Abstract
Necrotizing enterocolitis (NEC) is associated with the release of interferon-γ (IFN) by enterocytes and delayed intestinal restitution. Our laboratory has recently demonstrated that IFN inhibits enterocyte migration by impairing enterocyte gap junctions, intercellular channels that are composed of connexin43 (Cx43) monomers and that are required for enterocyte migration to occur. The mechanisms by which IFN inhibits gap junctions are incompletely understood. Lipid rafts are cholesterol-sphingolipid-rich microdomains of the plasma membrane that play a central role in the trafficking and signaling of various proteins. We now hypothesize that Cx43 is present on enterocyte lipid rafts and that IFN inhibits enterocyte migration by displacing Cx43 from lipid rafts in enterocytes. We now confirm our previous observations that intestinal restitution is impaired in NEC and demonstrate that Cx43 is present on lipid rafts in IEC-6 enterocytes. We show that lipid rafts are required for enterocyte migration, that IFN displaces Cx43 from lipid rafts, and that the phorbol ester phorbol 12-myristate 13-acetate (PMA) restores Cx43 to lipid rafts after treatment with IFN in a protein kinase C-dependent manner. IFN also reversibly decreased the phosphorylation of Cx43 on lipid rafts, which was restored by PMA. Strikingly, restoration of Cx43 to lipid rafts by PMA or by transfection of enterocytes with adenoviruses expressing wild-type Cx43 but not mutant Cx43 is associated with the restoration of enterocyte migration after IFN treatment. Taken together, these findings suggest an important role for lipid raft-Cx43 interactions in the regulation of enterocyte migration during exposure to IFN, such as NEC.
Keywords: gap junctions, connexin, necrotizing enterocolitis, cytokine, phorbol ester
necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in newborn infants (1). The pathological features that characterize NEC include patchy areas of mucosal inflammation, which develop in part due to the release of proinflammatory cytokines including interferon-γ (IFN) (14, 21). The importance of IFN to the development of NEC is highlighted by our recent observation that IFN-deficient mice are nearly completely protected from the development of experimental NEC, owing largely to the effects of IFN on repair of the injured gastrointestinal mucosa (32). Mucosal repair occurs through the process of intestinal restitution, which involves the rapid migration of enterocytes from healthy areas to areas of mucosal disruption (42, 51). Our laboratory has recently demonstrated that intestinal restitution is significantly impaired in experimental NEC (6), an effect that that can be modeled in vitro upon exposure of cultured enterocytes to IFN (32). Previous authors have also shown that intestinal restitution is decreased in other inflammatory states that are characterized by an increase in IFN, including hemorrhagic shock (11), sepsis (4), and intestinal ischemia (10). Impaired intestinal restitution and persistent mucosal disruption is thought to lead to bacterial translocation across the injured mucosa, which initiates and propagates a local and systemic inflammatory response that characterizes NEC (39). Taken together, these findings underscore the importance of studying enterocyte migration to further understand the factors that lead to the development, and resolution, of this devastating disease.
The mechanisms by which IFN and other proinflammatory molecules inhibit enterocyte migration remain incompletely understood. However, our laboratory recently reported the novel finding that enterocytes are interconnected via molecular channels called gap junctions (32), structures comprised of individual connexin monomers that allow for the passage of small molecules (under 1,000 Da) between adjacent cells (20, 29). We have shown that gap junction communication is required for enterocyte migration to occur and that IFN inhibits enterocyte migration in part through the disruption of gap junction communication between adjacent enterocytes (32). Although the precise mechanisms by which IFN impairs gap junction communication remain unclear, we have recently determined that IFN caused a rapid loss of the most abundant connexin isoform connexin43 (Cx43) from the enterocyte surface (32). This suggests the exciting possibility that IFN may also regulate molecules that are required for gap junction dynamics within the plasma membrane.
In understanding the mechanisms by which IFN could alter the trafficking of Cx43, we turned our attention to the study of lipid rafts, discrete regions of the plasma membrane that are characterized by the accumulation of cholesterol-sphingolipid rich microdomains and integral membrane proteins called caveolins (26). Lipid rafts are known to play a central role in the trafficking and signaling of various proteins within the cell (41). Connexins have been reported to be present on lipid rafts in some cells (30, 33), although their presence on lipid rafts in enterocytes has not been evaluated. We therefore now hypothesize that Cx43 is present on lipid rafts in enterocytes and that IFN inhibits enterocyte migration by displacing Cx43 from lipid rafts. Such a mechanism could account for the rapid redistribution of Cx43 from the surface of enterocytes after exposure to IFN, as well as the reversibility of the effect (32).
In the present paper, we now confirm our previous observations that intestinal restitution is impaired in NEC, and we demonstrate that Cx43 is present on lipid rafts in enterocytes. We also show for the first time that lipid rafts are required for enterocyte migration, that IFN displaces Cx43 from lipid rafts, and that the phorbol ester analog phorbol 12-myristate 13-acetate (PMA) restores Cx43 to lipid rafts after treatment with IFN in a PKC-dependent manner. Strikingly, restoration of Cx43 to lipid rafts by PMA exposure or by infection with adenoviruses expressing wild-type Cx43 but not mutant Cx43 is associated with the restoration of enterocyte migration after IFN treatment. Taken together, these findings suggest an important role for lipid raft-Cx43 interactions in the regulation of enterocyte migration during conditions characterized by exposure to IFN such as NEC.
MATERIALS AND METHODS
Cell culture, treatment, and reagents.
Cultured IEC-6 cells (small intestine cell line) were obtained from the American Type Culture Collection (Manassas, VA) and maintained as described (9, 23, 44). Where indicated, cells were treated with IFN (Sigma-Aldrich, St. Louis, MO; 1,000 IU/ml for 1–14 h, 37°C). The phorbol ester PMA or the nonactivating analog 4α-PMA (25 nM PMA, Calbiochem, San Diego, CA) were added for 1 h after incubation with IFN in the presence or absence of the PKC inhibitor calphostin (100 nM, 1 h, Calbiochem). Antibodies to the following proteins were obtained as follows: phospho-Cx43 and Cx43 from Chemicon (Temecula, CA); the lipid raft protein caveolin-1 (Cav-1) from BD Biosciences (San Diego, CA), the Golgi-membrane protein TGN38 from Calbiochem; the mitochondrial membrane protein Pmp-70 from Zymed (San Francisco, CA); the plasma membrane protein Na+-K+-ATPase from Upstate Biotechnologies (Lake Placid, NY).
Induction of experimental necrotizing enterocolitis.
The following experimental protocol was approved by the Animal Research and Care Committee of the Children's Hospital of Pittsburgh (protocol no. 0805). NEC was induced in 10-day-old Swiss-Webster mice (the Jackson Laboratory, Bar Harbor, ME) by the administration of enteral formula and the induction of hypoxia (5% oxygen for 10 min prior to each feeding) thrice daily for 4 days (7). We and others have demonstrated that this experimental protocol induces intestinal inflammation in animals that resembles clinical NEC (8, 13, 31, 38, 45). Control animals remained with their mothers and received breast milk. To measure enterocyte migration, animals were injected with 50 mg/kg 5′-bromodeoxyuridine (BrdU; Sigma Chemical, St. Louis MO) intraperitoneally then euthanized 18 h later. Samples of terminal ileum were then immunostained with anti-BrdU antibodies as described (24, 31). Enterocyte migration was expressed by measuring the distance from the bottom of the crypt to the foremost labeled enterocyte and is expressed both as a velocity and as a percentage traveled of each villus enumerated (100 villi per field, over 100 fields per experiment).
