Abstract
AMP-activated protein kinase (AMPK) is activated by increases in the intracellular AMP-to-ATP ratio and plays a central role in cellular responses to metabolic stress. Although activation of AMPK has been shown to have anti-inflammatory effects, there is little information concerning the role that AMPK may play in modulating neutrophil function and neutrophil-dependent inflammatory events, such as acute lung injury. To examine these issues, we determined the effects of pharmacological activators of AMPK, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) and barberine, on Toll-like receptor 4 (TLR4)-induced neutrophil activation. AICAR and barberine dose-dependently activated AMPK in murine bone marrow neutrophils. Exposure of LPS-stimulated neutrophils to AICAR or barberine inhibited release of TNF-α and IL-6, as well as degradation of IκBα and nuclear translocation of NF-κB, compared with findings in neutrophil cultures that contained LPS without AICAR or barberine. Administration of AICAR to mice resulted in activation of AMPK in the lungs and was associated with decreased severity of LPS-induced lung injury, as determined by diminished neutrophil accumulation in the lungs, reduced interstitial pulmonary edema, and diminished levels of TNF-α and IL-6 in bronchoalveolar lavage fluid. These results suggest that AMPK activation reduces TLR4-induced neutrophil activation and diminishes the severity of neutrophil-driven proinflammatory processes, including acute lung injury.
Keywords: adenosine 5′-monophosphate-activated protein kinase, neutrophil, nuclear factor-κB, cytokine
amp-activated protein kinase (AMPK) is a serine/threonine protein kinase consisting of three heterogenic subunits, a catalytic α-subunit as well as regulatory β- and γ-subunits that directly bind AMP (9, 24, 44, 52). AMPK activation is initiated by alterations in cellular metabolic status that result from inhibition of ATP generation, such as hypoxia, glucose deprivation, heat shock, or reduction in mitochondrial oxidative phosphorylation, or from increased ATP consumption, such as occurs during exercise or with increased cellular metabolism (15, 24, 56, 61). AMPK participates in the activation of ATP-generating pathways involving uptake and oxidation of glucose and fatty acids and inhibits intracellular events that consume ATP and are not essential for the short-term survival of the cell (22, 23, 27, 42, 55). Although AMPK was initially shown to be involved in regulation of fatty acid and cholesterol synthesis (14), there is recent evidence that AMPK participates in modulating acute inflammatory reactions. For example, activation of AMPK is associated with diminished nuclear translocation of NF-κB in TNF-α-stimulated endothelial cells (50).
Neutrophils play a central role in innate immune and inflammatory responses (7, 49). Although neutrophils have beneficial actions in eradicating microbial infections, excessive neutrophil activation, with resultant release of cytokines and other proinflammatory mediators, results in tissue injury and contributes to the development of organ dysfunction, such as acute lung injury (ALI) (2, 19, 37, 47). Although AMPK is present in neutrophils and has been shown to be involved in modulating respiratory burst (6), there is little additional information concerning the roles that AMPK may play in acute inflammatory processes, such as ALI, in which neutrophils play a major role.
In the present study, we examined the role that AMPK occupies in modulating Toll-like receptor 4 (TLR4)-induced activation of neutrophils and the development of ALI. We found that AMPK activation inhibited cytokine production, IκBα degradation, and nuclear translocation of NF-κB in LPS-stimulated neutrophils. Enhanced activation of AMPK also resulted in diminished severity of LPS-induced ALI in mice.
MATERIALS AND METHODS
Mice.
Male C57BL/6 mice (age 8–12 wk; The Jackson Laboratory, Bar Harbor, ME) were kept on a 12:12-h light-dark cycle with free access to chow and water. All experiments were conducted in accordance with institutional review board-approved protocols.
Materials.
