Abstract
Recent evidence supports a prominent role for Rho kinase (ROK)-mediated pulmonary vasoconstriction in the development and maintenance of chronic hypoxia (CH)-induced pulmonary hypertension. Endothelin (ET)-1 contributes to the pulmonary hypertensive response to CH, and recent studies by our laboratory and others indicate that pulmonary vascular reactivity following CH is largely independent of changes in vascular smooth muscle (VSM) intracellular free calcium concentration ([Ca2+]i). In addition, CH increases generation of reactive oxygen species (ROS) in pulmonary arteries, which may underlie the shift toward ROK-dependent Ca2+ sensitization. Therefore, we hypothesized that ROS-dependent RhoA/ROK signaling mediates ET-1-induced Ca2+ sensitization in pulmonary VSM following CH. To test this hypothesis, we determined the effect of pharmacological inhibitors of ROK, myosin light chain kinase (MLCK), tyrosine kinase (TK), and PKC on ET-1-induced vasoconstriction in endothelium-denuded, Ca2+-permeabilized small pulmonary arteries from control and CH (4 wk at 0.5 atm) rats. Further experiments examined ET-1-mediated, ROK-dependent phosphorylation of the regulatory subunit of myosin light chain phosphatase (MLCP), MYPT1. Finally, we measured ET-1-induced ROS generation in dihydroethidium-loaded small pulmonary arteries and investigated the role of ROS in mediating ET-1-induced, RhoA/ROK-dependent Ca2+ sensitization using the superoxide anion scavenger, tiron. We found that CH increases ET-1-induced Ca2+ sensitization that is sensitive to inhibition of ROK and MLCK, but not PKC or TK, and correlates with ROK-dependent MYPT1Thr696 phosphorylation. Furthermore, tiron inhibited basal and ET-1-stimulated ROS generation, RhoA activation, and VSM Ca2+ sensitization following CH. We conclude that CH augments ET-1-induced Ca2+ sensitization through ROS-dependent activation of RhoA/ROK signaling in pulmonary VSM.
Keywords: endothelin-1, fura 2, pulmonary hypertension, superoxide anion, protein kinase C, tyrosine kinase, myosin light chain phosphatase
pulmonary hypertension, associated with high altitude exposure and chronic obstructive pulmonary disease, is attributed to both structural remodeling as well as enhanced vasoconstriction in the pulmonary vasculature. Although it has been widely believed that vascular remodeling due to medial hypertrophy of pulmonary vascular smooth muscle (VSM) is the key mediator of increased vascular resistance following chronic hypoxia (CH), it is now evident that vasoconstriction may play a more prominent role than remodeling in the elevation of pulmonary vascular resistance and arterial pressure in rats (51). Consistent with this possibility, CH-induced pulmonary hypertension can be acutely reversed by inhibition of Rho kinase (ROK), a mediator of myofilament contractility (21, 41, 44), suggesting ROK-mediated vasoconstriction contributes substantially to increased pulmonary vascular resistance.
The 21-amino acid peptide endothelin (ET)-1 has both vasoactive and mitogenic properties and plays a significant role in the pathogenesis of CH-induced pulmonary hypertension (4, 7, 10). Under normal conditions, vasoconstrictor responses to ET-1 are mediated by Ca2+ influx through L-type Ca2+ channels and Ca2+ release from the sarcoplasmic reticulum (45). However, in pulmonary arterial myocytes isolated from CH rats, ET-1-induced contraction is largely independent of changes in [Ca2+]i (50), a phenomenon known as Ca2+ sensitization of the contractile apparatus. There are several possible signaling pathways responsible for Ca2+ sensitization, including PKC, tyrosine kinases (TK), and RhoA/ROK, all of which have been shown to be activated by ET-1 (39, 43, 45, 48). Recent studies have demonstrated that CH is associated with increased RhoA activation, ROK expression, and RhoA/ROK-dependent vasoconstriction (3, 5, 13, 26, 41, 44). Therefore, we first hypothesized that CH augments ET-1-induced Ca2+ sensitization through the RhoA/ROK signaling pathway.
Although studies from our laboratory and others have provided convincing evidence for upregulation of RhoA/ROK signaling following CH (3, 14, 17, 21, 26, 41), the cause of this upregulation is unknown. Hypoxia has been shown to increase production of reactive oxygen species (ROS), such as superoxide anion (O2−) and hydrogen peroxide, in rat pulmonary arterial VSM (30, 53, 55, 59) and endothelial (16, 60) cells. ROS are important modulators of vascular tone and can function as second messengers to activate multiple intracellular signaling cascades, including that of RhoA/ROK (29). Therefore, we further hypothesized that ROS mediate RhoA/ROK-dependent Ca2+ sensitization to ET-1 in pulmonary VSM following CH. In this study, we examined various signaling pathways that may be involved in ET-1-induced Ca2+ sensitization in pulmonary arteries following CH. Our findings demonstrate an effect of CH to augment ET-1-mediated myofilament Ca2+ sensitization through ROS-dependent activation of the RhoA/ROK signaling pathway in pulmonary VSM.
METHODS
All protocols employed in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico School of Medicine (Albuquerque, NM).
Experimental Groups
Male Sprague-Dawley rats (250–300 g body wt, Harlan Industries) were divided into two groups for each experiment. Animals designated for exposure to CH were housed in a hypobaric chamber with barometric pressure maintained at ∼380 mmHg for 4 wk. The chamber was opened three times per week to provide animals with fresh food, water, and clean bedding. Age-matched control animals were housed at ambient barometric pressure (∼630 mmHg). All animals were maintained on a 12:12-h light-dark cycle. We have previously shown that this model of CH produces many of the cardiopulmonary changes observed in chronic obstructive pulmonary disease and prolonged residence at high altitude, including right ventricular hypertrophy, pulmonary hypertension, arterial remodeling, and polycythemia (47).
Cannulation and Pressurization of Small Pulmonary Arteries for Assessment of Vasoreactivity
Rats were anesthetized with pentobarbital sodium (200 mg/kg ip), and the heart and lungs were exposed by midline thoracotomy. The left lung was removed and immediately placed in ice-cold physiological saline solution (PSS, pH 7.4) containing (in mM) 129.8 NaCl, 5.4 KCl, 0.5 NaH2PO4, 0.83 MgSO4, 19 NaHCO3, 1.8 CaCl2, and 5.5 glucose (all from Sigma). Fourth-order intrapulmonary arteries [100–200 μm inner diameter (ID)] of ∼1-mm length and without visible side branches were dissected free and transferred to a vessel chamber (CH-1, Living Systems). The proximal end of the artery was cannulated with a tapered glass pipette, secured in place with a single strand of silk ligature, and gently flushed to remove any blood from the lumen. The vessel was stretched longitudinally to approximate in situ length and pressurized with a servo-controlled peristaltic pump (Living Systems) to 12 mmHg. A transmural pressure of 12 mmHg was chosen for both control and CH vessels based on our earlier findings that NO-mediated vasodilation and associated VSM [Ca2+]i responses were similar between control and CH arteries whether studied at 12 or 35 mmHg (25). Arteries were required to hold a steady pressure on switching off the servo-control function to verify the absence of leaks; any vessel with apparent leaks was discarded. The vessel chamber was transferred to the stage of a Nikon Eclipse TS100 microscope where vessels were superfused with PSS equilibrated with a gas mixture containing 10% O2, 6% CO2, and balance N2. A vessel chamber cover was positioned to permit this same gas mixture to flow over the top of the chamber bath. Previous studies from our laboratory (25) indicate that this gas mixture yields an approximate superfusate pH = 7.40, Po2 = 57 mmHg, and Pco2 = 31 mmHg. Bright-field images were obtained with an IonOptix CCD100M camera, and dimensional analysis was performed by IonOptix SarcLen software to measure ID.
Verification of Endothelial Integrity/Disruption
For experiments in endothelium-intact vessels, endothelial integrity was assessed by preconstricting arteries with UTP (2–5 μM) and measuring the subsequent vasodilatory response to ACh (1 μM). To examine VSM reactivity to ET-1 without the complicating influence of ETB receptor (ETBR) activation on the endothelium, the majority of experiments were performed in endothelium-disrupted vessels. After cannulating the proximal end of the vessel and flushing out blood, the vessel lumen was rubbed with a strand of moose mane to disrupt the endothelium. The effectiveness of endothelial disruption was verified by the lack of a vasodilatory response to ACh. Following administration of ACh, vessels were rinsed in normal PSS and loaded with fura 2-AM as indicated below. These methods have been described in previous studies from our laboratory (24, 26, 27).
Measurement of VSM [Ca2+]i
Pressurized arteries were loaded abluminally with the cell-permeant, ratiometric, Ca2+-sensitive fluorescent indicator fura 2-AM (2 μM and 0.05% pluronic acid, Molecular Probes) as we have previously reported (24, 26, 27). Arteries were incubated in this solution for 45 min at room temperature in the dark and were equilibrated with the 10% O2 gas mixture during this loading period. Vessels were rinsed for 20 min with aerated PSS (37°C) after the loading period to wash out excess dye and to allow for hydrolysis of AM groups by intracellular esterases. Fura 2-loaded vessels were alternately excited at 340 and 380 nm at a frequency of 1 Hz with an IonOptix Hyperswitch dual-excitation light source, and the respective 510-nm emissions were collected with a photomultiplier tube. Background-subtracted 340/380 emission ratios were calculated with IonOptix Ion Wizard software and recorded continuously throughout the experiment, with simultaneous measurement of ID from red-wavelength bright-field images as described above.
