Abstract
We have previously reported the participation of mitogen-activated protein, Rho, and phosphoinositide-3 (PI3) kinases in separate pathways in serotonin (5-HT)-induced proliferation of pulmonary artery smooth muscle cells (SMCs). In this study, we investigated the possible participation of phospholipase D (PLD) and phosphatidic acid (PA) in this growth process. 5-HT stimulated a time-dependent increase in [3H]phosphatidylbutanol and PA generation. Exposure of SMCs to 1-butanol or overexpression of an inactive mutant of human PLD1R898R blocked 5-HT-induced proliferation. Furthermore, 1-butanol inhibited 5-HT activation of S6K1 and S6 protein, downstream effectors of mammalian target of rapamycin (mTOR), by 80 and 72%, respectively, and partially blocked activation of extracellular signal-regulated kinase (ERK) by 30% but had no effect on other associated signaling pathways. Exogenous PA caused cellular proliferation and revitalized cyclin D1 expression by 5-HT of the 1-butanol-treated cells. PA also reproduced activations by 5-HT of mTOR, S6K1, and ERK. Transfection with inactive human PLD1 reduced 5-HT-induced activation of S6K1 by ∼50%. Inhibition of 5-HT receptor 2A (R 2A) with ketaserin blocked PLD activation by 5-HT. Inhibition with PI3-kinase inhibitor failed to block either activation of PLD by 5-HT or PA-dependent S6K1 phosphorylation. Taken together, these results indicate that ligation of the 5-HTR 2A by 5-HT initiates PLD activation in SMCs, and that its product, PA, is an early signaling molecule in 5-HT-induced pulmonary artery SMC proliferation. Signaling by PA produces its downstream effects primarily through the mTOR/S6K1 pathway and to a lesser extent through the ERK pathway. Hydrolysis of cell membrane lipid may be important in vascular effects of 5-HT.
Keywords: pulmonary artery smooth muscle cell proliferation, serotonin, phospholipase D, phosphatidic acid, cell signal transduction
serotonin (5-HT), a smooth muscle cell (SMC) mitogen (7), has been increasingly associated with pulmonary hypertension (2, 4, 9, 30), and it is recognized that hyperplasia of SMCs accompanies the pathology of pulmonary hypertension (5, 10, 22). 5-HT initiates its SMC mitogenic action through both 5-HT receptors and the 5-HT transporter (5-HTT) (7) that are present on the cell membrane in close proximity to membrane structural lipids that include phosphatidylcholine. Through the receptor and transporter ligands, 5-HT produces SMC proliferation via combinatorial actions on the small G protein Rho, p 42/44 mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3-kinase)/protein kinase B (Akt), and platelet-derived growth factor-β receptor signaling pathways (17–20).
Lipid-derived second messengers generated by the action of phospholipases on membrane-associated phospholipids play an important role in cellular signal transduction in general (3). Phospholipase D (PLD), which catalyzes the hydrolysis of phosphotidylcholine to phosphatidic acid (PA) and choline, has been recognized to be an intermediate in the actions of a wide variety of agonists on cellular functions, including those that cause proliferation (8, 33). Agonists identified to activate PLD include neurotransmitters, growth factors, hormones, calcium ionophores, and reactive oxygen species (6, 24). The cell signaling associated with PLD activation occurs at least in part through actions of PA (14).
It previously was reported that 5-HT activates PLD in rat mesangial cells (15) and mesenteric SMCs, which associates the activation of PLD with contraction (11). Plevin and coworkers (27) demonstrated in 1994 that 5-HT activates PLD in cultured sheep pulmonary artery SMCs. However, there is no information currently available about the relationship between 5-HT, its receptors and transporter, and PLD in pulmonary artery SMC signaling and proliferation. In the present study, we show that 5-HT activates PLD of pulmonary artery SMCs via the 5-HT 2A receptor, leading to the generation of PA that promotes SMC proliferation through activations of mammalian target of rapamycin (mTOR), S6K1 and MAPK but not the Rho or PI3-kinase/Akt signaling pathways. We hypothesize that these observations link an important lipid component of the cell membrane, i.e., phosphatidyl choline, with cell signaling occurring via 5-HT ligands in SMCs.
MATERIALS AND METHODS
Reagents.
RPMI 1640 medium was purchased from GIBCO Laboratories (Grand Island, NY). 5-HT, 1-butanol, 2-butanol and tert-butanol, chloroform, methanol, acetic acid, ethyl acetate, and isooctane were purchased from Sigma Chemical (St. Louis, MO). PA (catalog no. 840861) and phosphatidyl butanol (catalog no. 860203C) were from Avanti Lipids (Alabaster, AL). Whatman LK6DF silica gel 60 thin layer chromatography plates were from Whatman (Kent, UK). [methyl-3H]thymidine (1 mCi/ml, sp act 6.7 Ci/mmol) and [9,10-3H]myristic acid (1 mCi/ml) were purchased from New England Nuclear Perkin-Elmer (Boston, MA). Rapamycin, LY-294002, and antibodies to phosphospecific p42/44 MAPK (202Thr/204Tyr), phospho-Akt (473Ser), Akt, phospho-p70 S6K1 (421Thr/424Ser), p70 S6K1, phospho-mTOR (2481Ser), total mTOR, and phospho-S6 ribosomal protein (235Ser/236Ser) were from Cell Signaling Technology (Beverly, MA). Anti-phospho-myosin phosphatase target subunit 1 (MYPT1) (696Thr) rabbit polyclonal antibody was purchased from Upstate Group (Lake Placid, NY). Anti-cyclin D1, anti-MYPT-1 and anti-extracellular signal-regulated kinase (ERK) rabbit polyclonal antibodies were from Santa Cruz Biotech (San Diego, CA). pCGN vector and plasmids inserted with wild-type human PLD1, inactive PLD1 mutant, wild-type mouse PLD2, and inactive mPLD2 mutant were gifts from Professor Michael Frohman of the State University of New York (Stony Brook, NY).
Cell culture.
SMCs from bovine pulmonary artery were isolated by a modification of the method of Ross (29a) as previously described (17) and were cultured in RPMI 1640 medium containing 10% FBS, 1% penicillin and 0.5% streptomycin. Cells from passages 3 to 10 were used in our study.