Measurement of enterocyte migration.
Enterocyte migration was determined according to standardized techniques published previously by our laboratory (5, 31). In brief, IEC-6 cells were grown on glass coverslips to 100% confluence, scraped with a cell scraper, and then transferred to the stage of an Olympus 1X71 fluorescent inverted microscope (Melville, NY) and perfused with DMEM + 10 mM HEPES pH 7.4 at 0.5 ml/h. Where indicated, cells were treated with the lipid raft inhibitor 2-hydroxypropyl-β-cyclodextrin (HPbCD, 5 mg/ml, Sigma), the gap junction inhibitor oleamide (10 μM), IFN (1,000 IU/ml), and/or PMA (25 nM) or where infected with adenoviruses that express either green fluorescent protein (GFP), wild-type Cx43, or dominant negative Cx43. Adequacy of ectopic Cx43 protein expression was assessed by evaluating GFP emission. Images were taken every 5 min for 18 h and analyzed using Metamorph software (Universal Imaging, Downingtown, PA). A calibration scale was obtained and the migration rate was determined by measuring the mean distance traveled by 15 cells per field over the course of the experiment. Measurements were obtained from cells that were selected both at the leading edge and several rows back. The migration rate was determined by measuring the mean distance traveled by 15 cells per field over the course of the experiment.
RNA preparation and PCR.
Total RNA was isolated from the mucosal scrapings of terminal ileum of breast-fed (control) and formula-fed (NEC) mice using RNeasy kit (Qiagen, Valencia, CA). RNA quality and integrity was verified by agarose gel electrophoresis. cDNA synthesis (0.5–1.0 μg RNA) was performed with the Quantitect Reverse Transcription kit (Qiagen). The following mouse-specific primer pairs were used: IFN (forward 5′-AGGCCATCAGCAACAACATAAGCG-3′, reverse 5′-TGGGTTGTTGACCTCAAACTTGGC-3′) and β-actin (forward 5′-CCACAGCTGAGAGGGAAATC-3′, reverse 5′-TCTCCAGGGAGGAAGAGGAT-3′). The following PCR cycles were used to amplify the cDNA: 94°C for 2-min hold and 30–35 cycles of 94°C for 15 s, 56°C for 30 s, and 72°C for 90 s, via a Bio-Rad MyCycler (Hercules, CA). PCR products were separated by electrophoresis on a 2.5% agarose gel stained with ethidium bromide. In all cases, water was used instead of cDNA to serve as a nontemplate control. Images were captured with a Kodak Gel Logic 100 Imaging System (New Haven, CT).
Generation of Cx43 adenoviruses.
Adenoviruses expressing either wild-type or dominant-negative (L90V) connexin 43 with the COOH terminal fused to GFP were constructed using the Adeno-X Expression System 2 from Clontech according to the manufacturer's protocol. Briefly, cDNAs encoding GFP, wild-type Cx43 (wtCx43), and dominant-negative Cx43 (dnCx43) (L90V) (generous gifts of Dr. Charles Murry, University of Washington, Seattle, WA and Dr. Dale Laird, University of Western Ontario, London, ON, Canada) (36, 46) were cloned into pDNR-CMV and integrated into Adeno-X acceptor vectors by using Cre-loxP site-specific recombination. The L90V mutation in Cx43 was recently characterized to be incapable or inefficient in forming functional gap junction channels and to be dominant negative when expressed in equal amounts in cells that also express wild-type endogenous Cx43 (36, 48). The resulting plasmids were linearized with PacI digestion and transfected into the adenovirus packaging cell line HEK293. Adenoviruses were further amplified and titered in HEK293 cells. In all experiments, IEC-6 cells were then infected with viruses at a multiplicity of infection of ∼20. At this ratio, all cells were found to express GFP-wtCx43 or GFP-dnCx43 as determined under fluorescent microscopy using an excitation wavelength of 390 nm and emission wavelength of 505 nm. The expression and distribution of GFP-Cx43 was similar to that of endogenous Cx43 in IEC-6 cells, as confirmed by confocal microscopy.
Isolation of lipid rafts.
To assess the subcellular distribution of Cx43, IEC-6 enterocytes were plated on 35-mm plates and grown to confluence. To isolate lipid rafts, we took advantage of their detergent insolubility properties at low temperatures in combination with their specific light buoyant density on sucrose gradients (49). Briefly, IEC-6 enterocytes plated on 35-mm plates were collected with 1% Triton X-100 and MES-NaCl buffer containing 25 mM of MES and 150 mM NaCl, pH 6.5. Extracts were homogenized on ice with protease inhibitors with a 22-gauge needle and mixed with an equal volume of 80% sucrose in MES-NaCl buffer containing 25 mM MES (pH 6.5) and 150 mM NaCl without Triton X-100, loaded at the bottoms of 12-ml ultracentrifuge tubes and overlaid with 8 ml of a 5 to 35% continuous sucrose gradient in MES-NaCl buffer. Samples were ultracentrifuged at 40,000 g for 21 h at 4°C with a swinging bucket rotor (SW41 Ti; Beckman Instruments, Fullerton, CA). Thirteen 0.9-ml fractions were collected from the top of each gradient. Protein samples were diluted with 2× sample loading buffer for 5 min and then subjected to immunoblot analysis. To investigate the interaction of Cx43 with Cav-1 in lipid rafts, sucrose gradient fractions 4–6 that contain these three proteins were collected and solubilized prior to immunoblot analysis.
Immunoprecipitation, SDS-PAGE, and immunofluorescence microscopy.
To assess for the biochemical interaction of the gap junction protein Cx43 with the lipid raft protein Cav-1, IEC-6 cells (106 cells per plate) were cultured on 6-cm dishes, washed with PBS, and solubilized in detergent solution containing 50 mM Tris, pH 8.0, 1% Nonidet P-40, 0.4% deoxycholate, 62.5 nM EDTA, and 1 μg/ml aprotinin. The extract was centrifuged for 5 min in an Eppendorf (USA) Microfuge model 5414 (10,000 g) at 4°C to remove insoluble material and nuclei, and the supernatant was recovered. Where indicated, an aliquot of 30 μl of lysate was added to Laemmli sample buffer, heated for 2 min at 90°C, and subjected to SDS-PAGE. Alternatively, the entire detergent extract was immunoprecipitated with anti-Cx43 antibodies, and antibody-antigen complexes were collected by using protein G-coupled Sepharose (Sigma) as described (37). An equivalent amount (30 μl lysate, 106 cells/well starting material) of lysates of IEC-6 cells were prepared as positive controls for the Cx43 antibodies. In parallel, immunoprecipitation experiments were performed with irrelevant IgG at equimolar concentrations and with uncoated beads. Samples were then electrophoresed on 8% SDS-PAGE gels and band density from radiographic film images was analyzed with Scion Image Beta4.03 [National Institutes of Health (NIH), Bethesda, MD]. For immunohistochemistry, cells were processed as described (6).