VSM Ca2+ Permeabilization
VSM [Ca2+]i was clamped in some experiments by permeabilizing with the Ca2+ ionophore, ionomycin, similar to that previously described (26). First, arteries were equilibrated in Ca2+-free PSS containing 3 mM EGTA and 3 μM ionomycin to permeabilize the vessels to Ca2+. Arteries were then equilibrated in PSS with a calculated free Ca2+ concentration of 300 nM [containing (in mM) 129.8 NaCl, 5.4 KCl, 0.5 NaH2PO4, 1.3 MgSO4, 19 NaHCO3, 6.8 CaCl2, 5.5 glucose, 8.2 EGTA, 0.003 ionomycin (Sigma)]. This free [Ca2+] was calculated using the Kd of EGTA for Ca2+ of 43.7 nM and the Kd of EGTA for Mg2+ of 3.33 mM at 37°C and pH 7.4 (52). This free [Ca2+]i produced optimal vasoconstrictor responsiveness to ET-1 with minimal effects on basal tone in Ca2+-permeabilized arteries in preliminary experiments.
Isolated Vessel Experiments
ET-1-mediated constriction in endothelium-intact and -disrupted arteries.
The effect of CH exposure on vasoreactivity to ET-1 was assessed by generating cumulative concentration-response curves to ET-1 (10−12 to 10−7 M, Sigma) in endothelium-intact and endothelium-disrupted, small pulmonary arteries from control and CH rats. Similar protocols were conducted in the presence of Y-27632 or tiron in endothelium-intact arteries to evaluate the contribution of ROK and ROS to altered vasoconstrictor reactivity following CH.
ET-1-mediated Ca2+ sensitization.
The effect of CH exposure on ET-1 (10−10 to 10−7 M)-mediated Ca2+ sensitization was assessed in endothelium-denuded, small pulmonary arteries from control and CH rats. In separate sets of experiments, we assessed the mechanism of Ca2+ sensitization by examining the effects of myosin light chain (MLC) kinase (MLCK), ROK, PKC, and TK inhibition on ET-1-mediated Ca2+ sensitization in small pulmonary arteries. After fura 2 loading and permeabilization, arteries were allowed to incubate 30 min with the MLCK inhibitor, ML-9 (100 μM, Biomol), the ROK inhibitor, Y-27632 (10 μM, Calbiochem), the broad-spectrum PKC inhibitor, GF-109203X (1 μM, Biomol), the TK inhibitor, genistein (100 μM, Calbiochem), or vehicle (PSS, DMSO, or 50% ethanol) before attaining a concentration-response curve to ET-1 (10−10 to 10−7 M). The concentrations and specificity of these inhibitors have been previously verified by our laboratory (26) or others (41, 57).
Role of ETA and ETB receptors in ET-1-mediated Ca2+ sensitization.
The role of ETAR and ETBR in ET-1-induced Ca2+ sensitization was assessed in permeabilized and endothelium-denuded small pulmonary arteries from control and CH rats. A cumulative concentration-response relationship to ET-1 (10−10 to 10−7 M) was assessed in arteries in the presence of the ETAR antagonist, BQ-123 (10 μM, Calbiochem), the ETBR antagonist, BQ-788 (10 μM, Calbiochem), a combination of both BQ-123 and BQ-788 (10 μM each), or vehicle (PSS or DMSO). We (27) have previously demonstrated that this concentration of BQ-123 attenuates ET-1-mediated constriction in small pulmonary arteries from control animals.
Role of ROS in ET-1-induced Ca2+ sensitization.
The contribution of ROS to ET-1-induced Ca2+ sensitization was assessed in permeabilized and endothelium-denuded small pulmonary arteries from control and CH rats. A cumulative concentration-response relationship to ET-1 (10−10 to 10−7 M) was assessed in arteries in the presence of the O2− scavenger, 4,5-dihydroxy-1,3-benzene disulfonic acid (tiron; 10 mM; Sigma) or vehicle (PSS). This concentration of tiron inhibits xanthine-oxidase-induced ROS generation in isolated pulmonary arteries as determined in previous studies by our laboratory (27).
Fluorescence Detection of O2− by Dihydroethidium in Isolated Small Pulmonary Arteries
Dihydroethidium (DHE; Molecular Probes) was used to evaluate effects of CH on ET-1-induced ROS production in isolated, pressurized arteries. Cells are permeable to DHE, which is converted to the fluorescent products ethidium and 2-hydroxyethidium in an O2−-dependent manner (8, 61). After the 30-min equilibration, the pressurized pulmonary arteries were loaded with DHE (10 μM DHE and 0.05% pluronic acid). Vessels were incubated in this solution for 30 min at room temperature in the dark and then rinsed for 5 min with PSS (37°C) to wash out excess dye. We obtained fluorescent images using a standard tetramethylrhodamine isothiocyanate (TRITC) filter (excitation ∼550 nm and emission ∼600 nm) before loading the vessel (for background subtraction) and after the 30-min incubation with DHE. ET-1 (10−9 M) was added to the superfusate, and DHE fluorescence was monitored for 10 min. This concentration of ET-1 resulted in approximately half-maximal constriction in both groups (Fig. 2). Images (1 per minute) were generated with a CCD camera (SenSys 1400) and processed with MetaFluor software (Universal Imaging). Normalized fluorescence intensity is defined as average gray-scale values for all pixels in the field above background. In separate experiments, vessels were pretreated with tiron (10 mM) before administration of ET-1.
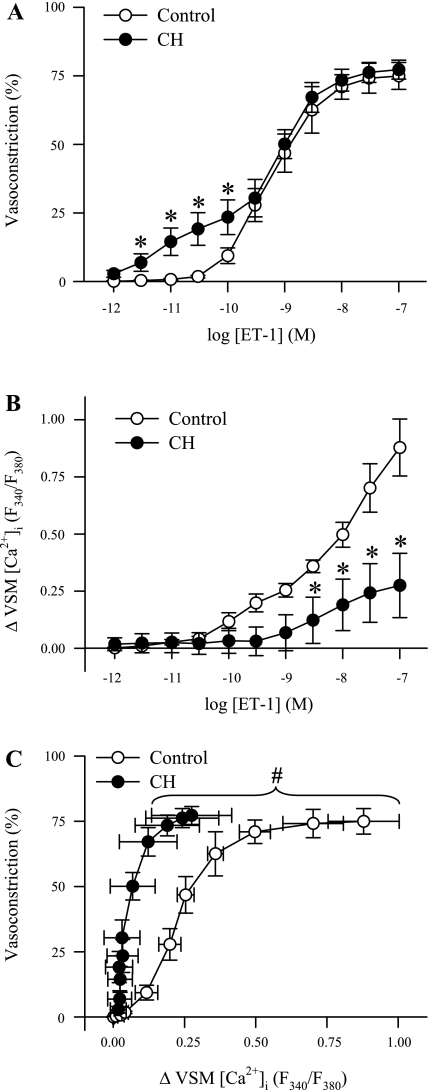
Fig. 2.
CH augments ET-1-induced vasoconstriction but impedes changes in vascular smooth muscle (VSM) [Ca2+]i in endothelium-disrupted pulmonary arteries. Percent vasoconstriction (A) and changes in VSM [Ca2+]i to ET-1 (10−12 to 10−7 M) (B) in endothelium-disrupted small pulmonary arteries from control (n = 6) and CH (n = 6) rats. C: percent vasoconstriction plotted as a function of changes in VSM [Ca2+]i. Values are means ± SE. *P ≤ 0.05 vs. control group. #P ≤ 0.05 for CH vs. control curves.
Western Blotting
Western blotting procedures were used to determine effects of CH on ET-1-mediated RhoA activation and phosphorylation of the myosin binding subunit (MYPT1) of MLC phosphatase (MLCP). To obtain sufficient tissue for analysis, intrapulmonary arteries (approximately 2nd through 5th order) from the left and right lungs were dissected from accompanying airways and surrounding lung tissue in a HEPES-based PSS (in MM, 130 NaCl, 4 KCl, 1.2 MgSO4, 4 NaHCO3, 1.8 CaCl2, 10 HEPES, 1.18 KH2PO4, 6 glucose, and 0.03 EDTA, pH adjusted to 7.4 with NaOH). A HEPES-based PSS was used to maintain physiological pH during dissection since the solution was not aerated with the 10% O2, 6% CO2, balance N2 gas mixture used for vasoreactivity protocols. Arteries were incubated at 37°C for 30 min in the presence of vehicle, Y-27632 (10 μM, for analysis of ROK-dependent phosphorylation of MYPT1) or tiron (10 mM, for analysis of ROS-mediated activation of RhoA). After 30 min, some arteries were stimulated with ET-1 (10−8 M) for 5 min before being snap-frozen in liquid N2. This concentration of ET-1 resulted in the greatest difference in vasoconstriction between groups in permeabilized arteries (Fig. 3). Each sample was homogenized in 10 mM Tris·HCl homogenization buffer containing 255 mM sucrose, 2 mM EDTA, 12 μM leupeptin, 1 μM pepstatin A, 0.3 μM aprotinin, and 1 mM phenylmethylsulfonyl fluoride (all from Sigma). Samples were centrifuged at 10,000 g for 10 min at 4°C to remove insoluble debris. The supernatant was collected, and sample protein concentrations were determined by the Bradford method (Bio-Rad Protein Assay). Control experiments were conducted using different concentrations of protein to ensure linearity of the densitometry curve.
