Abstract
The gastric pathogen Helicobacter pylori (H. pylori) is linked to peptic ulcer and gastric cancer, but the relevant pathophysiological mechanisms are unclear. We now report that H. pylori stimulates the expression of plasminogen activator inhibitor (PAI)-1, urokinase plasminogen activator (uPA), and its receptor (uPAR) in gastric epithelial cells and the consequences for epithelial cell proliferation. Real-time PCR of biopsies from gastric corpus, but not antrum, showed significantly increased PAI-1, uPA, and uPAR in H. pylori-positive patients. Transfection of primary human gastric epithelial cells with uPA, PAI-1, or uPAR promoters in luciferase reporter constructs revealed expression of all three in H+/K+ATPase- and vesicular monoamine transporter 2-expressing cells; uPA was also expressed in pepsinogen- and uPAR-containing trefoil peptide-1-expressing cells. In each case expression was increased in response to H. pylori and for uPA, but not PAI-1 or uPAR, required the virulence factor CagE. H. pylori also stimulated soluble and cell surface-bound uPA activity, and both were further increased by PAI-1 knockdown, consistent with PAI-1 inhibition of endogenous uPA. H. pylori stimulated epithelial cell proliferation, which was inhibited by uPA immunoneutralization and uPAR knockdown; exogenous uPA also stimulated proliferation that was further increased after PAI-1 knockdown. The proliferative effects of uPA were inhibited by immunoneutralization of the EGF receptor and of heparin-binding EGF (HB-EGF) by the mutant diphtheria toxin CRM197 and an EGF receptor tyrosine kinase inhibitor. H. pylori induction of uPA therefore leads to epithelial proliferation through activation of HB-EGF and is normally inhibited by concomitant induction of PAI-1; treatments directed at inhibition of uPA may slow the progression to gastric cancer.
Keywords: gastric cancer, primary gastric epithelial cells, cagE, HB-EGF
infection with the gastric bacterium Helicobacter pylori (H. pylori) is associated with inflammation of the gastric mucosa that, in some patients, progresses to tissue remodeling involving the development of a hyperproliferative condition and loss of some epithelial cell types, notably parietal cells (7, 8, 10, 11). These changes may lead eventually to gastric cancer (43, 44). Environmental and bacterial factors are both recognized to influence the progression of these changes with chronic infection. In addition, host factors that influence epithelial responses to infection have been identified including genetic background (18, 35), altered expression, and secretion of hormones, e.g., gastrin (33), growth factors such as heparin-binding epidermal growth factor (HB-EGF) (55), cytokines including IL-8 (12), and extracellular proteases such as matrix metalloproteinase (MMP)-7 (4, 13, 56). Although considerable progress has been made in recent years, the interactions between these systems are still poorly understood.
In many different tissues, the responses to infection and injury include induction of members of the urokinase plasminogen activator (uPA) system (2). The key components of this system are uPA itself, which mediates the conversion of plasminogen to plasmin, its receptor (uPAR), which localizes uPA on the cell surface, and several plasminogen activator inhibitors (PAI-1, -2, and -3). Stimulation of uPA/plasmin is implicated in fibrinolysis, degradation of extracellular matrix, activation of other proteases including multiple MMPs, and modulation of cell-matrix interactions (2, 36). In addition to inhibition of uPA, both PAI-1 and PAI-2 are recognized to have uPA-independent actions. In the case of PAI-1, these are thought, at least in part, to be a consequence of binding to vitronectin (14). Moreover, although PAI-2 is a secreted protein, its signal sequence is relatively weak and there is a substantial pool of intracellular PAI-2 that is thought to play a role in inhibiting apoptosis (15); the biology of PAI-3 is not well understood. Until recently the role of this system in gastric physiology and pathophysiology was relatively unknown.
Recently, we reported increased expression of PAI-2 in the gastric epithelium in patients infected with H. pylori (54). Previous studies have contributed to the view that uPA, PAI-1, and uPAR are all associated with an adverse outcome in gastric cancer (21, 23, 27, 40, 46), as well as other tumors (1, 48). However, the roles of these proteins in response to H. pylori infection in the normal epithelium are unknown. In the present study, we therefore asked whether there is H. pylori stimulation of uPA, uPAR, or PAI-1 in human gastric epithelial cells; we report here that there is and that induction is linked to a proliferative response to infection.
MATERIALS AND METHODS
Plasmids, drugs.
PAI-1 antisense oligonucleotides (ASO) and control scrambled oligonucleotides (CSO) were obtained from Biognostik (Gottingham, Germany). Human recombinant uPA, EGF, tissue inhibitor of metalloproteinases (TIMP)-1, and TIMP-3, and GM6001 were obtained from Calbiochem (Nottingham, UK), and uPA activity kit was purchased from Chemicon (Chandlers Ford, UK). 1.94-kb, 4.5-kb, and 2.3-kb fragments of the human uPA, PAI-1 and uPAR promoters, respectively, were generated by PCR of human genomic DNA and cloned into the firefly luciferase reporter construct pXP2 using methods previously reported (52). Antibodies to uPA, PAI-1, and uPAR were obtained from American Diagnostica (Stamford, CT), antibody to the EGF receptor was purchased from Oncogene (Cambridge, MA), and antibodies to TGF-α, amphiregulin, and HB-EGF were obtained from R&D Systems (Abingdon, UK). uPAR and control siRNA was obtained from Ambion (Austin, TX). Other chemicals were obtained from Sigma (Poole, Dorset, UK).
Patients.
Six endoscopic pinch biopsies of gastric corpus and antrum were obtained from patients referred with dyspepsia (n = 33, 18 men, mean age 54.2 ± 1.7 yr) and with H. pylori infection confirmed by serology, antral urease test (Prontodry; Med. Inst. Solothurn, Switzerland), and antral and corpus histology; in all cases, histology indicated superficial gastritis but not gastric atrophy or intestinal metaplasia. Patients were excluded if there was a previous history of neoplastic disease, present history of peptic ulcer, or elevated plasma gastrin concentrations (>30 pM). Dyspeptic patients (n = 90, 46 men, mean age 53.9 ± 1.3 yr) with normal fasting plasma gastrin concentrations (<30 pM), negative H. pylori status by serology, antral urease test, and antral and corpus histology were used as controls. The study was approved by the Ethics Committee of the Royal Liverpool and Broadgreen University Hospitals NHS Trust. All patients gave written, informed consent.
Real-time PCR.
