Abstract
Cholecystokinin, like many peptide hormones, is present as multiple molecular forms. CCK-58 has been identified as the dominant form in the circulation, whereas most of the studies of CCK-receptor interactions have been performed with CCK-8. Despite both sharing the pharmacophoric region of CCK, representing its carboxy terminal heptapeptide amide, studies in vivo have demonstrated biological diversity of action of the two peptides, with CCK-58, but not CCK-8, stimulating pancreatic fluid secretion and lengthening the interval between meals. Here, we have directly studied the ability of these two CCK peptides to bind to the type 1 CCK receptor and to stimulate it to elicit an intracellular calcium response. The calcium response relative to receptor occupation was identical for CCK-58 and CCK-8, with the longer peptide binding with approximately fivefold lower affinity. We also examined the ability of the two peptides to elicit receptor internalization using morphological techniques and to disrupt the constitutive oligomerization of the CCK receptor using receptor bioluminescence resonance energy transfer. Here, both full agonist peptides had similar effects on these regulatory processes. These data suggest that both molecular forms of CCK act at the CCK1 receptor quite similarly and elicit similar regulatory processes for that receptor, suggesting that the differences in biological activity observed in vivo most likely reflect differences in the clearance and/or metabolism of these long and short forms of CCK peptides.
Keywords: bioluminescence resonance energy transfer
cholecystokinin (CCK) is a peptide hormone that is critical for nutritional homeostasis, with biological actions to stimulate gallbladder contraction, pancreatic exocrine secretion, gastrointestinal motility, and even satiation (9) and postprandial satiety (2). It is present as a variety of length linear peptides, all sharing the pharmacophoric domain within the carboxy terminal heptapeptide amide. Although most studies of CCK receptor binding, biological activity, and regulation have been performed with sulfated CCK octapeptide, referred to here as CCK-8, CCK-58 is now considered to represent the predominant form of this hormone in the circulation of dog (8), rat (21), and human (6).
Although rat CCK-58 has been shown to bind to the transfected rat CCK1 receptor with affinity close to that of CCK-8 (7- to 12-fold less) (24) and human CCK-58 has similar biological activity to stimulate calcium responses in human pancreatic acinar cells as CCK-8 (19), rat CCK-58 has been reported to have in vivo biological effects that are qualitatively different from those of the shorter peptide. This includes the ability of CCK-58, but not CCK-8, to stimulate pancreatic fluid secretion in both anesthetized (24) and awake rats (28). Whereas concentrations of CCK-8 that stimulate maximal pancreatic protein output have been reported to inhibit pancreatic fluid secretion, these concentrations of CCK-58 have been described to be maximal for protein output and to stimulate fluid output (28). Similarly, whereas both CCK-8 and CCK-58 cause reduction in food intake, only CCK-58 causes lengthening of the interval between meals (20). It is important to note that both aspects of the pancreatic secretory response (28) and the feeding response (2, 26) to endogenous CCK are attenuated in a potent manner by the selective CCK1 receptor antagonist, devazepide. This suggests that these biological differences for different-length forms of CCK are mediated by the same CCK1 receptor subtype, making it particularly important to explore the molecular and physiological basis for the observed in vivo differences.
In this work, we have carefully studied CCK-58 binding to the CCK1 receptor and its ability to stimulate intracellular calcium and have examined for the first time the effects of this peptide on CCK1 receptor regulation, evaluating agonist-induced effects on receptor internalization and receptor oligomerization. Internalization was studied in a morphological assay tracking yellow fluorescent protein (YFP)-tagged CCK1 receptor expressed on Chinese hamster ovary (CHO) cells. Receptor homooligomerization (association of two or more CCK receptor molecules with each other) was studied using bioluminescence resonance energy transfer (BRET) between CCK receptor constructs tagged at the carboxy terminus with Renilla luciferase (donor) and YFP (acceptor).
METHODS
CCK peptides.
Natural rat sulfated CCK-58 was prepared in the laboratory of Dr. Reeve, as previously described (24). The CCK-58 peptide was greater than 90% pure, as analyzed by high-performance capillary electrophoresis. Synthetic sulfated CCK-8 was obtained from Bachem (Torrance, CA).
CCK receptor constructs.
Wild-type human and rat type 1 CCK (CCK1) receptor constructs were utilized for the present studies. Both have been shown to bind and to be activated by CCK-8, in manner not different from natural receptor expressed on rat pancreatic acinar cells (11). These were utilized, as noted, as transiently expressed on COS-1 cells after transfection or as stably expressed on CHO-K1 or HEK-293 cell lines (27). For BRET experiments, the human CCK1 receptor was tagged with Renilla luciferase (Rlu) or YFP at its carboxy terminus. We have previously validated that these constructs bound CCK and exhibited agonist-stimulated intracellular calcium responses that were similar to untagged wild-type CCK1 receptor (3).
