Abstract
Mediators of angiogenesis such as VEGFs and angiopoietins may regulate pulmonary vascular permeability under normal and pathological conditions. Ephrin family receptor tyrosine kinases are expressed in the vasculature and also regulate angiogenesis under some circumstances, but whether they also modulate lung vascular permeability is unknown. We hypothesized that stimulation of lung endothelial EphA receptors with ephrin-a1 ligand would alter pulmonary vascular permeability and tested this idea in vivo and in vitro. We found that ephrin-a1 ligand and EphA2 receptors are expressed in distal normal lung vasculature and that their expression is increased in injured lung, suggesting a link to mechanisms of increased permeability. Intravenous injection of ephrin-a1 caused a large increase in the leakage of labeled albumin into the lungs of rats within 30 min (293 ± 27 vs. 150 ± 6 ng/mg dry lung, P < 0.01), along with histological evidence of the formation of endothelial disruptions. In cultured lung vascular endothelial cells, stimulation with ephrin-a1 increased monolayer permeability by 44% (P < 0.01), a permeability change similar to that seen with VEGF stimulation of the same cells. Ephrin-a1 stimulation in vivo and in vitro was associated with histological evidence for disruptions of tight and adherens junctions. These observations describe a novel role for ephrin-a1 and EphA receptors in the regulation of vascular permeability in the lung.
Keywords: ephrin, EphA2, pulmonary edema
control of pulmonary vascular permeability is a critical factor in the proper functioning of the lung, but the mechanisms regulating permeability remain incompletely understood. One interesting area that has drawn recent attention is the possible link between angiogenesis and vascular permeability (18). Current concepts of angiogenesis suggest that this process requires initial disruption followed by repair and stabilization of the endothelial barrier, which in turn suggests that “proangiogenic” mediators may have endothelial barrier regulatory properties. A growing body of evidence indicates that this is indeed the case.
Three major families of receptor tyrosine kinases and ligands have been described to control the process of angiogenesis: VEGFs, angiopoietins, and ephrins. Members of two of these molecular families, VEGFs and angiopoietins, have been implicated in the control of vascular permeability and in the pathophysiology of human and experimental lung injuries (15, 17, 22, 26). Although several ephrins are known to be expressed in pulmonary vascular cells, their physiological role in the lung is not known, and, in particular, whether ephrin family members regulate pulmonary vascular permeability has not been investigated. However, the EphA2 receptor and its cognate ligand ephrin-a1 have been reported to mediate some aspects of angiogenesis, such as vascular assembly and endothelial cell migration, in lung microvascular endothelial cells (5). These findings suggested to us that “A”-class ephrins might also have a role in the control of endothelial permeability.
Most evidence indicates that endothelial barrier function in the lung and other organs is regulated in large part by the state of intracellular junctions between endothelial cells (1). Tight junctions are thought to form the principal barrier to paracellular fluid flux in vascular endothelium, with the integrity of the barrier dependent to a large extent on the expression and assembly of claudin family proteins. Adherens junctions also play an important role in mediating endothelial permeability changes, and disruption of these junctions has been shown to lead to microvascular damage in vivo (7). Of particular interest, a recent study demonstrated that EphA2 can directly phosphorylate claudin-4 in epithelial cells, leading to alterations in epithelial barrier function (25), and interactions between EphA2 and epithelial cadherins also have been described (8). Whether similar interactions occur in endothelial cells is unknown.
We hypothesized, then, that activation of EphA receptors by ephrin-a1 ligand in the pulmonary vasculature would lead to increases in endothelial permeability, possibly as a result of tight and adherens junction rearrangements. We investigated this possibility in cultured endothelial cells and in intact rats.
METHODS
Isolation and characterization of lung microvascular endothelial cells.
Human lung microvascular endothelial cells (hLMVEC) were purchased from Lonza (Walkersville, MD) and propagated per their instructions. Bovine pulmonary artery endothelial cells (bPAEC) were isolated from the lungs of 2-wk-old male Holstein calves. Briefly, small (1- to 5-μm-diameter) intrapulmonary arteries were manually dissected free of the lung and opened longitudinally, and the luminal surface was gently scraped. To reduce contamination with other cell types, the luminal scrapings were incubated with a bovine-reactive rabbit anti-vascular endothelial (VE)-cadherin antibody (Alexis, Lausanne, Switzerland) and magnetic microspheres coated with anti-rabbit antibody (Pierce, Rockford, IL) and then isolated using magnetic separation. Isolated cells were plated on 1% gelatin and further purified by selective isolation of groups of cells with classic cobblestone endothelial morphology using trypsin-soaked cloning disks. Endothelial cells were verified to be positive for diacylated LDL uptake and characterized by immunostaining as negative for smooth muscle myosin and smooth muscle α-actin and positive for the endothelial cell markers VEGF receptor type 2, Tie-2, and VE-cadherin (data not shown).
RT-PCR studies.
To determine which ephrin ligands and receptors are expressed by cultured bPAEC, we isolated RNA from quiescent cells grown on 1% gelatin as described above. Total cell RNA was reverse transcribed using oligo(dT) priming, and the resulting cDNA was amplified using primers specific for ephrin receptors and ligands and cycling conditions as described in Table 1.