Incorporation of [3H]thymidine.
SMCs seeded in 96-well plates were growth-arrested for 72 h in medium containing 0.1% FBS. Cells were incubated in medium with and without 1 μmol/l 5-HT for 20 h and then labeled with [methyl-3H]thymidine (20 μCi/ml) for 4 h. In some experiments, 0.3% 1-butanol was added 30 min before the 5-HT. After labeling, experiments were terminated by aspiration of medium, and the cells were harvested on 96-well microplate filter paper using a Tomtec harvester (Tomtec, Hamden, CT). Radioactivity was counted in a Trilux liquid scintillation and luminescence counter (Perkin Elmer Life Science).
Preparation of whole cell lysates.
Treated SMCs were rinsed with ice-cold PBS and then incubated for 15 min at 4°C in RIPA lysis buffer. Lysates were centrifuged at 14,000 g for 10 min to collect supernatants.
PLD plasmid transfections.
The wild-type human PLD1, inactive PLD1 R898R, and mouse PLD2 and PLD2 K758R plasmids inserted in pCGN vector were transfected in cells by using lipofectamine 2000 according to the instructions from the manufacturer (Invitrogen, Carlsbad, CA). Cells first were plated in 35-mm dishes in medium containing 10% serum. Ninety percent confluent cells in 35-mm dishes were transfected with PLD plasmid (1 μg)/lipofectamine 2000 (5 μl) in 0.5 ml OPTI-DMEM medium for 6 h. The medium was replaced with fresh 0.1% FBS medium and incubated at 37C° in 5% CO2 for 48 h before performing experiments.
Western blot analysis.
Phosphorylations of Akt, ERK, MYPT1, S6K1, and S6 were analyzed using phosphospecific rabbit polyclonal antibodies. Immunoreactive bands were bonded with horseradish peroxidase-conjugated secondary antibodies and visualized using an ECL Chemiluminescent Western Blotting Detection kit (Pierce, Rockford, IL). Quantification of bands was done for gel densitometry with UN-SCAN-IT gel analyzer software, and protein phosphorylation was normalized by total protein band densitometry.
PLD activity as measured by thin layer-chromatography.
Pulmonary artery SMCs were seeded in 60-mm tissue culture dishes. As they reached confluence, cells were prelabeled for 20 h in serum-free RPMI containing 0.2 mg/ml BSA with [3H]myristic acid at a concentration of 2 μCi/ml (25, 32). The reaction was initiated by addition to cells of 1 μmol/l 5-HT in serum-free RPMI medium containing 0.1% 1-butanol and terminated by rapid aspiration of medium from the dish followed by the addition of 0.5 ml ice-cold acidic methanol (methanol/0.1 M HCl 1:1). The cells then were scraped from dishes and transferred to centrifuge tubes. Labeled phospholipid products were extracted with 0.25 ml chloroform. The aqueous and organic phases were separated by centrifugation, and the lower chloroform layer was dried under vacuum. The dried extract was dissolved in 25 μl chloroform and applied to Whatman TLC plates. The resolving solvent was the upper phase of ethyl acetate/isooctane/acetic acid/water (13:2:3:10). Iodine-stained phosphobutanol (PBt) standards were used to identify the corresponding radiolabeled PBt on the TLC plate. The radioactivity of [3H]PBt was visualized by exposure to blue-sensitive film. Following exposure, the film was developed, and lipid quantification was done by densitometry.
Measurement of PA generation by thin-layer chromatography.
SMCs in culture were prelabeled with the [3H]myristic acid for 20 h and stimulated with 5-HT for periods up to 60 min in the absence of 1-butanol. The formation of [3H]PA in the cells was detected by TLC with the same protocol as for the PLD activity assay. PA (PA) standards were used to identify the corresponding radiolabeled PA on the TLC plate.
Statistical analysis.
Means ± SD were calculated, and statistically significant differences among groups were determined by one-way ANOVA followed by Turkey's post hoc comparisons. An effect was considered significant for P < 0.05.
RESULTS
5-HT stimulates PLD activity via 5-HT receptor 2A.
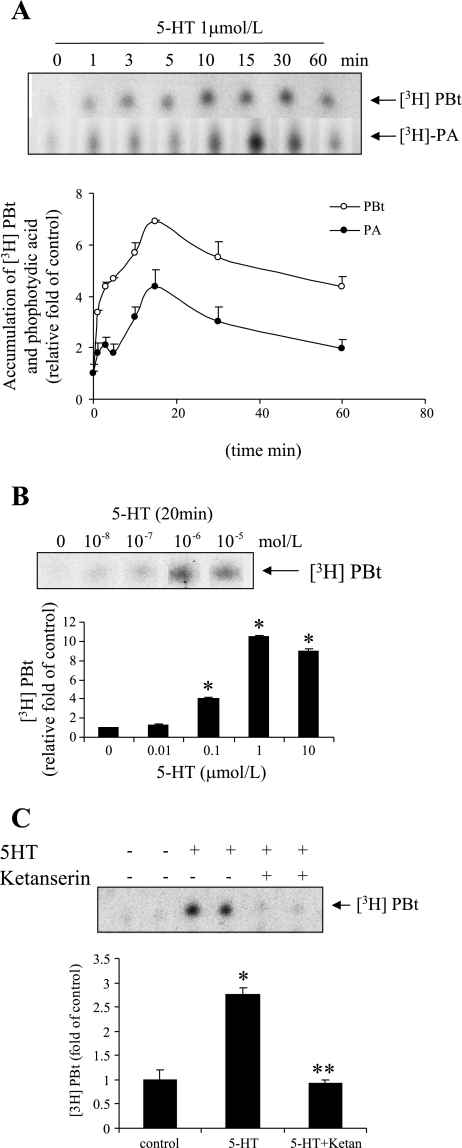
To investigate the effect of 5-HT on PLD activity, we measured the accumulation of PBt in cells continuously incubated with 5-HT. Figure 1A shows that 5-HT (1 μmol/l) stimulated rapid accumulation of [3H]PBt and [3H]PA that reached a peak at ∼15 min. Furthermore, 5-HT stimulated the accumulation of [3H]PBt dose dependently, but a maximum was reached at ∼1 μmol/l (Fig. 1B). The maximum stimulation of [3H]PBt formation by 5-HT was about sevenfold the basal rate.