RPMI 1640, l-glutamine, penicillin-streptomycin, barberine, and Escherichia coli 0111:B4 endotoxin (LPS) were obtained from Sigma-Aldrich (St. Louis, MO). FBS was purchased from Atlanta Biologicals (Norcross, GA). 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) was purchased from Toronto Research Chemicals (Toronto, Canada). Antibodies against phosphorylated-AMPK-α (Thr172), phosphorylated acetyl-CoA carboxylase (ACC) (Ser79), AMPK-α (Thr172), ACC (Ser79), and IκBα were purchased from Cell Signaling Technology (Beverly, MA), whereas anti-actin antibody was from Sigma-Aldrich. Goat anti-rabbit IgG horseradish peroxidase (HRP) conjugate was obtained from Bio-Rad. Custom antibody mixtures and negative selection columns for neutrophil isolation were purchased from Stemcell Technologies (Vancouver, British Columbia, Canada). Cytokine ELISA kits were obtained from R&D Systems (Minneapolis, MN).
Neutrophil isolation and culture.
Bone marrow neutrophils were isolated as previously described (53, 63). Neutrophil purity was consistently >97%, as determined by Wright-Giemsa-stained cytospin preparations. Neutrophils were cultured in RPMI 1640 medium containing 0.5% FBS and treated as indicated in the figure legends. Neutrophil viability under experimental conditions was determined using trypan blue staining and was consistently >99%.
Nuclear extract purification.
Purification of nuclei was performed as previously described (38, 63). Briefly, bone marrow neutrophils (4 × 106) were collected in PBS and centrifuged. The cell pellet was resuspended with 50 μl of hypotonic buffer [Tris, pH 7.5 (10 mM), NaCl (10 mM), MgCl2 (3 mM), Nonidet P-40 (0.05%), EGTA (1 mM), and protease/phosphatase inhibitors] and then centrifuged (2,700 g) for 5 min at 4°C. The nuclei were lysed in 50 μl of Nonidet P-40 lysis buffer [Tris, pH 7.5 (50 mM), NaCl (137 mM), Nonidet P-40 (0.1%), EDTA (2 mM), and protease/phosphatase inhibitors]. After 1-h incubation on ice, enriched nuclei fractions were collected by centrifugation (10,000 g) for 20 min at 4°C.
Western blot analysis.
Western blot analysis was performed as previously described (53, 63). In brief, samples obtained from neutrophils or lung homogenates were mixed with Laemmli sample buffer and boiled for 5 min. Equal amounts of protein were resolved by 8–10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Immobilon-P; Millipore, Billerica, MA). The membranes were probed with specific antibodies to IκBα, phospho-AMPKα, phospho-ACC, AMPKα, ACC, or actin, followed by detection with HRP-conjugated goat anti-rabbit IgG. Bands were visualized by enhanced chemiluminescence (ECL Plus, Amersham) and quantified by using AlphaEase FC software (Alpha Innotech, San Leandro, CA). Each experiment was carried out three or more times using cell populations or lung homogenates obtained from separate groups of mice.
EMSA.
Nuclear extracts were obtained from bone marrow neutrophils treated with AICAR with or without inclusion of LPS in the cultures, and EMSA were performed as reported previously (45, 53, 60). In brief, the κB DNA sequence of the Ig gene was used. Synthetic double-stranded sequences (with enhancer motifs underlined) were filled in and labeled with [32P]dATP (GE Healthcare) using Sequenase DNA polymerase: B sequence, 5′-GCCATGGGGGGATCCCCGAAGTCC-3′ (Geneka Biotechnology).
ALI model.
ALI was induced by intratracheal administration of 1 mg/kg LPS in 50 μl of PBS. With this model, ALI, as characterized by neutrophil infiltration into the lung interstitium and airways, development of interstitial edema, and increased pulmonary proinflammatory cytokine production, occurs after injection of LPS, with the greatest degree of injury being present 24 h after LPS exposure (4, 8, 41). Briefly, mice were anesthetized with isoflurane. The tongue was then gently extended, and the LPS solution was deposited into the pharynx (53). For the AICAR or vehicle pretreatment groups, mice were injected intraperitoneally with either 100 μl of 0.9% saline (controls) or AICAR (500 mg/kg body wt ip) dissolved in 0.9% saline 4 h before intratracheal administration of LPS. There were no deaths associated with AICAR and/or LPS administration.
Harvest of lungs and bronchoalveolar lavage.