RNA from human gastric biopsies was extracted in Trizol (Invitrogen, Paisley, UK) and DNase treated (Promega, Southampton, UK), and 5 μg were reverse transcribed with avian myeloblastosis virus reverse transcriptase (Promega) and random hexamers (Promega). Real-time PCR was carried out using TaqMan probe/primer sets specific for human uPA (forward primer 5′-ggaaaacctcatcctacacaagga; reverse primer 5′-cggatcttcagcaaggcaat; probe 5′-ctgacacgcttgctcaccacaacga), PAI-1 (forward primer 5′-ggctgacttcacgagtctttcag; reverse primer 5′-cgttcacctcgatcttcactttc; probe 5′-aagagcctctccacgtcgcgca), uPAR (forward primer 5′-cgaggttgtgtgtgggttagac; reverse primer 5′-ggcttcgggaataggtgaca; probe 5′-tgcaaccagggcaactctggcc), PAI-3 (forward primer 5′-tctgtgaccttatgctccacactaa; reverse primer 5′-gcatggtggctgttctttgtt; probe 5′-tctggcagagcctccgtttcctca), and an 18S rRNA control kit (Eurogentec, Southampton, UK). Reactions were performed in an Applied Biosystems (Warrington, UK) PrismTM7700 sequence detection system and quantified using a standard curve. uPA, uPAR, PAI-1, and PAI-3 mRNA abundances were determined relative to 18S in the same sample.
Gastrin radioimmunoassay.
Plasma samples were assayed for total amidated gastrin concentrations by radioimmunoassay using antibody L2 (which reacts with G17 and G34 but not progastrin or Gly-gastrins) as previously described (17).
Immunohistochemistry.
Formalin-fixed, paraffin-embedded tissue sections were processed for immunohistochemical detection of uPA, PAI-1, and uPAR after antigen recovery as previously described (53).
Cultured human gastric gland cells.
Adherent human gastric glands were prepared using the method previously described (56). Glands were cultured in DMEM/Hams F12 supplemented with 10% FBS and 1% antibiotic-antimycotic solution (Sigma) at 37°C in 5% CO2-95% O2.
Knockdown of PAI-1 and uPA activity assays.
Human gastric glands were cultured as monolayers and treated with 2 μM PAI-1 ASO sodium salt or the corresponding negative control oligonucleotide (CSO) sodium salt (Biognostik) for 72 h as previously described (37). Knockdown of PAI-1 was verified by immunocytochemistry. Cells were infected with H. pylori strain 60190 [American Type Culture Center (ATCC)] for 16 h as described earlier (49). uPA activity assay was performed from both media and cell extracts according the manufacturer's instruction and absorption measured at 340 nm.
Transient transfection and bacterial infection of primary human gland cultures.
Primary human gastric glands cultured for 48 h on 12-well plates were transfected with 2 μg firefly luciferase reporter construct per well together with a constitutively active Renilla luciferase reporter, phRL-SV40 (5 ng per well, Promega), using CombiMag (Oz Biosciences, Marseille, France) as previously described (49). Cells were infected with H. pylori strain 60190 (ATCC) and its isogenic mutants at a multiplicity of infection (MOI) of 1:150 for 16 h as described previously (3, 54). Luciferase activity was measured by dual luciferase assay (Promega) in a Lumat LB9507 luminometer (Berthold, Redbourne, UK). Results are presented as fold increase over unstimulated control, so a value of 1.0 signifies no change in luciferase activity.
Cellular targeting of the members of the uPA system in primary human adherent glands.
To study cellular targeting of uPA, uPAR, and PAI-1-luciferase (luc) expression, transfected primary human gastric glands were double immunostained with goat anti-luciferase antibody (Rockland Immunochemicals, Gilbertsville, PA) together with one of the following: rabbit anti-pepsinogen (gift f;rom Mike Samloff, Center for Ulcer Research, Los Angeles, CA), rabbit anti-H+/K+ATPase (Calbiochem), rabbit anti-vesicular monoamine transporter 2 (22), mouse anti-trefoil factor-1 (TFF-1; Dako, Glostrup, Denmark), and mouse anti-TFF-2 (NovoCastra, Newcastle-upon-Tyne, UK) antibodies with appropriate FITC or Texas red-conjugated secondary antibodies (Jackson Immunoresearch, Soham, UK), using Vectashield mounting medium with DAPI (Vector Laboratories, Peterborough, UK) to counterstain nuclei. Slides were examined using a Zeiss Axioplan-2 microscope (Zeiss Vision, Welwyn Garden City, UK) and images captured using a JVC-3 charge-coupled device camera and KS300 software combined with a deconvolution software (Imaging Associates, Bicester, UK). Ten fields from each of three patients were counted; results are expressed as a percentage of total cell number as reported earlier (49, 54).
BrdU labeling.
Primary gastric glands from H. pylori-negative and -positive patients were plated on cover slips in 12-well plates and cultured for 48 h. Glands were then serum starved for 24 h before addition of bromodeoxyuridine (BrdU) (3 ng/ml) with uPA (500 ng/ml) or H. pylori (MOI 1:150) and further incubated for 24 h at 37°C. Control scrambled and PAI-1 antisense oligonucleotides were present for 72 h as described above. Control or siRNA to uPAR were transfected using SilenceMag (Oz Biosciences) as previously described and cells incubated for a further 48 h (37). BrdU was detected with FITC-conjugated fast immune anti-BrdU/DNase (Becton Dickinson, Oxford, UK) using Vectashield mounting medium with DAPI (Vector Laboratories) to counterstain nuclei. Glands were counterstained with vimentin (RDI, Flanders, NJ) and cells with colocalized BrdU and vimentin-positive staining (myofibroblasts) were excluded. BrdU-positive cells were expressed as a percentage of total cell number as previously described (37).
Statistics.
Results are presented as means ± SE; comparisons were made using ANOVA or Student t-tests where appropriate and were considered significant at P < 0.05.
RESULTS
Increased expression of uPA, uPAR, and PAI-1 in human gastric corpus with H. pylori infection.