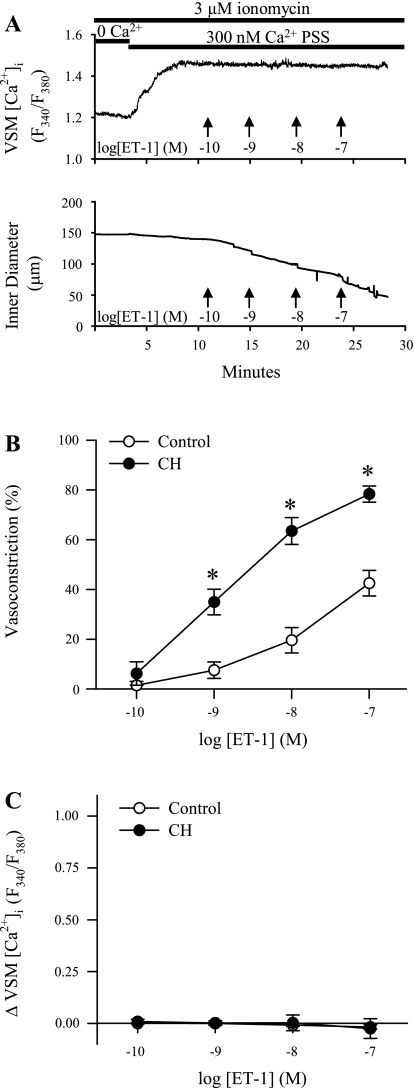
Fig. 3.
ET-1-induced pulmonary VSM Ca2+ sensitization is augmented following CH. A: traces of VSM [Ca2+]i and ID (μm) in an endothelium-disrupted, small pulmonary artery from a control rat following permeabilization to Ca2+ with ionomycin (3 μM). Switching to 300 nM Ca2+ physiological saline solution (PSS) caused an increase in the F340/F380 ratio. Addition of ET-1 (10−10 to 10−7 M) resulted in vasoconstriction without a change in VSM [Ca2+]i. Summary data for B are percent vasoconstriction and for C are changes in VSM [Ca2+]i to ET-1 in arteries from control (n = 5) and CH (n = 5) rats. Values are means ± SE. *P ≤ 0.05 vs. control group.
Phospho-MYPT1
ROK-dependent phosphorylation of MYPT1 was measured using antibodies specific for MYPT1 phosphorylated at Thr696 (pMYPT1Thr696) or Thr850 (pMYPT1Thr850) using a protocol similar to previously published work (13). Briefly, pulmonary artery lysates (35 μg per lane) were separated by SDS-PAGE (12% Tris·HCl gels, Bio-Rad) and transferred to polyvinylidene difluoride membranes. Blots were blocked for 1 h at room temperature with 5% BSA and 0.05% Tween 20 (Bio-Rad) in TBS containing 10 mM Tris·HCl and 50 mM NaCl (pH 7.5). Blots were then incubated overnight at 4°C with polyclonal anti-pMYPT1Thr696 (1:1,000, Upstate Biotechnology), anti-pMYPT1Thr850 (1:1,000, Upstate Biotechnology), or monoclonal anti-MYPT1 (1:500, BD Biosciences). For immunochemical labeling, blots were incubated for 1 h at room temperature with goat anti-rabbit IgG-horseradish peroxidase (HRP; 1:2,500, for pMYPT1Thr696 and pMYPT1Thr850; Bio-Rad) or goat anti-mouse IgG-HRP (1:2,500, for MYPT1; Bio-Rad). After chemiluminescence labeling (ECL, Amersham), pMYPT1Thr696, pMYPT1Thr850, and MYPT1 bands were detected by exposing the blots to chemiluminescence-sensitive film (Kodak). Quantification of the bands was accomplished by densitometric analysis of scanned images (SigmaGel software, SPSS). Bands for pMYPT1Thr696 and pMYPT1Thr850 were normalized to those of MYPT1.
RhoA Activation
RhoA activity was assessed using a Rho activation assay kit (Cytoskeleton) that detects levels of GTP-bound RhoA as previously published from our laboratory (26). Activated RhoA was purified from vessel homogenates by incubation for 1 h at 4°C with Rhotekin-Rho binding domain (RBD) glutathione affinity beads (0.5 mg of sample protein per 50 μg of Rhotekin-RBD beads), which specifically bind GTP-bound RhoA. Samples were pelleted by centrifugation, and the supernatant was discarded before washing the samples with a solution containing 25 mM Tris (pH 7.5), 30 mM MgCl2, 40 mM NaCl, and 150 mM EDTA. The pellet was resuspended in Laemmli buffer, separated by SDS-PAGE (15% Tris·HCl gels, Bio-Rad), and transferred to polyvinylidene difluoride membranes. Blots were blocked for 1 h at room temperature with 5% nonfat milk and 0.05% Tween 20 (Bio-Rad) in TBS. Blots were then incubated overnight at 4°C with a mouse monoclonal antibody for RhoA (1:500, Cytoskeleton). For immunochemical labeling, blots were incubated for 1 h at room temperature with goat anti-mouse IgG-HRP (1:5,000, The Jackson Laboratories). After chemiluminescence labeling (ECL, Amersham), RhoA (∼23 kDa) bands were detected by exposing the blots to chemiluminescence-sensitive film (Kodak). Total RhoA protein (25 μg per lane) was determined in separate Western blots and used to normalize GTP-bound RhoA densitometric units.
Calculations and Statistics
Vasoconstrictor responses were calculated as percentages of baseline ID. VSM [Ca2+]i is represented as a change in the fura 2 emission ratios (F340/F380) from baseline. pMYPT1 and GTP-bound RhoA were normalized to total MYPT1 and RhoA expression, respectively, for each sample. All data are expressed as means ± SE, and values of n refer to the number of animals in each group. A two-way ANOVA was used to make comparisons when appropriate. If differences were detected by ANOVA, individual groups were compared with the Student-Newman-Keuls test. A probability of P ≤ 0.05 was accepted as significant for all comparisons. To determine dependency of vasoconstriction on Ca2+, data were plotted as percent vasoconstriction as a function of the change in VSM [Ca2+]i (Fig. 2C). To address whether the curves between control and CH were different, we fit separate cubic spline models to each group where each spline had a single knot at 0.20 (R2 = 98.8%) and then tested the regression between groups.
RESULTS
Vessel ID (control 145 ± 6 μm, n = 47; and CH 155 ± 5 μm, n = 46) and basal VSM [Ca2+]i (control, 1.48 ± 0.05 F340/F380, n = 44; CH, 1.44 ± 0.05 F340/F380, n = 42) did not differ between groups. VSM [Ca2+]i ratios in permeabilized vessels maintained in 300 nM Ca2+ PSS were similar between groups (control, 1.02 ± 0.03 F340/F380, n = 39; CH, 1.01 ± 0.03 F340/F380, n = 41).
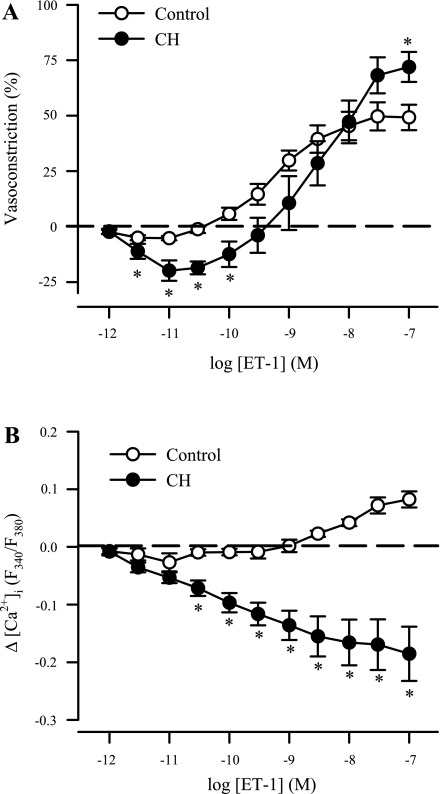
CH Reveals a Biphasic Vasoactive Response to ET-1 and an ET-1-Mediated Reduction in VSM [Ca2+]i in Endothelium-Intact Pulmonary Arteries
Vasodilation to lower concentrations of ET-1 (3 × 10−12 to 10−10 M) was present in endothelium-intact vessels from CH rats but not controls (Fig. 1A), presumably reflecting a reduction in CH-induced myogenic tone as recently reported (5). However, vasoconstriction was augmented at higher concentrations of ET-1 in arteries from CH rats compared with control vessels, with significance achieved at 10−7 M. In control arteries, [Ca2+]i was moderately elevated in response to ET-1 (3 × 10−9 to 10−7 M; Fig. 1B). In contrast, VSM [Ca2+]i progressively decreased by ET-1 in arteries from CH rats, consistent with the observed vasodilatory response in these vessels at lower concentrations. Interestingly, [Ca2+]i continued to fall in arteries from CH rats despite substantial vasoconstriction to ET-1 observed at higher concentrations (10−9 to 10−7 M), thus demonstrating an effect of CH to increase ET-1-induced myofilament Ca2+ sensitivity.