Fig. 1.
Serotonin (5-HT) induces phospholipase D (PLD) activation and phosphatidic acid (PA) formation in smooth muscle cells (SMCs). A: time courses of 5-HT-induced PLD activation and PA formation. As described more fully in materials and methods, bovine pulmonary artery SMCs were labeled with [3H]myristic acid for 20 h then treated with 1 μmol/l 5-HT. [3H]phosphatidylbutanol (PBt) and PA accumulations were measured in separate experiments; SDs are shown. B: for a dose response, cells were treated with 0.01–10 μmol/l of 5-HT for 20 min. C: washed cells were pretreated with 5-HT receptor 2A inhibitor ketanserin (5 μmol/l) for 30 min and stimulated with 5-HT (1 μmol/l) for 20 min in the presence of 0.1% 1-butanol. Accumulation of PBt was measured by TLC as noted in materials and methods. Each point in A–C represents the mean ± SD of triplicate determinations from three independent experiments. Representative autographic TLC blots are shown. *Significant difference from the controls at P < 0.05. **Significant difference from 5-HT-treated cells at P < 0.05.
We have previously concluded that 5-HT causes SMC proliferation through combined action of the 5-HTT and the 5-HT 1B/1D and 2A receptors in bovine pulmonary artery SMCs (16, 18–20). To assess if a 5-HT receptor or 5-HTT is required for PLD activation, we tested the effect of different receptor and transporter antagonists on PLD activity as measured by accumulation of [3H]PBt. Figure 1C shows that inhibition of the 5-HT 2 receptor with 5 μmol/l ketanserin completely blocked the increase in [3H]PBt produced by 5-HT. Neither the 5-HTT inhibitor imipramine nor the 5-HT 1B receptor inhibitor GR-55562 reduced the increase in [3H]PBt by 5-HT (data did not shown). These findings suggest that ligation of the 5-HT 2A receptor evokes PLD activation.
Inhibition of PLD activity reduces 5-HT-induced proliferation of SMCs.
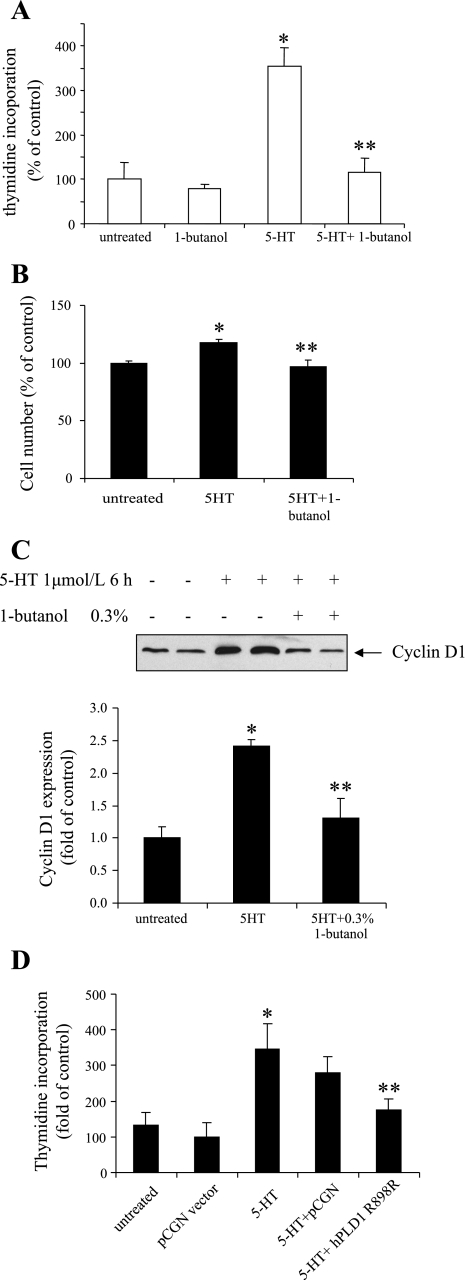
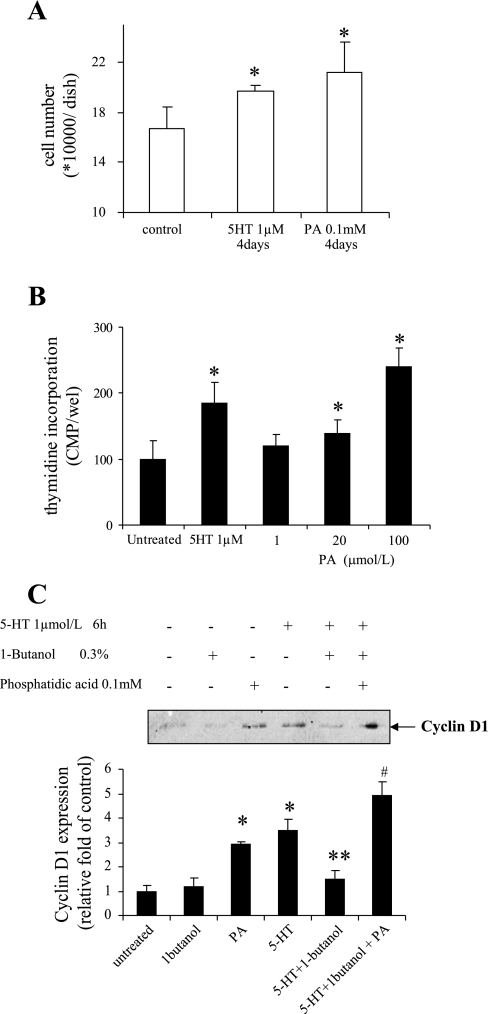
To further evaluate the function of PLD activation by 5-HT, we tested the impact of PLD activity inhibition on 5-HT-induced proliferation of pulmonary artery SMCs. The cellular proliferation response was measured by thymidine incorporation and measurement of cell number. Our results noted in Fig. 2, A and B, show that the stimulation by 5-HT resulted in a 350 ± 64% increase in thymidine incorporation and a 20 ± 4% increase in cell number. Both responses were blocked by treatment of cells with 0.3% 1-butanol, which diverts the PA generated by PLD to PBt. Similarly, 1-butanol also inhibited the expression of cyclin D1 caused by 5-HT, as shown in Fig. 2C. Furthermore, overexpression of an inactive mutant of human PLD1 R898R in SMCs significantly decreased the DNA synthesis caused by 5-HT (Fig. 2D). These results indicate that PLD activation participates in the cellular proliferation response to 5-HT.