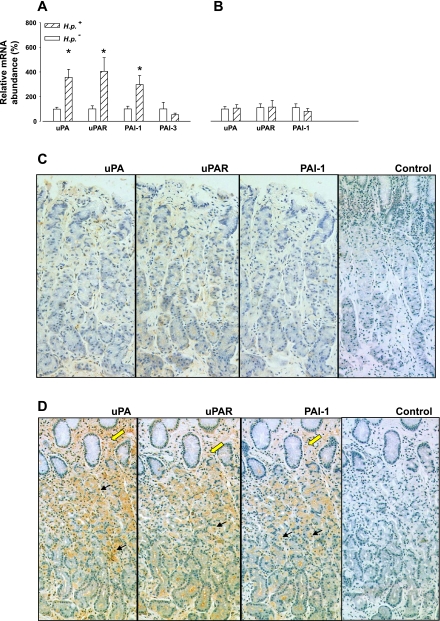
The results from real-time PCR indicated that uPA, uPAR, and PAI-1 mRNA abundances were significantly increased in human gastric corpus in patients infected with H. pylori (Fig. 1A). These changes appear to be specific in that there was no significant difference in the abundance of PAI-3 mRNA in gastric corpus, and neither was there a significant difference in the abundance of uPA, uPAR, or PAI-1 in the antrum of individuals infected with H. pylori compared with controls (Fig. 1B). Plasma gastrin concentrations in the patients infected with H. pylori used in this study (21.5 ± 1.6 pM) were within the normal range and were not significantly different from the control group (20.7 ± 1.7 pM), so the differences are not attributable to raised plasma gastrin. Immunohistochemical studies indicated that there was increased PAI-1, uPAR, and uPA in epithelial cells in H. pylori-positive subjects, including parietal cells. However, in the case of uPA and uPAR, there was also abundant expression in subepithelial cells with H. pylori infection (Fig. 1, C and D).
Fig. 1.
Increased gastric urokinase plasminogen activator (uPA), uPA receptor (uPAR) and plasminogen activator inhibitor (PAI)-1, but not PAI-3, expression in patients infected with Helicobacter pylori (H. pylori). A: real-time PCR normalized to 18S shows elevated uPA, uPAR, and PAI-1 mRNA expression in the gastric corpus in patients with H. pylori (H. p.) infection compared with uninfected individuals (n = 13–14, *P < 0.05, ANOVA). B: there was no increase in the antrum in uPA, uPAR, or PAI-1 mRNA abundance in patients with H. pylori infection compared with controls (n = 11–20). C: representative examples of low uPA, uPAR, and PAI-1 staining of serial sections of gastric corpus from an uninfected patient compared with a patient infected with H. pylori (D); control section: first antibody omitted. Black arrows, staining of epithelial cells; yellow arrows, stromal cells.
H. pylori increases uPA-, uPAR- and PAI-1-promoter/luciferase-reporter expression in primary cultured human gastric glands by distinct mechanisms.
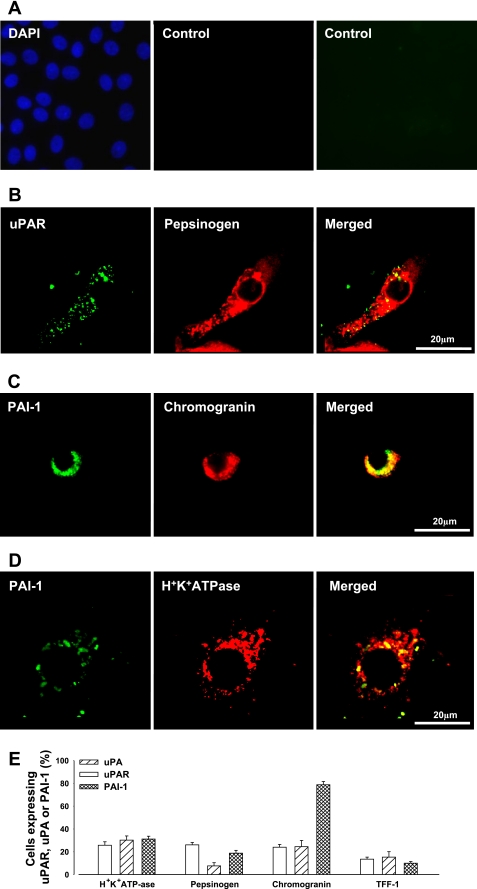
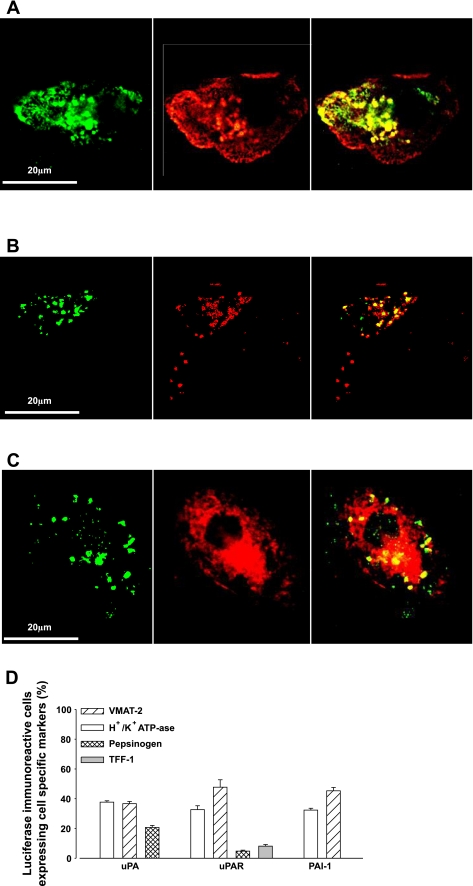
To determine whether H. pylori directly stimulated transcription of PAI-1, uPAR, and uPA in human gastric epithelial cells, we transfected cultured gastric glands with constructs of PAI-1, uPAR, and uPA promoters coupled to a luciferase reporter (i.e., PAI-1-luc, uPAR-luc, and uPA-luc). In untransfected cells, there was expression of wild-type uPA, uPAR, and PAI-1 in subsets of parietal cells (identified by H+/K+ATPase staining), chief cells (identified by pepsinogen staining), enhanced chemiluminescence (ECL) cells (identified by chromogranin staining), and mucus cells (identified by TFF-1 staining) (Fig. 2, A–E). Generally, around 25–30% of parietal, chief, or ECL cells expressed uPA, uPAR, or PAI-1; however, PAI-1 was expressed in a relatively high proportion of ECL cells, and uPA was expressed in less than 10% of chief cells (Fig. 2E). uPA, uPAR, or PAI-1 were expressed in less than 15% of mucus cells (Fig. 2E). In cells transfected with luciferase promoter-reporter vectors, dual labeling immunofluorescence using antibodies to luciferase and to markers of parietal and ECL cells indicated that expression of the constructs was predominantly targeted to these cells. In addition, however, there was some expression of uPA-luc in chief cells and relatively low expression of uPAR-luc in either chief or surface mucus cells (Fig. 3, A–D). However, the promoter sequences used in these vectors were not able to direct detectable expression of PAI-1-luc in chief cells or of PAI-1 or uPA-luc in mucus cells indicated by TFF-1 immunoreactivity, or of PAI-1-, uPA- or uPAR-luc in mucus neck cells indicated by TFF-2 expression. Importantly, expression of each luciferase vector was increased in response to H. pylori (Fig. 4, A–C).
Fig. 2.