Lungs were harvested 24 h after LPS administration. As described previously (53), bronchoalveolar lavage (BAL) was obtained by cannulating the trachea with a blunt 20-gauge needle and then lavaging the lungs three times with 1 ml of ice-cold PBS. Total cell counts were measured in the BAL fluid with a hemocytometer (Hausser Scientific).
BAL fluid samples were centrifuged onto glass slides at 500 rpm for 5 min using a Shandon Cytospin 4 (Thermo Fisher Scientific, Kalamazoo, MI). The slides were fixed and stained with Hema-3 stain (Thermo Fisher Scientific), and the number of total cells and neutrophils were counted.
Wet-to-dry lung weight ratios.
Wet-to-dry ratios were determined as reported previously (4). In brief, lungs were excised, rinsed with PBS, blotted, and then weighed for the “wet” weight. Lungs were then dried in an oven for 7 days at 80°C, and the “dry” weight was measured.
MPO assay.
MPO activity was quantified as reported previously with minor modifications (18, 53). In brief, lung homogenates collected in 1 ml of potassium phosphate buffer (50 mM), pH 6.0, and N-ethylmaleimide (10 mM) were centrifuged at 12,000 g for 30 min at 4°C. The pellet was then washed twice and sonicated on ice for 90 s in hexadecyltrimethylammonium bromide (HTAB) buffer [HTAB (0.5%), potassium phosphate, pH 6.0 (50 mM)]. The samples were incubated in a water bath for 2 h at 56°C and then centrifuged (12,000 g) for 10 min. The supernatants were collected for assay of MPO activity by measuring the H2O2-dependent oxidation of 3,3′-dimethoxybenzidine dihydrochloride (λ = 460 nm).
Cytokine ELISA.
ELISA were used to measure cytokines in BAL fluid or in cell culture supernatants from LPS-treated neutrophils as previously described (53). Levels of TNF-α and IL-6 were determined using commercial available ELISA kits (R&D Systems) according to the manufacturer's instructions.
Statistical analyses.
For each experiment, neutrophils were isolated and pooled from groups of mice (n = 3–6) and all conditions were studied at the same time. In studies examining LPS-induced ALI, each experimental group consisted of at least 6 mice. One-way ANOVA, the Tukey-Kramer multiple comparisons test (for multiple groups), or Student's t-test (for comparisons between 2 groups) were used. P < 0.05 was considered to be statistically significant.
RESULTS
AICAR activates AMPK in bone marrow neutrophils.
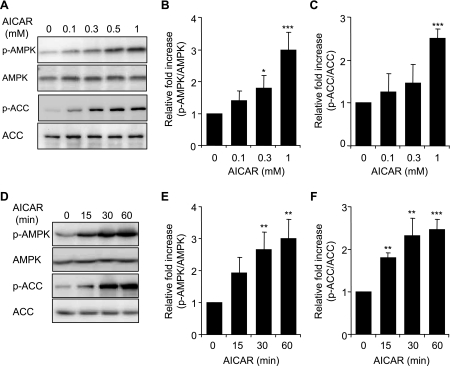
AICAR is a cell permeable compound that has been shown to activate AMPK in various cell populations, including human neutrophils (6, 12, 32). To confirm the ability of AICAR to activate AMPK in murine neutrophils, we determined AMPK Thr172 phosphorylation in bone marrow neutrophils incubated with increasing doses of AICAR. In addition to assessing AMPK Thr172 phosphorylation, AMPK activation was also confirmed by determining the levels of Ser79 phosphorylated ACC, a downstream target of AMPK.
As shown in Fig. 1, A–C, treatment of neutrophils with AICAR dose-dependently increased AMPK and ACC phosphorylation, an effect that reached maximal levels with the highest concentration (1 mM) of AICAR. AICAR-induced phosphorylation of AMPK and ACC was rapid, beginning within 15 min after addition of AICAR to neutrophil cultures, and reached maximum levels after 60 min (Fig. 1, D–F). LPS treatment did not affect AICAR-induced phosphorylation of AMPK or ACC (data not shown).
Fig. 1.