For the immunodetection of lipid rafts, IEC-6 cells were grown to 70% confluence on glass coverslips, washed with PBS, and then incubated in chilled IEC-6 growth medium supplemented with the lipid raft marker 1 mg/ml Alexa Fluor-555-conjugated cholera toxin, subunit B (Molecular Probes, Eugene, OR) at 4°C for 20 min. IEC-6 cells were then copiously washed with ice cold PBS, fixed with 4% paraformaldehyde, and analyzed by confocal microscopy. Where indicated, cells were immunostained with antibodies against phosphorylated Cx43 (Chemicon). The extent of colocalization between lipid rafts and GFP was determined by use of Image J software with the colocalization plug-in (NIH, Bethesda, MD).
Statistical analysis.
Data presented are means ± SE, and comparisons are by two-tailed Student's t-test or ANOVA, with statistical significance accepted for P < 0.05.
RESULTS
Enterocyte migration is reduced in experimental NEC and is also inhibited by IFN, by the inhibition of gap junctions, and by disruption of lipid rafts.
We first sought to confirm our recent observations regarding the rate and extent of enterocyte migration in experimental NEC. To do so, 7- to 10-day-old mice were subjected to intermittent hypoxia and formula gavage for 4 days, an experimental model that we and others have shown to result in the development of morphological and pathological features of NEC in ∼70% of mice (31). Control mice remained with their mothers and were breast-fed; they demonstrated healthy intact villi (see Fig. 1A), whereas animals with NEC developed blunted, irregular villi, neutrophil infiltration, and submucosal edema (Fig. 1B). As shown in Fig. 1C, experimental NEC was associated with an increase in the expression of IFN in the ileal mucosa as determined by RT-PCR (Fig. 1C). To assess the rate of enterocyte migration in experimental NEC, sections of the terminal ileum were harvested from breast-fed and NEC-induced animals 18 h after injection with BrdU, a nucleotide analog that accumulates in dividing cells within the crypts. Immunostaining of tissue using anti-BrdU antibodies reveals the rate of migration of enterocytes along the crypt-villus axis. As our laboratory (6) and others (12) have reported previously, in control mice, BrdU-stained enterocytes were observed to travel along the villi such that they reach the villus tips by ∼20 h (Fig. 1D). By contrast, in animals with experimental NEC, BrdU-stained enterocytes remained localized to the intestinal crypts and did not migrate toward the villi tips (Fig. 1E), indicating that enterocyte migration was impaired. When multiple tissue sections were evaluated, both the rate of enterocyte migration along the crypt-villus axis and the extent by which BrdU-positive enterocytes traveled along individual villi were found to be significantly reduced in experimental NEC compared with untreated mice (Fig. 1F), confirming our previous studies (6, 45).
Fig. 1.
Enterocyte migration is reduced in experimental necrotizing enterocolitis (NEC) and is also inhibited by interferon-γ (IFN), by the inhibition of gap junctions, and by disruption of lipid rafts. A and B: NEC was induced in 10-day-old mice by a combination of formula gavage and hypoxia for 4 days as described in methods, and the terminal ileum was harvested 1 cm proximal to the ileocecal valve. Shown are the terminal ileal histology in control, breast-fed mice (A) and mice with experimental NEC (B). C: expression of IFN in the terminal ileum of two samples obtained from each of mice with and without NEC by RT-PCR; the expression of the housekeeping gene β-actin is shown. D–F: 10-day-old Swiss-Webster mice were induced to develop NEC and injected intraperitoneally with bromodeoxyuridine (BrdU) 18 h prior to euthanasia and immunostained for BrdU (arrows indicate position of peroxidase staining). In control animals, most BrdU has accumulated at varying positions along the villi (D, arrows). In animals with NEC, BrdU uptake remains in the crypts (E), demonstrating a significant impairment in migration. F: rate of migration and percent of maximum crypt height reached are both significantly decreased in NEC compared with controls. Data are means ± SE of 4 separate experiments. *P < 0.05 vs. control. A minimum of 100 cells were counted per experiment. G: rate of migration of IEC-6 cells into a scraped wound in the presence or absence of interferon (IFN), the gap junction inhibitor oleamide (OLM), or upon adenoviral infection and overexpression of dominant negative connexin43 (Cx43; dnCx43), wild-type Cx43 (wtCx43), or the lipid raft inhibitor 2-hydroxypropyl-β-cyclodextrin (HpB). Representative of 4 separate experiments with over 100 cells per experiment counted; *P < 0.05 vs. control; **P < 0.05 vs. dnCx43.
In the next series of experiments we sought to further evaluate the mechanisms by which enterocyte migration may be impaired during intestinal inflammation and to test the possibility that IFN may inhibit enterocyte migration by displacing the gap junction protein Cx43 from lipid rafts. To study the various roles of IFN, Cx43, and lipid rafts on enterocyte migration directly, we first utilized a scrape-wounding assay, as we have previously described (6), in which IEC-6 enterocytes are grown to confluence and then wounded with a cell scraper and evaluated for their ability to migrate into and close the resulting wound. As is shown in Fig. 1G, the rate of enterocyte migration was significantly reduced upon exposure to IFN, consistent with our previous findings (32) and those of others (52). Importantly, inhibition of gap junctions with oleamide or upon infection of IEC-6 cells with adenoviruses that express dominant negative but not wild-type Cx43 significantly reduced the rate of enterocyte migration (Fig. 1G, open bars). Moreover, disruption of lipid rafts using the specific inhibitor HPbCD markedly reduced the rate of enterocyte migration (Fig. 1G, shaded bar). Taken together, these findings confirm our previous observations that IFN inhibits and gap junctions are required for enterocyte migration, and they identify an important role for lipid rafts in enterocyte migration. And although these findings are similar to previous findings from our group, they provide the necessary context and rationale for the present studies.
The gap junction protein Cx43 is expressed on lipid rafts in enterocytes.
In the next series of studies, we sought to determine whether Cx43 is localized on lipid rafts in enterocytes. To identify lipid rafts, we performed confocal immunomicroscopy on IEC-6 cells that had been treated with Alexa 555-labeled cholera toxin, an agent that intercalates with and therefore directly identifies lipid rafts in enterocytes (22) (Fig. 2A). The distribution of Cx43 was assessed by coimmunostaining with antibodies against Cx43 (Fig. 2B), in which significant colocalization between lipid rafts and Cx43 was observed (Fig. 2C; yellow staining indicates points of colocalization at arrows). To confirm these cellular-based results, a biochemical approach was taken to determine whether Cx43 is found on lipid rafts in enterocytes. As described in methods, lipid raft fractions were isolated from IEC-6 cells by density gradient membrane centrifugation, and the raft-enriched fractions were identified by using antibodies to the lipid raft protein Cav-1. The molecular characterization of other fractions was performed by assessing for the expression of PMP70 (mitochondria), Na+-K+-ATPase (plasma membrane), and TGN38 (Golgi). As is shown in Fig. 2D, Cav-1-enriched lipid raft fractions were identified in fractions 4–7 of the membrane preparation in enterocytes. By contrast, PMP70-rich fractions were detected in fractions 8–12, Na+-K+-ATPase enriched fractions were detected in fractions 8–12, and TGN38-enriched fractions were detected in fractions 9–13. Having identified a lipid raft-enriched fraction in IEC-6 cells, we next sought to examine whether Cx43 was present on this fraction. As is shown in Fig. 2E, Cx43 was detected by SDS-PAGE on the Cav-1 (lipid raft)-enriched fraction in enterocytes, and not on other fractions. Taken together, these findings demonstrate that the gap junction protein Cx43 is present on lipid rafts in enterocytes.
