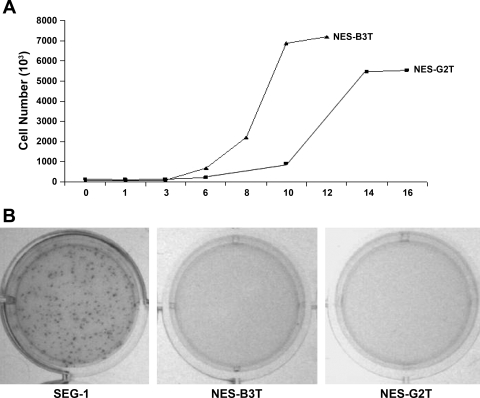
Immortalization can be the first step in in vitro transformation (14). Therefore, we determined the degree of in vitro tumorigenicity for our NES-B3T and NES-G2T cells. Unlike transformed cells, our cell lines demonstrate cell-contact inhibition (Fig. 3A). Transformed cells also exhibit anchorage independent growth, as evidenced by their ability to form colonies in soft agar. As shown in Fig. 3B, the SEG-1 cancer cell line developed multiple colonies in soft agar, whereas NES-B3T and NES-G2T cells showed no growth after 3 wk. These findings suggest that our long-term cultures of NES-B3T and NES-G2T cells are nontumorigenic although the ultimate evidence that cells are not transformed is the demonstration of their inability to form tumors after injection into athymic nude mice.
Fig. 3.
Telomerase-immortalized esophageal squamous cells exhibit contact inhibition and do not grow in soft agar. A: NES-G2T and NES-B3T cells demonstrate early log phase growth, but, with increasing confluence, the cells demonstrate a growth plateau. B: NES-G2T and NES-B3T cells show no colonies after 3 wk in soft agar in contrast to SEG-1 cancer cells, which exhibit numerous colonies.
NES-B3T and NES-G2T cells express p53 and p21 after UV irradiation.
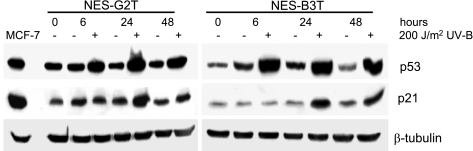
Viral oncoproteins used to immortalize human cells commonly disrupt the p53 cell cycle checkpoint (6). In contrast, telomerase-immortalized epidermal keratinocytes, mammary epithelial cells, esophageal squamous, and Barrett's epithelial cells maintain appropriate p53 cell cycle checkpoint responses (8, 13, 18). We determined whether our NES-B3T and NES-G2T cells could mount an appropriate p53 response to UV-induced DNA damage (Fig. 4). NES-B3T and NES-G2T cells were harvested at various time points after irradiation with 200 J/m2 of UVB. This single dose of UV exposure increased p53 protein expression levels within 6 h, and levels remained elevated at 48 h (Fig. 4). In both cell lines, expression of p21, a downstream effector of p53, increased at 24 h after UV exposure and remained elevated at 48 h after exposure (Fig. 4).
Fig. 4.
UVB irradiation induces expression of p53 and p21 in NES-G2T and NES-B3T cells. After irradiation, both cell lines show increased p53 expression by 6 h and increased p21 expression by 24 h; β-tubulin served as a loading control. MCF-7 cells treated with 0.2 μg/ml of doxorubicin served as a positive control for p53 and p21 expression.
Baseline levels of phosphorylated ERK1/2 are lower in NES-G2T than in NES-B3T cells, and acid exposure increases ERK1/2 phosphorylation only in NES-G2T cells.