Table 1.
Primers for PCR of ephrins
| Target |
Primer |
Annealing Temp, °C | ||
|---|---|---|---|---|
| Forward | Reverse | |||
| Bovine | ||||
| Ephrin-a1 | CCTGGAATCATCTGTCCTCATTACG | AAACCCTGTGAAGCGGTGGAAC | 57 | |
| Ephrin-a2 | ACTGGTGAAGATGGGCTCTGG | TCCCTGGGCTTTGAGTTCCG | 57 | |
| Ephrin-a5 | GATGTTGACGCCGCTGTTTC | TCCCACCTCTTGAACCCTTTGG | 57 | |
| EphA1 | TTTGGGGAAGTGTATCGGGG | AAGGCTCCATTCTCCATAAACTCC | 57 | |
| EphA2 | ACCCTGGCTGATTTTGACCC | ATCCTCTTGATGTCGTCGTTGG | 59 | |
| EphA3 | GCCAGCGATGTATGGAGTTACG | GGGTCTGTTGTTTCTGTCTTTCTGC | 56 | |
| EphA4 | GAACAACTGGCTGCGAACTGAC | AGCACCCACATCCTGAAAAGC | 50 | |
| EphA5 | GCTGACACTGGTGGAAGGAAAG | CATTACCTGCCTCAATCTCAAAGG | 55 | |
| EphA6 | CTGAAATGGATGAACACAACAGGC | ACCAAGGAGGAAGAATCAACCC | 55 | |
| Human | ||||
| Ephrin-a1 | CAAAATCACTCACAGTCCTCAGGC | GAGCGATGCTATGTAGAACCCG | 57 | |
| EphA2 | TGCCAGTGTCAGCATCAACCAG | AGTCTCCCTTCTTGCGGTAAGTG | 59 | |
| EphA5 | TGACCTTGGAGGATTTGAGACG | TGGCACCATTCCGTTTACCAGC | 55 | |
| EphA6 | TGGAGCCATTCTGGACTACGAG | AGACCTGTGATGATGACACTGGG | 53 | |
Lung injury studies.
The pulmonary vascular changes seen with the combination of recent viral infection and hypoxic exposure have been previously described (3). Sections from zinc formalin-fixed, paraffin-embedded lungs of those animals were immunostained with antibodies specific for ephrin-a1 and EphA2 (Santa Cruz Biotechnologies, Santa Cruz, CA) using standard techniques.
Bleomycin lung injury was generated as previously described (10, 20). Briefly, bleomycin (4 U/kg) was instilled via the oropharynx directly into the trachea of lightly anesthetized rats under direct vision. After 4 days, animals were killed, and the lungs were inflation fixed via the trachea with zinc formalin. Paraffin sections were immunostained as described above.
Experimental animals were pathogen-free weanling male Sprague-Dawley rats purchased from a commercial vendor (Harlan Sprague-Dawley, Indianapolis, IN). All animals were allowed free access to food and water and were subjected to a similar day-and-night light cycle. Animals were housed at Denver altitude (1,600 m) at all times. The University of Colorado Health Sciences Center Institutional Animal Care and Use Committee approved all procedures and animal use.
In vitro permeability studies.
bPAEC were seeded onto gelatin-coated Transwell inserts (0.4-μm pore size; Becton Dickinson, Franklin Lakes, NJ) placed in 24-well plates. Cells were grown to confluence in medium containing 10% FBS and then serum starved overnight in medium with 0.1% FBS before study. Transendothelial electrical resistance (TEER) was measured using a resistance meter (EVOM, World Precision Instruments, Sarasota, FL). Only inserts displaying >3.5 ohm·cm2 baseline resistance were used for permeability studies. Cells were stimulated with preclustered ephrin-a1-Fc or Fc alone at a concentration of 2.5 μg/ml, and TEER was measured serially over time.
For measurements of the movement of FITC-labeled dextran, bPAEC were grown on gelatin-coated Transwell inserts in a six-well format. Cells were grown to confluence in medium containing 10% FBS and then serum starved overnight in medium with 0.1% FBS before study. At the start of the assay, culture medium on both sides of the insert was changed to Optimem, and FITC-conjugated dextran (70 kDa; Sigma) was added to the upper chamber of the insert at a final concentration of 10 μM. After 30 min of equilibration, preclustered ephrin-a1-Fc (2.5 μg/ml) or control Fc was added to the insert, and medium from the lower chamber was collected 2 h later. The concentration of FITC in the lower chamber was measured in a benchtop fluorometer.
Evans blue dye-labeled albumin extravasation.
For measurements of vascular albumin extravasation in the lung, 5-wk-old Sprague-Dawley rats were anesthetized with ketamine and xylazine and then injected via the tail vein with preclustered ephrin-a1-Fc (50 μg/kg) or preclustered human Fc fragments in 0.9% saline. Ephrin-a1-Fc was allowed to circulate for 15, 30, or 60 min (n = 5 per group). Leakage of albumin out of the vasculature in the distal lung was then measured using Evans blue dye, as previously described (4).
Lectin labeling of sites of vascular leak.