Fig. 2.
The gap junction protein Cx43 is expressed on lipid rafts in enterocytes. A–C: IEC-6 cells were treated with Alexa555-cholera toxin as described in methods, then fixed, immunostained with anti-Cx43 antibodies, and evaluated by confocal microscopy. Shown are the distribution of lipid rafts (A), Cx43 (B), and the merged image (C), in which regions of colocalization of lipid areas and Cx43 are shown in yellow (arrows). D: subcellular fractionation of IEC-6 cells as obtained by sucrose density ultracentrifugation. Fractions were subjected to SDS-PAGE and immunoblotted for the indicated markers: caveolin-1 (Cav-1), lipid rafts; PMP-70, mitochondria; Na/K-ATPase, plasma membrane; TGN28, Golgi. Note that the lipid raft marker Cav-1 is most prominent on fractions 4–7. E: SDS-PAGE showing the expression of Cx43 on lipid raft enriched fractions from IEC-6 cells. Representative of at least 5 separate experiments.
IFN displaces Cx43 from enterocyte lipid rafts.
Having shown that Cx43 is expressed on lipid rafts in enterocytes (Fig. 2), and on the basis of our recent finding that IFN inhibits gap junction communication and migration in part by inhibiting Cx43 (32), we next sought to examine whether IFN could displace Cx43 from surface lipid rafts. As shown in Fig. 3A, treatment of IEC-6 cells with IFN led to a significant reduction in the association of Cx43 from lipid rafts in enterocytes, as demonstrated by SDS-PAGE analysis of lipid raft fractions (upper blot). IFN had no effect on the expression of Cx43 in whole cell lysates, excluding the possibility of protein degradation in response to IFN (Fig. 3A, lower blot; see quantification in B). As shown in Fig. 3C the lipid raft protein Cav-1 could be detected in Cx43-immunoprecipitates that were prepared from IEC-6 cell lysates (upper blot), providing further confirmation that Cx43 is found on lipid rafts in enterocytes (see quantification in Fig. 3D). However, pretreatment of IEC-6 cells with IFN led to a significant decrease in the extent of Cav-1 that was found to coimmunoprecipitate with Cx43 (Fig. 3C, upper blot), further supporting the finding that IFN displaces Cx43 from lipid rafts. In control experiments, IFN did not alter the expression of Cx43 that could be detected in immunoprecipitates that were prepared with the anti-Cx43 antibody, excluding the possibility that IFN simply interfered with the immunoprecipitation process (Fig. 3C, lower blot). The quantification of three such experiments is shown in Fig. 3D.
Fig. 3.
IFN displaces Cx43 from enterocyte lipid rafts; the phorbol ester PMA restores Cx43 to lipid rafts after treatment with IFN. A, upper blot: SDS-PAGE of Cx43 in lipid raft-enriched fractions that were purified from IEC-6 cells that had been either untreated (Ctrl), or treated with IFN in the absence (IFN) or presence of PMA (IFN + PMA) or PMA alone (PMA). Lower blot: SDS-PAGE showing the expression of Cx43 in whole cell lysate of IEC-6 cells treated as above. Representative of at least 5 separate experiments. B: quantification of 3 separate experiments similar to those in A, showing means and SE. *P < 0.05 vs. control by ANOVA. C, upper blot: SDS-PAGE showing the expression of Cav-1 in IEC-6 cells that had undergone immunoprecipitation with anti-Cx43 antibodies as described in methods. Cells had been treated with IFN and/or PMA as in A. Lower blot: the expression of Cx43 in the anti-Cx43 immunoprecipitate. Representative of at least 3 separate experiments. D: quantification of 3 separate experiments similar to those in C, showing means and SE. *P < 0.05 vs. control by ANOVA. E: expression of Cav-1 in whole cell lysates purified from IEC-6 cells under the conditions indicated, representative of 3 separate experiments. F: quantification of 3 separate experiments similar to those in E, showing means and SE. *P < 0.05 vs. control by ANOVA.
We next sought to determine whether IFN treatment led to fewer rafts or less Cx43 in each raft. To do so, we performed Western blot analysis of Cav-1 expression on IEC-6 cells that had been treated with IFN, PMA, or the combination of these agents. As is shown in Fig. 3, E and F, the total amount of Cav-1 was unaffected by IFN or PMA, either alone or in combination. Taken together, these findings demonstrate that IFN displaces Cx43 from lipid rafts in enterocytes.
To confirm the effects of IFN on displacing Cx43 from lipid rafts as determined biochemically, an immunohistochemical approach was undertaken. As shown in Fig. 4A, i–iii, the distributions of Cx43 (red staining) and Cav-1 (green staining) were found to significantly colocalize (see arrows, Fig. 4A, iii). This finding is consistent with that seen when enterocyte lipid rafts were detected by use of labeled cholera toxin, as shown in Fig. 2, and confirms the finding that Cx43 is found on enterocyte lipid rafts. Importantly, the treatment of IEC-6 cells with IFN resulted in a significant decrease in the extent of colocalization of Cx43 with Cav-1 (see Fig. 4A, iv–vi). Taken together, these findings suggest that IFN causes the displacement of Cx43 from lipid rafts between adjacent enterocytes. We next sought to determine the physiological significance of this finding by attempting to restore Cx43 to lipid rafts in the face of continued exposure to IFN and to assess the subsequent effects on enterocyte migration.
Fig. 4.
IFN reversibly displaces Cx43 from lipid rafts in IEC-6 cells and decreases the phosphorylation of Cx43 at lipid rafts; these events are reversed by PMA. A: IEC-6 cells were either untreated (i–iii), treated with IFN (iv–vi) or treated with IFN in the presence of PMA (vii–ix) as described in methods. Cells were stained with antibodies against Cx43 (red staining) and Cav-1 (green staining) along with the nuclear marker Draq-5 (blue staining) and were examined by immunoconfocal microscopy. Arrows indicate sites of colocalization of Cx43 and Cav-1 on the merged images. Representative of 5 separate experiments. B: confocal micrographs of IEC-6 cells that were either untreated (i) or were treated with IFN in the absence (ii) or presence (iii) of PMA, treated with cholera toxin and immunostained with antibodies against phosphorylated Cx43 as described in methods. The colocalization of phosphorylated Cx43 with lipid rafts is revealed by arrows. Representative of 3 separate experiments.