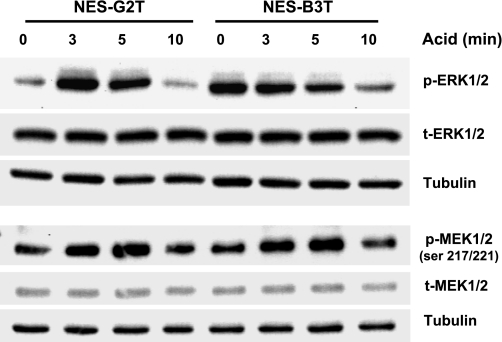
In an earlier study, we showed that the esophageal squamous epithelium of patients with GERD without Barrett's esophagus had lower baseline levels of phosphorylated ERK1/2 than that of patients with GERD with Barrett's esophagus (25). In addition, we found that esophageal acid perfusion activated ERK1/2 in the squamous epithelium of patients with GERD without Barrett's esophagus, but not in those with Barrett's esophagus (25). In the present study, we explored whether our NES-B3T and NES-G2T cells recapitulated those differences in vitro. In accordance with our in vivo data, we found that NES-G2T cells exhibited lower baseline levels of phosphorylated ERK1/2 than NES-B3T cells (Fig. 5). At 3 and 5 min of acid exposure, ERK1/2 phosphorylation increased in the NES-G2T cells; phospho-ERK1/2 levels were slightly decreased following 10 min of acid exposure (Fig. 5). In contrast, acid exposure decreased levels of phospho-ERK1/2 in NES-B3T cells in a duration-dependent manner (Fig. 5). Thus our esophageal cell lines appear to be good in vitro models that recapitulate the differences in baseline and acid-induced ERK1/2 phosphorylation that we had observed in our earlier study of primary biopsy tissues.
Fig. 5.
Acid increases MEK1/2 phosphorylation at activating sites (serines 217/221) in both cell lines, but acid increases ERK1/2 phosphorylation only in NES-G2T cells. Total ERK1/2, total MEK1/2, and β-tubulin were used as loading controls.
Acid exposure increases MEK1/2 phosphorylation at activating sites (ser217/221) in both NES-G2T and NES-B3T cells.
We next used our cells to investigate the mechanisms underlying the disparate effects of acid on ERK1/2 phosphorylation. We found no apparent differences in expression of MKP-1 at baseline or after acid exposure between NES-B3T and NES-G2T cells (data not shown). Thus differences in MKP-1 do not appear to underlie the differences in acid-induced phospho-ERK1/2 expression between the two cell lines. We next sought to determine the effects of acid on MEK1/2 phosphorylation sites (serines 217/221) that activate the kinase activity, using total MEK1/2 and tubulin as controls. Compared with nonacid exposed controls, we found a marked increase in phospho-MEK1/2 (ser 217/221) in both NES-G2T and NES-B3T cells at 3 and 5 min of acid exposure; there was no change in phospho-MEK1/2 expression levels by 10 min of acid exposure in either cell line (Fig. 5). These data suggest that failure of acid to cause MEK1/2 phosphorylation at activating sites is not the mechanism underlying the lack of acid-induced phosphorylation of ERK1/2 in NES-B3T cells.
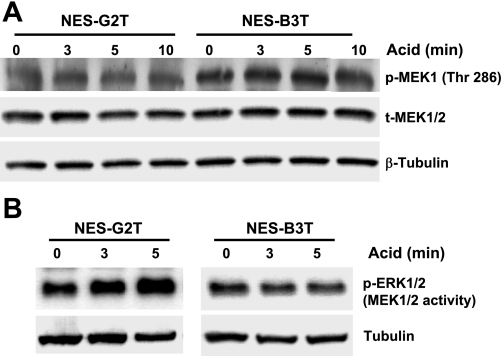
NES-B3T cells have higher levels of MEK1 phosphorylation at an inhibitory site (threonine 286) than NES-G2T cells.
