Abstract
This investigation demonstrates the presence and binding of the protein LC8 (described as “protein inhibitor of nNOS” or PIN in some reports) to different components of neuronal nitric oxide synthase (nNOS) in nitrergic varicosities of mice gut. Whole varicosity extracts showed three (320-, 250-, and 155-kDa) nNOS bands with anti-nNOS1422–1433 antibody and a 10-kDa band with anti-LC8 antibody. The LC8 immunoprecipitate (IP) showed three nNOS bands, suggesting that LC8 was bound with all three forms of nNOS but dissociated from them during SDS-PAGE. Studies using LC8 IP and supernatant and probed with anti-CaM showed that LC8 was not associated with CaM-bound 320-kDa nNOS but was present in the CaM-lacking fraction. Probing these fractions with anti-serine847-P-nNOS showed that 320-kDa serine847-phosphorylated-nNOS consisted of LC8-bound and LC8-lacking components. Subsequent studies with varicosity membrane and cytosolic fractions separately showed that membrane contained CaM-bound and CaM-lacking, serine847-phosphorylated 320-kDa nNOS; both these fractions lacked LC8. On the other hand, the cytosolic fraction contained CaM-lacking, serine847-phosphorylated 320-kDa, 250-kDa, and 155-kDa nNOS bands that were all associated with LC8. These studies, along with in vitro nitric oxide assays, show that in gut nitrergic nerve varicosities 1) all cytosolic serine847-phosphorylated nNOS was catalytically inactive and bound with LC8, and 2) membrane-associated nNOS consisted of catalytically active, CaM-bound and catalytically inactive, CaM-lacking, serine847-phosphorylated nNOSα dimers, both of which lacked LC8. These results suggest that LC8 may dissociate from the 320-kDa nNOSα dimer upon binding to membrane, thus supporting the view that LC8 may transport nNOSα dimer to the varicosity membrane for participation in nitrergic neurotransmission.
Keywords: protein inhibitor of nNOS (PIN), dynein light chain (DLC8 or LC8), nNOS; membrane-bound nNOS; cytosolic nNOS; nitrergic neurotransmission
protein inhibitor of nNOS (PIN), an 89-amino acid, 10-kDa protein, was so named because it was thought to negatively regulate neuronal nitric oxide synthase (nNOS) activity (8). PIN was shown to selectively bind and inhibit nNOS and not other isoforms of nitric oxide synthase (NOS) such as endothelial or inducible NOS (8). PIN was suggested to inhibit nNOS activity by promoting catalytically inactive nNOS monomer formation from catalytically active nNOS dimers. PIN was subsequently identified as a member of the light chains (LC8) of two motor proteins, namely, dynein and myosin Va (3, 9). Therefore the terms PIN and LC8 are used interchangeably. LC8 may play an important role in the intracellular transport of various subcellular proteins including nNOS and their targeting to their sites of action.
LC8 has ubiquitous cellular distribution and binds with multiple natural protein partners (11, 13). It has been proposed that LC8 may form a major hub for protein-protein interaction (1). In biological systems, LC8 exists as a dimer that has two binding groves (12, 22). Each of these grooves may bind a 17-amino acid protein stretch (228Met to 244His of nNOS at the NH2 terminal), leading to formation of a homodimer of nNOS (4). On the other hand, one groove of LC8 dimer may bind with the intermediate chain of dynein or myosin and the other one to nNOS and other cargo proteins being transported (3).
The role of LC8 in the regulation of nitrergic neurotransmission is not well understood. On one hand, it may inhibit nitrergic neurotransmission by negative regulation of nNOS activity (8). On the other, it may facilitate nitrergic neurotransmission by linking nNOS to the motor proteins and transporting it to the nerve terminals and the varicosity membrane (6, 13).
Although PIN was initially thought to be an inhibitor of nNOS, its effect on nNOS activity is controversial. Jaffrey and Snyder (8) had initially shown that addition of PIN or glutathione-S-transferase (GST)-PIN to cell extract with overexpressed nNOS resulted in decreased nNOS activity. Hemmens et al. (6) have shown that the inhibitory effect of GST-PIN on purified active nNOS was not selective as other types of NOS were also inhibited. Moreover, Rodriguez-Crespo et al. (17) found no effect of PIN on nNOS activity.
Effect of PIN on dimer-monomer state of nNOS is also controversial. Jaffrey and Snyder (8) had suggested that PIN may act by destabilizing nNOS dimers to catalytically inactive monomers. This conclusion was based on their findings that cell extract from nNOS-overexpressed cells had both 320-kDa dimers and 155-kDa monomers, but when GST-PIN was added, only the 155-kDa monomers were seen. nNOSα dimer-monomer equilibrium has been proposed as an important mechanism of regulation of nNOS activity (5). However, the role of PIN in destabilizing nNOS dimer to promote its monomerization is not supported by other studies (14, 17, 23).
Intracellular nNOS in vivo may exist in different forms based on alternative splicing of its mRNA, dimer vs. monomer state, binding with other proteins such as CaM, and its phosphorylation at serine847 (5, 7, 15, 16, 18). The different forms of nNOS may differ in their subcellular localization and their catalytic activity to produce nitric oxide (NO). Information on the association of LC8 with different forms nNOS may help understand the role of LC8 in nitrergic neurotransmission in the nerve terminals of gut. The present studies were performed to 1) determine whether LC8 was present in the nitrergic varicosities of mice gut, 2) identify the forms of nNOS that were associated with LC8, and 3) determine the nature of membrane-bound and cytosolic nNOS and their association with LC8. In addition, in vitro NO production assays were used to corroborate the functional status of LC8-bound and LC8-lacking fractions of nNOS.
MATERIALS AND METHODS
The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of Veterans Affairs Boston Healthcare System.
Antibodies and Chemicals
The antibodies used in the present study are as follows: anti-LC8 (anti-PIN FL-89), 1:75 (Santa Cruz), anti-COOH-terminal-nNOS1422–1433, 1:50 (Alexis), anti-serine847-P-nNOS, 1:50 (Santa Cruz), anti-CaM, 1:100 (Upstate), anti-synaptophysin, 1:25–1:100 (Santa Cruz), anti-lactate dehydrogenase, 1:25 (Santa Cruz). All species-appropriate secondary antibodies were obtained from Santa Cruz and were used at a three- to fivefold dilution to the primary antibody. Reagents for immunoblot and different chemicals used were from Bio-Rad, Sigma, and Cayman Chemical. Protein A/G was obtained from Santa Cruz.