The phorbol ester PMA restores Cx43 to lipid rafts after treatment with IFN.
Previous authors have demonstrated that phorbol esters including PMA can regulate the association of membrane proteins with lipid rafts (2). We therefore next tested the possibility that exposure of IEC-6 cells to PMA could restore the localization of Cx43 to lipid rafts. As is shown in Fig. 3, treatment of IEC-6 cells with IFN in the presence of PMA restored the association of Cx43 with lipid rafts as demonstrated by evaluation of lipid raft-rich fragments using SDS-PAGE (Fig. 3A, quantification in Fig. 3B). PMA treatment also restored the extent of coimmunoprecipitation of Cav-1 with Cx43 after IFN treatment (Fig. 3C, quantification in Fig. 3D). There was no effect of PMA on the total number of lipid rafts, as determined by SDS-PAGE of IEC-6 cell lysates (Fig. 3, E and F). PMA was also found to reverse the decrease in colocalization of Cx43 and Cav-1 as detected by immunoconfocal microscopy (Fig. 4A, vii–ix). There was no effect of the negative control phorbol ester (4α-PMA) on each of these assays, confirming the specificity of the effect (not shown). Taken together, these findings suggest that PMA treatment can restore Cx43 to lipid rafts in enterocytes after exposure to IFN.
Treatment of IEC-6 cells with IFN and PMA modulates the phosphorylation of Cx43 in lipid rafts.
We have previously demonstrated that the function and subcellular localization of Cx43 in enterocytes is associated with the protein's phosphorylation status (32). We therefore next investigated whether IFN and/or PMA would affect the phosphorylation of Cx43 in lipid rafts. Of note, treatment with IFN and/or PMA was indeed found to modulate the phosphorylation of Cx43 in the lipid rafts. Evidence for this is shown in Fig. 4B, in which IEC-6 cells were treated with IFN in the presence or absence of PMA, and the location of phosphorylated Cx43 on lipid rafts was determined by staining cells with the lipid raft marker cholera toxin (in red) and antibodies against phosphorylated Cx43 (green). As can be seen, in untreated cells, phosphorylated Cx43 is found at the intersection of adjacent cells on lipid rafts (Fig. 4B, i, see arrows). Treatment with IFN led to a loss of phosphorylation of Cx43 from lipid rafts (Fig. 4B, ii), whereas treatment with IFN in the presence of PMA restored the phosphorylation of Cx43 at lipid rafts (Fig. 4B, iii, arrows). Taken together with our previous findings (see Ref. 32), these data suggest that the phosphorylation of Cx43 in the lipid rafts may be linked with its ultimate loss from the raft fraction of cells, and the reversibility of this effect by PMA.
The phorbol ester PMA reverses the inhibitory effects of IFN on enterocyte migration.
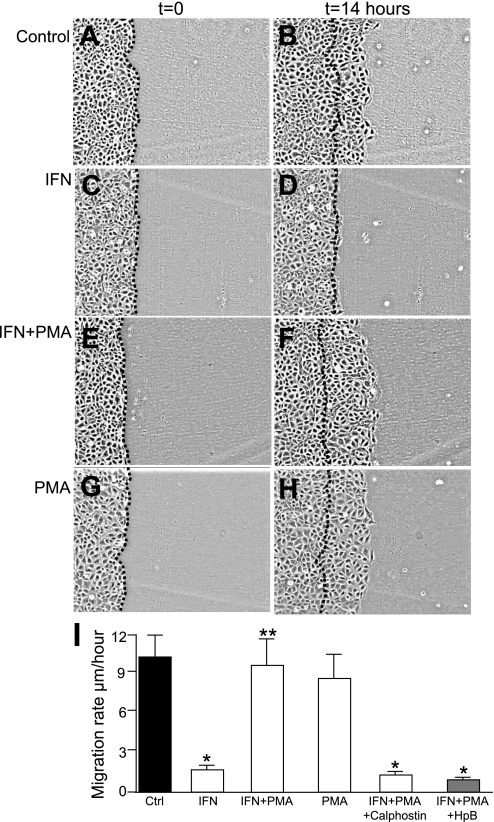
Having shown above that PMA could restore Cx43 to enterocyte lipid rafts after IFN treatment, we next sought to investigate whether PMA could restore enterocyte migration after IFN treatment. To do so, live cell videomicroscopy was performed to evaluate the migration of IEC-6 cells into a scraped wound in the presence of IFN and PMA. As shown in Fig. 5, A–D and I, IFN inhibited the migration of IEC-6 cells into a scraped wound compared with untreated cells, a finding consistent with recent findings from our group (32) and supported by the work of others (52). Strikingly, the pretreatment of enterocytes with PMA, which restores Cx43 to lipid rafts after IFN treatment (see Fig. 3), led to a restoration of the rate of enterocyte migration in the face of ongoing IFN exposure (Fig. 5, E–F and I). There was no effect of treatment of cells with PMA alone (Fig. 5, G–H and I) or of 4α-PMA (not shown) on enterocyte migration after IFN exposure, confirming the specificity of this effect. Since PMA is known to activate many intracellular pathways including those that involve PKC, we next sought to investigate whether the activity of PMA on enterocyte migration required PKC. To do so, we utilized the specific PKC inhibitor calphostin, which is known to inhibit PKC by competing at the binding site for diacylglycerol and phorbol esters (3, 27). As shown in Fig. 5I, calphostin prevented the ability of PMA to reverse the inhibitory effects of IFN on enterocyte migration. Addition of calphostin alone had no effects on enterocyte migration (not shown). Of note, PMA did not reverse the effects of IFN on enterocyte migration in cells that had been pretreated with lipid raft inhibitor HPbCD (Fig. 1G), indicating that intact lipid rafts are required for PMA to act to reverse the effects of IFN on enterocyte migration.
Fig. 5.
The phorbol ester PMA reverses the inhibitory effects of IFN on enterocyte migration. PMA reverses the inhibitory effects of IFN on enterocyte migration. IEC-6 cells were induced to migrate across a scraped wound as described in methods after being either untreated (A and B) or treated with IFN in the absence (C and D) or presence (E and F) of PMA or were treated with PMA alone (G and H). Shown are representative images from a typical experiment at the beginning (t = 0) of the experiment and 14 h later (t = 14 h), where the dotted line indicates the position of the cells at the edge of the scraped wound, at t = 0. I: quantification of migration rate. Where indicated, IEC-6 cells were treated with IFN in the presence or absence of PMA, as well as the PKC inhibitor calphostin (100 nm, 1 h) or the lipid raft inhibitor 2-hydroxypropyl-β-cyclodextrin (HpB, 5 mg/ml). Shown are means ± SE of 3 separate experiments with over 50 cells per condition, *P < 0.05 by ANOVA compared with untreated cells.
The overexpression of wild-type but not dominant-negative Cx43 restores the ability of enterocytes to migrate in the presence of IFN exposure.