For identification of the anatomic sites of vascular leak in the ephrin-a1-treated animals, rats (n = 4 per group) were anesthetized and injected with ephrin-a1-Fc or Fc control as described above. After 30 min, the chest was opened and the lungs were perfused at 20 cmH2O pressure via the pulmonary artery with PBS until clear of blood. The lungs were perfused for 5 min with 2% paraformaldehyde in PBS, flushed with PBS alone, and then perfused with rhodamine-conjugated Ricinus communis 1 (RCA) lectin (20 μg/ml) diluted in PBS. The RCA lectin was flushed out of the lungs with PBS after 10 min of incubation, and the lungs were inflated via the trachea with optimal cutting temperature compound (OCT) and stored at −70°C until cryosectioning. For examination, 15-μm-thick frozen sections were cut, and the rhodamine-conjugated RCA was visualized using a fluorescence microscope and appropriate filters.
Immunofluorescence microscopy.
Immunofluorescent studies of rat lungs for claudin-5 and VE-cadherin were performed on cryosections prepared as described above. Sections were rinsed in PBS, permeabilized in PBS + 0.1% Triton X-100 for 10 min, blocked with 2.5% bovine albumin in PBS for 10 min, and incubated in primary antibody diluted with PBS for 2 h at room temperature and then in fluorescent secondary antibody for 45 min. Sections were mounted in PBS-glycerol for examination.
For in vitro immunofluorescence studies, cells were cultured to confluence on chamber slides, stimulated with 2.5 μg/ml preclustered ephrin-a1-Fc for 30 min, and fixed in ice-cold methanol for 5 min. Cells were then permeabilized in PBS + 0.1% Triton X-100 for 2 min, blocked with 1% bovine albumin, and incubated with primary and secondary antibodies: rabbit anti-claudin-5 (Labvision, Fremont, CA) and goat anti-VE-cadherin (Santa Cruz Biotechnologies) and anti-rabbit Alexa 555 and anti-goat Alexa 488 (Invitrogen, Carlsbad, CA).
Cell fractionation studies.
To determine whether VE-cadherin or claudin-5 was shifting compartments within the cell after ephrin-a1 stimulation, hLMVEC were stimulated with ephrin-a1-Fc (2.5 μg/ml) for 0, 10, or 30 min and then lysed and fractionated using a commercial cell fractionation kit (Proteoextract S-PEK kit, Calbiochem, San Diego, CA). Membrane and cytoskeletal fractions were separated by standard Western blotting techniques, and the resulting blots were probed with antibodies for VE-cadherin and claudin-5. Experiments were repeated three times with similar results.
Statistical analysis.
Values are means ± SE unless otherwise noted. Comparisons between multiple groups were analyzed with one-way ANOVA and Tukey's multiple-comparison post testing. Statistical analyses were done with Prism 4.0 statistical analysis software (GraphPad Software, San Diego, CA). Results were considered significant when P < 0.05.
RESULTS
Expression of EphA receptors and ligands in the pulmonary vasculature.
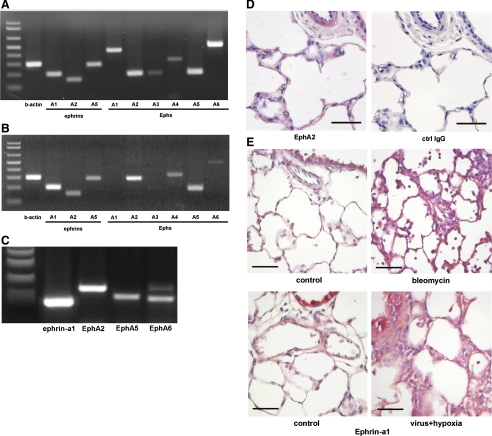
The expression of A-class ephrin ligands and EphA receptors in pulmonary vascular tissues remains relatively unexplored. To determine whether these molecules are expressed in locations consistent with a role in regulating permeability, we surveyed mRNA expression of A-class ephrins by RT-PCR in normal peripheral bovine lung tissue, as well as cultured bPAEC and hLMVEC. Our results showed that ephrin-a1, ephrin-a2, and ephrin-a5 mRNAs are expressed in the lung, as are mRNAs for EphA1, EphA2, EphA5, EphA6, and, to a lesser extent, EphA3 and EphA4 (Fig. 1A). Cultured bPAEC also expressed mRNA for ephrin-A ligands and EphA receptors, although in a more restricted pattern (Fig. 1B). In bPAEC, the predominant ephrin ligand transcript expressed was ephrin-a1, and EphA2 was the predominant Eph receptor. Cultured hLMVEC also express transcripts for ephrin-a1, EphA2, and, to a lesser extent, EphA5 and EphA6. (Fig. 1C).
Fig. 1.
Ephrin-a ligands and EphA receptors are expressed in normal and injured lungs. Multiple ephrin-a ligand and EphA receptor mRNAs are expressed in normal calf lung tissue (A), cultured bovine pulmonary artery endothelial cells (bPAEC, B), and cultured human lung microvascular endothelial cells (hLMVEC, C), as shown by RT-PCR. D: EphA2 protein (red) expression in microvasculature of normal distal calf lung. Ctrl, control. E: ephrin-a1 (red) expression in distal lung of normal rats. Note increased expression in inflamed areas associated with bleomycin- or hypoxia + viral infection-induced lung injury. Scale bar, 50 μm.