MEK1 and MEK2 are highly homologous proteins, but MEK1 has threonine located at position 286, whereas MEK2 has valine at that position. MEK1 phosphorylation at threonine 286 inhibits the kinase activity of the enzyme (19, 23). Compared with NES-G2T cells, NES-B3T cells exhibited higher levels of MEK1 phosphorylation at the threonine 286 inhibitory site at baseline and at all time points after acid exposure (Fig. 6A). Acid exposure had no apparent effect on the levels of this phosphorylated form of MEK1 in either cell line (Fig. 6A). These data suggest, but do not prove, that it is the high basal levels of phospho-MEK1 threonine 286 that prevent ERK1/2 phosphorylation following acid exposure in NES-B3T cells.
Fig. 6.
A: NES-B3T cells have higher levels of MEK1 phosphorylation at an inhibitory site than NES-G2T cells. Total MEK1/2 and β-tubulin were used as loading controls. B: acid exposure increases MEK1/2 activity (measured as ERK1/2 phosphorylation) in NES-G2T but not in NES-B3T cells. β-tubulin expression in whole cell lysates served as a control to confirm that equal amounts of protein were added in the immunoprecipitation reaction.
Acid exposure increases MEK1/2 activity in NES-G2T cells but not in NES-B3T cells.
To confirm that the acid-induced increase in ERK phosphorylation at 3 and 5 min that we observed in our NES-G2T cells is due to an acid-induced increase in MEK1/2 kinase activity, we immunoprecipitated the MEK1/2 kinases before and after acid exposure and performed an in vitro kinase assay using exogenous ERK2 as the substrate. As expected, acid increased MEK1/2 activity in NES-G2T cells but not in NES-B3T cells (Fig. 6B).
Acid exposure increases MEK1/2 phosphorylation at activating sites (serines 217/221) in squamous mucosa from patients with GERD with and without Barrett's esophagus.
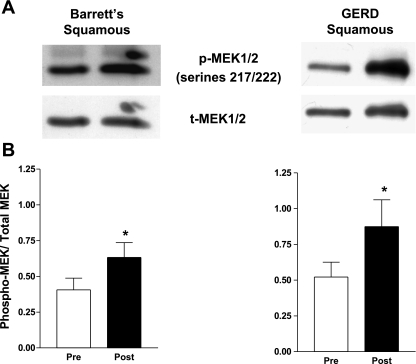
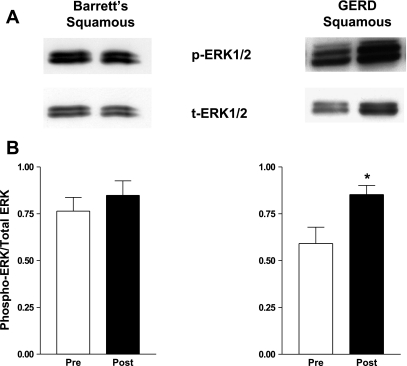
Having found that acid exposure increases MEK1/2 phosphorylation at activating sites (ser 217/221) in both the NES-G2T and NES-B3T cell lines, we sought to confirm that these same effects occur in vivo by obtaining endoscopic biopsy specimens of esophageal squamous epithelium before and after acid perfusion in seven patients with GERD with Barrett's esophagus and five patients with GERD without Barrett's esophagus. In agreement with our in vitro data, we found that acid significantly increased phospho-MEK1/2 levels at serines 217/221 in the esophageal squamous epithelium of patients with GERD both with [.41 ± .08 to .63 ± .28 relative intensity units (RIUs), P = .04] and without (.52 ± .10 to .87 ± .42 RIUs, P = .03) Barrett's esophagus (Fig. 7). Using the same patient samples, we determined MEK1/2 activity by performing Western blot for the active, diphosphorylated form of ERK1/2. ERK1/2 phosphorylation data from some of these patients were included in our previous publication (25). In agreement with our previous findings, we found that acid significantly increased phosphorylated ERK1/2 expression in the esophageal squamous mucosa from patients who had GERD without Barrett's esophagus (.59 ± .09 to .85 ± .05, P = .03) but not in those with Barrett's esophagus (.76 ± .07 to .85 ± .08, P = .43) (Fig. 8).
Fig. 7.