Cell culture.
Cell lines were acquired from the American Type Culture Collection (ATCC; Rockville, MD) and were grown in tissue culture plasticware in an environment containing 5% CO2 at 37°C. COS-7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 5% Fetal Clone II (Hyclone, Logan, UT). CHO cell lines were cultured in Ham's F-12 medium supplemented with 5% Fetal Clone II or fetal bovine serum (FBS) (GIBCO). HEK-293 cell lines and rat pancreatic AR42J cells (ATCC CRL 1492) were cultured in DMEM/F12 (Invitrogen, Carlsbad, CA) with 10% FBS. For transient expression studies, COS-1 cells were plated 24 h before transfection at a density of ∼0.5 × 106 cells/10-cm dish. Cells were transfected with 3 μg of total DNA per dish by the diethylaminoethyl-dextran method. For stable receptor expression, CHO-K1 or HEK-293 cells were transfected with 1 μg of plasmid DNA (pcDNA3, Invitrogen) containing cloned CCK receptors. Cells were then selected by G418 (500 μg/ml Geneticin, Invitrogen), and drug-resistant single cell-derived clones were screened by binding and calcium responses to CCK-8.
CCK receptor binding studies.
Stable CHO cells expressing wild-type rat CCK1 receptors, HEK-293 cells expressing wild-type human CCK1 receptors, and rat pancreatic AR42J cells were used in the binding studies. Ligand binding and competition assays were performed on intact cells with radiolabeled CCK-8. Cells were cultured in poly-l-lysine-coated 24-well plates and grown to a final density of near confluence (1 × 106 cells/well). Cells were rinsed twice with phosphate-buffered saline (PBS) and 1 ml of cell binding buffer (Waymouth's medium, 20 mM HEPES, pH 7.4, 0.1% bacitracin, and 0.2% bovine serum albumin) was added. Binding assays were then started by adding Bolton Hunter-labeled 125I-CCK-8 (40 pM, ∼2,000 Ci/mmol, Amersham, Buckinghamshire, UK) in the presence of increasing concentrations of unlabeled CCK peptides, as indicated. After 1-h incubation at room temperature, cells were washed twice with ice-cold PBS and then solubilized in 1 ml of 1% Triton X-100 in PBS. Bound (cell lysate) and free (medium) radioactivity was quantified and values were expressed as percentage of maximal binding (without unlabeled peptide). Data were analyzed by using Prism software (GraphPad, San Diego, CA) to generate binding graphs based on nonlinear regression and curve fitting in a one-site competition model.
Calcium imaging studies.
Cells were cultured for 48 h on 20-mm glass coverslips precoated with poly-l-lysine. Cells were preincubated with 5 μM Ca2+ indicator dye, fura 2-AM (Molecular Probes, Eugene, OR), for 30 min at 37°C. Coverslips were then mounted in a perfusion chamber with 0.9 ml HBSS containing 20 mM HEPES, pH 7.4. CCK-8 or CCK-58 was added to each disc to produce final concentrations ranging from 1 pM to 1 μM. A video imaging workstation consisting of a Zeiss 100TV inverted microscope with a ×40 objective and a computerized videomicroscopy system (Attofluor Digital Imaging System, Atto Instruments, Rockville, MD) was used. Ca2+-dependent fluorescence was obtained by exciting fura 2 at 340 and 380 nm. The indicator was calibrated before the measurements with saturated and Ca2+-free solutions, and accuracy was controlled with standard solutions of known Ca2+ concentrations. Basal calcium concentrations were recorded before the addition of peptides and the difference between the first predominant peak (after stimulation) and the basal values were calculated to represent values for intracellular calcium increase (Δ[Ca2+]i). At least 20 cells were selected for imaging each condition, and two experiments were performed for each stable receptor-expressing cell line.
Receptor internalization studies.