Fig. 1.
Endothelin-1 (ET-1) mediates a biphasic vasoactive response and a reduction in vessel wall free intracellular calcium concentration ([Ca2+]i) in endothelium-intact pulmonary arteries from chronically hypoxic (CH) rats. Vascular reactivity [percent baseline inner diameter (ID)] (A) and changes (Δ) in vessel wall [Ca2+]i expressed as fura 2 340-/380-nm emission ratios (F340/F380) to ET-1 (10−12 to 10−7 M) (B) in endothelium-intact small pulmonary arteries from control (n = 6) and CH (n = 6) rats. Values are means ± SE. *P ≤ 0.05 vs. control group.
CH Enhances ET-1-Mediated Vasoconstriction but Attenuates the VSM [Ca2+]i Response to ET-1 in Endothelium-Disrupted Pulmonary Arteries
Vasoconstriction to lower concentrations of ET-1 (3 × 10−12 to 10−10 M) was augmented in endothelium-disrupted vessels from CH rats compared with controls (Fig. 2A) and occurred without a detectable increase in VSM [Ca2+]i. Furthermore, the change in VSM [Ca2+]i to higher concentrations of ET-1 (3 × 10−9 to 10−7 M) was diminished in arteries from CH rats compared with control arteries (Fig. 2B). When data from Fig. 2, A and B, are plotted to illustrate vasoconstriction as a function of the change in VSM [Ca2+]i, the left-shifted curve in arteries from CH animals signifies that vasoconstriction is largely independent of a change in VSM [Ca2+]i and is significantly different [F(5,12) = 46.72; P < 0.001] compared with control animals (Fig. 2C). These findings provide additional evidence that CH reduces the dependency of ET-1-mediated vasoconstriction on changes in VSM [Ca2+]i.
ET-1-Induced Pulmonary VSM Ca2+ Sensitization is Augmented Following CH
To directly assess effects of CH on ET-1-mediated VSM Ca2+ sensitization, intracellular Ca2+ was clamped by permeabilizing the arteries with ionomycin. We then examined responses to ET-1 in endothelium-disrupted pulmonary arteries from each group of rats. Since VSM [Ca2+]i is clamped by the extracellular Ca2+ concentration in permeabilized vessels, any vasoconstrictor response is a function of myofilament Ca2+ sensitization. Figure 3A depicts representative traces of 340/380 emission ratios and ID responses to ET-1 from a Ca2+-permeabilized control artery. Switching from superfusion with Ca2+-free PSS to PSS containing a calculated Ca2+ concentration of 300 nM produced an increase in VSM [Ca2+]i (control, 0.14 ± 0.03, n = 44; CH, 0.13 ± 0.02, n = 42) and a modest vasoconstriction (control, 16% ± 2%, n = 44; CH, 8% ± 2%, n = 42). After stabilization of both vessel ID and VSM [Ca2+]i, a concentration-response relationship to ET-1 was performed, yielding vasoconstriction independent of a change in VSM [Ca2+]i. Consistent with evidence for enhanced Ca2+ sensitivity following CH in nonpermeabilized arteries (Figs. 1 and 2), ET-1-mediated vasoconstriction was greater in Ca2+-permeabilized arteries from CH rats vs. controls (Fig. 3B), whereas VSM [Ca2+]i remained stable in arteries from both groups (Fig. 3C).
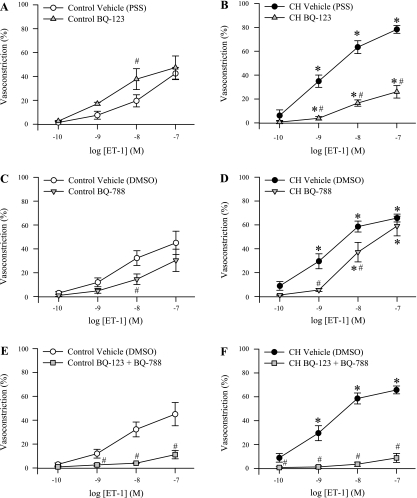
Augmented ET-1-Induced Pulmonary VSM Ca2+ Sensitization Following CH is Mediated Predominantly by ETAR Activation
In arteries from control animals, inhibition of ETAR with BQ-123 had little effect on ET-1-mediated constriction except for an unexpected augmentation of the response to 10−8 M ET-1 (Fig. 4A), whereas the ETBR antagonist, BQ-788, attenuated vasoreactivity to ET-1 only at the 10−8 M concentration (Fig. 4C). In contrast, inhibition of either ETAR or ETBR reduced ET-1-mediated constriction in CH arteries, although ETAR blockade (Fig. 4B) was more effective than ETBR inhibition (Fig. 4D). The combination of ETA/ETB inhibition nearly abolished ET-1 vasoconstrictor responses in both groups (Fig. 4, E and F).
Fig. 4.
Augmented ET-1-induced pulmonary VSM Ca2+ sensitization following CH is mediated largely by ETA receptor (ETAR) activation. Percent vasoconstriction to ET-1 (10−10 to 10−7 M) in Ca2+-permeabilized, endothelium-disrupted small pulmonary arteries from control (A, C, and E) and CH (B, D, and F) rats in the presence of the ETAR antagonist BQ-123 (10 μM; A and B; n = 4 per group), the ETBR antagonist BQ-788 (10 μM; C and D; n = 6 per group), both BQ-123 and BQ-788 (E and F; n = 4 per group), or vehicle (PSS or DMSO as indicated). Values are means ± SE. *P ≤ 0.05 vs. control group. #P ≤ 0.05 vs. corresponding vehicle group. For illustration purposes, the same vehicle group is used in C/E and D/F. Vehicle groups in A and B are additionally the same as those in Fig. 3.
Increased ET-1-Induced Pulmonary VSM Ca2+ Sensitization Following CH is Dependent on ROK but Not PKC or TK
The MLCK inhibitor ML-9 blunted vasoconstriction to ET-1 in Ca2+-permeabilized arteries from both groups, resulting in similar vasoreactivity between groups (Fig. 5, A and B). Consistent with effects of ML-9, ROK inhibition with Y-27632 markedly inhibited reactivity in CH vessels, effectively normalizing constriction to that of control arteries (Fig. 5, C and D). In contrast, only a modest effect of Y-27632 to inhibit Ca2+ sensitization was observed in arteries from control rats (Fig. 5C). The PKC inhibitor GF-109203X and the TK antagonist genistein were without effect on ET-1-induced constriction in permeabilized arteries from either control or CH rats (Fig. 5, E–H).
Fig. 5.
Enhanced ET-1-induced pulmonary VSM Ca2+ sensitization following CH is mediated by Rho kinase (ROK) but not PKC or tyrosine kinase. Percent vasoconstriction to ET-1 (10−10 to 10−7 M) in Ca2+-permeabilized, endothelium-disrupted small pulmonary arteries from control (A, C, E, and G) and CH (B, D, F, and H) rats in the presence of ML-9 (100 μM; A and B), Y-27632 (10 μM; C and D), GF-109203X (1 μM; E and F), genistein (100 μM; G and H), or vehicle [PSS, DMSO, or 50% ethanol (EtOH) as indicated]. Values are means ± SE. *P ≤ 0.05 vs. control group. #P ≤ 0.05 vs. corresponding vehicle group. n = 5 per group for all treatments. For purposes of illustration, the same vehicle group is used in C/E and D/F. Vehicle groups in A–F are additionally the same as those shown in Figs. 3 and 4.
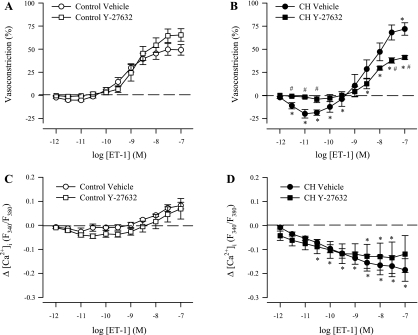
In agreement with responses to ROK inhibition in Ca2+-permeabilized vessels, Y-27632 attenuated vasoconstriction to ET-1 in endothelium-intact (nonpermeabilized) arteries from CH rats without altering reactivity in control vessels (Fig. 6, A and B). Furthermore, Y-27632 alone caused a significant reduction in basal tone in arteries from CH rats (15.5% ± 6.5%) but not in control arteries (0.2% ± 0.8%), thus supporting our recent findings that CH induces myogenic tone in these arteries through a ROK-dependent Ca2+ sensitization mechanism (5). Consequently, ROK inhibition similarly abolished the vasodilatory responses to lower concentrations of ET-1 in CH arteries (Fig. 6B). Y-27632 did not alter VSM [Ca2+]i responses to ET-1 in arteries from either group of rats (Fig. 6, C and D). Collectively, these data support a role for CH to augment ET-1-dependent VSM Ca2+ sensitization via the RhoA/ROK signaling pathway.
Fig. 6.