To verify the results of the mRNA analysis, immunohistochemical staining for ephrin-a1 and EphA2 was performed on paraffin sections of normal bovine and rat lung tissue (Fig. 1, D and E). These experiments demonstrated the presence of EphA2 in airway epithelium, vascular and airway smooth muscle, and the alveolar wall. In addition, sections of normal rat lung (Fig. 1E) demonstrate weak-to-moderate ephrin-a1 immunoreactivity in the alveolar wall, consistent with previous reports of ephrin-a1 expression in distal lung endothelial cells. Together with the PCR results, these results demonstrate that ephrin-A ligands and EphA receptors are expressed in the distal vasculature of the normal postnatal lung in anatomic locations, consistent with a role in regulating the endothelial barrier.
To determine whether the presence of ephrins in the pulmonary circulation could be of relevance to lung injuries, we also stained lung tissues from rats exposed to two previously described lung injury models. The early phase of bleomycin-induced lung injury is characterized by lung edema and increased vascular permeability (10, 20). As shown in Fig. 1E, lungs from animals 4 days after intratracheal bleomycin administration show a marked increase in ephrin-a1 and EphA2 protein expression in inflamed areas of lung parenchyma compared with control animals. We also studied animals exposed to the combination of a recent viral infection and hypoxia, a stimulus previously shown to increase vascular permeability and trigger early pulmonary edema formation (3, 4). As with bleomycin, the lungs injured by viral infection + hypoxia also demonstrated clear increases in ephrin-a1 and EphA2 immunostaining, most notably in areas with thickened edematous alveolar septae. These results suggest that ephrin-a1 and EphA2 are upregulated in the injured lung and that control of endothelial permeability by ephrins could have pathophysiological significance.
Ephrin-a1 increases lung albumin extravasation.
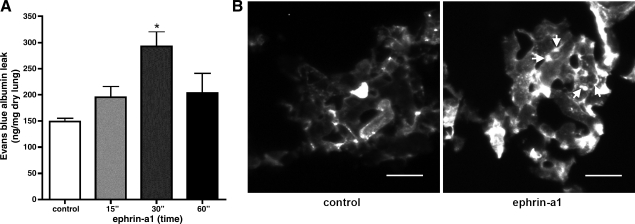
To determine whether activation of EphA receptors by ephrin-a1 ligand alters pulmonary vascular permeability in vivo, we measured the extravasation of labeled intravascular albumin into the distal rat lung over a time course following intravenous injection with the soluble ephrin ligand ephrin-a1-Fc. As shown in Fig. 2A, ephrin-a1-Fc caused a marked increase in labeled albumin extravasation into the lung by 30 min after injection compared with animals injected with control Fc fragment alone (293 ± 27 vs. 150 ± 6 ng/mg dry lung, P < 0.01). The albumin leak appeared to be subsiding by 60 min after injection but remained elevated in some animals (220 ± 40 ng/mg dry lung, P = 0.14 vs. untreated controls).
Fig. 2.
Ephrin-a1 increases endothelial permeability in intact rat lung. A: ephrin-a1 ligand injection (15, 30, or 60 min) increases labeled albumin extravasation into the distal lung. Values are means ± SE (n = 4 per group). *P = 0.01. B: endothelial disruptions (arrows) in distal lung following ephrin-a1-Fc injection as visualized by Ricinus communis (RCA) lectin (white) staining. Scale bar, 25 μm.
For determination of the anatomic site of the albumin leak, rats were injected with ephrin-a1-Fc ligand or Fc control, and, 30 min later, sites of endothelial disruption were marked by vascular perfusion with rhodamine-conjugated RCA lectin, a glycoprotein that binds weakly to intact endothelium but avidly to exposed basement membrane (7). The lungs of control animals demonstrated diffuse weak labeling of the vascular endothelium. In animals injected with ephrin-a1-Fc, however, discreet patches of intense lectin labeling were noted in the alveolar microvasculature, mostly in a pattern suggestive of gaps in cell-cell boundaries (Fig. 2B). No such labeling was seen in extra-alveolar vessels, suggesting that in vivo the predominant site of endothelial disruption in the lungs associated with ephrin-a1 injection is in the alveolar microvasculature.
Ephrin-a1 increases endothelial monolayer permeability in vitro.