Expression of uPA, uPAR, and PAI-1 in human cultured gastric glands. A: controls where primary antibody was omitted (left, DAPI staining of nuclei; middle, Texas red-labeled donkey anti-rabbit IgG; right, FITC-labeled donkey anti-mouse IgG). B: example of expression of uPAR (green, left) in a pepsinogen-positive cell (chief cell, red, middle; merged image, right). C: example of expression of PAI-1 (green, left) in a chromogranin-positive cell, [enhanced chemiluminescence (ECL) cell, red, middle; merged image, right]. D: expression of PAI-1 (green, left) in a H+/K+ ATPase-positive cell (parietal cell, red, middle; merged image, right). E: percentage (%) of H+/K+ATPase-, pepsinogen-, chromogranin-, and trefoil peptide (TFF)-1-positive cells expressing PAI-1, uPAR, or uPA (n = 3). Note that <3.0% of TFF-2-expressing cells expressed uPA, uPAR, or PAI-1.
Fig. 3.
uPA, uPAR, and PAI-1 promoter-reporter constructs target expression to specific cell types in human gastric glands. 1.94 kb of uPA promoter, 2.3 kb of uPAR promoter, and 4.5 kb of PAI-1 promoter coupled to a luciferase reporter were transfected into primary human gastric epithelial cells. A: expression of PAI-1-luc (green, left) in a H+/K+ ATPase-positive cell (parietal cell, red, middle; merged image, right). B: expression of PAI-luc (green, left) in a vesicular monoamine transporter (VMAT)-2-positive cell, (ECL cell, red, middle; merged image, right). C: expression of uPA-luc (green, left) in a pepsinogen-positive cell (chief cell, red, middle; merged image, right). D: percentage (%) of PAI-1, uPAR, and uPA luciferase-positive cells expressing H+/K+ATPase, VMAT-2, or pepsinogen (n = 3). Note that <1.0% of uPA- or PAI-1-luc-expressing cells also expressed TFF-1 and that <1.0% of cells expressing all 3 constructs also expressed TFF-2.
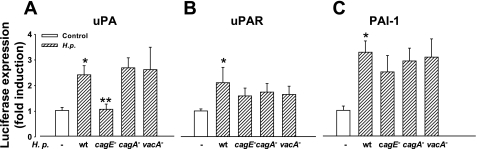
Fig. 4.
H. pylori stimulates uPA, but not uPAR, or PAI-luc through a CagE-dependent mechanism. A: H. pylori [16 h, multiplicity of infection (MOI) 1:150] stimulates expression of uPA-luc that is abolished by deletion of the cagE but not cagA and vacA virulence factors. B and C: H. pylori also stimulates expression of PAI-luc and uPAR-luc, respectively, but this is not decreased by deletion of the cagE, cagA, or vacA virulence factors; n = 3–15, P < 0.05. (*control vs. wild-type H. pylori; **wild-type H. pylori vs. cagE-null mutant). wt, wild-type.
To determine whether recognized major virulence factors of H. pylori were responsible for stimulation of uPA, uPAR, and PAI-1, isogenic mutants were studied in which the virulence genes cagA, cagE, and vacA were disrupted. Disruption of these virulence factors had no effect on H. pylori-stimulated PAI-1 or uPAR expression, but disruption of cagE virtually abolished the stimulatory effect of H. pylori on the uPA-luc construct (Fig. 4, A–C), indicating that H. pylori acts through separate mechanisms to stimulate PAI-1, uPA, and uPAR.
H. pylori increases uPA activity in cultured human gastric glands, which is enhanced by PAI-1 knockdown.
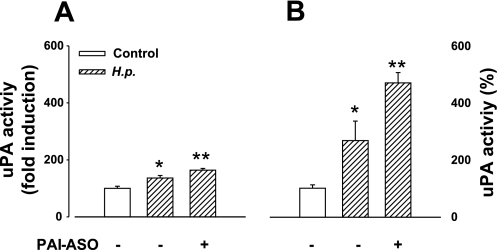
Since both uPA and its inhibitor PAI-1 were increased by H. pylori, we asked whether there were changes in uPA enzyme activity. Infection of cultured gastric glands with H. pylori increased both cell surface-bound and soluble uPA activity (Fig. 5). We then examined the consequences of PAI-1 knockdown. Immunohistochemistry confirmed that PAI-1 antisense oligonucleotide treatment significantly decreased the total number of cells in cultured gastric glands expressing endogenous PAI-1 (CSO, 10.0 ± 0.3% vs. ASO, 3.8 ± 0.2%). Moreover, PAI-1 knockdown (Fig. 5, A and B) increased both cell surface-bound and soluble uPA activity although the increase was more marked for the latter than the former.
Fig. 5.
H. pylori increases both cell surface-bound and soluble uPA activity, and PAI-1 knockdown increases activity still further. A: cell surface-bound uPA activity after H. pylori infection with or without PAI-1 antisense oligonucleotide (PAI-1-ASO, 2 μM) treatment expressed as % of control. B: soluble uPA activity after H. pylori infection with or without PAI-1-ASO treatment expressed as % of control. In control cells, the cell surface uPA amounted to 48 ± 7% of soluble activity; n = 5–13, P < 0.05, ANOVA (*control vs. H. pylori; **H. pylori alone vs. H. pylori with PAI-1 knockdown).
H. pylori acts via uPA to increase epithelial cell proliferation, and PAI-1 knockdown enhances this response.
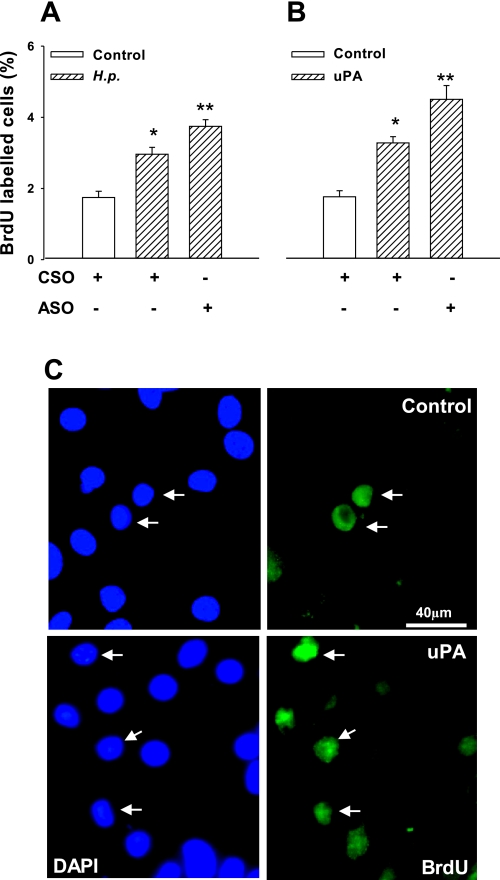
Previous work has linked proliferative responses to H. pylori with activation of the EGF receptor, including stimulation of the ligand HB-EGF and its liberation by proteolysis (29, 55). In primary human gastric glands, we showed that H. pylori increased BrdU incorporation and that the response was further enhanced by PAI-1 knockdown (Fig. 6A). Consistent with the idea that PAI-1 inhibited uPA in this system, we then showed that uPA itself increased BrdU incorporation, and the response was again further enhanced by PAI-1 knockdown (Fig. 6, B and C).