Fig. 2.
Inhibition of PLD activity reduces SMC proliferation produced by 5-HT. Quiescent SMCs were pretreated with the PLD inhibitor 1-butanol (0.3%) for 30 min. They were then incubated with 1 μmol/l 5-HT. A: DNA synthesis was determined at 24 h by monitoring [3H]thymidine incorporation. B: cell number was counted 4 days after 5-HT treatment. C: cyclin D1 expression was determined 6 h after 5-HT stimulation by immunoblotting with anti-cyclin D1 antibody. D: SMCs were transfected with inactive hPLD1 R898R mutant in pCGN vector for 48 h; transfected cells were treated with 1 μmol/l 5-HT. DNA synthesis was determined at 24 h by monitoring [3H]thymidine incorporation. Bar graphs represent means ± SD for n = 3. *Significant difference from the untreated controls at P < 0.05. **Significant difference from 5-HT-treated cells at P < 0.05.
Relationship of PLD activation by 5-HT with activations of S6K1, MAPK, and Rho kinase pathways.
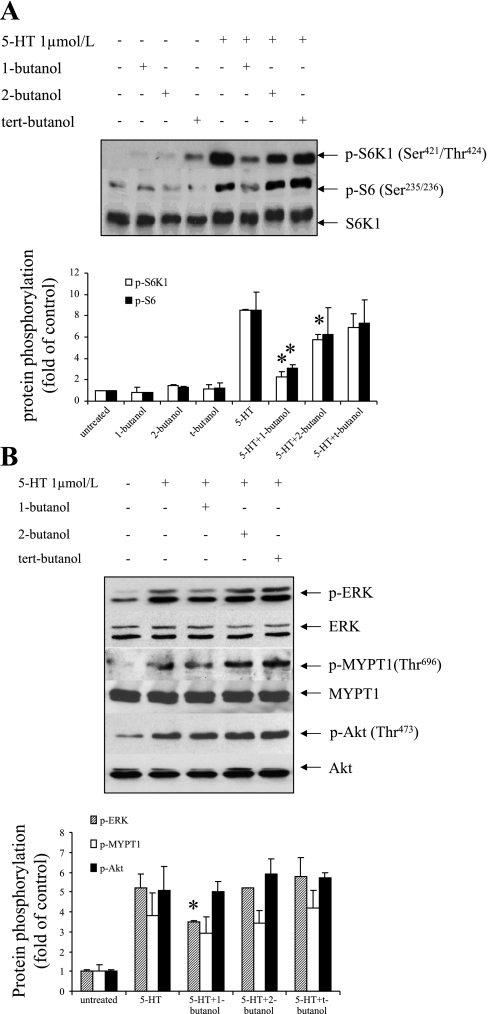
Our previous work has demonstrated that multiple signaling pathways concomitantly regulate 5-HT-mediated cellular proliferation (16, 18–20). We first determined the impact of inhibition of PLD activity with 1-butanol on the S6K1/S6 pathway activation, which we have previously shown to modulate 5-HT-induced mTOR signaling in pulmonary artery SMCs (18). As shown in Fig. 3A, 1-butanol inhibited 5-HT-induced phosphorylation of S6K1 and S6, whereas its analogs 2-butanol and tert-butanol, which are poor substrates for PLD, showed minor or no effects. The 5-HT-activated ERK MAPK pathway controls transcription processes and consequently regulates cell growth. Our results indicate that 1-butanol treatment reduced 40% of the phosphorylation by 5-HT of ERK MAPK (Fig. 3B). PLD inhibition did not alter 5-HT-induced PI3-kinase/Akt and Rho kinase (ROCK) activations as measured by the phosphorylations of Akt and MYPT1, respectively. These results suggest that 5-HT-induced PLD activation is upstream to the mTOR/S6K1/S6 pathway and PLD activation also modulates ERK MAPK activation to some extent in pulmonary artery SMCs.
Fig. 3.
Influence of PLD activation on S6K1, mitogen-activated protein kinase (MAPK), Rho kinase (ROCK), and protein kinase B (Akt) pathways. Quiescent SMCs were pretreated with 0.1% 1-butanol, 2-butanol, and tert-butanol for 30 min and then incubated with 1 μmol/l 5-HT for 10 min. Phosphorylation of S6K1 (421Thr/424Ser), S6 ribosomal protein (235Ser/236Ser), extracellular signal-regulated kinase (ERK) (Thr202/Tyr204), MYPT1 (696Thr), and Akt (473Ser) was determined by Western blot analysis using phosphospecific antibodies. The levels of total S6K1 (A) and ERK, MYPT1, and Akt (B) in the whole cell lysates were determined by Western blot analysis using antibodies to these molecules for loading control. All experiments were repeated three times and presented as a representative blot. The band densitometries were calculated as the relative fold in band intensity compared with untreated controls. The bar graphs for blots of A and B represent means ± SD for n = 3. *Significant difference from 5-HT-treated cells (P < 0.05).
PLD1 is responsible for PLD activation by 5-HT.