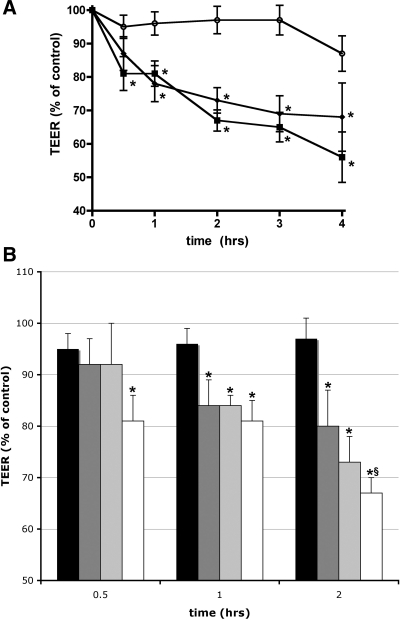
We next investigated whether activation of EphA receptors by ephrin-a1 ligand binding alters vascular endothelial permeability in vitro. bPAEC were grown on gelatin-coated Transwell inserts, and TEER was measured as a marker of endothelial permeability before and after exposure to ephrin-a1-Fc (2.5 μg/ml). By 30 min after ephrin-a1 stimulation, TEER had dropped to 80% of baseline values (P < 0.01 vs. control cells) and by 4 h to 56% of baseline values (P < 0.01 vs. control cells; Fig. 3). For comparison, duplicate inserts were treated with VEGF (100 ng/ml), which caused a reduction in TEER to 78% of control values at 1 h and 68% at 4 h. Experiments using hLMVEC yielded similar results, with ephrin-a1-Fc causing a 50% reduction in TEER compared with control after 2 h of stimulation (P < 0.01, n = 4). Additional experiments (Fig. 3B) with bPAEC demonstrated that the effect of ephrin-a1 on TEER was dose dependent. Ephrin-a1-Fc concentrations of 0.5 or 1.5 μg/ml did not significantly change permeability by 30 min, while 2.5 μg/ml did, and by 2 h after stimulation, all three concentrations of ephrin-a1 caused significant increases in permeability, with greater effect at higher concentrations. Finally, as an alternate measure of permeability, macromolecular movement of FITC-labeled dextran (70 kDa) across bPAEC monolayers was also measured in response to stimulation with ephrin-a1-Fc. Dextran movement across the monolayer increased by 49% (P = 0.02 vs. control cells) after ephrin-a1-Fc stimulation compared with Fc control alone. These findings demonstrate that stimulation with ephrin-a1 ligand causes a substantial increase in the permeability of cultured endothelial monolayers.
Fig. 3.
Ephrin-a1 increases endothelial permeability in cultured bPAEC. A: decrease in monolayer resistance [transendothelial electrical resistance (TEER)] over time following ephrin-a1 (2.5 μg/ml) or VEGF (100 ng/ml) stimulation. ○, Control cells; ▪, ephrin-a1-stimulated cells; ⧫, VEGF-stimulated cells. Values are means ± SE (n = 8 per group). *P < 0.01 vs. control. B: effect of ephrin-a1 on TEER is dose dependent. Solid bars, control; dark-gray bars, 0.5 μg/ml ephrin-a1; light-gray bars, 1.5 μg/ml ephrin-a1; open bars, 2.5 μg/ml ephrin-a1. *P < 0.05 vs. control. §P < 0.05 vs. 0.5 μg/ml ephrin-a1.
Ephrin-a1 ligand causes tight and adherens junction rearrangements.
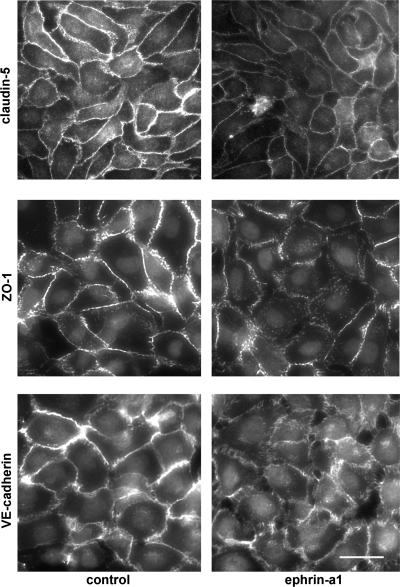
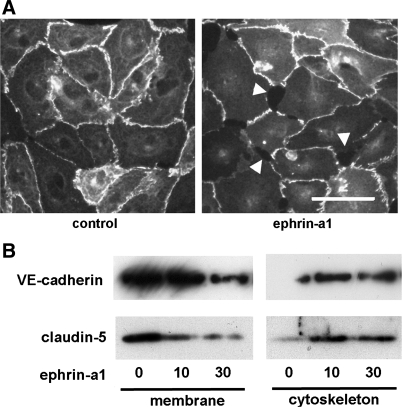
Paracellular permeability is controlled by the state of cell-cell junctions, principally tight junctions and adherens junctions. To determine whether these changes in endothelial monolayer permeability were associated with changes in the localization of tight and adherens junction proteins, bPAEC were stimulated with ephrin-a1-Fc or Fc control for 30 min and then fixed. Immunofluorescent staining for the tight junction proteins claudin-5 and zona occludens (ZO)-1 demonstrated intense staining at cell-cell boundaries in control cells but a marked reduction in immunoreactive claudin-5 and ZO-1 at cell boundaries after ephrin-a1 stimulation (Fig. 4). Immunofluorescence for VE-cadherin demonstrated a very similar pattern, with marked loss of cell junction staining after ephrin-a1 stimulation. Staining for claudin-5 was also repeated in hLMVEC with similar results (Fig. 5A).
Fig. 4.