Receptor internalization studies were performed using CHO cells stably expressing YFP-tagged CCK receptor, as described previously (11, 12). CHO-CCK1-YFP cells grown on coverslips for at least 48 h were washed twice with PBS, pH 7.4, containing 0.08 mM CaCl2 and 0.1 mM MgCl2. They were then incubated with 1 μM peptide (CCK-8 or CCK-58) at 4°C for 90 min. After incubation, the cells were then washed with ice-cold PBS and incubated further with prewarmed PBS at 37°C for varying periods of time, ranging between 3 and 30 min. At each time point, the cells on the coverslips were fixed with 2% paraformaldehyde, washed twice with PBS, and then mounted on slides using Vectashield (Vector Laboratories, Burlingame, CA). Fluorescence was observed via a Zeiss LSM510 (Thornwood, NY) confocal microscope (pinhole diameter of 210 μm with a plan-apochromat 63 × 1.3 numerical aperture oil objective) configured to capture YFP emission (excitation at 488 nm by argon laser and emission through a 505-nm long-pass filter). Background-subtracted images were assembled with Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA). Receptor on the cell surface as a percentage of cellular complement of receptor was quantified with Metamorph 6.32 (Molecular Devices, Sunnyvale, CA).
BRET assays.
COS cells transiently expressing Rlu- and YFP-tagged CCK1 receptor constructs were used for the BRET studies, as described previously (4). Transfected COS cells were detached by using nonenzymatic cell dissociation solution (Sigma) 48 h after transfection and resuspended in Krebs-Ringer-HEPES (KRH) medium containing the following: 25 mM HEPES, pH 7.4, 104 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM KH2PO4, and 1.2 mM MgSO4, with 0.2% bovine serum albumin and 0.01% soybean trypsin inhibitor. Approximately 25,000 receptor-bearing cells were incubated with varying concentrations of peptide (CCK-8 or CCK-58) at room temperature for 30 min. After incubation, the BRET assay was started by the addition 5 μM coelentrazine h (Biotium, Hayward, CA), the substrate for Renilla luciferase, to the cell suspension in a 96-well Optiplate (PerkinElmer Life Sciences). Bioluminescence and fluorescence intensities were quantified using the 2103 Envision fluorescence plate reader (PerkinElmer Life Sciences). The instrument was set with a mirror (<700 nm) and emission filter sets for luminescence (460 nm, bandwidth 25 nm) and fluorescence (535 nm, bandwidth 25 nm), which allowed the calculation of the BRET ratio as previously described (13).
Saturation BRET studies were performed as described previously (12). For this, COS cells were transiently transfected with a fixed concentration of Rlu-tagged CCK1 receptor (1.5 μg DNA/dish) construct and increasing amounts of YFP-tagged CCK1 receptor (0.3 μg to 6.0 μg DNA/dish). Cells were detached by use of nonenzymatic cell dissociation medium 48 h after transfection and were incubated with peptides (CCK-8 or CCK-58) at a concentration of 1 μM in KRH medium at room temperature for 30 min. BRET data were collected by using the Envision fluorescence plate reader as described above. Acceptor-donor ratios were plotted relative to the BRET ratios. Curves were fit to one-site exponential and linear functions via Prism 3.0 (GraphPad, San Diego, CA). Differences were evaluated using the t-test, with P < 0.05 considered to be statistically significant.
RESULTS
Binding and biological activity studies.
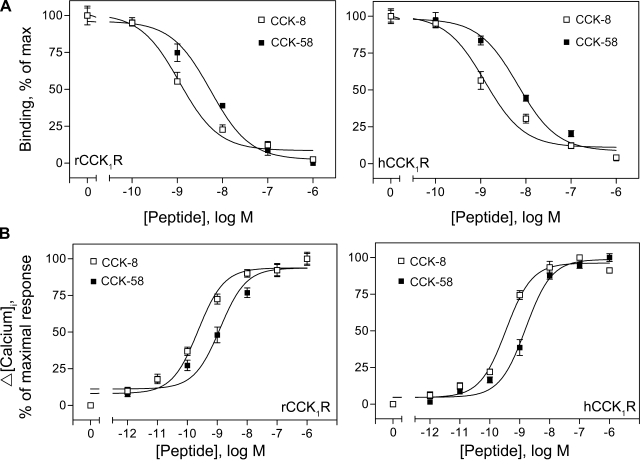
Binding studies were performed on CHO or HEK-293 cell lines expressing comparable levels of wild-type rat or human CCK1 receptors, respectively. Binding of radiolabeled CCK-8 to CCK1 receptors could be displaced competitively by CCK-8 with similar high affinities of 1.1 ± 0.1 nM in rat and 1.2 ± 0.2 nM in human receptor. Displacement curves of 125I-CCK-8 binding by CCK-58 in both rat and human CCK1 receptors were shifted toward lower affinity, with Ki values of 5.4 ± 0.4 nM and 6.7 ± 0.3 nM, respectively (Fig. 1A and Table 1). Therefore, at equal molar concentrations, CCK-58 showed about fivefold reduced binding affinities compared with CCK-8. This was also confirmed with a rat pancreatic AR42J cell line that naturally expresses CCK receptors, where the IC50 values for CCK-8 and CCK-58 were 4 ± 1 and 26 ± 3 nM, respectively. We then compared calcium responses of CCK1 cells following stimulation with CCK-8 or CCK-58 at increasing concentrations (1 pM to 1 μM). Typical transient Δ[Ca2+]i values were observed in both rat and human CCK1 receptor-expressing cell lines in concentration-dependent manner (Fig. 1B). EC50 values were estimated to range from 0.23–0.35 nM for CCK-8 and 1.2–1.5 nM for CCK-58 to act at the human and rat CCK1 receptors. Maximal increases of Δ[Ca2+]i induced by either CCK peptide were similar in each type of cell (CHO: 200–250 nM, HEK-293: 300–400 nM).