The above findings suggest the possibility that PMA may reverse the effects of IFN on enterocyte migration by restoring Cx43 to lipid rafts. To test this directly, a viral transfection approach was undertaken. Specifically, we generated adenoviruses that expressed either GFP alone, GFP-tagged wild-type Cx43 (wild-type), or GFP-tagged Cx43 bearing a dominant negative L90V mutation in its polypeptide backbone (dominant negative). This mutation leads to the inhibition of the function of endogenous Cx43 (36). Representative confocal micrographs revealing GFP emission are displayed in Fig. 6 and indicate that, compared with the diffuse cytoplasmic appearance of cells infected with adenoviruses that express GFP alone (Fig. 6A), both wild-type-Cx43 (Fig. 6B) and mutant Cx43 (Fig. 6C) were localized at sites between adjacent enterocytes as well as in the cytoplasm. To assess the roles, if any, of the ectopic expression of Cx43 on enterocyte migration after treatment with IFN, IEC-6 cells were infected with each of these adenoviruses and then treated with IFN and assessed for the ability of cells that overexpress either wild-type or mutant Cx43 to migrate into a scraped wound. As expected, in control experiments in which cells infected with GFP alone were treated with IFN, the rate of enterocyte migration was significantly reduced compared with untreated cells (Fig. 6D, see open vs. solid bars). Strikingly, the overexpression of wild-type Cx43 restored the ability of enterocytes to migrate in the presence of IFN treatment compared with cells that overexpressed GFP alone (Fig. 6D, see shaded vs. open bars). It is noteworthy that the overexpression of mutant Cx43 could not “rescue” the ability of enterocytes to migrate after IFN treatment, confirming the specific role of wild-type Cx43 in mediating this effect (Fig. 6D, see checkered vs. open bars). Taken in aggregate, these findings indicate that wild-type Cx43 is necessary to reverse the inhibitory effects of IFN on enterocyte migration and, in conjunction with the results shown in Figs. 3 and 4, raise the possibility that the “recovery” in enterocyte migration occurs through the restoration of ectopic Cx43 to lipid rafts.
Fig. 6.
Overexpression of wild-type but not dominant-negative Cx43 restores the ability of enterocytes to migrate in the presence of IFN exposure. IEC-6 cells were infected with adenoviruses expressing either green fluorescent protein (GFP) alone, GFP-wild-type Cx43 (GFP-wtCx43), or GFP-dominant negative Cx43 (GFP-dnCx43), then treated with IFN and induced to migrate into a scraped wound. A–C: confocal images demonstrating the GFP emission of IEC-6 cells expressing GFP alone (A), GFP-wtCx43 (B), or GFP-dnCx43 (C). D: rate of enterocyte migration of IEC-6 cells after transfection with the above constructs in the absence (solid bars) or presence of IFN (nonsolid bars). Shown are means ± SE; representative of 3 separate experiments with over 100 cells counted in each group; *P < 0.05 by ANOVA vs. GFP alone (open vs. solid bars, checkered vs. solid bars); **P < 0.05 by ANOVA vs. IFN + GFP and IFN + dnCx43 (shaded vs. open bars; shaded vs. checkered bars).
Cx43 overexpression restores Cx43 to lipid rafts in IFN-treated enterocytes.
We next sought to determine whether the exogenously expressed wild-type Cx43 was found on lipid rafts after IFN treatment, a finding that would strongly suggest that a loss of Cx43-lipid raft interactions mediates the impairment in enterocyte migration induced by IFN. To do so, IEC-6 cells were infected with either GFP alone, GFP-tagged wild-type Cx43, or GFP-tagged dominant-negative Cx43 and then treated with IFN and assessed for the extent of the association of Cx43 with lipid rafts as determined by confocal microscopy using the lipid raft marker Alexa 555-labeled cholera toxin. In non-IFN-treated, GFP-infected cells (Fig. 7, A–D), cholera toxin was detected at the expected sites between adjacent cells and on the cell surface (A), whereas GFP was found in a diffuse cytoplasmic distribution (B) and did not colocalize with lipid rafts to any detectable degree (C and D). Treatment of GFP-infected cells with IFN (Fig. 7, E–H) led to the loss of detectable lipid rafts from the regions between adjacent cells (E), which expectedly did not localize with GFP to any detectable degree (F–H). Strikingly, overexpression of wild-type Cx43 in IEC-6 cells that were treated with IFN (Fig. 7, I–L) led to enhanced expression of Cx43 on the cell surface and between adjacent cells at sites of lipid rafts, a finding that did not occur in IEC-6 cells treated with dominant negative Cx43 (Fig. 7, M–P). Taken in conjunction with the studies presented in Fig. 5 in which overexpression of wild-type Cx43 restores enterocyte migration after IFN treatment, these findings provide strong evidence in support of the notion IFN prevents enterocyte migration by disrupting the association between Cx43 and lipid rafts in enterocytes.
Fig. 7.
Cx43 overexpression restores Cx43 to lipid rafts in IFN-treated enterocytes. IEC-6 cells were infected with adenoviruses expressing either GFP alone (GFP), GFP-wild-type Cx43 (wtCx43), or GFP-dominant negative Cx43 (dnCx43), then treated with IFN as in methods. Cells were then treated with Alexa555-cholera toxin to label lipid rafts, then fixed and evaluated by confocal microscopy for the localization of lipid rafts (red) and the distribution of the transfected GFP or Cx43 (green). The extent of colocalization (green + red) was determined by using Image J software. A–D: IEC-6 cells were infected with adenoviruses expressing GFP alone and were untreated. There is no measurable colocalization between the GFP signal and the lipid rafts; E–H: IEC-6 cells were infected with adenoviruses expressing GFP alone and were treated with IFN, leading to a loss of lipid rafts from the cell surface. There is no measurable colocalization between the GFP signal and the lipid rafts. I–L: IEC-6 cells were infected with adenoviruses expressing GFP-tagged wild-type Cx43 and were treated with IFN, leading to a restoration of lipid rafts to the cell surface at sites where Cx43 was detected. Note the colocalization between the GFP (wtCx43) signal and the lipid rafts (arrows in K–L). M–P: IEC-6 cells were infected with adenoviruses expressing GFP-tagged dominant negative Cx43 and were treated with IFN, leading to a failure of restoration of lipid rafts to the cell surface, at sites where Cx43 was detected. Note the lack of colocalization between the GFP (wtCx43) signal and the lipid rafts. Representative of 3 separate experiments.
DISCUSSION
In the present study, we report the novel observations that Cx43 is found on lipid rafts in enterocytes, that IFN, a proinflammatory cytokine released in the ileal mucosa in NEC, inhibits enterocyte migration in vitro by disrupting the association between lipid rafts and gap junctions and that the redistribution of Cx43 to lipid rafts through the use of either phorbol esters or the induced overexpression of Cx43 results in a reversal of the inhibitory effects of IFN on enterocyte migration. The physiological relevance of the present findings to the pathogenesis of NEC lie in our recent observations that the expression of Cx43 in enterocytes in vivo is decreased and Cx43 becomes internalized from the surface of enterocytes in the small bowel when NEC is induced in neonatal mice (32). Taken together, these findings provide insights into the mechanisms by which IFN modulates enterocyte migration through effects on gap junctions and provide insights into why mucosal healing may be impaired in diseases associated with high levels of local IFN release, such as experimental NEC.