Tissue Dissection
Adult male C57BL/6j mice (25–30 g each) (Jackson Laboratories) were euthanized by carbon dioxide (CO2) inhalation in an airtight chamber. Gastrointestinal tissues from six mice were pooled for each experiment. The entire gastrointestinal tract from the stomach to colon was dissected quickly and the gut lumen was cleaned in ice-cold homogenization buffer (0.3 M sucrose with 0.1 M sodium phosphate). Small pieces of intestine were placed in a plastic tube in 10 volumes of the homogenization buffer and were pulverized into a homogenous extract with a Brinkmann homogenizer, and the tube was cooled on ice before further processing. The homogenization buffers contained protease and phosphatase inhibitors. The phosphatase inhibitor was used at a concentration of 1 ml/100 ml of homogenization buffer. The protease inhibitor was used at a concentration of 1 ml/20 g wet tissue. The complete gastrointestinal tract from each animal had an average mass of 2.8 g, n = 6 mice. The protease inhibitor (P8340, Sigma) contained 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, aprotinin, bestatin, E-64, leupeptin hemisulfate, and pepstatin. The phosphatase inhibitor contained cantharidin and microcystin LR (P2850, Sigma) that specifically inhibited serine phosphatase PP2A.
Subcellular Fractionation
Samples were centrifuged at 1,000 g for 10 min at 4°C to remove undissociated tissue (pellet P1) that was washed once in buffer; the pellet was discarded, and the combined supernatants were further centrifuged at 4,000 g. The pellet P2 obtained after spinning at 4,000 g represented the nuclear fraction, and the supernatant was the cytoplasmic fraction. This supernatant was subjected to ultracentrifugation at 25,000 rpm at 4°C for 30 min in an Optima TLX cold ultracentrifuge. The pellet P3 was the varicosity fraction, and the supernatant represented the microsomal fraction. Pellet P3 was resuspended in 400 μl of Krebs buffer (111 mM NaCl, 26.2 mM NaHCO3, 1.2 mM NaH2PO4, 4.7 mM KCl, 1.8 mM CaCl2, 1.2 mM MgCl2, 11 mM glucose) and subjected to further purification. The P3 extract was layered on a 0.8/1.2 M sucrose gradient and subjected to sucrose gradient ultracentrifugation at 58,000 rpm for 1 h at 4°C. Intact varicosities that formed a cloudy or ringlike structure at the interface of the two differing sucrose concentrations were carefully collected with a 200-μl pipette tip, diluted in Krebs buffer, and centrifuged at 12,000 rpm for 5 min at 4°C to pellet down varicosities. Varicosities were stored at −80°C until further experiments.
Separation of Membrane and Cytosolic Fractions of Synaptosomes
The purified varicosity lysate obtained after sucrose-gradient centrifugation was incubated in a two-volume solution of 0.5 mM sodium phosphate (pH = 8.1) and 0.1 mM magnesium sulfate for 6 h on ice. This protocol was adapted from previously standardized methodology of preparation of unfolded red blood cell membrane by incubation in chilled alkaline buffer of very low ionic strength (21). The divalent magnesium ions facilitated nonsealing of membranes. After incubation, the lysate was subjected to high-velocity differential centrifugation, as described earlier for membrane protein preparation (20), at a speed of 70,000 rpm for 1 h at 4°C. The supernatant represented the cytosolic fraction whereas the yellowish-white pellet represented only membranes of the varicosities.
Preparation for Western Blots
The extracts were processed at low temperature (4°C) or heat treated at 37°C for 10 min. For the low-temperature processing, 60–80 μg of protein in standard Laemmli buffer at 4°C was used for SDS-PAGE. The low-temperature process was used to identify nNOS dimers and monomers in the native state as low temperature is known to prevent monomerization of nNOS dimers (13). Heat-treated samples were processed as follows: protein was treated with Laemmli buffer for 10 min at 37°C and immediately subjected to electrophoresis; 35 μl of protein samples were then loaded into each lane during electrophoresis.
SDS-PAGE
Electrophoresis was carried out with Bio-Rad mini-protean II system gel casting system. Experiments were carried using 7.5% glycine gels. For detection of proteins with molecular weight < 20 (PIN and CaM), 10–20% tricine peptide gels were used, since tricine gels have been reported to provide enhanced resolution of very low molecular weight proteins (19). For tricine gel experiments, the sample buffer used was 10% Tris-tricine-SDS, and SDS-glycine buffer was used during electrophoresis. Electrophoresis was started at 60 V and stepped up to 90 V after the samples crossed the stacking gel. The run time was determined based on the migration pattern of the molecular weight markers. Extensive pilot experiments were performed for standardization of the type of gel, the run time, the concentrations of the primary and secondary antibodies, and the incubation times.
Total protein concentrations were measured by the Bradford method at 595λ optical density. For almost every experiment, 60 μg of protein was loaded for low-temperature SDS-PAGE. The samples were subjected to SDS-PAGE for a variable period of time (between 2 and 5 h, depending on the migration of the molecular weight marker) at 90 V in a cold room at 4°C. For all electrophoresis, Precision Plus (Bio-Rad) molecular weight marker was used to identify migration patterns. All experiments were performed with appropriate positive controls, and loading controls were evaluated for homogeneity of results. Additionally, for each experiment, three different sets of varicosity extracts were always used to compare results (each extract from a set of 6 mice) and electrophoresis was repeated twice to confirm the findings.
After all electrophoresis, the separated proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad) overnight at 30 V at 4°C in a glycine buffer medium and the efficiency of immunoblotting was checked by Ponceau staining. Blots were washed with Tris-buffered saline (Bio-Rad) with Tween (TBST) for 10 min and blocked for 1 h in 5% BSA at room temperature. The primary antibody was added in the blocking solution at differing dilutions and the blot was probed with this primary antibody on a rocking platform overnight at 4°C. The blot was subsequently washed with washing buffer for a few times (the steps were standardized according to the individual experiments) and an appropriate secondary antibody conjugated to chemiluminescent horseradish peroxidase was added and incubated for an hour on a rocking platform at room temperature. After the blot was washed with TBST thrice for ∼20 min each/wash, immunolabeled blots were developed by use of an enhanced chemiluminescent developer ECL reagent (GE Healthcare) or a Visualizer spray (Millipore). Blots were exposed to a chemiluminescent detection film in the dark for variable time periods and processed in an X-ray developer machine (Kodak X-OMAT 2000A).