Fig. 6.
Increased gastric epithelial cell proliferation in response to H. pylori and uPA. A: H. pylori stimulated bromodeoxyuridine (BrdU) incorporation of human primary gastric epithelial cells, and this was enhanced by PAI-1 knockdown. B: addition of uPA (500 ng/ml) mimicked the stimulatory effect of H. pylori on BrdU incorporation, and this was also enhanced by PAI-1 knockdown. C: representative examples of BrdU-labeled cells (right, arrows) in control (top) and uPA-treated glands; DAPI staining of all nuclei shown on left; n = 4, P < 0.05, ANOVA (*control vs. H. pylori or uPA; **H. pylori or uPA along compared with PAI-1). CSO, control scrambled oligonucleotides.
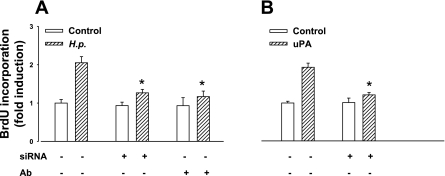
Treatment of cells with a neutralizing antibody to uPA inhibited the proliferative effect of H. pylori, suggesting a role for endogenous uPA in this response (Fig. 7A). A role for uPAR in the response was indicated by the observation that knockdown of uPAR with siRNA significantly inhibited H. pylori-induced proliferation (Fig. 7A). Immunohistochemistry confirmed that siRNA treatment significantly decreased the number of uPAR-expressing cells in cultured gastric glands (control, 9.1 ± 0.5% of positive cells; uPAR knockdown, 3.0 ± 0.4%), whereas PAI-1 expression was not altered (8.4 ± 0.5% vs. 8.8 ± 0.4%). Consistent with these observations, the increased BrdU incorporation in response to exogenous uPA was also decreased by uPAR knockdown (Fig. 7B). The specificity of the latter effect is indicated by the fact that there was no decrease in BrdU incorporation after uPAR knockdown in response to an exogenous EGF receptor (EGF-R) ligand (EGF, 50 ng/ml) compared with control (EGF alone, 2.14 ± 0.05-fold increase in BrdU incorporation over control; EGF and uPAR knockdown, 2.36 ± 0.03-fold).
Fig. 7.
Increased gastric epithelial cell proliferation in response to H. pylori is mediated by uPA. A: H. pylori-stimulated BrdU incorporation of human primary gastric epithelial cells was inhibited by treatment with neutralizing uPA antibody or knockdown of uPAR with siRNA (60 nM) B: uPA (500 ng/ml)-stimulated BrdU incorporation was also inhibited by uPAR knockdown; n = 3, *P < 0.05, ANOVA. Ab, antibody.
uPA increases epithelial cell proliferation via metalloproteinase release of HB-EGF.
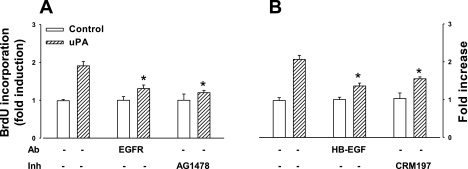
Since there was increased BrdU incorporation in response to exogenous EGF, we asked whether the proliferative response to uPA was linked to activation of the EGF pathway. Neutralizing antibody against EGF-R significantly reduced BrdU incorporation induced by uPA, and so too did the EGF-R tyrosine kinase inhibitor, AG1478 (Fig. 8A). Moreover, neutralizing antibody to HB-EGF inhibited BrdU incorporation in response to uPA (Fig. 8B), and, in agreement with the idea that HB-EGF mediated responses to uPA, the mutant diphtheria toxin CRM197 also reduced uPA-mediated BrdU incorporation (Fig. 8B). Interestingly, however, responses to uPA were not influenced by neutralization of other EGF-R ligands such as amphiregulin and TGF-α that are known to be expressed in gastric mucosa (uPA, 1.90 ± 0.12-fold increase over control; uPA and amphiregulin immunoneutralization, 1.74 ± 0.15-fold over control; uPA and TGF-α immunoneutralization, 2.10 ± 0.15-fold over control). Since it is known that HB-EGF is released by metalloproteinases (47), we examined the effect of a broad spectrum inhibitor, GM6001, and two tissue inhibitors of MMPs (TIMP-1 and TIMP-3) on proliferative response to uPA. At a concentration of 100 nM, TIMP-1 inhibited BrdU incorporation by 21 ± 5% and TIMP-3 inhibited by 69 ± 10% (P < 0.05), whereas GM6001 inhibited uPA responses by 70 ± 12% (P < 0.05).
Fig. 8.
uPA acts through heparin-binding EGF (HB-EGF) to stimulate epithelial cell proliferation. A: uPA (500 ng/ml)-stimulated BrdU incorporation was inhibited by neutralizing Ab to the EGF receptor (EGF-R) (2 μg/ml) and by receptor tyrosine kinase inhibitor (Inh) AG1478 (2 ng/ml). B: uPA-stimulated BrdU incorporation was also inhibited by neutralizing Ab to HB-EGF (5 μg/ml) and by the mutant diphtheria toxin CRM197 (5 μg/ml); n = 3–4, *P < 0.05, ANOVA.
DISCUSSION
The present data show that H. pylori infection of the human gastric corpus is associated with increased expression of uPA, its receptor uPAR, and its inhibitor PAI-1. It seems probable that separate mechanisms are associated with increased expression of uPA, uPAR, and PAI-1. Thus within the epithelium, both parietal and ECL cells have the capacity to increase uPA, uPAR, and PAI-1 expression in response to H. pylori, whereas chief cells can also express uPA, and mucus cells may express uPAR. Moreover, the CagE virulence factor is required for uPA responses to H. pylori but not PAI-1 or uPAR. A role for uPA and uPAR in H. pylori-stimulated cell proliferation was identified through mechanisms that required HB-EGF and EGF-R activation and were at least partly held in check by PAI-1. The data implicate activation of uPA in the preneoplastic hyperproliferative condition that is associated with H. pylori infection. Control of uPA activity may therefore present a new target in arresting H. pylori-stimulated progression to cancer.