Effects of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) on AMP-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC) phosphorylation (p-) in bone marrow neutrophils. Neutrophils were cultured with AICAR (0, 0.1, 0.3, or 1 mM) for 60 min (A–C) or with 1 mM AICAR for the indicated time periods (D–F). Cell lysates were subjected to SDS-PAGE and Western blot analysis using antibodies specific for phospho-AMPK (Thr172), total AMPK, phospho-ACC (Ser79), and total ACC. A and D show representative Western blots, whereas B, C, E, and F demonstrate mean band optical density obtained from 3 individual experiments. Data are expressed as p-AMPK/AMPK or p-ACC/ACC ratios. Means ± SE, n = 3; *P < 0.05; **P < 0.01; ***P < 0.001 compared with control (untreated cells).
AMPK activation inhibits LPS-induced NF-κB activation and cytokine production.
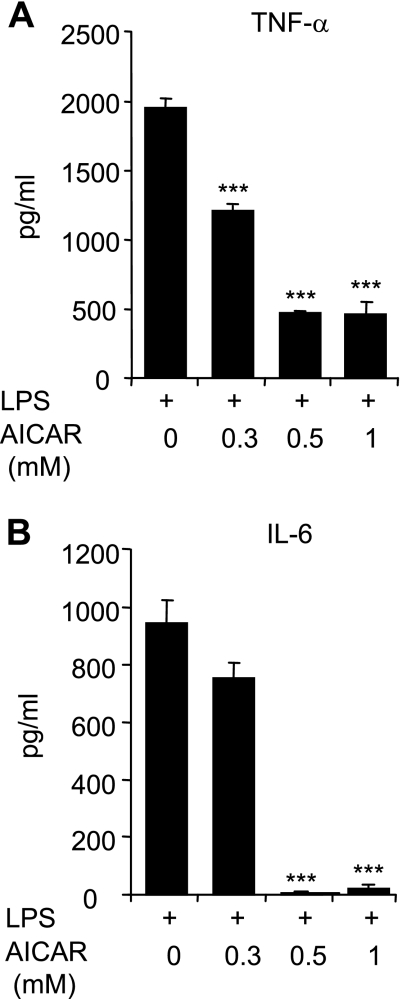
To investigate the effects of AMPK activation on LPS-induced cytokine production by neutrophils, cells were cultured with a range of AICAR concentrations (0.1 to 1 mM) for 1 h, followed by treatment with LPS (100 ng/ml) for 4 h. As shown in Fig. 2, A and B, significant increases in TNF-α and IL-6 secretion, to 900 pg/ml or greater, were produced by exposure of neutrophils to LPS. Production of both cytokines by LPS-stimulated neutrophils was decreased in a dose-dependent manner by AICAR.
Fig. 2.
AICAR inhibits LPS-induced TNF-α and IL-6 production by neutrophils. Cells were treated with the indicated doses of AICAR for 1 h followed by LPS (0 or 100 ng/ml) for 4 h. Levels of TNF-α (A) and IL-6 (B) in the culture supernatants were determined using ELISA assays. Values are expressed as means ± SE using results from 3 independent experiments. ***P < 0.001 compared with LPS-treated cells.
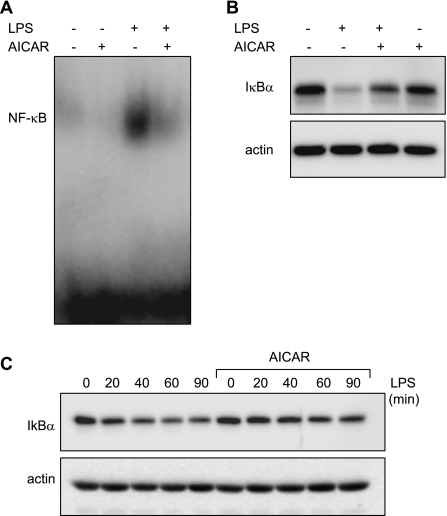
To investigate if the inhibitory effects of AMPK activation on proinflammatory cytokine production might occur through altering NF-κB activation, nuclear translocation of NF-κB was determined in LPS-stimulated neutrophils cultured with or without AICAR. As shown in Fig. 3A, nuclear accumulation of NF-κB was increased in LPS-stimulated neutrophils but was markedly attenuated when the cells were pretreated with AICAR.
Fig. 3.