Immunoprecipitation studies
Solubilized varicosity extracts, the separated membranes and cytosolic fractions of varicosities were diluted to a starting protein concentration of 1 mg/ml using a buffer that resembled composition of the standard RIPA buffer. This buffer contained protease and phosphatase inhibitors and 50 mM Tris·HCl pH 7.5 (5 ml), 150 mM NaCl (3.75 ml), 1% Nonidet P40, and 0.5% sodium deoxycholate (2.5 ml); 50 μl of a homogenous suspension of protein G-agarose (A/G) was added to 1 ml varicosity extract and incubated overnight at 40% speed at 2–8°C on a rotating rocker (Glas-Col, Terre Haute, IN). Varicosity extracts with beads were centrifuged at 12,000 g for 20 s in a microfuge. After the supernatant was transferred to fresh tubes, 10 μg of the specific antibody was added and gently rocked overnight at 2–8°C. After the IPs were collected by centrifugation at 12,000 g for 20 s in a microfuge, the supernatant was carefully aspirated and stored for further experiments. The beads were washed thrice with 1 ml of lysis buffer and spun at 12,000 g for 2 min each time and 2× loading buffer (the Laemmli buffer) was added to these protein A/G beads on ice to isolate protein from the beads. Immunoprecipitated protein was subjected to different types of electrophoresis and probed with different antibodies.
Quantification of blots
After developing, certain blots were scanned with unaltered luminosity so that the optical density remained unaffected. The peak intensity of a user-identified band was evaluated by using the NIH program ImageJ. The band intensity was expressed as arbitrary units and mean expression was obtained by averaging intensity values of three different lanes for each experiment, each lane representing gut varicosity extract from six mice. Expression values of these signals were represented as means ± SE. Comparison of means was performed with t-statistics using MS Excel.
Functional Studies of In Vitro NO Production
NO production by LC8-bound and LC8-lacking fractions of nNOS (using immunoprecipitated gut nerve varicosity extracts with anti-LC8 antibody, and the supernatants) was measured by using fluorescence of diaminofluorescein diacetate (DAF-2-DA) at 495/515 nm in a spectrofluorometer (Biomate, Thermo Fischer Scientific). One hundred microliters of immunoprecipitated protein samples was mixed with 100 μl of assay buffer. The assay buffer consisted of 55 mM HEPES, 20 mM Tris·HCl, pH 7.4, 2 mM l-arginine, 0.8 mM dithiothreitol, 0.8 μM NADPH, 1 mM MgCl2, 1 mM CaCl2, 0.5 μM CaM, 0.8 μM riboflavin monophosphate, 6 μM (6R)-5,6,7,8-tetrahydrobiopterin (BH4), 0.8 μM flavin-adenine dinucleotide, and protease inhibitors. DAF-2 (10 μM) was added to this reaction mixture at the time of incubation. The assays were performed in triplicates under different experimental conditions by using the native protein, preincubating with the nNOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME), and omitting l-arginine in the buffer. The fluorescence intensity produced by known concentrations of the NO donor Nor-3 was used for plotting a standard graph and NO production of the unknown biological samples were calculated as molar equivalents of Nor-3 and expressed per milligram of protein samples.
RESULTS
Studies in Whole Varicosity Extracts
LC8 is present in nitrergic nerve terminals.
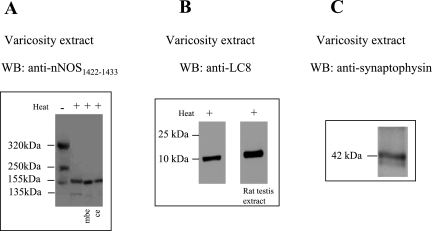
Varicosity extracts processed at low temperature showed three bands of 320-kDa, 250-kDa, and 155-kDa reactive with nNOS1422–1433. The 320-kDa and 250-kDa bands were dimers as evidenced by the fact that they were converted to 155-kDa and 135-kDa monomers on heat treatment of the extract at 37°C in SDS containing Laemmli solution (Fig. 1A). It is known that SDS at warm temperature monomerizes nNOS dimer. Western blots of varicosity extracts probed with anti-nNOS1422–1433 (COOH-terminal) antibody identify all isoforms of nNOS because the COOH-terminal regions of all isoforms of nNOS are identical. Western blots of the varicosity extracts using anti-LC8 antibody showed no LC8 staining with bands in the region of the nNOS. However, a 10-kDa band was visualized with this anti-LC8 antibody (Fig. 1B). These observations suggest that LC8 was present in the varicosities but was dissociated from its other protein partners during SDS-PAGE. The extracts used for the above experiments showed a 42-kDa band reacting with anti-synaptophysin antibody, indicating that the samples used were obtained from nerve varicosities (Fig. 1C).
Fig. 1.
LC8 is present in nitrergic nerve varicosities. A: varicosity extracts processed at 4°C showed 3 bands of 320 kDa, 250 kDa, and 155 kDa reactive with nNOS1422–1433. The 320-kDa and 250-kDa bands were dimers because they were converted to 155-kDa and 135-kDa monomers on warming the extract at 37°C (Fig. 1A, right). The 155-kDa signal of neuronal nitric oxide synthase (nNOS) in mouse brain extract (mbe) and rat cerebellum (ce) served as positive controls. B: after tricine gel electrophoresis, Western blots (WB) using anti-LC8 antibody showed a 10-kDa band. Rat testis extract, a tissue rich in dynein light chain, served as a positive control. C: nerve varicosities were confirmed by the 42-kDa band reactive with synaptophysin antibody.
LC8 is associated with nNOS in the varicosities.