Immunofluorescence microscopy of bPAEC shows loss of claudin-5, zona occludens (ZO)-1, and vascular endothelial (VE)-cadherin staining at cell-cell junctions after 30 min of ephrin-a1 (2.5 μg/ml) stimulation. Scale bar, 15 μm.
Fig. 5.
A: immunofluorescence microscopy of hLMVEC shows loss of claudin-5 staining at cell-cell junctions and endothelial gap formation (arrowheads) after 30 min of ephrin-a1 (2.5 μg/ml) stimulation. Scale bar, 15 μm. B: ephrin-a1 stimulation of hLMVEC (0, 10, or 30 min) causes subcellular redistribution of claudin-5 and VE-cadherin out of membrane fraction and into cytoskeletal fraction.
To confirm that ephrin-a1 stimulation causes tight and adherens junction proteins to move away from the cell surface, hLMVEC monolayers were stimulated with ephrin-a1-Fc (2.5 μg/ml) for 0, 10, or 30 min and then separated into subcellular fractions. Membrane and cytoskeletal fractions were analyzed by Western blotting for VE-cadherin and claudin-5 content, inasmuch as previous reports showed that permeability-inducing stimuli can cause a shift of both of those proteins from the membrane fraction to a cytoskeletal fraction (11, 16). As shown in Fig. 5B, ephrin-a1 stimulation caused levels of VE-cadherin and claudin-5 in the membrane fraction to fall while corresponding levels of each protein in the cytoskeletal fraction increased. These results suggest that, consistent with the results of immunofluorescent staining, ephrin-a1 stimulation triggers a shift of at least some portion of the membrane pools of VE-cadherin and claudin-5 away from the membrane and into the cell.
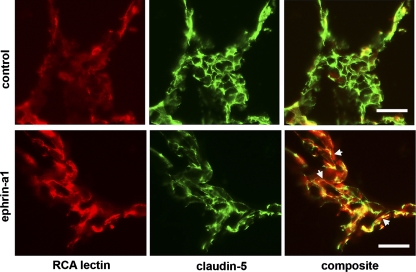
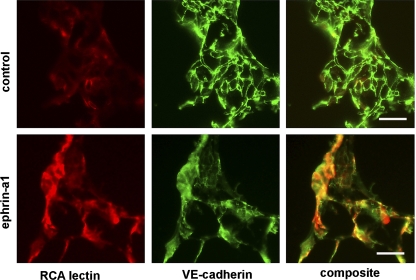
Finally, to determine whether ephrin-a1 stimulation also alters claudin-5 and VE-cadherin localization in vivo, we performed immunofluorescence microscopy on frozen lung sections from rats injected with ephrin-a1 or vehicle control, again using rhodamine-conjugated RCA lectin to label sites of vascular leak. As shown in Fig. 6, in control animals claudin-5 expression is clearly visible outlining cell-cell boundaries throughout the pulmonary vasculature, and minimal RCA lectin labeling is present. In contrast to the control animals, rats injected with ephrin-a1-Fc showed a much more diffuse claudin staining pattern with less clearly visible tight junctions. In these lungs, RCA lectin staining was visible in intense patches, and a strong correlation was observed between areas of increased lectin staining and areas of reduced claudin-5 staining, suggesting that the changes in claudin localization were associated with disruptions of the endothelial barrier in vivo. Parallel studies staining for VE-cadherin demonstrated a similar pattern (Fig. 7).
Fig. 6.
Immunofluorescent staining of distal rat lung for claudin-5. Ephrin-a1 injection causes loss of claudin-5 (green) from cell-cell junctions in association with areas of endothelial disruption. Red, RCA lectin staining; arrows, endothelial disruptions with reduced claudin staining. Scale bars, 25 μm.
Fig. 7.
Immunofluorescent staining of distal rat lung for VE-cadherin. Ephrin-a1 injection causes loss of VE-cadherin (green) from cell-cell junctions in association with areas of endothelial disruption. Red, RCA lectin staining. Scale bars, 25 μm.
DISCUSSION
These experiments demonstrate that ephrin-a1 stimulation of the pulmonary vasculature leads to increases in vascular albumin leak in vivo and increases in endothelial permeability in vitro, associated in both settings with disruptions of tight and adherens junctions. These results are the first demonstration of a role for ephrin family receptor tyrosine kinases and ligands in the control of vascular permeability and suggest that these molecules could contribute to the regulation of the endothelial barrier in the lung.
Ephrins are a large family of receptor tyrosine kinases and ligands with demonstrated roles in neural and vascular development. These molecules are grouped into two classes, A and B, with relatively promiscuous receptor-ligand binding within each class. Relatively little is known about A-class ephrin expression in the postnatal lung. The ephrin-a1 ligand is expressed by endothelial cells, including cells isolated from the postnatal lung microvasculature (9), and its cognate receptor EphA2 has also been reported to be expressed in systemic vascular endothelium and by pulmonary microvascular endothelial cells in culture (2, 12). We used mRNA analysis and immunohistochemical staining to confirm these findings and demonstrate that A-class ephrin ligands and receptors are expressed in the normal intact rat and calf pulmonary vasculature and in cultured pulmonary vascular endothelial cells. Using two previously described experimental lung injuries associated with increased vascular permeability, intratracheal bleomycin injury and hypoxia exposure following viral infection (4, 20, 23), we also examined ephrin-a1 and EphA2 expression in the injured lung. In both settings, we found evidence of upregulation of ephrin-a1 in the distal vasculature of the injured lung, suggestive of a role for ephrins in the pathogenesis of lung injury deserving of further investigation. These findings are consistent with previous reports that ephrin-a1 and EphA2 expression are induced by certain mediators associated with acute lung injury. For example, endothelial ephrin-a1 is upregulated by TNF-α and VEGF (6, 21), and EphA2 is upregulated by hypoxia and by bacterial endotoxin (13, 27).