Acid perfusion induces MEK1/2 phosphorylation on serines 217/222 in esophageal squamous epithelium of patients with GERD with and without Barrett's esophagus in vivo. A: representative Western blot demonstrating MEK1/2 phosphorylation and total MEK1/2 expression before and after esophageal acid perfusion. B: MEK1/2 phosphorylation relative to total MEK1/2 increases significantly following acid perfusion in esophageal squamous epithelium of patients with GERD with and without Barrett's esophagus (*P < 0.05). The bar graphs show the mean ± SE of esophageal squamous epithelial biopsies from 7 patients with GERD with Barrett's esophagus and 5 patients with GERD without Barrett's esophagus before and after acid perfusion.
Fig. 8.
Acid perfusion induces ERK1/2 phosphorylation in esophageal squamous epithelium in patients who have GERD without Barrett's esophagus. A: representative Western blot demonstrating ERK1/2 phosphorylation before and after acid perfusion. B: ERK 1/2 phosphorylation relative to total ERK 1/2 increased significantly following acid exposure in esophageal squamous epithelium from patients who had GERD without Barrett's esophagus but not in those with Barrett's esophagus (*P < 0.05). The bar graphs show the mean ± SE of squamous epithelial biopsies from 7 patients with GERD with Barrett's esophagus and 5 patients with GERD without Barrett's esophagus before and after acid perfusion.
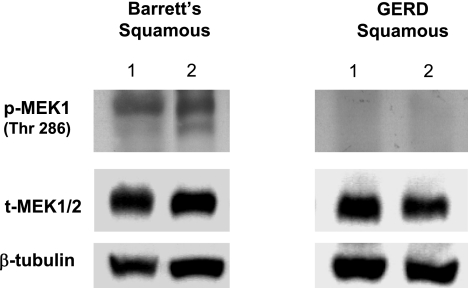
Phosphorylated levels of MEK1 at threonine 286 are found in the esophageal squamous epithelium of patients with GERD with Barrett's esophagus but not of those without Barrett's esophagus.
Our in vitro data demonstrated that levels of phospho-MEK1 at the inhibitory site (thr 286) were not altered by acid exposure. We detected this inhibitory form of phospho-MEK1 in esophageal squamous epithelial biopsies from eight patients who had GERD with Barrett's esophagus but not in eight patients who had GERD without Barrett's esophagus (Fig. 9).
Fig. 9.
Phosphorylation of MEK1 on threonine 286 in esophageal squamous epithelium from 2 representative patients with GERD with Barrett's esophagus and 2 representative patients with GERD without Barrett's esophagus. Note expression of the inhibitory form of phospho-MEK1 only in the patients with Barrett's esophagus. β-tubulin served as a loading control.
DISCUSSION
This study adds support to our hypothesis that, in esophageal squamous epithelial cells, there are differences among individuals in signal transduction pathways activated by acid reflux that might underlie the development of Barrett's esophagus. To explore that hypothesis, we established stable, telomerase-immortalized, but not transformed, esophageal squamous epithelial cell lines from patients with GERD with and without Barrett's esophagus. We have shown that our cells maintain morphological and immunohistochemical characteristics of esophageal squamous epithelium, including the expression of squamous epithelial cell markers like cytokeratins 4 and 13 (3, 5, 20). Unlike transformed cells, our cells maintain growth inhibition with cell-to-cell contact, anchorage-dependent growth, and an intact p53 cell cycle checkpoint. Thus our cell lines maintain a number of normal features desirable for an in vitro model of nonneoplastic esophageal squamous epithelial cells with which to study early events in the pathogenesis of metaplasia.