ROK inhibition diminishes ET-1-mediated vasoconstriction in endothelium-intact pulmonary arteries from CH rats. Vascular reactivity (A and B) and changes in vessel wall [Ca2+]i to ET-1 (10−12 to 10−7 M; C and D) in the presence of Y-27632 (10 μM) in arteries from control (n = 6; A and C) and CH (n = 6; B and D) rats. Values are means ± SE. *P ≤ 0.05 vs. control group. #P ≤ 0.05 vs. CH vehicle group. Vehicle groups are the same as those shown in Fig. 1.
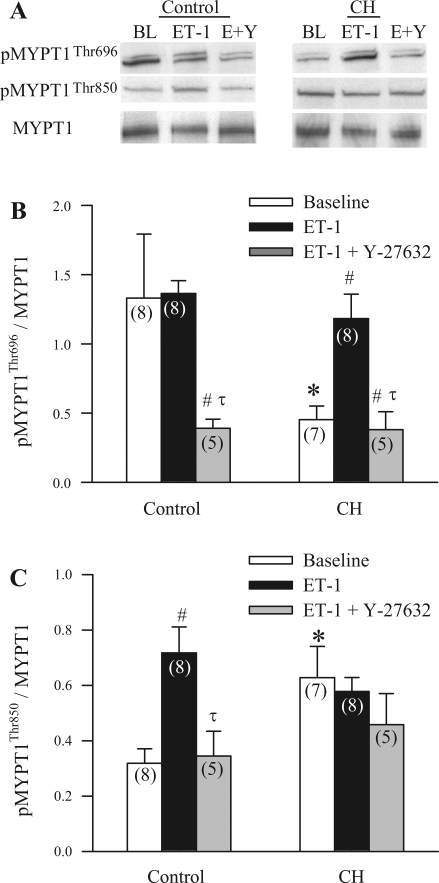
ET-1 Stimulates ROK-Dependent MYPT1 Phosphorylation in Control and CH Arteries
Basal levels of pMYPT1Thr696 were greater in control vs. CH arteries (Fig. 7A), whereas basal pMYPT1Thr850 was increased following CH (Fig. 7B). ET-1 increased levels of pMYPT1Thr696 in CH arteries (Fig. 7A) and pMYPT1Thr850 in control arteries (Fig. 7B). Furthermore, this ET-1-stimulated phosphorylation was inhibited by preincubation with Y-27632 in both groups (Fig. 7). Neither ET-1 nor Y-27632 altered pMYPT1Thr850 in CH arteries.
Fig. 7.
ET-1 mediated, ROK-dependent phosphorylation of the myosin binding subunit (MYPT1) of myosin light chain (MLC) phosphatase (MLCP) in control and CH arteries. Western blot images (A) and mean densitometric data for MYPT1 phosphorylated at Thr696 (pMYPT1Thr696)/total MYPT1 (B) and pMYPT1Thr850/total MYPT1 (C) in intrapulmonary arteries from control and CH rats in the presence of vehicle (baseline, BL), ET-1 (10−8 M), or ET-1 and Y-27632 (10−5 M; E+Y). n values are indicated in each bar. Values are means ± SE. *P ≤ 0.05 vs. corresponding control group. #P ≤ 0.05 vs. baseline. τP ≤ 0.05 vs. ET-1-stimulated group.
ROS Generation Contributes to ET-1-Induced Pulmonary VSM Ca2+ Sensitization Following CH
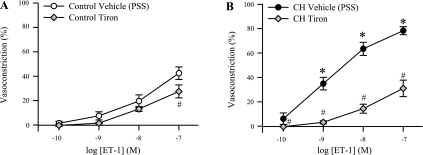
Similar to effects of ROK inhibition (Fig. 5D), the O2− scavenger tiron largely inhibited ET-1 vasoconstriction in Ca2+-permeabilized CH arteries (Fig. 8B) and normalized vasoconstrictor responses between groups while having little inhibitory effect in control arteries (Fig. 8A).
Fig. 8.
Reactive oxygen species (ROS) generation contributes to ET-1-induced pulmonary VSM Ca2+ sensitization following CH. Percent vasoconstriction (percent baseline ID) to ET-1 (10−10 to 10−7 M) in isolated, permeabilized, endothelium-disrupted small pulmonary arteries from control (A) and CH (B) rats in the presence of tiron (10 mM; n = 4 per group). Values are means ± SE. *P ≤ 0.05 vs. control group. #P ≤ 0.05 vs. corresponding vehicle group. For purposes of illustration, the same vehicle groups are used as those shown in Fig. 3.
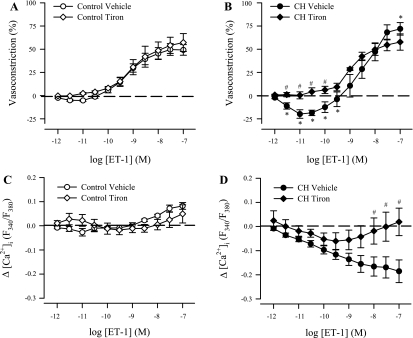
In endothelium-intact, nonpermeabilized arteries, tiron abolished the ET-1-mediated vasodilation in CH vessels (Fig. 9B) similar to that observed with Y-27632 (Fig. 6B), thus supporting a contribution of O2− to CH-induced basal tone. Furthermore, tiron tended to reduce the vasoconstrictor response to higher concentrations of ET-1 in CH arteries, although this effect did not reach statistical significance. Although tiron attenuated the fall in VSM [Ca2+]i in response to ET-1 in arteries from CH rats (Fig. 9D), the O2− inhibitor did not alter either vasoconstrictor responses (Fig. 9A) or changes in VSM [Ca2+]i (Fig. 9C) to ET-1 in control arteries.
Fig. 9.
Effects of O2− scavenging on responses to ET-1 in endothelium-intact pulmonary arteries from control and CH rats. Vascular reactivity (A and B) and changes in vessel wall [Ca2+]i (C and D) to ET-1 (10−12 to 10−7 M) in the presence of tiron (10 mM) in arteries from control (n = 6; A and C) and CH (n = 6; B and D) rats. Values are means ± SE. *P ≤ 0.05 vs. control group. #P ≤ 0.05 vs. CH vehicle group. Vehicle groups are the same as those shown in Fig. 1.
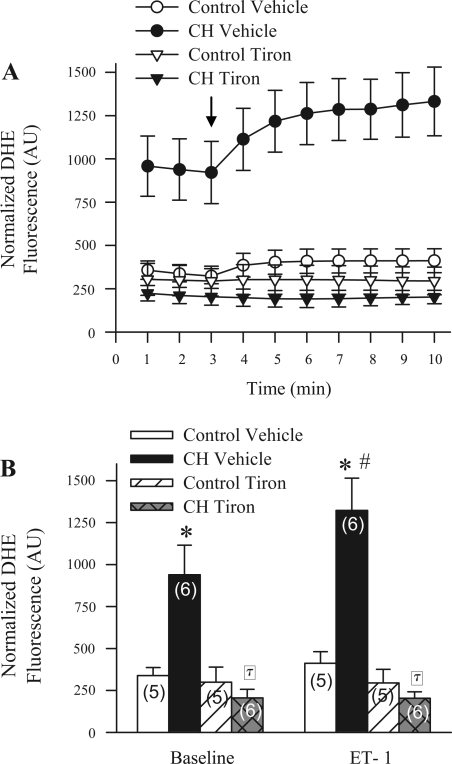
CH Increases Basal and ET-1-Stimulated O2− Levels in Pulmonary Arteries
Basal DHE fluorescence was significantly elevated in endothelium-disrupted small pulmonary arteries from CH rats compared with controls (Fig. 10, A and B). Addition of ET-1 increased DHE fluorescence in arteries from CH but not control animals. As expected, tiron abolished both the CH-induced elevation in basal ROS and ET-1-stimulated ROS production without altering DHE fluorescence in control arteries.
Fig. 10.
CH increases both basal and ET-1-stimulated superoxide anion levels in pulmonary arteries. A: dihydroethidium (DHE; 10 μM) fluorescence normalized to background [arbitrary units (AU)] as a function of time in isolated small pulmonary arteries from control (n = 5 per treatment) and CH (n = 6 per treatment) rats in the presence of vehicle or tiron (10 mM). Arrow indicates addition of ET-1 (10−9 M). B: summary data of average baseline and ET-1-stimulated (5 min postadministration) values for control and CH arteries in the presence of vehicle or tiron. Values are means ± SE. *P ≤ 0.05 vs. corresponding control group. #P ≤ 0.05 vs. baseline. τP ≤ 0.05 vs. CH vehicle.
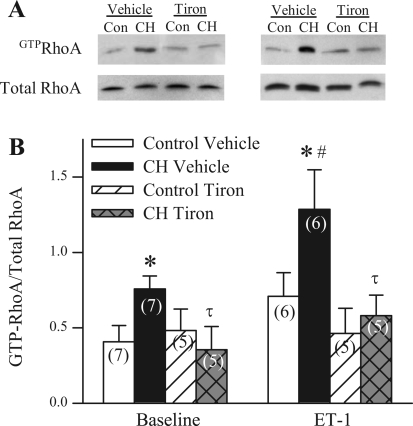
ROS Mediate CH-Induced Increases in Both Basal and ET-1-Stimulated RhoA Activity
As previously reported (26), CH was associated with enhanced basal arterial RhoA activity (Fig. 11, A and B). In addition, ET-1 caused an increase in GTP-bound RhoA levels in arteries from CH but not control animals (Fig. 11B). Consistent with an inhibitory influence of tiron on ET-1-mediated Ca2+ sensitization in CH arteries (Fig. 8B), tiron abolished both the CH-induced elevation in basal GTP-bound RhoA and ET-1-induced RhoA activation in pulmonary arterial homogenates (Fig. 11B). There was no effect of tiron in control arteries.