Fig. 1.
CCK-58 and CCK-8 receptor binding and biological activity. Shown are competition-binding curves (A) and stimulated intracellular calcium response (Δ[Calcium]i) curves (B) to CCK-8 and CCK-58 in rat and human CCK1 receptor-bearing cells. All values represent the means ± SE of data from at least 3 independent experiments (for binding) and duplicate determinations (for calcium). rCCK1R and hCCK1R, rat and human type 1 CCK receptors, respectively; Max, maximum; brackets denote concentration.
Table 1.
Binding and activation of cell signaling by CCK peptides
|
CCK-8 |
CCK-58
|
Bmax | |||
|---|---|---|---|---|---|
| Binding Kd, nM | Δ[Ca2+]i EC50, nM | Binding Ki, nM | Δ[Ca2+]i EC50, nM | ||
| rCCK1 | 1.1±0.1 | 0.23±0.1 | 5.4±0.4 | 1.2±0.1 | 4.2±0.2 |
| hCCK1 | 1.2±0.2 | 0.35±0.1 | 6.7±0.3 | 1.5±0.2 | 4.4±0.8 |
Receptor kinetic binding constants (Kd and Ki , nM) represent the means ± SE of data from 3 separate experiments. Maximum binding capacity (Bmax) values were calculated by Scatchard analysis of stably transfected cells and expressed as expressed as no. × 105 sites/cell. Concentrations of peptides stimulating half-maximal calcium responses (EC50, nM) were determined in duplicate from at least 2 separate experiments. rCCK1 and hCCK1, human and rat type 1 CCK, respectively; Δ[Ca2+]i, intracellular calcium increase.
Receptor internalization studies.
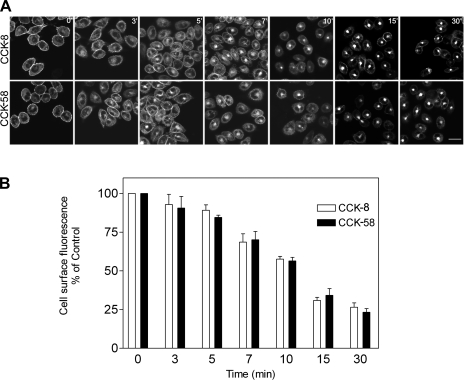
We previously demonstrated that agonist occupation of the CCK receptor expressed on CHO cells stimulates its internalization (25). This proceeds predominantly via clathrin-mediated endocytosis (25). Here, we have examined CCK receptor internalization in the same cellular context after occupation by CCK-58. We tracked the fluorescently tagged receptor after exposure to unlabeled CCK ligands. Like CCK-8, CCK-58 stimulated prompt and extensive internalization of the CCK receptor (Fig. 2). Of note, kinetics of internalization were also similar after occupation with the two distinct forms of CCK (Fig. 2B). In both cases, the fluorescently tagged receptors were observed as punctate structures under the plasma membrane within 5 min and were observed in internalized endosomal structures within 10 min.
Fig. 2.
Agonist-induced CCK receptor internalization. A: representative confocal microscopic images demonstrating internalization of yellow fluorescent protein (YFP)-tagged CCK1 receptors expressed on Chinese hamster ovary (CHO) cells after their occupation with nonfluorescent CCK peptides. Time points after stimulation are noted. Like CCK-8, CCK-58 stimulated prompt and extensive CCK receptor internalization, with similar patterns of distribution. The kinetics of internalization were also similar after stimulation with the 2 CCK peptides. Scale bar = 25 μm. B: quantitation of the receptor on the cell surface under these conditions. Results reflect means ± SE of data from 3 independent experiments.
BRET studies.