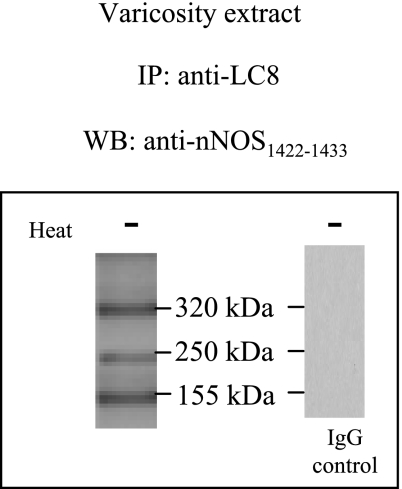
To study whether LC8 was associated with nNOS in the varicosities, IPs of varicosities with anti-LC8 antibody were probed with anti-nNOS1422–1433 antibody. SDS-PAGE of LC8-IPs at low temperature showed three bands at 320, 250, and 155 kDa (Fig. 2). These results indicated that LC8 was associated with both dimers and monomers of nNOS.
Fig. 2.
LC8 is associated with nNOS in the varicosities. Immunoprecipitates (IPs) of varicosities with LC8 antibody probed with anti-nNOS1422–1433 antibody at low temperature showed 3 bands at 320, 250, and 155 kDa, respectively. The IgG control experiment is shown at right.
LC8 is not associated with CaM-bound nNOS.
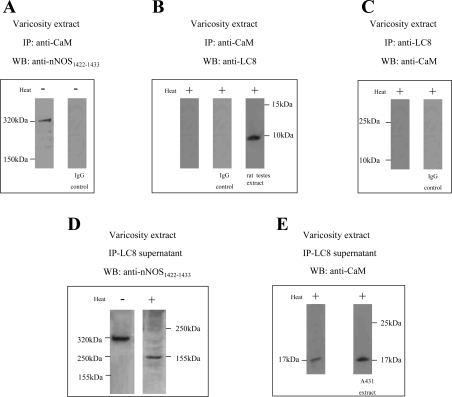
It has been shown that a fraction of nNOS in the varicosities exists as CaM-associated active nNOS (14). To identify whether LC8 was associated with CaM-bound fractions of nNOS, varicosity extracts were immunoprecipitated with CaM antibody. The CaM IP was probed with anti-nNOS1422–1433 and anti-LC8 antibodies. The CaM IPs showed a 320-kDa nNOS reactive band (Fig. 3A) but no LC8-reactive band (Fig. 3B).
Fig. 3.
LC8 is not associated with calmodulin (CaM)-bound nNOS. Varicosity extracts immunoprecipitated with CaM antibody and probed with anti-nNOS1422–1433 and anti-LC8 antibodies showed a 320-kDa nNOS-reactive band (A) but no LC8-reactive band (B). Varicosity extracts immunoprecipitated with LC8 antibody also did not react with anti-CaM antibody (C). Appropriate controls are shown. The LC8-lacking fraction obtained in the supernatant after immunoprecipitation with LC8 antibody showed a 320-kDa nNOS band during cold SDS-PAGE when probed with an anti-nNOS1422–1433 antibody and a 155-kDa band when the samples were warmed at 37°C (D). The supernatant also reacted with anti-CaM antibody and showed a 17-kDa band (E). A431 cell extract served as a positive control for the calmodulin signal.
In reverse experiments, varicosity extracts were immunoprecipitated with LC8 antibody. The LC8 IP did not react with the anti-CaM antibody (Fig. 3C). These observations show that LC8 was not associated with CaM-bound nNOS dimers.
The LC8-lacking fraction obtained after immunoprecipitation with LC8 antibody showed a 320-kDa nNOS band during cold SDS-PAGE when probed with an anti-nNOS1422–1433 antibody and a 155-kDa band when the samples were warmed at 37°C prior to loading (Fig. 3D). This suggested that a component of the 320-kDa nNOSα may not be associated with LC8. It also reacted with anti-CaM antibody and showed a 17-kDa band (Fig. 3E). These observations suggest that LC8 does not associate with CaM-bound nNOS in the nerve terminals.
LC8 is associated with CaM-lacking nNOS.
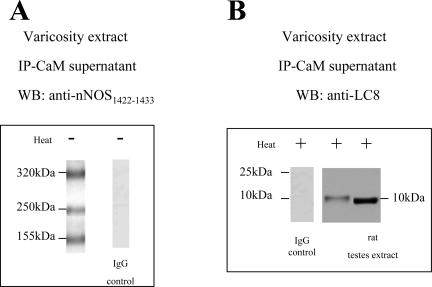
To identify whether LC8 associated with CaM-lacking fractions of nNOS, the CaM-free supernatant was examined after immunoprecipitation of varicosity extracts with CaM. The CaM-lacking fraction was probed with anti-nNOS and anti-LC8 antibodies; it showed three bands of 320-kDa, 250-kDa, and 155-kDa nNOS bands during low-temperature SDS-PAGE (Fig. 4A). Warm SDS-PAGE of the CaM-lacking fraction showed a 10-kDa LC8-reactive band (Fig. 4B). These observations suggested that PIN was associated with CaM-lacking nNOS bands.
Fig. 4.
LC8 is associated with CaM-lacking nNOS. CaM-lacking fraction probed with anti-nNOS and anti-LC8 antibodies showed 3 bands of 320-kDa, 250-kDa, and 155-kDa nNOS bands during low-temperature SDS-PAGE (A). Warm SDS-PAGE of the CaM-lacking fraction showed a 10-kDa LC8-reactive band (B); rat testis extract was a positive control for LC8. Appropriate IgG controls are also shown.
In reverse experiments, varicosity extracts were immunoprecipitated with LC8 antibody and probed with anti-CaM and anti-nNOS1422–1433 antibodies (Figs. 3C and 2, respectively). These results showed that LC8-bound nNOS fractions included 320-kDa, 250-kDa, and 155-kDa bands and none of these bands reacted with anti-CaM antibody, further showing that LC8-bound nNOS was not CaM bound.
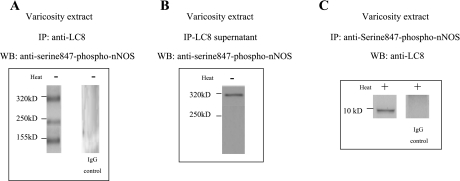
LC8 is associated with a component of serine847-phosphorylated nNOS.