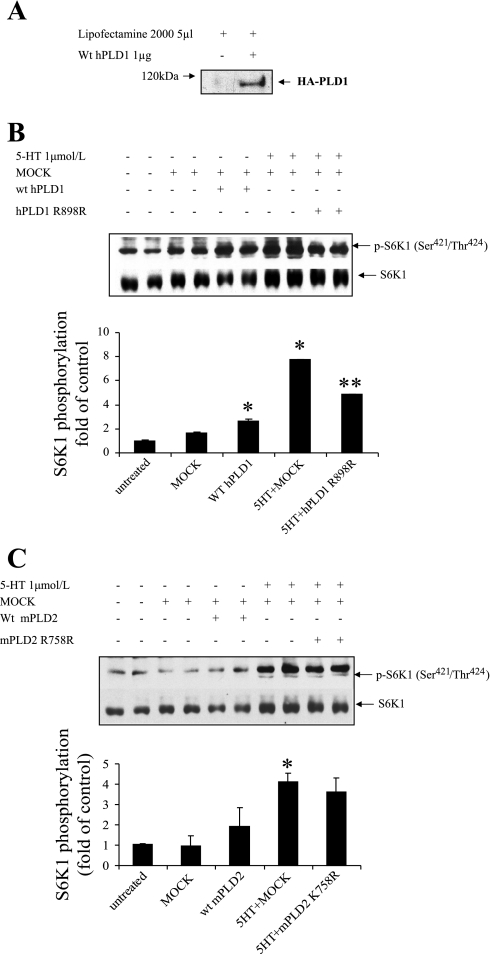
To date, two PLD isoforms have been cloned and characterized. PLD1 has a low basal activity and is upregulated by the small G proteins ADP-ribosylation factor (ARF), Rho, Ral, and protein kinase C (PKC) as well as by phosphatidylinositol 4,5-bisphosphate (PIP2) (6). PLD2 has a high basal activity, requires PIP2, and is upregulated by ARF and PKC (21). We investigated which PLD isoform contributes to PLD activation by 5-HT. We first examined the overexpression of transfected human PLD1 in primary cultured bovine pulmonary artery SMCs. As shown in Fig. 4A, transfection of SMCs with human PLD1 resulted in a significant amount of the transfected PLD1 protein indicated as HA-tagged PLD1 occurring in transfected SMCs. When wild-type human PLD1 is overexpressed in the SMCs, phosphorlyation of S6K1 was upregulated by 50%, and the inactive PLD1 K898R mutant inhibited 50% of the p-S6K1 activation by 5-HT (Fig. 4B). In contrast, overexpression of mouse wild-type PLD2 and its inactive mutant PLD2 K758R had no significant effect on p-S6K1 activation by 5-HT (Fig. 4C). These results suggest that the PLD1 isoform is a significant contributor to PLD activation by 5-HT.
Fig. 4.
Effect of overexpression of wild-type (wt) PLDs and inactive mutant PLDs on 5-HT-induced S6K1 phosphorylation. A: overexpression of human (h) PLD1 in cells is indicated by representative immunoblots of HA-tagged PLD1 by using anti-HA antibody in Western blot analysis. SMCs were transfected with wild-type human PLD1, inactive hPLD1 R898R mutant (B), and mouse PLD2, inactive mouse PLD2 K758R mutant plasmids (C) and pCGN vector with lipofectamine 2000 for 6 h. After 48 h incubation in 0.1% FBS medium, cells were treated with 1 μmol/l 5-HT for 10 min. The phosphorylation of S6K1 (421Thr/424Ser) and total S6K1 was detected by Western blot analysis. The bar graphs represent means ± SD for n = 3. *Significant difference from untreated cells (P < 0.05). **Significant difference from 5-HT-treated cells (P < 0.05).
Exogenous PA stimulates cell growth and signal activation in pulmonary artery SMCs.
PA is generally recognized to be the signaling product of PLD and functions as an effector in multiple physiological processes. To further clarify the possible role of PA in 5-HT-induced SMC proliferation, we tested the influence of exogenous PA on cell growth of SMCs. Our results in Fig. 5A show that incubation with 100 μmol/l PA for 4 days resulted in a 27 ± 14% increase in the number of SMCs. PA also dose-dependently increased the thymidine labeling of bovine pulmonary artery SMCs (Fig. 5B). Furthermore, reconstitution of PA in 1-butanol-treated cells revitalized 5-HT-induced cyclin D1 expression (Fig. 5C). These data suggest that PA may be a key component mediating PLD-regulated proliferation.
Fig. 5.
Exogenous PA stimulates cell growth in SMCs. Quiescent SMCs were incubated with 100 μmol/l PA. A: cell number was counted 4 days after the PA treatment. B: DNA synthesis was determined 24 h after PA treatment by monitoring [3H]thymidine incorporation as noted in materials and methods. C: growth-arrested SMCs were preincubated with 100 μmol/l PA in the presence or absence of 0.3% 1-butanol for 30 min. Cells were then treated with 1 μmol/l 5-HT for 6 h. Cyclin D1 expression was detected by Western blot. Bar graphs represent means ± SD for n = 3. In A and B, * denotes a significant difference from the untreated controls at P < 0.05. In C, * denotes a significant difference from the untreated controls at P < 0.05. **Significant difference from 5-HT treated control and #significant difference from 5-HT + 1-butanol-treated cells.