Previous work has established that uPA, uPAR, and PAI-1 are increased in gastric cancer and in chronic atrophic gastritis (6, 19, 21, 23, 27, 28, 40, 46); moreover, increased expression in cancer is associated with an adverse outcome. There is expression of uPA, uPAR, and PAI-1 in both stromal and tumor cells, and it is generally thought that the expression of these proteins is associated with progression of the disease through increased cell invasion, metastasis, and angiogenesis. In the case of uPA, binding to uPAR generates a focal point of proteolytic activity that facilitates cell invasion (1). In addition to inhibition of uPA through accelerated internalization of the uPA/uPAR complex, PAI-1 also exhibits uPA-independent actions. Specifically, by binding vitronectin it increases cell dissociation from extracellular matrix and accelerates invasion (14). It has been suggested that the latter effects account for the association of increased PAI-1 in gastric and other cancers with adverse outcomes, which is sometimes referred to as “paradoxical” given that uPA is also associated with disease progression. In spite of the work on uPA and PAI-1 in gastric cancer, little attention has been given to how this system responds to H. pylori infection and how it might be implicated in the tissue remodeling that characterizes the preneoplastic condition.
We previously showed that PAI-2 was increased in gastric epithelial cells in response to H. pylori via activation of NF-κB (54). A pool of PAI-2 is retained within cells and is associated with inhibition of apoptosis, whereas some is secreted and has been shown to be involved in inhibition of invasion of a cancer cell line (54). The present data now make it clear that infection with H. pylori also induces the main members of the system, uPA, uPAR, and PAI-1 in primary, untransformed, gastric epithelial cells; there was no evidence that a third inhibitor, PAI-3, was changed with H. pylori infection, and we did not study this further. Previous work using microarrays to identify H. pylori-regulated genes demonstrated increased uPA in gastric cancer cell lines (32), and there has been work using gastric cancer cell lines that indicates H. pylori stimulation of uPA, uPAR, and PAI-1 expression (24, 30, 31). However, given the nature of cancer cell lines, it remained uncertain how these observations related to expression of members of the uPA system in primary gastric epithelial cells. Our data show that with infection there is the capacity to increase uPA, uPAR, and PAI-1 expression in two cell types associated with the gastric corpus, namely the acid-secreting parietal cell and the histamine-releasing enterochromaffin-like cell; the restriction of both cell types to the gastric corpus is consistent with the observation that there was no difference in expression of uPA, uPAR, and PAI-1 in the antral region of the stomach (where these cell types are absent). In contrast, PAI-2 is prominently expressed in mucin- and pepsinogen-expressing cells and is only occasionally found in parietal or ECL cells (54); it is also increased in the antrum in H. pylori-positive patients (S. Kenny and A. Varro, unpublished observations). Interestingly, only subsets of the major differentiated gastric epithelial cell types expressed uPA, uPAR, or PAI-1; the basis of this requires further work because the different subsets might, for example, vary in terms of age. It is clear that promoters of the length we used in the luciferase reporter vectors do not precisely mimic the patterns of expression of the wild-type gene, indicating that information outside these sequences is required for faithful cell-restricted expression. Even so, at a qualitative level the general patterns of expression of the wild-type proteins and the promoter-reporter vectors were similar. Importantly, the increased abundance of uPA transcripts in response to H. pylori was associated with increased enzyme activity in normal gastric epithelial cells that was enhanced still further by blocking PAI-1 expression compatible with a dynamic equilibrium between enzyme and its inhibitor, and with the idea that the changes in transcript abundance were functionally relevant.
H. pylori increases plasma gastrin concentrations, and parietal and ECL cells express the CCK-2 receptor at which gastrin acts (16, 33). However, increased abundance of uPA, uPAR, and PAI-1 transcripts was found in patients infected with H. pylori with plasma gastrin concentrations in the normal range, and H. pylori stimulation of promoter-reporter constructs occurred in epithelial cells in vitro (in the absence of gastrin) so that the effects of H. pylori are not secondary to increased gastrin. Although stimulation of uPAR and PAI-1 was independent of the pathogenicity island, we found that a cagE isogenic mutant was defective in uPA induction. Disruption of cagE means that H. pylori cannot make a functional type IV secretory apparatus. This has two effects: first, it blocks Nod1-NF-κB signaling, and second, it blocks delivery of CagA into epithelial cells and the signaling consequent on this. Since disruption of cagA had no effect on uPA induction, it is likely that the deficit seen in the cagE mutant is due to lack of stimulation of Nod1-NF-κB. This is compatible with the already described role of NF-κB in regulation of uPA transcription (20, 28, 38, 42). It is worth noting that, in MKN-45 and KATO III cell lines, H. pylori stimulation of uPA expression was reported to be abolished by mutation of CagA (24); differences between cancer cell lines and primary, untransformed, cells used in the present study may well account for this difference.
The data link H. pylori induction of uPA to one of the key characteristics of preneoplastic, H. pylori-infected epithelium, namely increased cell proliferation. Previous work has shown that H. pylori stimulates proliferation and that this includes transactivation of the EGF receptor and stimulation of HB-EGF cleavage (29, 45, 55). We demonstrate that the proliferative response of normal human gastric epithelial cells to uPA is mediated by EGF-R. It is possible that there are interactions between ligand-bound uPAR and EGF-R that result in proliferation (25). However, the cells expressing uPAR in gastric glands (mainly parietal and ECL cells) are distinct from the proliferating cells, and, instead, the data suggest that uPA stimulates proliferation via the release of an EGF-R ligand, HB-EGF. This system is therefore distinct from prostatic DU-145 cells in which uPA-stimulated invasion is mediated by HB-EGF in an autocrine mechanism (9). Other ligands of EGF-R produced in the gastric epithelium include amphiregulin and TGF-α; however, in contrast to HB-EGF, immunoneutralization of amphiregulin and TGF-α did not inhibit the proliferative responses to uPA. It is known that HB-EGF is cleaved from its cell-bound precursor via proteases that include TNF-α-converting enzyme (ADAM-17) (50), MMP-3 and MMP-7 (51, 57), and matrilysin (41). There is also a considerable body of evidence indicating that in different systems there are cascades of proteolytic activation involving uPA, plasmin, and MMPs (34, 39). The data show GM6001, TIMP-1, and TIMP-3 inhibit the action of uPA, consistent with a role for metalloproteinases downstream of uPA although the precise sequence of events by which uPA activates HB-EGF remains to be determined. In this context, it is worth noting, however, that, although uPA activates MMPs and MMPs are known to be increased with H. pylori infection, the localization of MMPs in the gastrointestinal mucosa is for the most part subepithelial. Within the normal gastric epithelium, MMP-7 is the predominant representative of this group, and we have already shown that this does not stimulate proliferation in cultured gastric glands (37).