The present study represents a departure from current thinking by demonstrating that the association between Cx43 and lipid rafts in enterocytes plays an important role in the regulation of enterocyte migration. The finding that Cx43 is found on lipid rafts in enterocytes is novel and is supported by previous studies showing that Cx43 is detected on lipid rafts in keratinocytes (30). There are several possible mechanisms by which gap junctions may participate in the regulation of enterocyte migration. Since gap junctions are known to facilitate the intercellular connectivity of adjacent cells, the present findings raise the possibility that enterocyte migration is governed by the gap junction-mediated interchange of critical molecules between adjacent cells. The exact molecule(s) that are exchanged between gap junctions to regulate enterocyte migration remain unknown, although possibilities include ATP (19) or ions that are required for migration, such as calcium and magnesium (25, 35). Alternatively, and perhaps more likely, Cx43 may act as a docking platform for a variety of proteins that themselves are required for enterocyte migration. Specifically, Cx43 has been shown to interact directly with a host of actin binding and intracellular signaling proteins (16–18, 40), that themselves could lead to the cytoskeletal changes required for migration to occur. Indeed, Cx43 has been shown to be a target of the Rho signaling cascade in astrocytes (47), a protein that we have shown to play a pivotal role in the regulation of enterocyte migration (6). Cx43 has also been shown to interact with kinases including c- and v-Src kinase, PKC, and mitogen-activated protein kinase (16), each of which has been shown to play a role in the regulation of migration (43). The localization of Cx43 to lipid rafts in enterocytes may thus facilitate the initiation of a signaling cascade that is required to allow enterocytes to migrate; as such the loss of Cx43 from lipid rafts in response to IFN would be expected to prevent migration from occurring. It is tempting to speculate that this could also explain our observation that the inhibition of lipid rafts led to a marked disruption in enterocyte migration independent of other treatment. By restoring Cx43 to lipid rafts either pharmacologically or through overexpression of wild-type Cx43, the capacity of the signaling cascade to initiate migration is recovered.
It should be acknowledged that the ability of GFP-Cx43 to bypass the effect of IFN does raise the possibility that these results could represent either an artifact of the GFP tag or some degree of mislocalization due to the overexpression system. However, the fact that the dominant negative GFP-Cx43 did not rescue the inhibitory effects of IFN-despite being GFP-tagged and expressed at similar levels to the wild-type protein-argue strongly for a specific effect of Cx43 being relocalized to lipid rafts in mediating this effect and emphasize the importance of the role of Cx43 on lipid rafts in the regulation of enterocyte migration.
We readily acknowledge that IFN may affect other pathways that impair migration. For instance, IFN has been shown to prevent wound healing by causing endocytosis of β1-integrin focal adhesions (52) and to disrupt tight junctions(34) in vitro. These findings therefore raise the possibility that the disruption of gap junctions by IFN between enterocytes may be a secondary consequence to disruption of other junctional/cell adhesion complexes. However, the present findings that overexpression of Cx43 rescues the effects of IFN on enterocyte migration and that overexpression of Cx43 restores Cx43 to lipid rafts upon IFN treatment lead us to conclude that lipid raft-Cx43 interactions must play a major role. It is also noteworthy that the adenovirus-based induced expression of Cx43 as used in the present work also has several limitations, including the possibility of virus-induced effects independent of the expression of Cx43. However, the fact that adenoviral infection with GFP alone had no measurable effect on either migration or lipid raft dynamics as well as the fact that wild-type Cx43 had opposite effects to those observed when dominant negative Cx43 was expressed strongly argue in favor of a specific effect of the virally induced protein in rescuing the inhibition of IFN on enterocyte migration.
The present studies raise the important question as to how IFN actually disrupts the association between lipid rafts and Cx43 in enterocytes in the first place. Although a definitive explanation remains lacking, we now speculate that the binding of IFN to its receptor may induce a conformational change in the enterocyte plasma membrane, leading to biophysical alterations in the binding ability of lipid rafts such that they lose affinity for Cx43 and potentially other components of gap junction channels. Evidence for this possibility may be found in a recent study demonstrating that IFN can lead to changes in the plasma membrane receptor ifnar1, with effects on signaling (28, 50). Alternatively, IFN may exert its effects on the association between lipid rafts and Cx43 by acting through parallel signaling pathways, such as those involving MAPK or the JAK-STAT signaling pathway, and these pathways could lead to secondary effects on the assembly of lipid rafts in enterocytes and their association with Cx43. This latter possibility is supported by our observation that the loss of Cx43 from lipid rafts induced by IFN could be reversed by phorbol esters, which are known to activate the MAPK and STAT signaling pathways (15). It is also noteworthy that expression of dominant negative Cx43 was not able to restore the localization of Cx43 to lipid rafts, indicating a specific effect of IFN on functional gap junction-lipid raft interactions that cannot be reversed by a mutant protein.
In summary, we have now shown that IFN inhibits enterocyte migration by reversibly disrupting the association between the gap junction protein Cx43 and lipid rafts. Given that necrotizing enterocolitis is characterized by the release of IFN and the loss of enterocyte migration, we now propose that a loss of Cx43-lipid raft interactions may represent an early event in the impairment in enterocyte migration that characterizes NEC.
GRANTS
D. J. Hackam is supported by 1R01-GM078238-01 from the National Institutes of Health and the Children's Hospital of Pittsburgh Research Foundation. This work was also supported by the Resident Research Award from the American College of Surgeons to S. C. Gribar, as well as a Loan Repayment Award from the National Institutes of Health to S. C. Gribar, C. L. Leaphart, and W. Richardson.