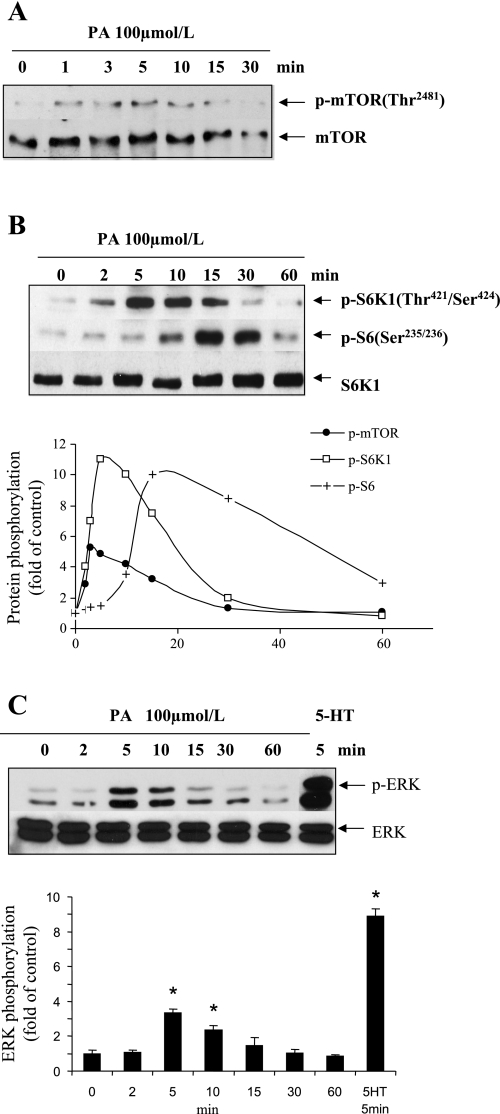
As shown in Fig. 3A, diversion of PA produced by PLD with 1-butanol primarily blocked the mTOR/S6K1/S6 pathway. We further confirmed the relationship between PLD/PA and the mTOR/S6K1 pathway in SMCs by testing mTOR/S6K1/S6 activation with exogenous PA. As shown in Fig. 6A, PA (100 μmol/l) stimulated rapid and transient mTOR (2481Thr) phosphorylation in the cells. Phosphorlyation of S6K1 and S6 occurred downstream to mTOR phosphorylation (Fig. 6B). Exogenous PA (100 μmol/l) produced a 3.8-fold increase in ERK MAPK phosphorylation as seen in Fig. 6C, which is 42% of the stimulation of p-ERK by 1 μmol/l 5-HT. These data combined with the results observed with 1-butanol inhibition suggest a partial regulation of ERK MAPK by PLD-derived PA.
Fig. 6.
PA stimulates the activation of mTOR/S6K1/S6 and MAPK pathways. Quiescent SMCs were incubated with 100 μmol/l PA for indicated time periods or with 1 μmol/l 5-HT for 5 min. The phosphorylations of mTOR (A) (2481Ser), S6K1 (421Thr/424Ser), S6 ribosomal protein (235Ser/236Ser) (B), and ERK (C) (202Tyr/204Thr) were detected by Western blot analysis. The total mTOR, S6K1, and ERK were reblotted with the individual stripped membrane to normalize the signal of phosphoproteins. The bar graphs for the blot in C represent means ± SD for n = 3. *Significant difference from untreated cells (P < 0.05).
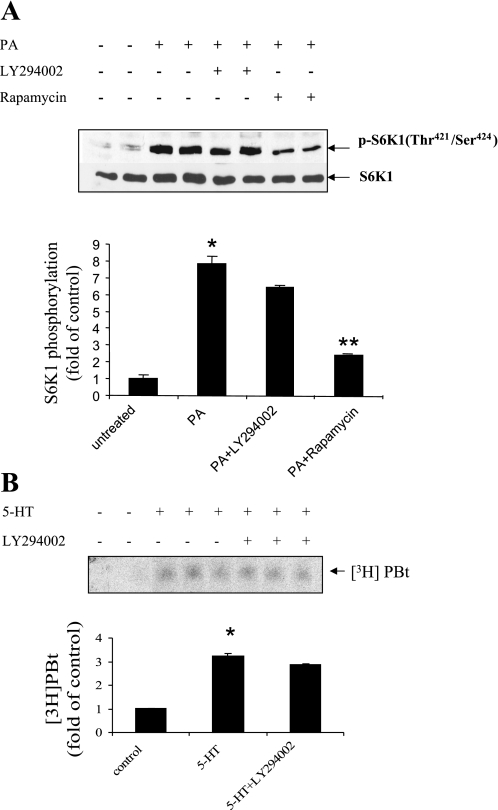
Our previous study demonstrated that PI3-kinase/Akt is upstream of mTOR in mediating 5-HT-induced S6K1 phosphorylation (18). We therefore investigated whether PA regulates mTOR/S6Ks via PI3-kinase/Akt signaling. As shown in Fig. 7A, inhibition of PI3-kinase with LY-294002 failed to reduce the p-S6K1 activation caused by PA. Furthermore, this pharmacological inhibitor of PI3-kinase failed to block 5-HT-induced PLD activation (Fig. 7B). These results suggest that PLD/PA and PI3-kinase/Akt regulate mTOR/S6K1 signaling in a parallel manner rather than being in series.
Fig. 7.
PA stimulates mammalian target of rapamycin (mTOR)/S6K1/S6 pathway independent of phosphatidylinositol 3-kinase (PI3-kinase) activation. A: inhibition of PI3-kinase failed to significantly block S6K1 phosphorylation induced by PA. Quiescent SMCs were pretreated with 10 μmol/l LY-294002 and 100 nmol/l rapamycin for 30 min and incubated with 100 μmol/l PA for 10 min. The phosphorylation of S6K1 (421Thr/424Ser) and total S6K1 was detected by Western blot analysis. B: inhibition of PI3-kinase does not reduce 5-HT-induced PLD activation. After cells were prelabeling with [3H]myristic acid for 18 h, SMCs were treated with LY-294002 (10 μmol/l) for 30 min and then stimulated with 5-HT for 20 min. The accumulation of [3H]PBt was measured as noted in materials and methods for the cellular lipid extract from each group. The bar graphs for blots in A and B represent means ± SD for n = 3. *Significant difference from untreated cells (P < 0.05). **Significant difference from 5-HT-treated cells (P < 0.05) in A.
DISCUSSION
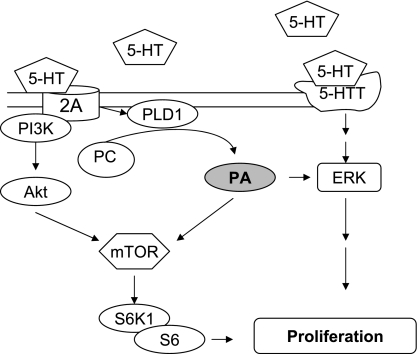
We have previously demonstrated that the MAPK, Rho/ROCK, and PI3-kinase/Akt cell signaling pathways all participate in the SMC mitogenic response of pulmonary artery SMCs to 5-HT (16, 18, 20). These signaling actions by 5-HT occur in parallel as opposed to being in series and are promulgated by ligation of different cell surface molecules. The MAPK response depends upon activation of the 5-HTT and formation of reactive oxygen species (16); the Rho/ROCK response depends on ligation of the 5-HT 1B receptor (20); and the PI3-kinase/Akt response relies upon ligation of the 5-HT 2A receptor (18). The signaling process by 5-HT is made more complex by the ability of the 5-HTT to transactivate the tyrosine kinase receptor for platelet-derived growth factor (19). Thus activation of cell signaling by 5-HT in SMCs is a much more complex process than has been previously recognized and illustrates for the first time combinatorial actions of multiple 5-HT ligands and signaling pathways in 5-HT-induced pulmonary artery SMC proliferation (Fig. 8).