We first set out to determine whether our squamous cell lines could recapitulate our in vivo finding from an earlier study that acid causes activation of ERK1/2 in the esophageal squamous epithelium of patients who have GERD without Barrett's esophagus but not in patients with Barrett's esophagus. As in that study, we found that baseline levels of the active, diphosphorylated form of ERK1/2 were lower in squamous cells from a patient who had GERD without Barrett's esophagus (NES-G2T cells) than in those from a patient with Barrett's esophagus (NES-B3T cells), and that acid exposure increased phospho- ERK1/2 levels in NES-G2T but not in NES-B3T cells. This in vitro recapitulation of in vivo findings supports the use of our telomerase-immortalized cells as reasonable models for investigating mechanisms underlying differences in acid-induced ERK1/2 phosphorylation in patients with and without Barrett's esophagus.
Even if acid exposure causes ERK1/2 phosphorylation in esophageal squamous cells, ERK1/2 could be rapidly dephosphorylated, and thus inactivated, if the cells have high levels of MKPs such as MKP-1 (24). There is a strong, inverse correlation between levels of MKP-1 and phospho- ERK1/2, and MKP-1 expression can be induced by a variety of cell stresses (10, 11, 27). We found that acid exposure caused no apparent change in MKP-1 expression in either NES-B3T or NES-G2T cells, suggesting that differences in MKP-1 expression are not responsible for the differences that we observed in acid-induced ERK1/2 phosphorylation. Nevertheless, our findings do not exclude the possibility that other types of MKPs (e.g., serine/threonine phosphatases such as PP2A, protein Tyr phosphatases such as PTP-SL) may affect acid-stimulated ERK1/2 phosphorylation levels in esophageal squamous cells. Further investigations are needed in this area (1, 17, 27).
ERK1/2 is phosphorylated by MEK1/2, whose activity also is regulated by phosphorylation (24). MEK1/2 phosphorylation at serines 217/221 activates, whereas MEK1 phosphorylation at threonine 286 inhibits the catalytic activity of the enzymes. In both NES-G2T and NES-B3T cells, acid increased MEK1/2 phosphorylation at serines 217/221. This suggests that differences in acid-induced ERK1/2 phosphorylation between the cell lines do not result from failure of acid to induce phosphorylation at MEK1/2 activating sites. At the threonine 286 inhibitory site of MEK1, however, NES-B3T cells exhibited higher levels of phosphorylation than NES-G2T cells at baseline, and the levels did not change with acid exposure. This suggests that the lack of acid-induced ERK1/2 phosphorylation in NES-B3T cells is not due to acid-induced increases in MEK1 phosphorylation at threonine 286. We speculate that the high basal levels of phospho-MEK1 threonine 286 in the NES-B3T cells interfere with the efficiency of the MEK1 kinase in phosphorylating and activating ERK1/2 when it is exposed to acid. In support of this contention, MEK1 phosphorylation at threonine 286 in NIH3T3 and HeLa cells has been shown to decrease their levels of phospho-ERK1/2 (19, 23). Nevertheless, our studies do not prove that it is the high levels of threonine 286 phosphorylation that underlie the differences between the cell lines in the degree of phosphorylation of ERK1/2 after acid exposure.
Signals transduced by ERK can result in cellular proliferation, differentiation, or apoptosis depending on the cell type, the duration of the signal, and the degree of MEK activity. In PC12 cells, for example, transient activation of ERK1/2 signaling by epidermal growth factor causes proliferation, whereas sustained ERK1/2 activation by neural growth factor leads to differentiation (15, 22, 29). Alternatively, if ERK1/2 activation by neural growth factor is inhibited, then apoptosis ensues (22). Other investigators have found that sustained ERK1/2 activation also can induce apoptosis in PC12 cells (31). Moreover, alterations in MEK1 phosphorylation at threonine 286 have been shown to alter ERK1/2 activity and its effects on apoptosis (22, 31).