Acknowledgments
We thank Dr. Matthias M. Falk (Lehigh University, Bethlehem, PA) for GFP-tagged Cx43 cDNA, Dr. Dale Laird (The University of Western Ontario, London, ON, Canada) for provision of wild-type and mutant Cx43 cDNA constructs, and Dr. Chuck Murry (University of Washington, Seattle, WA) for GFP adenoviruses.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anand RJ, Leaphart CL, Mollen KP, Hackam DJ. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock 27: 124–133, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Botto L, Masserini M, Palestini P. Changes in the composition of detergent-resistant membrane domains of cultured neurons following protein kinase C activation. J Neurosci Res 85: 443–450, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bruns RF, Miller FD, Merriman RL, Howbert JJ, Heath WF, Kobayashi E, Takahashi I, Tamaoki T, Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochem Biophys Res Commun 176: 288–293, 1991. [DOI] [PubMed] [Google Scholar]
- 4.Bu HF, Zuo X, Wang X, Ensslin MA, Koti V, Hsueh W, Raymond AS, Shur BD, Tan XD. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest 117: 3673–3683, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cetin S, Dunklebarger J, Li J, Boyle P, Ergun O, Qureshi F, Ford H, Upperman J, Watkins S, Hackam DJ. Endotoxin differentially modulates the basolateral and apical sodium/proton exchangers (NHE) in enterocytes. Surgery 136: 375–383, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Cetin S, Ford HR, Sysko LR, Agarwal C, Wang J, Neal MD, Baty C, Apodaca G, Hackam DJ. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J Biol Chem 279: 24592–24600, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Cetin S, Leaphart CL, Li J, Ischenko I, Hayman M, Upperman J, Zamora R, Watkins S, Ford HR, Wang J, Hackam DJ. Nitric oxide inhibits enterocyte migration through activation of RhoA-GTPase in a SHP-2-dependent manner. Am J Physiol Gastrointest Liver Physiol 292: G1347–G1358, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Clark JA, Lane RH, MacLennan NK, Holubec H, Dvorakova K, Halpern MD, Williams CS, Payne CM, Dvorak B. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 288: G755–G762, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MB, Jensen NJ, Hawkins JA, Mann EA, Thompson MR, Lentze MJ, Giannella RA. Receptors for Escherichia coli heat stable enterotoxin in human intestine and in a human intestinal cell line (Caco-2). J Cell Physiol 156: 138–144, 1993. [DOI] [PubMed] [Google Scholar]
- 10.El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology 129: 609–625, 2005. [DOI] [PubMed] [Google Scholar]
- 11.El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery 142: 234–242, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Feng J, Besner GE. Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J Pediatr Surg 42: 214–220, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Feng J, El-Assal ON, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin Pediatr Surg 14: 167–174, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Frost BL, Jilling T, Caplan MS. The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Semin Perinatol 32: 100–106, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiger M, Wrulich OA, Jenny M, Schwaiger W, Grunicke HH, Uberall F. Defining the human targets of phorbol ester and diacylglycerol. Curr Opin Mol Ther 5: 631–641, 2003. [PubMed] [Google Scholar]
- 16.Giepmans BN Gap junctions and connexin-interacting proteins. Cardiovasc Res 62: 233–245, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol 8: 931–934, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Giepmans BN, Verlaan I, Hengeveld T, Janssen H, Calafat J, Falk MM, Moolenaar WH. Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol 11: 1364–1368, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg GS, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem 277: 36725–36730, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Goodenough DA, Golliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem 65: 475–502, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Hackam DJ, Upperman JS, Grishin A, Ford HR. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg 14: 49–57, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Hansen G, Pedersen E, Immerdal L, Niels-Christiansen L, Danielsen E. Anti-glycosyl antibodies in lipid rafts of the enterocyte brush border: a possible host defense against pathogens. Am J Physiol Gastrointest Liver Physiol 289: G1100–G1107, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Hanski C, Stolze B, Riecken EO. Tumorigenicity, mucin production and AM-3 epitope expression in clones selected from the HT-29 colon carcinoma cell line. Int J Cancer 50: 924–929, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Houle VM, Park YK, Laswell SC, Freund GG, Dudley MA, Donovan SM. Investigation of three doses of oral insulin-like growth factor-I on jejunal lactase phlorizin hydrolase activity and gene expression and enterocyte proliferation and migration in piglets. Pediatr Res 48: 497–503, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Isakson BE, Evans WH, Boitano S. Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am J Physiol Lung Cell Mol Physiol 280: L221–L228, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson K, Muoritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol 9: 7–14, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (DCN-1028c), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 159: 548–553, 1989. [DOI] [PubMed] [Google Scholar]
- 28.Krause CD, Lavnikova N, Xie J, Mei E, Mirochnitchenko OV, Jia Y, Hochstrasser RM, Pestka S. Preassembly and ligand-induced restructuring of the chains of the IFN-gamma receptor complex: the roles of Jak kinases, Stat1 and the receptor chains. Cell Res 16: 55–69, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Laird D Life cycle of connexins in health and disease. Biochem J 394: 527–543, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol Biol Cell 19: 912–928, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 179: 4808–4820, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Leaphart CL, Qureshi F, Cetin S, Li J, Dubowski T, Batey C, Beer-Stolz D, Guo F, Murray SA, Hackam DJ. Interferon-γ inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology 132: 2395–2411, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Lin D, Lobell S, Jewell A, Takemoto DJ. Differential phosphorylation of connexin46 and connexin50 by H2O2 activation of protein kinase Cγ. Mol Vis 10: 688–695, 2004. [PubMed] [Google Scholar]
- 34.Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest 83: 724–727, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maghazachi AA Intracellular signaling events at the leading edge of migrating cells. Int J Biochem Cell Biol 32: 931–943, 2000. [DOI] [PubMed] [Google Scholar]
- 36.McLachlan E, Manias JL, Gong XQ, Lounsbury CS, Shao Q, Bernier SM, Bai D, Laird DW. Functional characterization of oculodentodigital dysplasia-associated Cx43 mutants. Cell Commun Adhes 12: 279–292, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Mohan S, Bruns JR, Weixel KM, Edinger RS, Bruns JB, Kleyman TR, Johnson JP, Weisz OA. Differential current decay profiles of epithelial sodium channel subunit combinations in polarized renal epithelial cells. J Biol Chem 279: 32071–32078, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res 92: 71–77, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol 176: 3070–3079, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Park DJ, Freitas TA, Wallick CJ, Guyette CV, Warn-Cramer BJ. Molecular dynamics and in vitro analysis of Connexin43: a new 14-3-3 mode-1 interacting protein. Protein Sci 15: 2344–2355, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parton RG, Hanzal-Bayer M, Hancock JF. Biogenesis of caveolae: a structural model for caveolin-induced domain formation. J Cell Sci 119: 787–796, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Podolsky DKV Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol Gastrointest Liver Physiol 277: G495–G499, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Polk D Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology 114: 493–502, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 80: 248–265, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qureshi FG, Leaphart CL, Cetin S, Jun L, Grishin A, Watkins S, Ford HR, Hackam DJ. Increased expression and function of integrins in enterocytes by endotoxin impairs epithelial restitution. Gastroenterology 128: 1012–1022, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Reinecke H, Minami E, Virag JI, Murry CE. Gene transfer of connexin43 into skeletal muscle. Hum Gene Ther 15: 627–636, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Rouach N, Pebay A, Meme W, Cordier J, Ezan P, Etienne E, Giaume C, Tence M. S1P inhibits gap junctions in astrocytes: involvement of G and Rho GTPase/ROCK. Eur J Neurosci 23: 1453–1464, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M, Musa H. Functional characterization of connexin43 mutations found in patients with oculodentodigital dysplasia. Circ Res 96: e83–e91, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Sprenger RR, Horrevoets A. The ins and outs of lipid domain proteomics. Proteomics 7: 2895–2903, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Strunk JJ, Gregor I, Becker Y, Li Z, Gavutis M, Jaks E, Lamken P, Walz T, Enderlein J, Piehler J. Ligand binding induces a conformational change in ifnar1 that is propagated to its membrane-proximal domain. J Mol Biol 377: 725–739, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Sturm A, Dignass A. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol 14: 348–353, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tong Q, Vassilieva EV, Ivanov AI, Wang Z, Brown GT, Parkos CA, Nusrat A. Interferon-γ inhibits T84 epithelial cell migration by redirecting transcytosis of β1 integrin from the migrating leading edge. J Immunol 175: 4030–4038, 2005. [DOI] [PubMed] [Google Scholar]