AMPK inhibits LPS-induced IκBα degradation and NF-κB nuclear accumulation in LPS-treated neutrophils. Neutrophils were treated with AICAR (0 or 1 mM) for 1 h followed by LPS (0 or 100 ng/ml) for 1 h. A: representative EMSA showing inhibitory effects of AICAR on LPS-induced nuclear accumulation of NF-κB. B: IκBα levels in LPS-stimulated neutrophils treated or untreated with AICAR. C: neutrophils were pretreated with AICAR (0 or 1 mM) for 1 h, and degradation of IκBα was determined after at baseline and 20, 40, 60, and 90 min after the addition of LPS (100 ng/ml) to the cultures. A representative Western blot of IκBα and actin are shown. A 2nd experiment with neutrophils derived from a separate group of mice provided similar results.
NF-κB exists as a p65/p50 heterodimer and is retained in cytoplasm through association with IκBα (1, 3, 46). Phosphorylation of IκBα by the upstream kinase IKK, with subsequent ubiquitination and proteasomal degradation of IκBα, leads to exposure of the nuclear localization sequence in NF-κB and translocation of NF-κB to the nucleus (11, 20). To determine whether the inhibition of LPS-induced NF-κB nuclear translocation by AICAR was due to effects on IκBα degradation, we examined levels of IκBα in AICAR-treated neutrophils. As shown in Fig. 3, B and C, degradation of IκBα produced by neutrophil exposure to LPS was prevented by AICAR pretreatment.
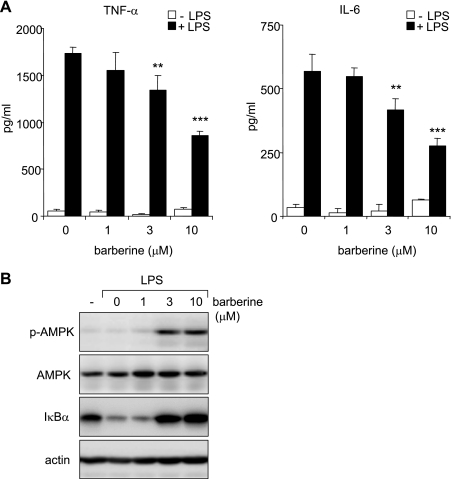
Although the primary role of AICAR is in activating AMPK, AICAR has been reported to affect additional intracellular signaling pathways, such as phosphatidylinositol 3-kinase and Akt (29). Therefore, to confirm that AMPK activation has inhibitory effects on neutrophil activation, we treated LPS-stimulated neutrophils with barberine, which activates AMPK through inhibiting mitochondrial respiratory complex I (54).
As shown in Fig. 4A, barberine dose-dependently decreased LPS-induced cytokine production by neutrophils. As previously reported in other cell types (36, 54), barberine activated AMPK in neutrophils (Fig. 4B). Similar to the effects of AICAR, pretreatment of neutrophils with barberine (3 or 10 μM) inhibited IκBα degradation in LPS-stimulated neutrophils (Fig. 4B).
Fig. 4.
Barberine-dependent activation of AMPK inhibits LPS-induced IκBα degradation and cytokine production. A: neutrophils were cultured with barberine (0, 1, 3, or 10 μM) for 60 min followed by stimulation with LPS (0 or 100 ng/ml) for 4 h. Levels of TNF-α and IL-6 in the culture media were measured using ELISA assays. Means ± SE, n = 3; ***P < 0.001 or **P < 0.01 compared with LPS-treated cells. B: representative Western blots show levels of phospho-AMPK (Thr172), total AMPK, IκBα, or actin in cell extracts obtained from neutrophils treated with barberine (0, 1, 3, or 10 μM) for 60 min and then LPS (100 ng/ml) for an additional 1 h. A 2nd experiment with neutrophils obtained from a separate group of mice provided similar results.
Effects of AMPK activation on the severity of LPS-induced ALI.
Activated neutrophils that accumulate in the lungs and that produce cytokines and other proinflammatory mediators play a central role in the pathogenesis of LPS-induced ALI (2, 5, 60). To determine if AMPK activation modulates LPS-induced proinflammatory response in vivo, mice were injected with saline or saline containing AICAR (500 mg/kg ip) 4 h before intratracheal administration of LPS, and then parameters of lung injury were determined 24 h after LPS exposure.