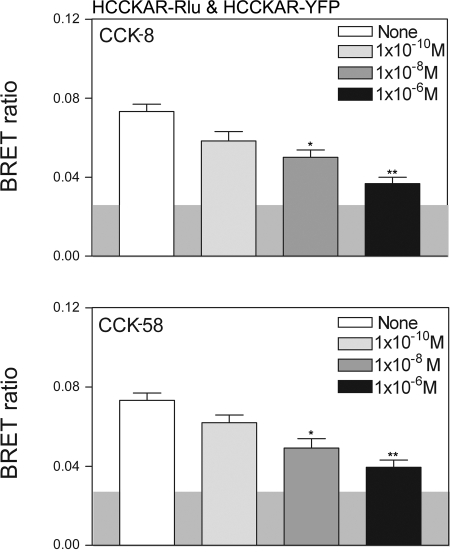
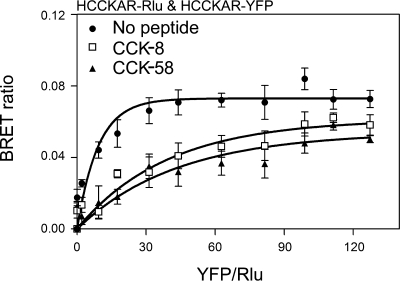
We previously demonstrated that the CCK receptor exists as constitutive oligomers on the cell surface and that agonist stimulation results in dissociation of these complexes (4). Here, we have evaluated the oligomerization status of this receptor in the presence of CCK-58. Receptor BRET results show that, like CCK-8, CCK-58 can dissociate CCK receptor oligomers in a concentration-dependent manner (Fig. 3). To further confirm these results, we utilized saturation BRET assays to distinguish between specific molecular association events and random interactions between bystander molecules that are mobile in the plasma membrane. In the absence of ligand, the saturation BRET results supported the presence of a specific oligomeric complex, with the BRET signal being saturated and reaching an asymptote. This was clearly different from the curves observed after both CCK-8 and CCK-58 that were not different from a linear fit to the data (Fig. 4). This supports the interpretation that the decrease in the receptor BRET signal observed after exposure to CCK-8 and CCK-58 represents dissociation of a significant oligomeric complex.
Fig. 3.
CCK-58 and CCK-8 disrupt CCK1 receptor oligomerization. Shown are bioluminescence resonance energy transfer (BRET) ratios obtained from COS cells coexpressing Renilla luciferase (Rlu)-and YFP-tagged human CCK1 receptors (HCCKAR). Cells expressing the CCK receptor constructs were incubated with increasing concentrations of either CCK-8 or CCK-58 prior to measurement of the BRET signals. Concentration-dependent decreases in the BRET signals were observed after stimulation with each of the peptides. The shaded area represents the background BRET ratio that can be measured upon expression of structurally unrelated receptors on the cell surface or upon expression of a donor-acceptor pair with one in the plasma membrane and the other in the cytosol. Signals above this are considered to be significant. Data represent means ± SE of 4 independent experiments. *P < 0.05, **P < 0.01 compared with BRET signals obtained without exposure to CCK peptides.
Fig. 4.
Saturation BRET analysis. Shown are BRET saturation curves plotted as the ratios of YFP fluorescence to Rlu luminescence obtained when COS cells coexpressing fixed concentrations of Rlu-tagged CCK1 receptors (donor) and increasing the concentrations of YFP-tagged CCK1 receptors (acceptor) were exposed to the noted peptides. For control cells not exposed to CCK, the BRET ratios generated exponential curves that increased until values reached saturation, as reflected in an asymptote. In contrast, CCK-8 and CCK-58 stimulation yielded BRET saturation curves that were not statistically different from linear fits. Data are represented as means ± SE of 4 independent experiments.
DISCUSSION
Hormones exert their physiological effects on the organism through receptor interactions and activation of postreceptor effector cascades. However, it is widely appreciated that distinct molecular forms of a given hormone that interact with the same receptor can exert quite distinct biological effects. This often results from differences in the binding affinity or biological potency or biological efficacy of the different forms of the hormone acting at the receptor. However, in the situation that is the focus of the present report, the different length forms of cholecystokinin (CCK-58 and CCK-8) have been reported to have similar binding properties (with slightly lower binding affinity for rat CCK-58) (24) and with similar calcium responses to human CCK-58 in pancreatic cells in vitro (19), yet rat CCK-58 has distinct biological effects in vivo (20, 24, 28). This suggests that the differences could be prereceptor, based on differences in hormone metabolism or clearance, or could occur after receptor binding and activation, at the level of regulation of the CCK1 receptor. In the present report, we have carefully evaluated events stimulated by CCK-58 that occur at the level of the CCK1 receptor, exploring ligand binding, initiation of signaling, and receptor regulation, comparing each of these to events stimulated by CCK-8.