Fig. 11.
ROS mediate CH-induced increases in both basal and ET-1-stimulated RhoA activity. Representative images (A) and mean densitometric data (B) for GTP-bound activated RhoA/total RhoA in homogenates of intrapulmonary arteries from control (Con) and CH rats in the presence of vehicle, tiron (10 mM), ET-1 (10−8 M), or ET-1 and tiron. Values are means ± SE. *P ≤ 0.05 vs. corresponding control group. #P ≤ 0.05 vs. baseline group. τP ≤ 0.05 vs. CH vehicle.
DISCUSSION
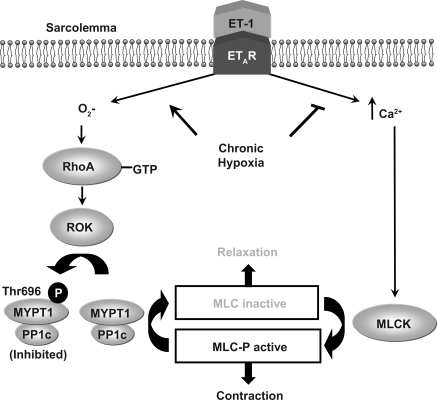
The present study examined the hypothesis that ROS mediate increased ET-1-induced, ROK-dependent pulmonary VSM Ca2+ sensitization following CH. The major findings from this study are: 1) CH attenuates ET-1-dependent increases in VSM [Ca2+]i and mediates a shift in ET-1 signaling toward enhanced myofilament Ca2+ sensitization in isolated, pressurized, small pulmonary arteries; 2) CH increases ET-1-induced VSM Ca2+ sensitization through a ROK-dependent signaling mechanism; 3) elevated VSM Ca2+ sensitivity to ET-1 following CH is associated with ROK-dependent phosphorylation of MYPT1Thr696; 4) ET-1-induced Ca2+ sensitization in arteries from CH rats occurs predominantly through ETAR stimulation; and 5) ROS scavenging inhibits basal and ET-1-stimulated O2− generation, RhoA activation, and ET-1-dependent Ca2+ sensitization following CH (Fig. 12). These results suggest that CH promotes ET-1-mediated myofilament Ca2+ sensitization through ROS-dependent activation of the RhoA/ROK signaling pathway in small pulmonary arteries.
Fig. 12.
Summary of major findings: CH attenuates ET-1-dependent increases in pulmonary VSM [Ca2+]i and mediates a shift in ET-1 signaling to promote myofilament Ca2+ sensitization predominantly through ETAR stimulation; CH augments ET-1-induced Ca2+ sensitization through O2−-dependent activation of RhoA/ROK signaling and resultant phosphorylation of MYPT1Thr696. PP1c, catalytic subunit of MLCP; MLCK, MLC kinase; P, inorganic phosphate.
ET-1 is an important mediator of CH-induced pulmonary hypertension, given that administration of ET-1 receptor antagonists prevents and reverses pulmonary hypertension in CH rats (4, 7, 10). CH is associated with increases in ET-1, ETAR, and ETBR expression in the lung (11, 33) and augments ET-1-dependent pulmonary vasoconstriction (9, 38, 54). However, binding of ET-1 to ETBR on endothelial cells can lead to endothelium-dependent vasodilation, which is also augmented following CH (40, 46). A novel aspect of the current study is that we have isolated VSM responses to ET-1 by endothelium disruption of pressurized small pulmonary arteries. In this setting, we found that CH augments vasoconstriction to ET-1 at lower concentrations (3 × 10−12 to 10−10 M), although reactivity to ET-1 converges between control and CH groups at higher concentrations (≥3 × 10−10 M). Interestingly, these responses correlate with a marked reduction in ET-1-induced VSM [Ca2+]i mobilization following CH, suggesting a myofilament Ca2+-sensitization mechanism is enhanced in the hypertensive pulmonary circulation. Such divergence between ET-1-dependent vasoconstriction and Ca2+ mobilization is consistent with studies by Shimoda et al. (50), demonstrating that ET-1-induced contraction of pulmonary arterial smooth muscle cells from CH rats occurs by a mechanism primarily independent of changes in [Ca2+]i. The mechanism by which CH inhibits agonist-induced VSM [Ca2+]i mobilization is largely unknown but may involve reduced Ca2+ entry through voltage-gated Ca2+ channels (42, 50). Furthermore, recent data from our laboratory indicate that CH impairs both store-operated and receptor-operated Ca2+ entry in pulmonary VSM (24). An additional unique aspect of our preparation is that we are able to clamp VSM [Ca2+]i with the selective Ca2+ ionophore, ionomycin, while simultaneously measuring vasoconstrictor responses to ET-1. This approach allows us to specifically isolate Ca2+-sensitization mechanisms that regulate vascular tone independent of changes in VSM [Ca2+]i. Following permeabilization, we observed a substantial increase in vasoconstrictor sensitivity to ET-1 in arteries from CH animals compared with controls, thus demonstrating a clear effect of CH to upregulate signaling mechanisms that increase myofilament Ca2+ sensitivity in the pulmonary circulation.
Several protein kinase pathways that inhibit MLCP activity could potentially be responsible for enhanced ET-1-induced Ca2+ sensitization following CH. Consistent with our hypothesis, inhibition of ROK with Y-27632 reduced ET-1-mediated Ca2+ sensitization in arteries from CH rats and normalized responses between groups. However, neither inhibition of PKC nor TK had an effect on ET-1-induced Ca2+ sensitization. These results are similar to recent findings by other investigators (57) demonstrating that contraction induced by ET-1 in intrapulmonary arteries from CH rats requires activation of ROK but not PKC. Furthermore, a recent study by Homma et al. (20) suggests that endogenous ET-1 contributes to increased RhoA/ROK-mediated Ca2+ sensitization in the pulmonary circulation of CH rats. In contrast, TK was found to play an important role in ET-1-induced contraction of intrapulmonary arterial rings from CH rats (57). Moreover, Nagaoka et al. (41) also demonstrated that RhoA/ROK-mediated Ca2+ sensitization is involved in sustained vasoconstriction and increased vasoreactivity in CH-induced pulmonary hypertension but failed to detect a role for either PKC or TK. These studies along with previous findings from our laboratory (26) demonstrating that CH increases basal and stimulated levels of GTP-bound RhoA, ROK expression, and UTP-induced RhoA/ROK Ca2+ sensitization suggest a central role for RhoA/ROK signaling in mediating CH-induced pulmonary hypertension. Our present observations linking ET-1 to ROK-mediated Ca2+ sensitization following CH, together with earlier reports that both ET-1 and ROK play a vital role in the development and maintenance of CH-induced pulmonary hypertension, further support the possibility that ET-1 contributes to pulmonary hypertension through stimulation of RhoA/ROK in pulmonary VSM.
In previous studies from our laboratory (26), inhibition of ROK abolished UTP-induced Ca2+ sensitization in arteries isolated from both control and CH rats. In contrast to these observations, however, Y-27632 attenuated but did not prevent the vasoconstrictor response to ET-1, suggesting either incomplete blockade of ROK by Y-27632 or possible involvement of other kinases. Indeed, it is now known that several different serine-threonine protein kinases, such as the recently described zipper-interacting protein (ZIP) kinase or integrin-linked kinase (ILK; Ref. 23), can catalyze the phosphorylation of MYPT1. It is not clear whether ROK is a proximal mediator of ZIP kinase or ILK signaling in VSM, but each of these kinases (ROK, ZIP kinase, and ILK) can also directly phosphorylate the MLC to increase cross bridge cycling independent of the Ca2+-dependent MLCK (23).
To address the possible contribution of Ca2+-independent MLC phosphorylation to enhanced ET-1-mediated Ca2+ sensitization following CH, we further examined the effect of MLCK inhibition with ML-9 on this response. We found that ML-9 prevented the increase in ET-1 Ca2+ sensitization following CH and normalized responses between groups, similar to ROK inhibition. These results suggest that a basal level of Ca2+-dependent MLCK activity is requisite for this augmented constriction and are consistent with Ca2+ sensitization via inhibition of MLCP mediating enhanced VSM sensitivity to ET-1 following CH, as opposed to a mechanism involving Ca2+-independent phosphorylation of the MLC. However, we cannot exclude the possibility that such inhibitory effects of ML-9 result from nonspecific actions of the compound to inhibit ROK or other relevant kinases (2). Interestingly, as with Y-27632, ML-9 did not abolish ET-1 Ca2+ sensitization in either group. Again, this residual constriction may be due to incomplete inhibition of MLCK or rather direct phosphorylation of the MLC by ZIP kinase, ILK, or ROK.