It has been reported that CaM-lacking nNOS may be serine847 phosphorylated (14). To test whether LC8-bound nNOS was serine847 phosphorylated, varicosity extracts were immunoprecipitated with LC8 antibody. The IP and the supernatants were probed with anti-serine847-P-nNOS antibody. The LC8 IP showed three phospho-nNOS bands (Fig. 5A). Interestingly, the supernatant also showed a 320-kDa phospho-nNOS (Fig. 5B).
Fig. 5.
LC8 is associated with a component of serine847-phosphorylated nNOS. Varicosity extracts were immunoprecipitated with LC8 antibody, and the immunoprecipitate and the supernatants were probed with anti-serine847-P-nNOS antibody. The LC8 immunoprecipitate showed 3 phospho-nNOS bands (A), whereas the supernatant also showed a 320-kDa phospho-nNOS (B). Varicosity extract immunoprecipitated with serine847-P-nNOS antibody and probed with anti-LC8 antibody showed a 10-kDa signal (C). Appropriate IgG controls are shown.
In reverse experiments, the varicosity extract was immunoprecipitated with serine847-P-nNOS antibody and probed with anti-LC8 antibody. Anti-LC8 antibody identified a 10-kDa band (Fig. 5C).
These observations suggested that in the varicosity there were two fractions of serine847-phosphorylated 320-kDa nNOS: one of these fractions was associated with LC8 and the second component was not associated with LC8.
Studies in Membrane-Bound and Cytosolic Fractions
The LC8-associated and LC8-unassociated components of 320-kDa nNOS may be related to the association of the nNOS components with the varicosity membrane. Therefore, we performed studies on membrane-bound and cytosolic nNOS and their association with LC8.
Confirmation of separation of membrane-bound and cytosolic fractions.
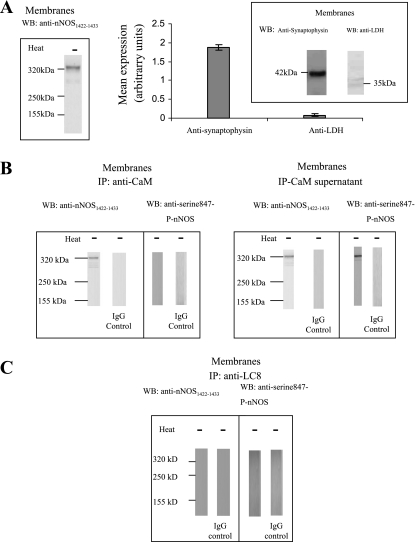
The purity of plasma membrane fractions was assessed by performing Western blotting against lactate dehydrogenase (a cytosolic marker), and purity of fractions was over 95%. Purity of cytosolic fractions was assessed by performing Western blotting against synaptophysin (a plasma membrane marker), and purity of fractions was 97% (Figs. 6A and 7A).
Fig. 6.
Membrane-bound nNOS isoforms do not bind with LC8. A: the membrane fraction of the varicosities showed only a 320-kDa band when probed with anti-nNOS1422–1433 antibody. Purity assessment was done by immunodetection of nerve membrane (synaptophysin) and cytosol [lactate dehydrogenase (LDH)]-specific markers. B: CaM-IP probed with total nNOS antibody showed 320-kDa band that did not react with serine847-P-nNOS antibody. The supernatant obtained after CaM-IP also showed a 320-kDa band that reacted with serine847-P-nNOS antibody. C: LC8-IP probed with total nNOS antibody did not show any signal.
Fig. 7.
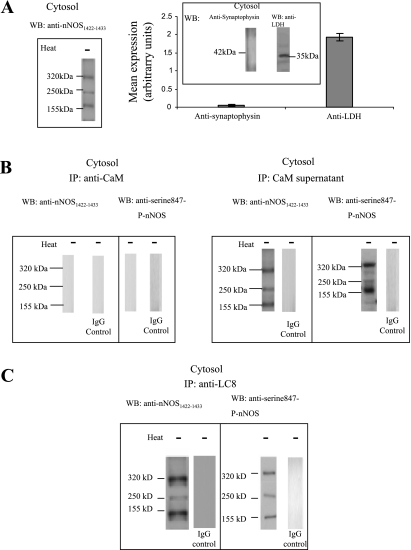
All cytosolic nNOS isoforms bind with LC8. A: the membrane fraction of the varicosities showed 320-, 250-, and 155-kDa bands when probed with anti-nNOS1422–1433 antibody. Purity assessment was done by immunodetection of nerve membrane- (synaptophysin) and cytosol (LDH)-specific markers. B: CaM-IP probed with total nNOS antibody showed no signal; the supernatant obtained after CaM-IP showed 320-, 250-, and 155-kDa bands that reacted with total nNOS and serine847-P-nNOS antibodies. C: LC8-IP probed with total nNOS and serine847-phospho-nNOS antibodies showed that all cytosolic nNOS isoforms were bound to LC8.
Membrane-bound nNOS isoforms.
The nature of the membrane-bound and cytosolic nNOS isoforms was first investigated. The membrane fraction of the varicosities showed only a 320-kDa band when probed with anti-nNOS1422–1433 antibody (Fig. 6A). This band represented the dimer of nNOSα. Subsequently, it was examined whether this 320-kDa band was associated with CaM or not. The membrane extracts were immunoprecipitated with anti-CaM antibody and both the IP and the supernatant were probed with anti-nNOS1422–1433 antibody. Both the anti-CaM IP and the CaM-lacking supernatant of membrane extract showed 320-kDa nNOS bands (Fig. 6B). The CaM-bound and CaM-lacking fractions were also probed for reactivity with anti-serine847-phosphorylated nNOS. The CaM-bound nNOS 320-kDa band did not react with anti-phospho-nNOS antibody, whereas the CaM-lacking fraction showed 320-kDa phospho-nNOS band (Fig. 6B). These observations suggest that the membrane of varicosities contains two different types of nNOSα dimer: a 320-kDa band that is CaM bound and another 320-kDa band that lacks CaM and is phosphorylated at serine847.
LC8 is not associated with either CaM-bound or CaM-lacking nNOS fractions bound to varicosity membrane.