Although the expression of ephrin-a1 and EphA2 by pulmonary endothelial cells has been previously reported, the physiological roles of these molecules in the pulmonary circulation remain uncertain. Interestingly, recent evidence has implicated ephrin-a1 and EphA2 in the regulation of postnatal angiogenesis (5, 12). In addition, previous work has implicated other proangiogenic mediators in the control of vascular permeability. For example, VEGF is well known not only for its role as an endothelial cell growth factor critical in blood vessel development but also for its profound effect in increasing endothelial permeability in many vascular beds (14, 19). The angiopoietin family ligands Ang1 and Ang2 also are critical modulators of angiogenesis, and recent work shows that they too can modulate endothelial barrier function (17, 22). These findings suggested to us the possibility that, as angiogenic mediators, ephrin-a1 stimulation of EphA receptors in the lung circulation might alter endothelial barrier function.
We first tested whether ephrin-a1 ligand can modulate permeability in vivo by administering soluble ephrin-a1 ligand intravenously to normal rats. We found ephrin-a1 to cause large increases in the extravasation of albumin from the vasculature into the distal lung, a finding consistent with increases in vascular permeability. To determine the likely anatomic site of this extravasation, additional studies were performed using a fluorescently tagged lectin that binds preferentially to sites of endothelial disruption (7). Consistent with the finding of increased albumin leak into the distal lung, we found that ephrin-a1 administration led to endothelial disruptions primarily localized to the alveolar microvasculature. To corroborate this finding in vitro, we also used ephrin-a1 to stimulate EphA receptors in cultured endothelial monolayers and found increases in monolayer permeability on a similar time course and of similar magnitude to those caused by VEGF, which is well known as a potent stimulus of angiogenesis and of increased vascular permeability. Taken together, these results represent the first demonstration that ephrin-a1 ligand can act directly on pulmonary vascular endothelial cells to reduce endothelial barrier function and increase pulmonary vascular permeability.
We next considered the mechanism by which ephrin-a1 might exert this effect. Although the control of endothelial barrier function is complex and not completely understood, paracellular fluid flux is believed to be largely regulated by the state of intercellular junctions. A major component of the junctional complex in endothelial cells is the tight junction, and claudin family proteins are among the major barrier-regulating proteins in tight junctions (1). Claudin-5 is the principal claudin isoform expressed in vascular endothelial cells, and loss of claudin-5 from tight junctions has been associated with increases in barrier permeability (24). In addition, VE-cadherin is a major structural protein in adherens junctions, and disruption of those junctions has also been reported to alter vascular permeability (7, 16). These factors led us to postulate that the effect of ephrin-a1 ligand on the lung endothelial barrier could be a result of EphA receptor-mediated rearrangements of claudin-5 and/or VE-cadherin, leading to reduced tight and/or adherens junction integrity. To test this idea, we used immunofluorescence microscopy to demonstrate the loss of claudin-5, ZO-1, and VE-cadherin from cell-cell junctions of cultured endothelial cells after ephrin-a1 stimulation. Consistent with these observations, biochemical analysis of subcellular fractions after ephrin-a1 stimulation also showed a redistribution of VE-cadherin and claudin-5 away from the membrane fraction and into a cytoskeletal fraction. We then used immunofluorescent staining of frozen sections of rat lung from animals injected with ephrin-a1 to confirm these in vitro findings in the intact animal. Indeed, intravenous injection of ephrin-a1 caused a patchy loss of claudin-5 and VE-cadherin immunoreactivity in the rat lung microvasculature, associated with focal areas of vascular damage as marked by RCA lectin extravasation. Taken together, these results suggest that ligand stimulation of endothelial EphA receptors leads to the disruption of tight and adherens junctions in the endothelium, resulting in reduced endothelial barrier integrity.
The signaling mechanisms underlying these effects of ephrin-a1 on endothelial permeability remain unexplored. Previous work has shown, however, that the proangiogenic effects of ephrin-a1 and EphA2 on endothelial cells are mediated, at least in part, via Rho family GTPases and Vav guanine exchange factors (5, 12). Additional studies are needed to determine whether ephrin-a1 effects on endothelial permeability use similar signaling pathways and whether the permeability effects result solely from changes in junctional proteins or whether Rho-GTPase-mediated effects on the actin cytoskeleton contribute as well.