Further supporting a role for ROK-dependent inhibition of MLCP in mediating ET-1-induced Ca2+ sensitization, we found that ROK inhibition prevented phosphorylation of the MYPT1 subunit of MLCP in response to ET-1. Activation of the RhoA/ROK pathway results in the phosphorylation of at least two distinct residues, Thr696 (pMYPT1Thr696), which inhibits the catalytic activity of MYPT1, and Thr850 (pMYPT1Thr850), which reduces binding of MYPT1 to myosin (36). In control arteries, ET-1 resulted in ROK-dependent phosphorylation of pMYPT1Thr850 but not pMYPT1Thr696. This is similar to recent findings in primary cultures of rat aortic smooth muscle cells, rabbit portal vein, and rabbit vas deferens smooth muscle in which ET-1 had little effect on pMYPT1Thr696 levels but increased pMYPT1Thr850 (31, 58). However, in contrast to control arteries, ET-1 stimulation of pulmonary arteries from CH animals resulted in ROK-dependent phosphorylation of pMYPT1Thr696 but not pMYPT1Thr850. Although phosphorylation of either residue has been correlated with diminished phosphatase activity, CH appears to mediate a shift in ET-1-induced, ROK-dependent phosphorylation of MYPT1 from Thr850 to Thr696. Whether this switch in targeting of MYPT1 phosphorylation sites contributes to the changes in VSM Ca2+ sensitivity following CH remains to be determined.
In addition to differences in the phosphorylation of MYPT1 by ET-1, we also observed marked changes in the receptor subtype responsible for mediating ET-1-induced Ca2+ sensitization between control and CH vessels. Whereas ETBR blockade provided a slight but significant inhibition in arteries from both groups, ETAR inhibition attenuated vasoconstriction only in arteries from CH animals. These data suggest that greater ROK-dependent Ca2+ sensitization following CH is predominantly mediated by the ETAR. This observation is consistent with recent findings demonstrating that stimulation of ETAR, but not ETBR, is involved in RhoA activation in rabbit basilar arteries (39), rat bronchial smooth muscle (6), and rabbit circular and longitudinal smooth muscle (19). It is unknown how CH mediates this shift to an ETAR- and ROK-dependent Ca2+-sensitization response to ET-1, however, it may involve an upregulation of ETAR expression (33) or rather an uncoupling of the receptor subtypes that results in a change in distal signaling mechanisms. Indeed, recent studies in HEK-293 cells demonstrated, by use of immunoprecipitation and fluorescence resonance energy transfer (FRET), that the ETAR and ETBR form heterodimers (15). In addition, Gregan et al. (15) showed that prolonged incubation with an ETBR agonist resulted in the dissociation of the ETAR/ETBR heterodimers. Furthermore, it has been postulated that ETAR/ETBR heterodimers exist in renal (12, 22) and pulmonary VSM (49).
Similar to the predominant role of the ETAR in activation of RhoA, it appears that the ETAR, but not the ETBR, is coupled to O2− production (12, 32, 34, 35). Hypoxia increases vascular ROS synthesis (16, 30, 55), and ET-1 has been shown to be a potent activator of O2− generation in VSM (34, 56). A recent study by Jin et al. (29) suggests that ROS-induced Ca2+ sensitization in rat aorta is mediated by activation of RhoA and a subsequent increase in ROK activity but not Ca2+-independent PKC activity. Therefore, we hypothesized that ROS mediate enhanced ROK-dependent Ca2+ sensitization following CH. Consistent with this hypothesis, we found that the O2− scavenger tiron inhibited ET-1-induced Ca2+ sensitization in CH arteries similar to that observed following ROK inhibition. In addition, the increased basal and ET-1-stimulated DHE fluorescence observed in CH arteries was normalized to that of control vessels by pretreatment with tiron, suggesting that CH increases basal O2− and facilitates ET-1 generated ROS.
We next questioned whether increased ROS generation following CH mediates elevated basal and ET-1-induced RhoA activity in pulmonary arteries. Supporting this possibility is evidence that ROS can enhance GDP release from Rho GTPases and thus modulate their activity (18). The redox sensitivity of these Rho GTPases is due to the presence of a redox-active motif (18). To address whether ROS activate RhoA, we examined basal and stimulated levels of GTP-bound RhoA in the presence or absence of tiron in intrapulmonary arteries from control and CH rats. Similar to previous results (26), we found that CH increased both basal and ET-1 stimulated RhoA activity. Furthermore, these responses to CH were abolished by pretreatment with tiron, thus demonstrating a novel role for endogenous ROS to mediate increased basal and ET-1-stimulated RhoA activity following CH.
VSM ROS production appears to be important in ET-1-mediated Ca2+ sensitization since tiron diminished responses in endothelium-denuded CH arteries in our preparation. However, whether ROS are generated by NADPH oxidase, xanthine oxidase, or the mitochondrial respiratory chain remains to be determined. Inhibitors of each of these sources have been shown to attenuate ROS-induced RhoA activation (1, 28, 29). Furthermore, a recent study by Liu et al. (37) demonstrates that CH (10% O2 for 3 wk) causes a significant increase in O2− production in intrapulmonary arteries of wild-type mice as measured by lucigenin-enhanced chemiluminescence, whereas this response to CH is absent in NADPH oxidase (gp91phox) knockout mice. Interestingly, they showed that the pathological changes associated with CH-induced pulmonary hypertension (elevated mean right ventricular pressure, medial wall thickening of small pulmonary arteries, and right heart hypertrophy) are completely abolished in NADPH oxidase (gp91phox) knockout mice, suggesting that NADPH oxidase provides the major source of ROS that contribute to CH-induced pulmonary hypertension (37). Further investigation is needed to identify the source and mechanistic basis for both elevated basal and ET-1-stimulated ROS production in arteries from CH rats.
Consistent with recent evidence that CH induces ROK-dependent myogenic tone in small pulmonary arteries (5) are findings from the present study that lower concentrations of ET-1 caused pronounced vasodilation in endothelium-intact arteries from CH rats but not controls. Whether this response to ET-1 reflects a reduction in CH-induced basal tone resulting from ETBR-mediated NO synthesis (25) remains to be determined. However, such a contribution of NO could account for the coincident fall in VSM [Ca2+]i in response to ET-1 in CH arteries. A role for ROK in mediating CH-induced basal vasomotor tone is further supported by our current observations that Y-27632 elicited a substantial vasodilatory response under basal conditions only in endothelium-intact arteries from CH rats and prevented ET-1-mediated vasodilation. Interestingly, this vasodilatory response to ET-1 was similarly prevented by tiron, thus providing intriguing evidence for a critical contribution of O2− to ROK-dependent myogenic reactivity following CH.
Despite abundant evidence for a contribution of ET-1 to the development of pulmonary hypertension in rats (4, 7, 10), our present findings that CH enhances ET-1-mediated vasoconstriction only at higher concentrations in endothelium-intact arteries may question the relevance of ET-1 to the vasoconstrictor component of CH-induced pulmonary hypertension. However, caution should be exercised in interpreting responses of isolated arteries to exogenous ET-1 in the context of the intact animal. Indeed, the local concentrations of ET-1 to which the VSM is exposed in vivo are presently unknown. Furthermore, it is possible that endogenous ET-1 is preferentially released toward the VSM, which could minimize endothelium-dependent vasodilatory influences of ET-1 on VSM tone. Nevertheless, it is becoming increasingly evident that ROK-mediated vasoconstriction plays a central role in the pulmonary hypertensive response to CH in rats (3, 17, 26, 41). It is perhaps likely that other receptor-mediated agonists that signal through ROK but lack the profound endothelium-dependent vasodilatory capacity of ET-1 (20, 46), along with inherent changes of the VSM that lead to ROK-dependent myogenic tone (5, 41), function synergistically to promote vasoconstriction in the hypertensive pulmonary vasculature. Future studies are therefore necessary to evaluate the contribution of ROS-mediated RhoA activation to CH-induced myogenicity and enhanced vasoreactivity to other agonists that signal through this pathway.
In conclusion, the present study demonstrates a novel role for CH-induced ROS generation to augment ET-1-mediated pulmonary VSM Ca2+ sensitization through upregulation of RhoA/ROK signaling. This response to CH may have important implications for the regulation of pulmonary vascular resistance and resultant pulmonary hypertension associated with CH.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-77876 and HL-88192 to T. C. Resta, HL-58124 and HL-07736 to B. R. Walker, and HL-92598 to N. L. Jernigan and the American Heart Association Grant-In-Aid 0755775Z to T. C. Resta.