To assess whether membrane-bound nNOS was associated with LC8, solubilized membrane proteins were immunoprecipitated with anti-LC8 antibody. Subsequent probing with an anti-nNOS1422–1433 antibody showed no signal (Fig. 6C). To confirm lack of LC8 association with membrane-bound phospho-nNOS, membranes were immunoprecipitated with LC8 antibody and probed with anti-serine847-P-nNOS antibody. As anticipated, this blotting of membrane fractions did not show any signal (Fig. 6C).
Cytosolic nNOS isoforms.
Like the membrane-bound nNOS, the nature of the cytosolic nNOS was investigated. The cytosolic fraction of the varicosities showed 320-, 250-, and 155-kDa bands when probed with anti-nNOS1422–1433 antibody. This band represented the dimer and monomer of nNOSα and dimer of nNOSβ (Fig. 7A). Subsequently, it was examined whether cytosolic nNOS was bound to CaM or not. The cytosolic extracts were immunoprecipitated with anti-CaM antibody and both the IP and the supernatant were probed with anti-nNOS1422–1433 antibody. The cytosolic fraction showed no nNOS in CaM IP (Fig. 7B) but showed 320-, 250-, and 155-kDa nNOS bands in the floating fraction (Fig. 7B). These results show that all of the cytosolic nNOS bands were CaM lacking. Furthermore, probing the cytosolic CaM IP and supernatant with anti-serine847-P-nNOS showed three 320-, 250-, and 155-kDa nNOS bands in the floating fraction only, indicating that all the CaM-lacking cytosolic nNOS bands were phosphorylated at serine 847 (Fig. 7B).
LC8 binding with cytosolic nNOS fractions.
To assess whether cytosolic nNOS was associated with LC8, cytosolic extracts were immunoprecipitated with anti-LC8 antibody. Subsequent probing with anti-nNOS1422–1433 antibody showed 320-, 250-, and 155-kDa bands, indicating that all cytosolic nNOS were associated with LC8 (Fig. 7C). Furthermore, cytosolic extracts immunoprecipitated with LC8 antibody and probed with anti-serine847-P-nNOS antibody showed 320-, 250-, and 155-kDa bands (Fig. 7C). This confirmed that cytosolic nNOS isoforms, which were all serine847 phosphorylated, were bound to LC8.
Thus, although the membrane-bound 320-kDa-phosphorylated nNOSα dimer lacked LC8, the cytosolic 320-kDa-phosphorylated nNOSα was associated with LC8, suggesting that the membrane binding of nNOS influences LC8 binding to nNOSα dimer.
NO Production by LC8-Bound and LC8-Lacking nNOS Isoforms
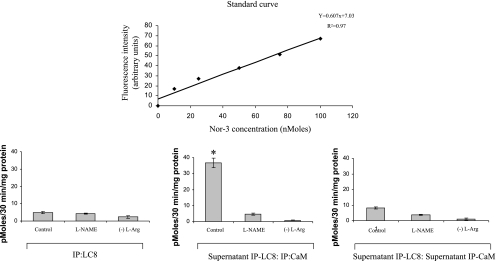
As shown in Fig. 8, IPs of varicosity extracts with LC8 showed that the fraction of LC8-bound nNOS produced negligible quantities of NO in an in vitro assay (5 ± 0.58 pmol·30 min−1·mg protein−1). This NO production was almost unchanged during preincubation with l-NAME (P < 0.05), a drug that inhibits all forms of nNOS enzyme. NO was almost not produced when l-arginine was removed from the assay mixture. These studies show that LC8-associated nNOS was catalytically inactive. As shown in Fig. 2, these fractions of nNOS represent all isoforms localized to the cytosol of the nerve terminals.
Fig. 8.
Quantification of nitric oxide production by LC8-bound and LC8-lacking fractions of nNOS in mice gut nerve varicosities in vitro. Note that LC8-bound nNOS, representing all cytosolic nNOS isoforms, is catalytically inactive. Also note that LC8-lacking fractions (supernatant IP-LC8) have 2 components: a CaM-bound, catalytically active form and a CaM-lacking (supernatant IP-CaM) catalytically inactive form. Whereas the former represents the CaM-bound nNOSα dimer in the membrane, the later fraction represents membrane-bound CaM-lacking, serine847-phospho-nNOSα dimer. Top: the standard curve obtained using known concentrations of the nitric oxide donor Nor-3. DAF-2 DA (diaminofluorescein diacetate) conjugate with nitric oxide at 495/515 nm was used to measure levels of nitric oxide. l-NAME, NG-nitro-l-arginine methyl ester.
In contrast, precipitates of the IP: LC8 supernatant with anti-CaM antibody showed robust NO production (36.67 ± 2.96 pmol·30 min−1·mg protein−1) (Fig. 8). This fraction represented the membrane-bound 320-kDa CaM-bound nNOSα dimer that lacked LC8 binding (Fig. 3E). The nNOS inhibitors l-NAME significantly inhibited NO production in these samples (P < 0.01, paired 2-tailed t-test). On the other hand, the floating fraction obtained after CaM-IP of IP: LC8 supernatant showed scant production of NO in the in vitro assay (Fig. 8), showing that the CaM-lacking nNOS in the LC8-lacking fraction of varicosity extract could not generate NO. This fraction represented the membrane-bound 320-kDa phospho-nNOSα dimer that lacked LC8 binding (Fig. 5B).
DISCUSSION
The present studies show that 1) LC8 is present in the nitrergic nerve terminals of mice gut and is associated with nNOS; 2) LC8 was not associated with CaM-bound nNOSα; 3) LC8 was associated with CaM-lacking nNOSα and only a component of serine847-P-nNOSα; 4) membrane-bound nNOS consisted of CaM-bound as well as CaM-lacking, serine847-P-nNOSα dimers; both these components were devoid of LC8 binding; 5) cytosolic nNOS included nNOSα dimer and monomers and nNOSβ dimers, all of which lacked CaM binding and were serine847-phosphorylated; all cytosolic nNOS fractions were associated with LC8; and 6) LC8-bound fractions of nNOS were catalytically inactive, whereas LC8-lacking fractions were both catalytically active and inactive. All the LC8-bound fractions were in the cytosol, whereas the catalytically active LC8-lacking fraction was located only at the membrane of the nerve varicosity.