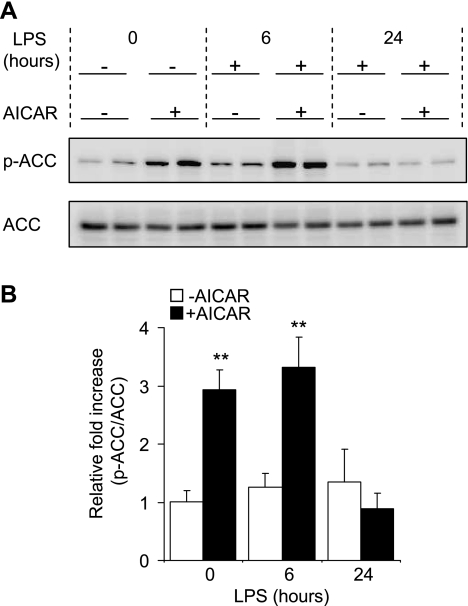
Significant increases in Ser79 phosphorylated ACC, a major downstream target of activated AMPK (24, 57, 59), were consistently present in lung homogenates from AICAR-treated mice (Fig. 5A). Phosphorylation of ACC was significantly increased 4 and 10 h after AICAR administration. The effects of AICAR on AMPK activation, as determined by phosphorylation of ACC, had largely dissipated by 28 h after its administration (Fig. 5).
Fig. 5.
Effects of AICAR on ACC phosphorylation in vivo. Mice were injected intraperitoneally with saline or AICAR (500 mg/kg) in saline 4 h before intratracheal PBS or PBS with LPS (1 mg/kg) administration. Lung homogenates were collected in mice treated with AICAR or saline 4 h previously or 6 or 24 h after LPS treatment, and samples were subjected to Western blot analysis using anti-phosphorylated-ACC (Ser79) and anti-total ACC specific antibodies. A: representative Western blots. B: quantitative densitometry of p-ACC/ACC ratios (means ± SE) with n = 3 mice/group. **P < 0.01 comparing control (LPS/PBS) to LPS/AICAR-treated mice.
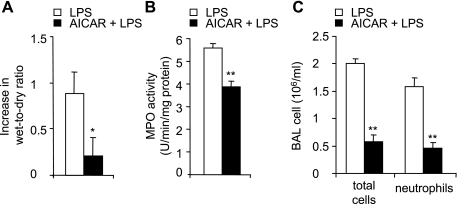
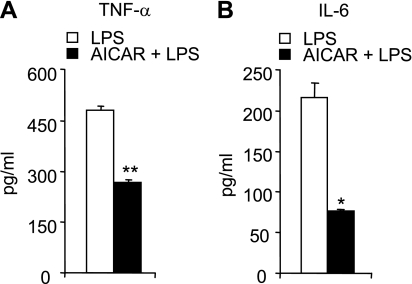
The severity of LPS-induced lung injury was reduced in AICAR-treated mice, as shown by significantly decreased wet-to-dry ratios, a measure of interstitial pulmonary edema (Fig. 6A). Neutrophil accumulation into the lung parenchyma after LPS administration was also reduced by AICAR pretreatment (Fig. 6B). LPS instillation resulted in increased numbers of total cells and neutrophils in BAL (Fig. 6C), and these effects were diminished by AICAR administration. AICAR pretreatment also significantly attenuated LPS-induced increases in bronchoalveolar concentrations of TNF-α and IL-6 (Fig. 7). AICAR alone did not affect wet-to-dry ratio or pulmonary MPO levels and had no effect on total cell or neutrophil counts or cytokine concentrations in BAL fluid (data not shown).
Fig. 6.