We have previously shown that CCK-58 and CCK-8 have different tertiary structures in solution (15, 16). It is noteworthy that this does not seem to interfere with the ability of these peptides to recognize and bind to the CCK1 receptor or to activate it. In the present report, the apparent binding affinity of rat CCK-58 was approximately fivefold lower than that of CCK-8 with transfected rat CCK1 receptors. However, consistent with this, the potency of CCK-58 to elicit an intracellular calcium response was approximately fivefold lower than that of CCK-8, whereas both peptides had similar efficacy. Thus the biological response relative to receptor occupation was identical for both CCK-58 and CCK-8. We were careful to control for the peptide concentration in solution, determined directly by radioimmunoassay (22). It is still possible that the much more basic longer peptide (CCK-58) exhibited more nonspecific adsorption to the glassware or to nonreceptor components of the cell than the shorter, more acidic peptide (CCK-8), resulting in less CCK-58 being available at the level of the receptor for binding and stimulation of biological activity. Such differential nonspecific adsorption events seem unlikely, since canine CCK-58 is reported to have a higher binding affinity to mouse CCK1 receptors than CCK-8 and since these peptides are reported to have identical affinities for binding to the mouse CCK2 receptor (23). These observations are also consistent with data recently reported by Murphy et al. (19) demonstrating that CCK-58 and CCK-8 do not differ in their ability to stimulate intracellular calcium responses at low concentrations and global calcium release at high concentrations in human pancreatic acinar cells. Although an increase in intracellular calcium is only one signaling event stimulated by CCK occupation of its receptor, it has always been considered to represent a component of the major physiological signaling pathway reflecting Gq coupling to this receptor. Other secondary signaling events can also be affected by ligands acting at the CCK receptor and there continues to be the possibility that CCK-58 and CCK-8 influence these in different ways. Such alternative signaling components have not been directly studied after CCK-58 stimulation.
Receptor internalization represents a cellular mechanism for reducing the number of cell surface receptors, diminishing the opportunity for circulating hormone to stimulate a biological response. CCK-8 is well known to stimulate prompt and extensive internalization of the CCK receptor (25). This occurs predominantly via clathrin-mediated endocytosis (25). Molecular mechanisms thought to play a role in internalization include a change in receptor conformation, exposing an epitope that can bind to the lattice of clathrin-coated pits, and receptor phosphorylation that leads to arrestin binding and its interaction with the same cellular machinery. Clearly, if CCK-58 and CCK-8 are equally efficacious, as was observed, it seems quite likely that they would also stimulate similar CCK receptor internalization. Indeed, that is what was observed in this study. Both forms of CCK stimulated prompt and extensive receptor internalization that was quantitatively similar.
Another more recently recognized form of receptor regulation involves the dimerization or oligomerization of G protein-coupled receptors (1). The CCK1 receptor has been shown to be constitutively oligomerized on the cell surface in the absence of agonist (4). CCK-8 has been shown to disrupt such complexes (4). This could be a critical step in the downregulation or desensitization of the CCK receptor. It was, therefore, important to evaluate whether the greater in vivo biological effects of CCK-58 could be due to failure to disrupt such oligomeric complexes. Again, CCK-58 behaved similarly to CCK-8 in regard to this mechanism of receptor regulation.
These results focus more attention on the prereceptor handling of various molecular forms of CCK. It has been demonstrated that CCK-8 is extracted from the hepatic portal circulation extremely efficiently, whereas CCK-33 is not (10). Consistent with this, CCK-33 has been reported to reduce meal size when it is infused into portal blood at doses at which CCK-8 has no effect (7). These observations are consistent with the shorter half-life of CCK-8 than of CCK-33 (17). Whereas no studies have been performed in rat, CCK-58 has a longer half-life than CCK-8 in dogs (14). Additionally, CCK-8 has been shown to be rapidly digested by endopeptidase 24.11, whereas CCK-58 is completely resistant to this action (23). This could conceivably result in CCK-8 not being able to get into blood after its release into duodenal interstitial fluid because of digestion by enzymes found in interstitial fluid. This is supported by the fact that no CCK-8 is detected in rat blood after its release is stimulated by a trypsin inhibitor (21) or by protein (24). The short half-life of CCK-8 coupled with its possible ease of degradation before it enters the circulation or after it leaves the blood suggests that CCK-8 may not reach some CCK receptors that CCK-58 can bind and activate in vivo.