Fig. 8.
5-HT-induced PLD activation and PA formation in the proliferative response of pulmonary artery SMCs; downstream actions on mTOR/S6K1/S6 and ERK MAPK.
The present study demonstrates that cell membrane lipid also participates in 5-HT signaling in SMCs and implicates PLD in this signaling through an mTOR pathway unrelated to PI3-kinase and Akt. We have previously demonstrated a PI3-kinase-dependent activation of mTOR in these SMCs through the 5-HT 2A receptor in 5-HT-induced stimulation of SMC proliferation (18). Our present studies show that the 5-HT 2A receptor also activates cellular PLD that catalyzes the formation of PA from phosphatidyl choline. PA, in turn, activates mTOR and S6K1, signals that participate in cellular proliferation. Several lines of evidence support our hypothesis that 5-HT activates mTOR signaling through a PLD-dependent increase in PA. First, 5-HT stimulated PLD activation, PA accumulation, and mTOR signaling. Second, exogenous PA was sufficient for the activation of mTOR signaling. Third, a mutant of the isoenzyme of PLD1 inhibited S6K1 phosphorylation induced by 5-HT. Fourth, pharmacological inhibition of PLD blocked 5-HT-induced proliferation and activation of mTOR signaling. Finally, inhibition of PI3-kinase and Akt failed to block mTOR signaling in PA-stimulated SMCs, suggesting that 5-HT-induced activation of mTOR signaling resulted from independent modulation of PLD and PI3-kinase pathways.
The present study also noted a modulation of the ERK MAPK pathway by PLD and exogenous PA. Inhibition of PLD activity with 1-butanol reduced by 30% activation of ERK MAPK by 5-HT. Furthermore, exogenous PA (100 μmol/l) stimulated a transient elevation of ERK MAPK activation. In studies by others, PA has been demonstrated to play a key role in the regulation of membrane traffic and activation of MAPK (29). A critical event in the regulation of MAPK is the recruitment of the protein kinase Raf-1 to cell membranes where it is activated by the small G protein Ras or by other protein kinases (28). Hypothetically, PA generated by PLD activation may facilitate the recruitment of Raf-1 from the cytoplasm and the formation of endocytic vesicles. Once bound to the plasma membrane, Raf-1 may interact with Ras and subsequently activate the Ras/mitogen/extracellular signal-regulated kinase/MAPK complex (28).
In this study, the 5-HT receptor 2A inhibitor ketanserin, but not an inhibition of the 5-HTT or other 5-HT receptors, completely blocked the activation of PLD by 5-HT. A study by Johnson et al. (12) previously demonstrated the role of interaction between the 5-HT 2A receptor and ARF in the activation of PLD in COS-7 cells. However, 5-HT may activate PLD through different cell surface receptors that are tissue specific. For example, 5-HT 3 receptor activates a PKC-dependent PLD pathway in human T cells (13). A 5-HT 1B receptor has been reported to be involved in PLD activation in rabbit mesenteric SMCs (11), and PLD is activated by 5-HT 2C receptors in rat choroid plexus epithelial cells (23). Moreover, a 5-HT 1A receptor has been found to be responsible for PLD stimulation in brain (26). Hence, there may be no common mechanism by which 5-HT receptors mediate PLD activation.
Although our results indicate that PLD is the enzyme responsible for the 5-HT-induced increase in PA, we cannot exclude the potential contribution of additional PA regulatory enzymes. Such enzymes might include the PA phosphatases (PAP), which dephosphorylate PA to diacylglycerol (DAG), and the DAG kinases (DAGK) generating PA through the phosphorylation of DAG (1). In fact, previous studies have shown that PLD-derived PA can be rapidly converted to DAG by PAP and that DAG can, in turn, be transformed back to PA by DAGK (31). Our finding that 5-HT promotes an increase in PLD activity raises questions about the mechanism(s) involved in this process.
In summary, the results of this study indicate that ligation by 5-HT of the 5-HT receptor 2A initiates PLD activation in pulmonary artery SMCs. PLD and its product PA are early signaling molecules in the 5-HT-induced pulmonary artery SMC proliferative response. As shown in a schematic Fig. 8, they may produce their downstream effects primarily through the mTOR/S6K1 pathway. Thus membrane lipid hydrolysis by PLD activation by 5-HT via the 5-HT 2A receptor contributes to SMC signaling by 5-HT that leads to cellular proliferation.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Research Grant HL-32723 (B. L. Fanburg) and by an American Heart Association Fellowship (Y. Liu).