AICAR attenuates LPS-induced acute lung injury in vivo. Mice were treated intratracheally with either PBS or PBS with LPS (1 mg/kg) or were given intraperitoneal saline or saline with AICAR (500 mg/kg) followed 4 h later with either PBS or PBS with LPS (1 mg/kg) intratracheally. Lungs and bronchoalveolar lavage (BAL) were obtained 24 h after LPS administration. A: effects of AICAR treatment on LPS-induced increase in lung wet-to-dry ratios. B: lung MPO levels. C: number of total white cells and neutrophils in BAL fluid. Means ± SE are shown using results from n = 6 mice per group; *P < 0.05 or **P < 0.01 when comparing AICAR/LPS to mice treated with LPS alone.
Fig. 7.
AICAR decreases LPS-induced pulmonary cytokine levels in vivo. BAL levels of TNF-α (A) and IL-6 (B). BAL fluid was obtained 24 h after LPS administration in mice treated intraperitoneally with either saline or saline with AICAR (500 mg/kg) 4 h before intratracheal LPS. Means ± SE are shown. n = 6 mice in each group; *P < 0.05 and **P < 0.01 comparing the AICAR/LPS group to mice treated with PBS/LPS.
DISCUSSION
In the present study, we found that activation of AMPK inhibits LPS-induced proinflammatory responses in murine neutrophils and also diminishes the severity of ALI, an inflammatory process in which neutrophils play an important role (51, 60). AMPK activation in LPS-treated neutrophils was associated with decreased degradation of IκBα and diminished nuclear translocation of NF-κB as well as reduced release of TNF-α and IL-6. Such results indicate that the inhibitory effects of AMPK on TLR4-induced neutrophil activation are likely to be through events upstream of IκBα degradation, including activation of IKK or more proximal TLR4-associated kinases such as IL-1 receptor-associated kinase-1 (IRAK-1).
Our results demonstrate that treatment of murine neutrophils with AICAR or barberine produces significant increases in phosphorylation of Thr172 in the AMPK α-subunit. Although the effects of AICAR or barberine on AMPK activation in murine neutrophils have not previously been described, the ability of AICAR and barberine to activate AMPK is consistent with results from previous studies in other cell populations, including human neutrophils (6). In the intracellular milieu, AICAR is phosphorylated to AICA riboside monophosphate (ZMP), which binds to and activates AMPK by mimicking effects of AMP. Binding of ZMP to AMPK produces allosteric alterations in AMPK, making the enzyme a better substrate for the upstream serine/threonine kinase 11 (STK11, also called LKB1) (21, 31). Barberine activates AMPK through inhibiting mitochondrial complex I (54), a different mechanism than is involved with AICAR-induced phosphorylation of AMPK.
Anti-inflammatory effects of AMPK activation have been previously reported in nonmyeloid cell populations (17, 29, 35). In murine models of experimental autoimmune encephalomyelitis (39) and asthma (34), AICAR treatment reduced proinflammatory cytokine production and decreased histological alterations associated with inflammatory injury. In additional studies, antidiabetic drugs of the biguanide class, such as metformin, were used to activate AMPK and decreased NF-κB activation and cytokine release in vascular endothelial cells (25, 28). Biguanides activate AMPK through mechanisms that differ from those used by AICAR. In particular, whereas metformin produces activation of AMPK through inhibiting mitochondrial complex I activity, in a manner similar to barberine, and increasing mitochondrial production of reactive oxygen species (10, 33, 62), AICAR does not affect mitochondrial function (52, 58).
In the present study, AICAR and barberine significantly enhanced AMPK activity, as shown by phosphorylation of the downstream target ACC, and also increased the levels of phosphorylated AMPK in mouse bone marrow neutrophils. These findings are in contrast to reports indicating that AICAR had no effects on AMPK activation in RAW 264.7 macrophages (29, 35) but are consistent with previous reports that AICAR can increase AMPK activity in human peripheral blood neutrophils (6). Such results indicate that the ability of AICAR to activate AMPK, and presumably to exert anti-inflammatory actions, is cell population specific.
To understand the molecular mechanisms underlying the anti-inflammatory effects of AMPK activation, we studied the ability of AICAR and barberine to modify LPS-induced activation of NF-κB and degradation of IκBα. We found that AICAR and barberine alone had no effects on nuclear accumulation of NF-κB in resting neutrophils. In contrast, AICAR as well as barberine inhibited LPS-induced degradation of IκBα and also decreased nuclear translocation of NF-κB, consistent with previous reports that AICAR can inhibit LPS-induced NF-κB activation and the expression of NF-κB-dependent genes, such as TNF-α and inducible nitric oxide synthase (35, 48).