The well-established association of uPA with pericellular proteolysis in tissue damage and repair raises the prospect that induction of the system in H. pylori infection is part of a host response mechanism relevant to the tissue remodeling characteristic of this infection. Previous work has established a variety of different ways that bacteria interact with the uPA system (5). In the case of H. pylori, two plasminogen-binding proteins have been identified that are proposed to coat the organism, and, with the subsequent activation of bound plasminogen to plasmin, these proteins are thought to increase virulence (26). The present data suggest a further role for this system in stimulation of cell proliferation. With prolonged infection, we suggest that the increased proliferation favors the acquisition of mutations leading to cancer. The present data therefore raise the possibility that inhibition of uPA might be therapeutically useful in those patients at risk of progression from gastric atrophy to cancer.
GRANTS
This work was supported by Medical Research Council, The Wellcome Trust, and Wolfson Foundation.
Acknowledgments
We are grateful to Mrs. Debbie Sales for culturing H. pylori, Mr. Andy Dodson for technical help with the immunohistochemistry, and Dr. Fiona Campbell for help with the interpretation of uPA, uPAR, and PAI-1 staining.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci 57: 25–40, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 72: 1–22, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Argent RH, Thomas RJ, Letley DP, Rittig MG, Hardie KR, Atherton JC. Functional association between the Helicobacter pylori virulence factors VacA and CagA. J Med Microbiol 57: 145–150, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Bebb JR, Letley DP, Thomas RJ, Aviles F, Collins HM, Watson SA, Hand NM, Zaitoun A, Atherton JC. Helicobacter pylori upregulates matrilysin (MMP-7) in epithelial cells in vivo and in vitro in a Cag dependent manner. Gut 52: 1408–1413, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann S, Hammerschmidt S. Fibrinolysis and host response in bacterial infections. Thromb Haemost 98: 512–520, 2007. [PubMed] [Google Scholar]
- 6.Beyer BC, Heiss MM, Simon EH, Gruetzner KU, Babic R, Jauch KW, Schildberg FW, Allgayer H. Urokinase system expression in gastric carcinoma: prognostic impact in an independent patient series and first evidence of predictive value in preoperative biopsy and intestinal metaplasia specimens. Cancer 106: 1026–1035, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res 55: 2111–2115, 1995. [PubMed] [Google Scholar]
- 8.Bodger K, Crabtree JE. Helicobacter pylori and gastric inflammation. Br Med Bull 54: 139–150, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Caceres M, Tobar N, Guerrero J, Smith PC, Martinez J. c-jun-NH2JNK mediates invasive potential and EGFR activation by regulating the expression of HB-EGF in a urokinase-stimulated pathway. J Cell Biochem 103: 986–993, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Correa P The biological model of gastric carcinogenesis. IARC Sci Publ 157: 301–310, 2004. [PubMed] [Google Scholar]
- 11.Correa P Precursors of gastric and esophageal cancer. Cancer 50: 2554–2565, 1982. [PubMed] [Google Scholar]
- 12.Crabtree JE, Wyatt JI, Trejdosiewicz LK, Peichl P, Nichols PH, Ramsay N, Primrose JN, Lindley IJ. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol 47: 61–66, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford HC, Krishna US, Israel DA, Matrisian LM, Washington MK, Peek RM Jr. Helicobacter pylori strain-selective induction of matrix metalloproteinase-7 in vitro and within gastric mucosa. Gastroenterology 125: 1125–1136, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J Cell Biol 134: 1563–1571, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickinson JL, Bates EJ, Ferrante A, Antalis TM. Plasminogen activator inhibitor type 2 inhibits tumor necrosis factor alpha-induced apoptosis. Evidence for an alternate biological function. J Biol Chem 270: 27894–27904, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Dockray G, Dimaline R, Varro A. Gastrin: old hormone, new functions. Pflügers Arch 449: 344–355, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Dockray GJ, Hamer C, Evans D, Varro A, Dimaline R. The secretory kinetics of the G cell in omeprazole-treated rats. Gastroenterology 100: 1187–1194, 1991. [PubMed] [Google Scholar]
- 18.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF Jr, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404: 398–402, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Farinati F, Herszenyi L, Plebani M, Carraro P, De Paoli M, Cardin R, Roveroni G, Rugge M, Nitti D, Grigioni WF, D'Errico A, Naccarato R. Increased levels of cathepsin B and L, urokinase-type plasminogen activator and its inhibitor type-1 as an early event in gastric carcinogenesis. Carcinogenesis 17: 2581–2587, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Guillemin K, Salama NR, Tompkins LS, Falkow S. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc Natl Acad Sci USA 99: 15136–15141, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heiss MM, Babic R, Allgayer H, Gruetzner KU, Jauch KW, Loehrs U, Schildberg FW. Tumor-associated proteolysis and prognosis: new functional risk factors in gastric cancer defined by the urokinase-type plasminogen activator system. J Clin Oncol 13: 2084–2093, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Hussain I, Bate GW, Henry J, Djali P, Dimaline R, Dockray GJ, Varro A. Modulation of gastrin processing by vesicular monoamine transporter type 1 (VMAT1) in rat gastrin cells. J Physiol 517: 495–505, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito H, Yonemura Y, Fujita H, Tsuchihara K, Kawamura T, Nojima N, Fujimura T, Nose H, Endo Y, Sasaki T. Prognostic relevance of urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitors PAI-1 and PAI-2 in gastric cancer. Virchows Arch 427: 487–496, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto J, Mizokami Y, Takahashi K, Nakajima K, Ohtsubo T, Miura S, Narasaka T, Takeyama H, Omata T, Shimokobe K, Ito M, Takehara H, Matsuoka T. Expressions of urokinase-type plasminogen activator, its receptor and plasminogen activator inhibitor-1 in gastric cancer cells and effects of Helicobacter pylori. Scand J Gastroenterol 40: 783–793, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Jo M, Thomas KS, Marozkina N, Amin TJ, Silva CM, Parsons SJ, Gonias SL. Dynamic assembly of the urokinase-type plasminogen activator signaling receptor complex determines the mitogenic activity of urokinase-type plasminogen activator. J Biol Chem 280: 17449–17457, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Jonsson K, Guo BP, Monstein HJ, Mekalanos JJ, Kronvall G. Molecular cloning and characterization of two Helicobacter pylori genes coding for plasminogen-binding proteins. Proc Natl Acad Sci USA 101: 1852–1857, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneko T, Konno H, Baba M, Tanaka T, Nakamura S. Urokinase-type plasminogen activator expression