The biological consequences of our observation that there are differences in acid-induced MEK-ERK activation between esophageal squamous cells from patients with and without Barrett's esophagus are not clear. However, it is conceivable that those differences underlie the development of Barrett's metaplasia. Proproliferative signals transduced by ERK1/2 appear to facilitate the healing of ulcerations in the gastrointestinal tract (28). Therefore, a squamous epithelium that responds to acid exposure by activating ERK1/2 might be predisposed to heal acid-peptic injury through the regeneration of new squamous epithelial cells (a proliferative response). Conversely, a squamous epithelium that does not experience ERK1/2 activation with acid exposure might be unable to heal its acid-peptic injuries through proliferation and instead might be predisposed to heal through the process of metaplasia, in which squamous cells are replaced by columnar ones. Given the key role that this pathway plays in cell survival, further studies to elucidate the consequences of acid-induced differences in its activation are warranted.
To confirm our in vitro findings, we studied the effects of esophageal acid perfusion during endoscopic examination on MEK1/2 phosphorylation levels in the esophageal squamous epithelium of patients with GERD with and without Barrett's esophagus. As in our cells lines, acid exposure significantly increased MEK1/2 phosphorylation at serines 217/221 in the squamous epithelium of both patient groups, but phospho-MEK1 expression at threonine 286 was detected only in the patients with Barrett's esophagus. These observations further support the utility of our NES-G2T and NES-B3T cell lines as models for studying GERD effects.
In conclusion, we have shown that acid exposure, both in vitro and in vivo, increases ERK1/2 phosphorylation in esophageal squamous cells from patients with GERD without Barrett's esophagus but not with Barrett's esophagus. We have also shown that esophageal squamous cells from patients with GERD with Barrett's esophagus have higher levels of MEK1 phosphorylation at an inhibitory site and have decreased MEK1/2 activity. We speculate that differences in reflux-induced activation of molecular pathways that regulate cellular proliferation and differentiation in esophageal squamous cells contribute to the pathogenesis of Barrett's metaplasia.
GRANTS
This work was supported by the Office of Medical Research, Department of Veteran's Affairs, Dallas, TX (to R. Souza), the Harris Methodist Health Foundation, Dr. Clark R. Gregg Fund (to R. Souza, K. Hormi-Carver), and the National Institutes of Health (DK63621 to R. Souza).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alessi DR, Gomez N, Moorhead G, Lewis T, Keyse SM, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol 5: 283–295, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Ali I, Rafiee P, Hogan WJ, Jacob HJ, Komorowski RA, Haasler GB, Shaker R. Dickkopf homologs in squamous mucosa of esophagitis patients are overexpressed compared with Barrett's patients and healthy controls. Am J Gastroenterol 101: 1437–1448, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Boch JA, Shields HM, Antonioli DA, Zwas F, Sawhney RA, Trier JS. Distribution of cytokeratin markers in Barrett's specialized columnar epithelium. Gastroenterology 112: 760–765, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Feagins LA, Zhang HY, Zhang X, Hormi-Carver K, Thomas T, Terada LS, Spechler SJ, Souza RF. Mechanisms of oxidant production in esophageal squamous cell and Barrett's cell lines. Am J Physiol Gastrointest Liver Physiol 294: G411–G417, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Glickman JN, Chen YY, Wang HH, Antonioli DA, Odze RD. Phenotypic characteristics of a distinctive multilayered epithelium suggests that it is a precursor in the development of Barrett's esophagus. Am J Surg Pathol 25: 569–578, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature 400: 464–468, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Holt SE, Norton JC, Wright WE. Comparison of the telomeric repeat amplification protocol (TRAP) to the new TRAP-eze telomerase detection kit. Methods Cell Sci 18: 237–248, 1996. [Google Scholar]