In summary, then, these studies describe a novel role for ephrins in endothelial barrier regulation in the lung. Although the pathophysiological significance of these results and the molecular mechanisms underlying them await further study, our findings of increased ephrin-a1 and EphA2 immunoreactivity in two distinct models of lung injury provide provocative evidence to suggest that these effects of ephrin-a1 on endothelial permeability may be relevant to the pathophysiology of acute lung injuries. We conclude that EphA receptors in the pulmonary vasculature regulate endothelial permeability and could contribute to pulmonary edema formation during acute lung injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL-077743 (T. C. Carpenter).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bazzoni G Endothelial tight junctions: permeable barriers of the vessel wall. Thromb Haemost 95: 36–42, 2006. [PubMed] [Google Scholar]
- 2.Brantley-Sieders DM, Fang WB, Hicks DJ, Zhuang G, Shyr Y, Chen J. Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression. FASEB J 19: 1884–1886, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter TC, Reeves JT, Durmowicz AG. Viral respiratory infection increases susceptibility of young rats to hypoxia-induced pulmonary edema. J Appl Physiol 84: 1048–1054, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter TC, Schomberg S, Stenmark KR. Endothelin-mediated increases in lung VEGF content promote vascular leak in young rats exposed to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol 289: L1075–L1082, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Cheng N, Brantley DM, Liu H, Lin Q, Enriquez M, Gale N, Yancopoulos G, Cerretti DP, Daniel TO, Chen J. Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor-induced angiogenesis. Mol Cancer Res 1: 2–11, 2002. [PubMed] [Google Scholar]
- 6.Cheng N, Chen J. Tumor necrosis factor-α induction of endothelial ephrin A1 expression is mediated by a p38 MAPK- and SAPK/JNK-dependent but nuclear factor-κB-independent mechanism. J Biol Chem 276: 13771–13777, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA 96: 9815–9820, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang WB, Ireton RC, Zhuang G, Takahashi T, Reynolds A, Chen J. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci 121: 358–368, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Favre CJ, Mancuso M, Maas K, McLean JW, Baluk P, McDonald DM. Expression of genes involved in vascular development and angiogenesis in endothelial cells of adult lung. Am J Physiol Heart Circ Physiol 285: H1917–H1938, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Folkesson HG, Nitenberg G, Oliver BL, Jayr C, Albertine KH, Matthay MA. Upregulation of alveolar epithelial fluid transport after subacute lung injury in rats from bleomycin. Am J Physiol Lung Cell Mol Physiol 275: L478–L490, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Gillrie MR, Krishnegowda G, Lee K, Buret AG, Robbins SM, Looareesuwan S, Gowda DC, Ho M. Src-family kinase dependent disruption of endothelial barrier function by Plasmodium falciparum merozoite proteins. Blood 110: 3426–3435, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter SG, Zhuang G, Brantley-Sieders D, Swat W, Cowan CW, Chen J. Essential role of Vav family guanine nucleotide exchange factors in EphA receptor-mediated angiogenesis. Mol Cell Biol 26: 4830–4842, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanov AI, Steiner AA, Scheck AC, Romanovsky AA. Expression of Eph receptors and their ligands, ephrins, during lipopolysaccharide fever in rats. Physiol Genomics 21: 152–160, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, Crystal RG. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol 22: 657–664, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Kosmidou I, Karmpaliotis D, Kirtane AJ, Barron HV, Gibson CM. Vascular endothelial growth factors in pulmonary edema: an update. J Thromb Thrombolysis 25: 259–264, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Lim MJ, Chiang ET, Hechtman HB, Shepro D. Inflammation-induced subcellular redistribution of VE-cadherin, actin, and γ-catenin in cultured human lung microvessel endothelial cells. Microvasc Res 62: 366–382, 2001. [DOI] [PubMed] [Google Scholar]
- 17.McCarter SD, Mei SH, Lai PF, Zhang QW, Parker CH, Suen RS, Hood RD, Zhao YD, Deng Y, Han RN, Dumont DJ, Stewart DJ. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med 175: 1014–1026, 2007. [DOI] [PubMed] [Google Scholar]
- 18.McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal 17: 131–139, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Mura M, dos Santos CC, Stewart D, Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol 97: 1605–1617, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Mutsaers SE, Foster ML, Chambers RC, Laurent GJ, McAnulty RJ. Increased endothelin-1 and its localization during the development of bleomycin-induced pulmonary fibrosis in rats. Am J Respir Cell Mol Biol 18: 611–619, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-α-induced angiogenesis. Science 268: 567–569, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 3: e46, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-β is a critical mediator of acute lung injury. J Clin Invest 107: 1537–1544, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction “opening”: signaling via Rho and Rho kinase. J Cell Sci 116: 4615–4628, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of claudin-4 and mediates paracellular permeability. J Biol Chem 280: 42375–42382, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Thickett DR, Armstrong L, Christie SJ, Millar AB. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am J Respir Crit Care Med 164: 1601–1605, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Vihanto MM, Plock J, Erni D, Frey BM, Frey FJ, Huynh-Do U. Hypoxia up-regulates expression of Eph receptors and ephrins in mouse skin. FASEB J 19: 1689–1691, 2005. [DOI] [PubMed] [Google Scholar]