correlates with tumor angiogenesis and poor outcome in gastric cancer. Cancer Sci 94: 43–49, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawasaki K, Hayashi Y, Wang Y, Suzuki S, Morita Y, Nakamura T, Narita K, Doe W, Itoh H, Kuroda Y. Expression of urokinase-type plasminogen activator receptor and plasminogen activator inhibitor-1 in gastric cancer. J Gastroenterol Hepatol 13: 936–944, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Keates S, Sougioultzis S, Keates AC, Zhao D, Peek RM Jr, Shaw LM, Kelly CP. cag+ Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J Biol Chem 276: 48127–48134, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Kim MH, Yoo HS, Chang HJ, Hong MH, Kim HD, Chung IJ, Shin BA, Cho MJ, Ahn BW, Jung YD. Urokinase plasminogen activator receptor is upregulated by Helicobacter pylori in human gastric cancer AGS cells via ERK, JNK, and AP-1. Biochem Biophys Res Commun 333: 874–880, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Kim MH, Yoo HS, Kim MY, Jang HJ, Baek MK, Kim HR, Kim KK, Shin BA, Ahn BW, Jung YD. Helicobacter pylori stimulates urokinase plasminogen activator receptor expression and cell invasiveness through reactive oxygen species and NF-kappaB signaling in human gastric carcinoma cells. Int J Mol Med 19: 689–697, 2007. [PubMed] [Google Scholar]
- 32.Kitadai Y, Sasaki A, Ito M, Tanaka S, Oue N, Yasui W, Aihara M, Imagawa K, Haruma K, Chayama K. Helicobacter pylori infection influences expression of genes related to angiogenesis and invasion in human gastric carcinoma cells. Biochem Biophys Res Commun 311: 809–814, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Levi S, Beardshall K, Haddad G, Playford R, Ghosh P, Calam J. Campylobacter pylori and duodenal ulcers: the gastrin link. Lancet 1: 1167–1168, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Lijnen HR Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost 86: 324–333, 2001. [PubMed] [Google Scholar]
- 35.Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Castro AC, Campos ML, Van Doorn LJ, Caldas C, Seruca R, Carneiro F, Sobrinho-Simoes M. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology 125: 364–371, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Madsen CD, Sidenius N. The interaction between urokinase receptor and vitronectin in cell adhesion and signalling. Eur J Cell Biol. In press. [DOI] [PubMed]
- 37.McCaig C, Duval C, Hemers E, Steele I, Pritchard DM, Przemeck S, Dimaline R, Ahmed S, Bodger K, Kerrigan DD, Wang TC, Dockray GJ, Varro A. The role of matrix metalloproteinase-7 in redefining the gastric microenvironment in response to Helicobacter pylori. Gastroenterology 130: 1754–1763, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Mitsuno Y, Yoshida H, Maeda S, Ogura K, Hirata Y, Kawabe T, Shiratori Y, Omata M. Helicobacter pylori induced transactivation of SRE and AP-1 through the ERK signalling pathway in gastric cancer cells. Gut 49: 18–22, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy G, Gavrilovic J. Proteolysis and cell migration: creating a path? Curr Opin Cell Biol 11: 614–621, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Nekarda H, Schmitt M, Ulm K, Wenninger A, Vogelsang H, Becker K, Roder JD, Fink U, Siewert JR. Prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in completely resected gastric cancer. Cancer Res 54: 2900–2907, 1994. [PubMed] [Google Scholar]
- 41.Nishi E, Hiraoka Y, Yoshida K, Okawa K, Kita T. Nardilysin enhances ectodomain shedding of heparin-binding epidermal growth factor-like growth factor through activation of tumor necrosis factor-alpha-converting enzyme. J Biol Chem 281: 31164–31172, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Novak U, Cocks BG, Hamilton JA. A labile repressor acts through the NFkB-like binding sites of the human urokinase gene. Nucleic Acids Res 19: 3389–3393, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2: 28–37, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Peek RM, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol 208: 233–248, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Peek RM, Moss SF, Tham KT, Perez-Perez GI, Wang S, Miller GG, Atherton JC, Holt PR, Blaser MJ. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst 89: 863–868, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Plebani M, Herszenyi L, Carraro P, De Paoli M, Roveroni G, Cardin R, Tulassay Z, Naccarato R, Farinati F. Urokinase-type plasminogen activator receptor in gastric cancer: tissue expression and prognostic role. Clin Exp Metastasis 15: 418–425, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402: 884–888, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Reuning U, Magdolen V, Wilhelm O, Fischer K, Lutz V, Graeff H, Schmitt M. Multifunctional potential of the plasminogen activation system in tumor invasion and metastasis (Review). Int J Oncol 13: 893–906, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Steele IA, Dimaline R, Pritchard DM, Peek RM Jr, Wang TC, Dockray GJ Varro A. Helicobacter and gastrin stimulate Reg1 expression in gastric epithelial cells through distinct promoter elements. Am J Physiol Gastrointest Liver Physiol 293: G347–G354, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Sunnarborg SW, Hinkle CL, Stevenson M, Russell WE, Raska CS, Peschon JJ, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, Lee DC. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem 277: 12838–12845, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem 272: 31730–31737, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Varro A, Hemers E, Archer D, Pagliocca A, Haigh C, Ahmed S, Dimaline R, Dockray GJ. Identification of plasminogen activator inhibitor-2 as a gastrin- regulated gene: role of Rho GTPase and menin. Gastroenterology 123: 271–280, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Varro A, Kenny S, Hemers E, McCaig C, Przemeck S, Wang TC, Bodger K, Pritchard DM. Increased gastric expression of MMP-7 in hypergastrinemia and significance for epithelial-mesenchymal signaling. Am J Physiol Gastrointest Liver Physiol 292: G1133–G1140, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Varro A, Noble PJ, Pritchard DM, Kennedy S, Hart CA, Dimaline R, Dockray GJ. Helicobacter pylori induces plasminogen activator inhibitor 2 (PAI-2) in gastric epithelial cells through NF-kB and RhoA: implications for invasion and apoptosis. Cancer Res 64: 1695–1702, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Wallasch C, Crabtree JE, Bevec D, Robinson PA, Wagner H, Ullrich A. Helicobacter pylori-stimulated EGF receptor transactivation requires metalloprotease cleavage of HB-EGF. Biochem Biophys Res Commun 295: 695–701, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Wroblewski LE, Noble PJ, Pagliocca A, Pritchard DM, Hart CA, Campbell F, Dodson AR, Dockray GJ, Varro A. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J Cell Sci 116: 3017–3026, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Yu WH, Woessner JF Jr, McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev 16: 307–323, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]