The presence of LC8 in the nitrergic nerve terminals in mice gut has not been demonstrated before. This is the first study showing the presence of LC8 in the nerve varicosities of the gut and its association with nNOS. The nerve varicosity extracts showed 320-kDa, 250-kDa, and 155-kDa nNOS bands that did not react with anti-LC8 antibody in Western blots during SDS-PAGE. The 320-kDa nNOS dimer band could be visualized due its SDS-resistant nature (10). The binding between LC8 and nNOS may be weak that was disrupted during denaturing SDS-PAGE. However, association of LC8 and nNOS could be demonstrated in Western blots with anti-nNOS antibody using LC8 immunoprecipitated samples of varicosities.
LC8 was associated with three different nNOS bands: 320-kDa, 250 kDa, and 155 kDa. The 320-kDa and 250-kDa nNOS bands represent two different splice variants of nNOS, namely nNOSα and nNOSβ; LC8 was associated with dimers of both nNOSα and nNOSβ. 155-kDa band represents monomer of nNOSα that was also associated with LC8. Thus LC8 was associated with both dimers and monomers of nNOSα. These results are similar to those reported before that also showed that LC8 binds with both dimers and monomers of purified nNOS (17). Moreover, in structural studies, LC8 dimer has been shown to bind with two molecules of nNOS (12, 22). These observations do not support the view that PIN binding to nNOS dimer destabilizes the dimer and promote monomer formation that was initially suggested as a plausible explanation for the inhibitory effect of PIN on nNOS (8).
LC8 also bound with nNOSβ isoforms. nNOSβ isoform in the mouse lacks the first 241 amino acids, at its NH2-terminal end, that are present in nNOSα (2). The nNOSβ starts with the sequence 1MRGLGSRDLD10. Although this sequence is different from the LC8-interacting residues in nNOSα, 229KDTGIQVDRDLD240, both contain the sequence RDLD that may interact with PIN. The negatively charged aspartic acid (D) of the RDLD sequence in the NH2-terminal region of nNOSβ can form ionic interactions with the positively charged amino acids at the rim of known interacting surface of PIN. Furthermore, the hydrophobic amino acids like leucine (L) can form hydrophobic interactions with the hydrophobic protein-binding surface of PIN. PIN has been predicted to be able to bind to multiple targets with different amino acid sequences using this solvent-exposed hydrophobic surface and positively charged rim (22). nNOSα and nNOSβ can very likely form heterodimers. This might also explain that during immunoprecipitation with anti-LC8 antibodies, nNOSβ might be immunoprecipitated as well. Further studies are needed to identify the exact LC8-binding domain of nNOSβ.
One of the interesting findings of this study was the observation that LC8 was not associated with CaM-bound nNOS in the nerve varicosity. This study demonstrated that CaM-bound nNOS was a 320-kDa dimer of nNOSα. This result is similar to that reported in an earlier study that also showed that CaM-bound nNOSα dimer was the only active form of nNOS in the nerve terminal (16). Thus it appears that LC8 was not associated with active nNOS. The reason why LC8 was not associated with CaM-bound nNOSα is not clear. However, this may be related to the fact that CaM-associated nNOS is associated with varicosity membrane and that this association may be responsible for lack of LC8 binding to CaM bound nNOS.
Another novel finding of this study was that there were two components of serine847-P-nNOS as regards their association with LC8. LC8 was found to be associated with only one of the components of serine847-P-nNOS whereas the other component was not associated with LC8. Previous studies have shown that CaM-lacking nNOS was serine 847 phosphorylated. Serine847 phosphorylation of nNOS interferes with CaM binding to the molecule and thus renders it inactive with regards to NO synthesis (16). Thus, in the nerve varicosities, LC8 was found to be associated with only a fraction of nNOSα.
The reason why LC8 was not bound to a fraction of CaM-lacking, serine847-phosphorylated nNOSα dimer was perplexing. However, it is possible that this finding may be related to membrane binding of the fraction of serine847-phosphorylated nNOSα. Both CaM-bound nNOSα and serine847-phosphorylated nNOSα contain a NH2-terminal PDZ binding domain that is essential for binding to varicosity membrane. PDZ is a structural domain that is found in postsynaptic density protein (PSD95), Drosophila disc large tumor suppressor (DlgA), and zonula occludens-1 protein (ZO-1). The PDZ binding domain of nNOSα allows its binding to the varicosity membrane via PSD95.
To further address these issues, the distribution and the nature of nNOS types in varicosity membranes and cytosol was examined. The membrane and the cytosolic fractions were separated by a combination of osmotic lysis and high velocity-gradient centrifugation and checked for purity. Furthermore, the association of LC8 with the membrane-bound and cytosolic fragments of nNOS was investigated.
The membrane-bound nNOS was found to consist of 320-kDa nNOSα dimers of two types: a CaM-bound form and a CaM-lacking, serine847-phosphorylated form. Both these forms were found to lack LC8 association. On the other hand, the cytosolic nNOSα dimer lacked CaM and was serine847 phosphorylated; it was associated with LC8. Cytosolic fractions also contained nNOSα monomer and nNOSβ dimer that were also serine847 phosphorylated. All these cytosolic nNOS forms were associated with LC8.
These studies show that PIN was not associated with membrane-bound nNOSα dimer but was associated with only cytosolic nNOS fractions including nNOSα dimers and monomers. These observations suggest that binding of nNOSα dimer to PSD95 in the membrane may dissociate LC8 from the nNOSα dimer. This hypothesis remains to be tested.