Acknowledgments
We thank Dr. Michael Frohman (State University of New York, Stony Brook, NY) for the generous gift of human and mice PLD constructs. In addition, we thank Dr. Viswanathan Natarajan (The University of Chicago, Chicago, IL) for technical counseling with the PLD activity assay.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abdelghaffar H, Vazifeh D, Labro MT. Erythromycin A-derived macrolides modify the functional activities of human neutrophils by altering the phospholipase D-phosphatidate phosphohydrolase transduction pathway: L-cladinose is involved both in alterations of neutrophil functions and modulation of this transductional pathway. J Immunol 159: 3995–4005, 1997. [PubMed] [Google Scholar]
- 2.Adnot S, Eddahibi S. Genetics and physiopathology of primary or secondary pulmonary artery hypertension. Bull Acad Natl Med 187: 1529–1525, 2003. [PubMed] [Google Scholar]
- 3.Cummings R, Parinandi N, Wang L, Usatyuk P, Natarajan V. Phospholipase D/phosphatidic acid signal transduction: role and physiological significance in lung. Mol Cell Biochem 234–235: 99–109, 2002. [PubMed] [Google Scholar]
- 4.Eddahibi S, Adnot S. Serotonin and pulmonary arterial hypertension. Rev Mal Respir 99: 621–625, 2006. [PubMed] [Google Scholar]
- 5.Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest 108: 1141–1150, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Exton JH Regulation of phospholipase D. FEBS Lett 531: 58–61, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Fanburg BL, Lee SL. A new role for an old molecule: serotonin as a mitogen. Am J Physiol Lung Cell Mol Physiol 272: L795–L806, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Foster DA, Xu L. Phospholipase D in cell proliferation and cancer. Mol Cancer Res 1: 789–800, 2003. [PubMed] [Google Scholar]
- 9.Herve P, Drouet L, Dosquet C, Launay JM, Rain B, Simonneau G, Caen J, Duroux P. Primary pulmonary hypertension in a patient with a familial platelet storage pool disease: role of serotonin. Am J Med 89: 117–120, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Herve P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, Poubeau P, Cerrina J, Duroux P, Drouet L. Increased plasma serotonin in primary pulmonary hypertension. Am J Med 99: 249–254, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Hinton JM, Adams D, Garland CJ. 5-hydroxytryptamine stimulation of phospholipase D activity in the rabbit isolated mesenteric artery. Br J Pharmacol 126: 1601–1608, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson MS, Robertson DN, Holland PJ, Lutz EM, Mitchell R. Role of the conserved NPxxY motif of the 5-HT2A receptor in determining selective interaction with isoforms of ADP-ribosylation factor (ARF). Cell Signal 18: 1793–1800, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Khan NA, Hichami A. Ionotrophic 5-hydroxytryptamine type 3 receptor activates the protein kinase C-dependent phospholipase D pathway in human T-cells. Biochem J 344: 199–204, 1999. [PMC free article] [PubMed] [Google Scholar]
- 14.Ktistakis NT, Delon C, Manifava M, Wood E, Ganley I, Sugars JM. Phospholipase D1 and potential targets of its hydrolysis product, phosphatidic acid. Biochem Soc Trans 31: 94–97, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Kurscheid-Reich D, Throckmorton DC, Rasmussen H. Serotonin activates phospholipase D in rat mesangial cells. Am J Physiol Renal Fluid Electrolyte Physiol 268: F997–F1003, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Lee SL, Wang WW, Finlay GA, Fanburg BL. Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am J Physiol Lung Cell Mol Physiol 277: L282–L291, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Lee SL, Wang WW, Lanzillo JJ, Fanburg BL. Regulation of serotonin-induced DNA synthesis of bovine pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 266: L53–L60, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Fanburg BL. Serotonin-induced growth of pulmonary artery smooth muscle requires activation of phosphatidylinositol 3-kinase/serine-threonine protein kinase B/mammalian target of rapamycin/p70 ribosomal S6 kinase 1. Am J Respir Cell Mol Biol 34: 182–191, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Li M, Warburton RR, Hill NS, Fanburg BL. The 5-HT transporter transactivates the PDGFbeta receptor in pulmonary artery smooth muscle cells. Faseb J 21: 2725–2734, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res 95: 579–586, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Lopez I, Arnold RS, Lambeth JD. Cloning and initial characterization of a human phospholipase D2 (hPLD2). ADP-ribosylation factor regulates hPLD2. J Biol Chem 273: 12846–12852, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res 94: 1263–1270, 2004. [DOI] [PubMed] [Google Scholar]
- 23.McGrew L, Chang MS, Sanders-Bush E. Phospholipase D activation by endogenous 5-hydroxytryptamine 2C receptors is mediated by Galpha13 and pertussis toxin-insensitive Gbetagamma subunits. Mol Pharmacol 62: 1339–1343, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Morris AJ Regulation of phospholipase D activity, membrane targeting and intracellular trafficking by phosphoinositides. Biochem Soc Symp: 247–257, 2007. [DOI] [PubMed]
- 25.Natarajan V, Garcia JG. Agonist-induced activation of phospholipase D in bovine pulmonary artery endothelial cells: regulation by protein kinase C and calcium. J Lab Clin Med 121: 337–347, 1993. [PubMed] [Google Scholar]
- 26.Palomaki VA, Laitinen JT. The basic secretagogue compound 48/80 activates G proteins indirectly via stimulation of phospholipase D-lysophosphatidic acid receptor axis and 5-HT1A receptors in rat brain sections. Br J Pharmacol 147: 596–606, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plevin NAKR, Wakelam MJ, Wadsworth R. Regulation by hypoxia of endothelin-1-stimulated phospholipase D activity in sheep pulmonary artery cultured smooth muscle cells (Abstract). Br J Pharmacol 112: 5, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzo M, Romero G. Pharmacological importance of phospholipase D and phosphatidic acid in the regulation of the mitogen-activated protein kinase cascade. Pharmacol Ther 94: 35–50, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Rizzo MA, Kraft CA, Watkins SC, Levitan ES, Romero G. Agonist-dependent traffic of raft-associated Ras and Raf-1 is required for activation of the mitogen-activated protein kinase cascade. J Biol Chem 276: 34928–34933, 2001. [DOI] [PubMed] [Google Scholar]
- 29a.Ross R The smooth muscle cell: II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol 50: 172–186, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circulation 100: 869–875, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Salvador GA, Ilincheta de Boschero MG, Pasquare SJ, Giusto NM. Phosphatidic acid and diacylglycerol generation is regulated by insulin in cerebral cortex synaptosomes from adult and aged rats. J Neurosci Res 81: 244–252, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Walker SJ, Brown HA. Measurement of G protein-coupled receptor-stimulated phospholipase D activity in intact cells. Methods Mol Biol 237: 89–97, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Cummings R, Zhao Y, Kazlauskas A, Sham JK, Morris A, Georas S, Brindley DN, Natarajan V. Involvement of phospholipase D2 in lysophosphatidate-induced transactivation of platelet-derived growth factor receptor-beta in human bronchial epithelial cells. J Biol Chem 278: 39931–39940, 2003. [DOI] [PubMed] [Google Scholar]