Indeed, the most likely explanation for the observed differences between CCK-8 and CCK-58 in feeding and pancreatic secretion relates to differences in prereceptor handling of these peptides. These differences would be expected to yield higher levels of CCK-58 for longer periods of time after the administration or secretion of similar amounts of both molecular forms of this hormone. This would clearly explain the prolonged interval between meals effected by CCK-58 (2). Similarly, differential prereceptor handling of these molecular forms of this hormone could explain the apparently unique effect of CCK-58 to stimulate pancreatic fluid secretion (28).
The pharmacophoric domain of CCK is the carboxy terminal heptapeptide amide, shared by all molecular forms of this hormone (18). Current understanding of the molecular basis of CCK binding to the CCK1 receptor shows that this region of CCK interacts closely with extracellular loop and tail domains of this receptor (5). It is noteworthy that this provides a route for amino terminal extensions of the peptide to move away from the binding cleft and to not have direct interactions with the receptor. It is important that any model of CCK binding to its receptor should include space to accommodate the amino terminal extension present in the dominant and fully biologically active form of this hormone, CCK-58. Indeed, this report extends our understanding of the CCK1 receptor interactions, biological effects, and regulatory impact of the different forms of this hormone, whereas it emphasizes the physiological importance of hormone metabolism and clearance.
GRANTS
This work was supported by grants from the National Institutes of Health, DK-32878 (L. J. Miller), DK-33850 (J. R. Reeve), DK-41301 (Peptidomic, Radioimmunoassay, and Proteomic Core of the CURE Digestive Diseases Center, J. R. Reeve), and P50-AA011999 (a pilot study supported by the Research Center for Alcoholic Liver and Pancreatic Diseases, S. V. Wu), as well as by the Veterans Affairs Research Administration at Veterans Affairs Greater Los Angeles Healthcare System (J. R. Reeve), the Fiterman Foundation (L. J. Miller), and the Mayo Clinic (L. J. Miller).
Acknowledgments
The authors thank F. J. Ho for expertise in the synthesis, cleavage, and purification of CCK-58, P. Chew and Moon Yang for assistance with receptor binding and calcium mobilization studies, and L. Bruins for assistance in BRET and internalization studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Angers S, Salahpour A, Bouvier M. Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42: 409–435, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Burton-Freeman B, Gietzen DW, Schneeman BO. Cholecystokinin and serotonin receptors in the regulation of fat-induced satiety in rats. Am J Physiol Regul Integr Comp Physiol 276: R429–R434, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Cheng ZJ, Harikumar KG, Holicky EL, Miller LJ. Heterodimerization of type A and B cholecystokinin receptors enhance signaling and promote cell growth. J Biol Chem 278: 52972–52979, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Cheng ZJ, Miller LJ. Agonist-dependent dissociation of oligomeric complexes of G protein-coupled cholecystokinin receptors demonstrated in living cells using bioluminescence resonance energy transfer. J Biol Chem 276: 48040–48047, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Dong M, Ding XQ, Thomas SE, Gao F, Lam PC, Abagyan R, Miller LJ. Role of lysine187 within the second extracellular loop of the type A cholecystokinin receptor in agonist-induced activation. Use of complementary charge-reversal mutagenesis to define a functionally important interdomain interaction. Biochemistry 46: 4522–4531, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberlein GA, Eysselein VE, Hesse WH, Goebell H, Schaefer M, Reeve JR Jr. Detection of cholecystokinin-58 in human blood by inhibition of degradation. Am J Physiol Gastrointest Liver Physiol 253: G477–G482, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Eisen S, Phillips RJ, Geary N, Baronowsky EA, Powley TL, Smith GP. Inhibitory effects on intake of cholecystokinin-8 and cholecystokinin-33 in rats with hepatic proper or common hepatic branch vagal innervation. Am J Physiol Regul Integr Comp Physiol 289: R456–R462, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Eysselein VE, Eberlein GA, Hesse WH, Singer MV, Goebell H, Reeve JR Jr. Cholecystokinin-58 is the major circulating form of cholecystokinin in canine blood. J Biol Chem 262: 214–217, 1987. [PubMed] [Google Scholar]
- 9.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 84: 488–495, 1973. [DOI] [PubMed] [Google Scholar]