- 8.Jaiswal KR, Morales CP, Feagins LA, Gandia KG, Zhang X, Zhang HY, Hormi-Carver K, Shen Y, Elder F, Ramirez RD, Sarosi GA Jr, Spechler SJ, Souza RF. Characterization of telomerase-immortalized, nonneoplastic, human Barrett's cell line (BAR-T). Dis Esophagus 20: 256–264, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Karin M The regulation of AP-1 activity by mitogen-activated protein kinases. Curr Opin Cell Biol 270: 16483–16486, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Keyse SM, Emslie EA. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature 359: 644–647, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Kim GS, Choi YK, Song SS, Kim WK, Han BH. MKP-1 contributes to oxidative stress-induced apoptosis via inactivation of ERK1/2 in SH-SY5Y cells. Biochem Biophys Res Commun 338: 1732–1738, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Locke GR, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 112: 1448–1456, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Morales CP, Gandia KG, Ramirez RD, Wright WE, Shay JW, Spechler SJ. Characterisation of telomerase immortalised normal human oesophageal squamous cells. Gut 52: 327–333, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newbold RF, Overell RW, Connell JR. Induction of immortality is an early event in malignant transformation of mammalian cells by carcinogens. Nature 299: 633–635, 1982. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen TT, Scimeca JC, Filloux C, Peraldi P, Carpentier JL, Van Obberghen E. Co-regulation of the mitogen-activated protein kinase, extracellular signal-regulated kinase 1, and the 90-kDa ribosomal S6 kinase in PC12 cells. Distinct effects of the neurotrophic factor, nerve growth factor, and the mitogenic factor, epidermal growth factor. J Biol Chem 268: 9803–9810, 1993. [PubMed] [Google Scholar]
- 16.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 97: 142–146, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Pulido R, Zuniga A, Ullrich A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J 17: 7337–7350, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez RD, Morales CP, Herbert BS, Rohde JM, Passons C, Shay JW, Wright WE. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev 15: 398–403, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossomando AJ, Dent P, Sturgill TW, Marshak DR. Mitogen-activated protein kinase kinase 1 (MKK1) is negatively regulated by threonine phosphorylation. Mol Cell Biol 14: 1594–1602, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salo JA, Kivilaakso EO, Kiviluoto TA, Virtanen IO. Cytokeratin profile suggests metaplastic epithelial transformation in Barrett's oesophagus. Ann Med 28: 305–309, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Seabright M A rapid banding technique for human chromosomes. Lancet 2: 971–972, 1971. [DOI] [PubMed] [Google Scholar]
- 22.Sharma M, Hanchate NK, Tyagi RK, Sharma P. Cyclin dependent kinase 5 (Cdk5) mediated inhibition of the MAP kinase pathway results in CREB down regulation and apoptosis in PC12 cells. Biochem Biophys Res Commun 358: 379–384, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Veeranna Sharma M, Amin ND, Sihag RK, Grant P, Ahn N, Kulkarni AB, Pant HC. Phosphorylation of MEK1 by cdk5/p35 down-regulates the mitogen-activated protein kinase pathway. J Biol Chem 277: 528–534, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta 1773: 1213–1226, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Souza RF, Shewmake KL, Shen Y, Ramirez RD, Bullock JS, Hladik CL, Lee EL, Terada LS, Spechler SJ. Differences in ERK activation in squamous mucosa in patients who have gastroesophageal reflux disease with and without Barrett's esophagus. Am J Gastroenterol 100: 551–559, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Spechler SJ Clinical practice. Barrett's esophagus. N Engl J Med 346: 836–842, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75: 487–493, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Tarnawski AS, Pai R, Wang H, Tomikawa M. Translocation of MAP (Erk-1 and -2) kinases to cell nuclei and activation of c-fos gene during healing of experimental gastric ulcers. J Physiol Pharmacol 49: 479–488, 1998. [PubMed] [Google Scholar]
- 29.Traverse S, Seedorf K, Paterson H, Marshall CJ, Cohen P, Ullrich A. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol 4: 694–701, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med 74: 589–607, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Zheng YL, Li BS, Kanungo J, Kesavapany S, Amin N, Grant P, Pant HC. Cdk5 Modulation of mitogen-activated protein kinase signaling regulates neuronal survival. Mol Biol Cell 18: 404–413, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]