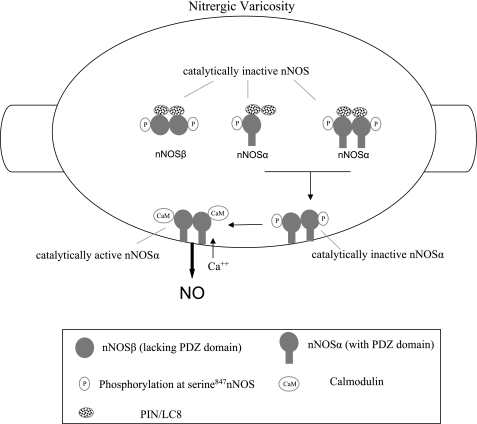
Figure 9 summarizes the key findings of the present study. LC8 binds with cytosolic nNOSα dimers and monomers and also nNOSβ dimers. All these nNOS forms are phosphorylated at serine847 and are catalytically inactive. LC8 is not associated with either the CaM-bound active or the serine847-phosphorylated membrane-associated nNOSα dimers. We propose that cytosolic LC8-associated inactive nNOS may represent the reserve pool of nNOSα in the nerve terminals and the pool of nNOSα in transit to the varicosity membrane. Association of inactive nNOSα dimer with varicosity membrane leads to dissociation of LC8 from the enzyme. The membrane-bound serine847-phosphorylated nNOSα may then get dephosphorylated and bind with CaM in a calcium-dependent fashion. As such, LC8 may be involved in targeting of nNOS to the varicosity membrane and thus play a positive role in maintenance of nitrergic neurotransmission. Future studies are needed to further define the role of LC8 and serine847 phosphorylation and dephosphorylation of membrane-bound nNOSα dimer in nitrergic neurotransmission.
Fig. 9.
Cartoon of gut nerve varicosity summarizing key findings of the present study. The cytosol of the nerve varicosity has 3 different nNOS isoforms: nNOSα monomer, nNOSα dimer, and nNOSβ dimer. All these isoforms are phosphorylated at serine847 and do not bind CaM. All these fractions of nNOS in the cytosol are catalytically inactive. The membrane of the nerve varicosity has two types of nNOS: 1) catalytically active, CaM-bound nNOSα dimer and 2) catalytically inactive, CaM-lacking, serine847-phospho-nNOSα dimer. LC8 is associated with only cytosolic nNOS isoforms. These findings indicate that LC8 plays a role in storage or transport of different nNOS isoforms in the nerve varicosity. NO, nitric oxide; PIN, protein inhibitor of nNOS.
GRANTS
This work was supported by a National Institute of Diabetes and Digestive and Kidney Diseases grant (DK-062867) and a Merit Review Award from the Office of Research and Development, Medical Research Services, Department of Veterans Affairs.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barbar E Dynein light chain LC8 is a dimerization hub essential in diverse protein networks. Biochemistry 47: 503–508, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84: 757–767, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Espindola FS, Suter DM, Partata LB, Cao T, Wolenski JS, Cheney RE, King SM, Mooseker MS. The light chain composition of chicken brain myosin-Va: calmodulin, myosin-II essential light chains, and 8-kDa dynein light chain/PIN. Cell Motil Cytoskeleton 47: 269–281, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Fan JS, Zhang Q, Li M, Tochio H, Yamazaki T, Shimizu M, Zhang M. Protein inhibitor of neuronal nitric-oxide synthase, PIN, binds to a 17-amino acid residue fragment of the enzyme. J Biol Chem 273: 33472–33481, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol 292: G725–G733, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemmens B, Woschitz S, Pitters E, Klosch B, Volker C, Schmidt K, Mayer B. The protein inhibitor of neuronal nitric oxide synthase (PIN): characterization of its action on pure nitric oxide synthases. FEBS Lett 430: 397–400, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Huber A, Saur D, Kurjak M, Schusdziarra V, Allescher HD. Characterization and splice variants of neuronal nitric oxide synthase in rat small intestine. Am J Physiol Gastrointest Liver Physiol 275: G1146–G1156, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Jaffrey SR, Snyder SH. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science 274: 774–777, 1996. [DOI] [PubMed] [Google Scholar]
- 9.King SM, Barbarese E, Dillman JF III, Patel-King RS, Carson JH, Pfister KK. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved Mr 8,000 light chain. J Biol Chem 271: 19358–19366, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Klatt P, Schmidt K, Lehner D, Glatter O, Bächinger HP, Mayer B. Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and l-arginine in the formation of an SDS-resistant dimer. EMBO J 14: 3687–3695, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lajoix AD, Gross R, Aknin C, Dietz S, Granier C, Laune D. Cellulose membrane supported peptide arrays for deciphering protein-protein interaction sites: the case of PIN, a protein with multiple natural partners. Mol Divers 8: 281–290, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Liang J, Jaffrey SR, Guo W, Snyder SH, Clardy J. Structure of the PIN/LC8 dimer with a bound peptide. Nat Struct Biol 6: 735–740, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Navarro-Lerida I, Martinez Moreno M, Roncal F, Gavilanes F, Albar JP, Rodriguez-Crespo I. Proteomic identification of brain proteins that interact with dynein light chain LC8. Proteomics 4: 339–346, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Paige JS, Jaffrey SR. Pharmacologic manipulation of nitric oxide signaling: targeting NOS dimerization and protein-protein interactions. Curr Top Med Chem 7: 97–114, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Panda K, Ghosh S, Stuehr DJ. Calmodulin activates intersubunit electron transfer in the neuronal nitric-oxide synthase dimer. J Biol Chem 276: 23349–23356, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Rao YM, Chaudhury A, Goyal RK. Active and inactive pools of nNOS in the nerve terminals in mouse gut: implications for nitrergic neurotransmission. Am J Physiol Gastrointest Liver Physiol 294: G627–G634, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Crespo I, Straub W, Gavilanes F, Ortiz de Montellano PR. Binding of dynein light chain (PIN) to neuronal nitric oxide synthase in the absence of inhibition. Arch Biochem Biophys 359: 297–304, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Saur D, Paehge H, Schusdziarra V, Allescher HD. Distinct expression of splice variants of neuronal nitric oxide synthase in the human gastrointestinal tract. Gastroenterology 118: 849–858, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Schagger H Tricine-SDS-PAGE. Nat Protoc 1: 16–22, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Skou JC, Esmann M. Preparation of membrane Na+,K+-ATPase from rectal glands of Squalus acanthias. Methods Enzymol 156: 43–46, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Steck TL, Kant JA. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol 31: 172–180, 1974. [DOI] [PubMed] [Google Scholar]
- 22.Tochio H, Ohki S, Zhang Q, Li M, Zhang M. Solution structure of a protein inhibitor of neuronal nitric oxide synthase. Nat Struct Biol 5: 965–969, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y, Berlowitz CO, Zweier JL. PIN inhibits nitric oxide and superoxide production from purified neuronal nitric oxide synthase. Biochim Biophys Acta 1760: 1445–1449, 2006. [DOI] [PubMed] [Google Scholar]