- 10.Gores GJ, LaRusso NF, Miller LJ. Hepatic processing of cholecystokinin peptides. I. Structural specificity and mechanism of hepatic extraction. Am J Physiol Gastrointest Liver Physiol 250: G344–G349, 1986. [DOI] [PubMed] [Google Scholar]
- 11.Hadac EM, Ghanekar DV, Holicky EL, Pinon DI, Dougherty RW, Miller LJ. Relationship between native and recombinant cholecystokinin receptors - role of differential glycosylation. Pancreas 13: 130–139, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Harikumar KG, Dong M, Cheng Z, Pinon DI, Lybrand TP, Miller LJ. Transmembrane segment peptides can disrupt cholecystokinin receptor oligomerization without affecting receptor function. Biochemistry 45: 14706–14716, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harikumar KG, Pinon DI, Miller LJ. Transmembrane segment IV contributes a functionally important interface for oligomerization of the Class II G protein-coupled secretin receptor. J Biol Chem 282: 30363–30372, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann P, Eberlein GA, Reeve JR Jr, Bünte RH, Grandt D, Goebell H, Eysselein VE. Comparison of clearance and metabolism of infused cholecystokinins 8 and 58 in dogs. Gastroenterology 105: 1732–1736, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Keire DA, Solomon TE, Reeve JR Jr. Identical primary sequence but different conformations of the bioactive regions of canine CCK-8 and CCK-58. Biochem Biophys Res Commun 266: 400–404, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Keire DA, Solomon TE, Reeve JR Jr. NMR evidence for different conformations of the bioactive region of rat CCK-8 and CCK-58. Biochem Biophys Res Commun 293: 1014–1020, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Liddle RA Cholecystokinin. In: Gut Peptides: Biochemistry and Physiology, edited by Walsh JH and Dockray GJ. New York: Raven, 1994, p. 175–216.
- 18.Miller LJ, Lybrand TP. Molecular basis of agonist binding to the type A cholecystokinin receptor. Pharmacol Toxicol 91: 282–285, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Murphy JA, Criddle DN, Sherwood M, Chvanov M, Mukherjee R, McLaughlin E, Booth D, Gerasimenko JV, Tepikin AV, Green GM, Reeve JR Jr, Petersen OH, Sutton R. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology. In Press. [DOI] [PubMed]
- 20.Overduin J, Reeve JR Jr, Gibbs J. Comparison of the satiety actions of CCK-8 and CCK-58 (Abstract). Appetite 35: 303, 2000. [Google Scholar]
- 21.Reeve JR Jr, Green GM, Chew P, Eysselein VE, Keire DA. CCK-58 is the only detectable endocrine form of cholecystokinin in rat. Am J Physiol Gastrointest Liver Physiol 285: G255–G265, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Reeve JR Jr, Liddle RA, McVey DC, Vigna SR, Solomon TE, Keire DA, Rosenquist G, Shively JE, Lee TD, Chew P, Green GM, Coskun T. Identification of nonsulfated cholecystokinin-58 in canine intestinal extracts and its biological properties. Am J Physiol Gastrointest Liver Physiol 287: G326–G333, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Reeve JR Jr, McVey DC, Bunnett NW, Solomon TE, Keire DA, Ho FJ, Davis MT, Lee TD, Shively JE, Vigna SR. Differences in receptor binding and stability to enzymatic digestion between CCK-8 and CCK-58. Pancreas 25: e50–e55, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Reeve JR Jr, Wu SV, Keire DA, Faull K, Chew P, Solomon TE, Green GM, Coskun T. Differential bile-pancreatic secretory effects of CCK-58 and CCK-8. Am J Physiol Gastrointest Liver Physiol 286: G395–G402, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Roettger BF, Rentsch RU, Pinon D, Holicky E, Hadac EM, Larkin JM, Miller LJ. Dual pathways of internalization of the cholecystokinin receptor. J Cell Biol 128: 1029–1042, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan CN, Raboin SJ, Gulley S, Sinzobahamvya NT, Green GM, Reeve JR Jr, Sayegh AI. Endogenous cholecystokinin reduces food intake and increases Fos-like immunoreactivity in the dorsal vagal complex but not in the myenteric plexus by CCK1 receptor in the adult rat. Am J Physiol Regul Integr Comp Physiol 292: R1071–R1080, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Wu SV, Yang M, Avedian D, Birnbaumer M, Walsh JH. Single amino acid substitution of serine-82 to asparagine in first intracellular loop of human cholecystokinin (CCK)-B receptor confers full cyclic AMP responses to CCK and gastrin. Mol Pharmacol 55: 795–803, 1999. [PubMed] [Google Scholar]
- 28.Yamamoto M, Reeve JR Jr, Keire DA, Green GM. Water and enzyme secretion are tightly coupled in pancreatic secretion stimulated by food or CCK-58 but not by CCK-8. Am J Physiol Gastrointest Liver Physiol 288: G866–G879, 2005. [DOI] [PubMed] [Google Scholar]