Acknowledgments
We thank Drs. Yuansheng Gao and J. Usha Raj at the University of California Los Angeles for their assistance with pMYPT1 Western blotting protocols; Dr. Ed Bedrick, Professor of Mathematics and Statistics at the University of New Mexico, for assistance with statistical analyses; Charles Norton for assistance in tissue sample preparation for Western analysis; and Minerva Murphy for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol 289: H243–H250, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J 371: 199–204, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barman SA Vasoconstrictor effect of endothelin-1 on hypertensive pulmonary arterial smooth muscle involves Rho-kinase and protein kinase C. Am J Physiol Lung Cell Mol Physiol 293: L472–L479, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bonvallet ST, Zamora MR, Hasunuma K, Sato K, Hanasato N, Anderson D, Sato K, Stelzner TJ. BQ123, an ETA-receptor antagonist, attenuates hypoxic pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol 266: H1327–H1331, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 294: L797–L806, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Chiba Y, Sakai H, Misawa M. Endothelin-1-induced translocation of RhoA is mediated by endothelin ET(A) receptors in rat bronchial smooth muscle. Eur J Pharmacol 517: 182–185, 2005. [DOI] [PubMed] [Google Scholar]
- 7.DiCarlo VS, Chen SJ, Meng QC, Durand J, Yano M, Chen YF, Oparil S. ETA-receptor antagonist prevents and reverses chronic hypoxia-induced pulmonary hypertension in rat. Am J Physiol Lung Cell Mol Physiol 269: L690–L697, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49: 717–727, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eddahibi S, Raffestin B, Braquet P, Chabrier PE, Adnot S. Pulmonary vascular reactivity to endothelin-1 in normal and chronically pulmonary hypertensive rats. J Cardiovasc Pharmacol 17, Suppl 7: S358–S361, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Eddahibi S, Raffestin B, Clozel M, Levame M, Adnot S. Protection from pulmonary hypertension with an orally active endothelin receptor antagonist in hypoxic rats. Am J Physiol Heart Circ Physiol 268: H828–H835, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Elton TS, Oparil S, Taylor GR, Hicks PH, Yang RH, Jin H, Chen YF. Normobaric hypoxia stimulates endothelin-1 gene expression in the rat. Am J Physiol Regul Integr Comp Physiol 263: R1260–R1264, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Fellner SK, Arendshorst W. Endothelin-A and -B receptors, superoxide, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol 292: F175–F184, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Portugal AD, Negash S, Zhou W, Longo LD, Usha Raj J. Role of Rho kinases in PKG-mediated relaxation of pulmonary arteries of fetal lambs exposed to chronic high altitude hypoxia. Am J Physiol Lung Cell Mol Physiol 292: L678–L684, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Girgis RE, Mozammel S, Champion HC, Li D, Peng X, Shimoda L, Tuder RM, Johns RA, Hassoun PM. Regression of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Lung Cell Mol Physiol 292: L1105–L1110, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Gregan B, Jurgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem 279: 27679–27687, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Grishko V, Solomon M, Breit JF, Killilea DW, Ledoux SP, Wilson GL, Gillespie MN. Hypoxia promotes oxidative base modifications in the pulmonary artery endothelial cell VEGF gene. FASEB J 15: 1267–1269, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Guilluy C, Sauzeau V, Rolli-Derkinderen M, Guerin P, Sagan C, Pacaud P, Loirand G. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br J Pharmacol 146: 1010–1018, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heo J, Campbell SL. Superoxide anion radical modulates the activity of Ras and Ras-related GTPases by a radical-based mechanism similar to that of nitric oxide. J Biol Chem 280: 12438–12445, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hersch E, Huang J, Grider JR, Murthy KS. Gq/G13 signaling by ET-1 in smooth muscle: MYPT1 phosphorylation via ETA and CPI-17 dephosphorylation via ETB. Am J Physiol Cell Physiol 287: C1209–C1218, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Homma N, Nagaoka T, Morio Y, Ota H, Gebb SA, Karoor V, McMurtry IF, Oka M. Endothelin-1 and serotonin are involved in activation of RhoA/Rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharmacol 50: 697–702, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Inscho E, Imig J, Cook A, Pollock DM. ETA and ETB receptors differentially modulate afferent and efferent arteriolar responses to endothelin. Br J Pharmacol 146: 1019–1026, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito M, Nakano T, Erdodi F, Hartshorne D. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem 259: 197–209, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Jernigan NL, Broughton BR, Walker BR, Resta TC. Impaired NO-dependent inhibition of store- and receptor-operated calcium entry in pulmonary vascular smooth muscle after chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 290: L517–L525, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Jernigan NL, Resta TC, Walker BR. Contribution of oxygen radicals to altered NO-dependent pulmonary vasodilation in acute and chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 286: L947–L955, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Jernigan NL, Walker BR, Resta TC. Chronic hypoxia augments protein kinase G-mediated Ca2+ desensitization in pulmonary vascular smooth muscle through inhibition of RhoA/Rho kinase signaling. Am J Physiol Lung Cell Mol Physiol 287: L1220–L1229, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Jernigan NL, Walker BR, Resta TC. Endothelium-derived reactive oxygen species and endothelin-1 attenuate NO-dependent pulmonary vasodilation following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L801–L808, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Jin L, Ying Z, Hilgers RH, Yin J, Zhao X, Imig JD, Webb RC. Increased RhoA/Rho-kinase signaling mediates spontaneous tone in aorta from angiotensin II-induced hypertensive rats. J Pharmacol Exp Ther 318: 288–295, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol 287: H1495–H1500, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Killilea DW, Hester R, Balczon R, Babal P, Gillespie MN. Free radical production in hypoxic pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 279: L408–L412, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol 546: 879–889, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laplante M, Wu R, Moreau P, de Champlain J. Endothelin mediates superoxide production in angiotensin II-induced hypertension in rats. Free Radic Biol Med 38: 589–596, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Elton TS, Chen YF, Oparil S. Increased endothelin receptor gene expression in hypoxic rat lung. Am J Physiol Lung Cell Mol Physiol 266: L553–L560, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF. Endothelin-1 increases vascular superoxide via endothelinA-NADPH oxidase pathway in low-renin hypertension. Circulation 107: 1053–1058, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Watts SW, Banes AK, Galligan JJ, Fink GD, Chen AF. NADPH oxidase-derived superoxide augments endothelin-1-induced venoconstriction in mineralocorticoid hypertension. Hypertension 42: 316–321, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Lincoln TM Myosin phosphatase regulatory pathways: different functions or redundant functions? Circ Res 100: 10–12, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006. [DOI] [PubMed] [Google Scholar]
- 38.MacLean MR, McCulloch KM, Baird M. Effects of pulmonary hypertension on vasoconstrictor responses to endothelin-1 and sarafotoxin S6C and on inherent tone in rat pulmonary arteries. J Cardiovasc Pharmacol 26: 822–830, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Miao L, Dai Y, Zhang J. Mechanism of RhoA/Rho kinase activation in endothelin-1-induced contraction in rabbit basilar artery. Am J Physiol Heart Circ Physiol 283: H983–H989, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Muramatsu M, Oka M, Morio Y, Soma S, Takahashi H, Fukuchi Y. Chronic hypoxia augments endothelin-B receptor-mediated vasodilation in isolated perfused rat lungs. Am J Physiol Lung Cell Mol Physiol 276: L358–L364, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Naik JS, Earley S, Resta TC, Walker BR. Pressure-induced smooth muscle cell depolarization in pulmonary arteries from control and chronically hypoxic rats does not cause myogenic vasoconstriction. J Appl Physiol 98: 1119–1124, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Ohanian J, Ohanian V, Shaw L, Bruce C, Heagerty AM. Involvement of tyrosine phosphorylation in endothelin-1-induced calcium-sensitization in rat small mesenteric arteries. Br J Pharmacol 120: 653–661, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. FASEB J 9: 1196–1204, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Resta TC, Walker BR. Chronic hypoxia selectively augments endothelium-dependent pulmonary arterial vasodilation. Am J Physiol Heart Circ Physiol 270: H888–H896, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Resta TC, Chicoine LG, Omdahl JL, Walker BR. Maintained upregulation of pulmonary eNOS gene and protein expression during recovery from chronic hypoxia. Am J Physiol Heart Circ Physiol 276: H699–H708, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Sato A, Hattori Y, Sasaki M, Tomita F, Kohya T, Kitabatake A, Kanno M. Agonist-dependent difference in the mechanisms involved in Ca2+ sensitization of smooth muscle of porcine coronary artery. J Cardiovasc Pharmacol 35: 814–821, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Sauvageau S, Thorin E, Caron A, Dupuis J. Endothelin-1-induced pulmonary vasoreactivity is regulated by ETA and ETB receptor interactions. J Vasc Res 44: 375–381, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. L-type Ca2+ channels, resting [Ca2+]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol 279: L884–L894, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Stenmark KR, McMurtry IF. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension: a time for reappraisal? Circ Res 97: 95–98, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Tsien R, Pozzan T. Transport: Membrane Isolation and Characterization. San Diego, CA: Academic Press, 1989.
- 53.Wang X, Tong M, Chinta S, Raj JU, Gao Y. Hypoxia-induced reactive oxygen species downregulate ETB receptor-mediated contraction of rat pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 290: L570–L578, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Wanstall J, O'Donnell S. Endothelin and 5-hydroxytryptamine on rat pulmonary artery in pulmonary hypertension. Eur J Pharmacol 176: 159–168, 1990. [DOI] [PubMed] [Google Scholar]
- 55.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res 88: 1259–1266, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Wedgwood S, Dettman RW, Black SM. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 281: L1058–L1067, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Weigand L, Sylvester JT, Shimoda LA. Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 290: L284–L290, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Woodsome TP, Polzin A, Kitazawa K, Eto M, Kitazawa T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J Cell Sci 119: 1769–1780, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Wu W, Platoshyn O, Firth AL, Yuan JX. Hypoxia divergently regulates production of reactive oxygen species in human pulmonary and coronary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 293: L952–L959, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Yang W, Block E. Effect of hypoxia and reoxygenation on the formation and release of reactive oxygen species by porcine pulmonary artery endothelial cells. J Cell Physiol 164: 414–423, 1995. [DOI] [PubMed] [Google Scholar]
- 61.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34: 1359–1368, 2003. [DOI] [PubMed] [Google Scholar]