Administration of AICAR to mice resulted in activation of AMPK in the lungs and diminished severity of LPS-induced ALI. These results are consistent with previous reports that AICAR is able to reduce lung injury in a model of hemorrhagic shock (13). Neutrophils play a major role in the early development of TLR2, TLR4, or hemorrhage-induced ALI, as shown by diminished severity of pulmonary injury after the induction of neutropenia (5, 26, 43). Therefore, it is likely that the beneficial effects of AMPK activation on the severity of LPS-induced ALI, as well as ALI associated with hemorrhage, result from diminished release of proinflammatory mediators by neutrophils, consistent with our in vitro results. In addition to modifying TLR4-induced neutrophil activation, systemic administration of AICAR is likely to affect additional pulmonary and extrapulmonary cell populations, including endothelial and epithelial cells, which contribute to the severity of ALI. In particular, previous studies in each of these cell populations found that AMPK activation was anti-inflammatory (16, 40, 64).
The present results, showing that AICAR or barberine treatment reduced TLR4-induced activation of isolated neutrophils and that AICAR administration diminished pulmonary injury after exposure to LPS, are consistent with previous studies demonstrating that AMPK activation has potent anti-inflammatory effects (13, 25, 28). These findings suggest that modulation of AMPK activation may be an appropriate pharmacological target in the treatment of ALI as well as other neutrophil-driven acute inflammatory processes.
GRANTS
This work was supported, in part, by National Heart, Lung, and Blood Institute Grants HL-62221, HL-76206, and HL-068743 to E. Abraham and by the Société Française d'Anesthésie et de Réanimation and the University Hospital of Amiens (France) to E. Lorne.
Acknowledgments
We thank Youhong Zhang for excellent technical support.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abraham E Alterations in cell signaling in sepsis. Clin Infect Dis 41, Suppl 7: S459–S464, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Abraham E Neutrophils and acute lung injury. Crit Care Med 31: S195–S199, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Abraham E NF-kappaB activation. Crit Care Med 28: N100–N104, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol 165: 2950–2954, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 279: L1137–L1145, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Alba G, El Bekay R, Alvarez-Maqueda M, Chacon P, Vega A, Monteseirin J, Santa Maria C, Pintado E, Bedoya FJ, Bartrons R, Sobrino F. Stimulators of AMP-activated protein kinase inhibit the respiratory burst in human neutrophils. FEBS Lett 573: 219–225, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol 172: 2522–2529, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Asti C, Ruggieri V, Porzio S, Chiusaroli R, Melillo G, Caselli GF. Lipopolysaccharide-induced lung injury in mice. I. Concomitant evaluation of inflammatory cells and haemorrhagic lung damage. Pulm Pharmacol Ther 13: 61–69, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Baron SJ, Li J, Russell RR 3rd, Neumann D, Miller EJ, Tuerk R, Wallimann T, Hurley RL, Witters LA, Young LH. Dual mechanisms regulating AMPK kinase action in the ischemic heart. Circ Res 96: 337–345, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhausl W, Furnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes 53: 1052–1059, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZJ Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol 7: 758–765, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558–565, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Davis KA, Fabian TC, Ragsdale DN, Trenthem LL, Croce MA, Proctor KG. Combination therapy that targets secondary pulmonary changes after abdominal trauma. Shock 15: 479–484, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Dolinsky VW, Dyck JR. Role of AMP-activated protein kinase in healthy and diseased hearts. Am J Physiol Heart Circ Physiol 291: H2557–H2569, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P, Kinnear NP, Hardie DG. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem 280: 41504–41511, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Gaskin FS, Kamada K, Yusof M, Korthuis RJ. 5′-AMP-activated protein kinase activation prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol 292: H326–H332, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci 24: 479–487, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol 59: 1978–1985, 1985. [DOI] [PubMed] [Google Scholar]
- 19.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol 23: 821–852, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE: re13, 2006. [DOI] [PubMed]
- 21.Hardie DG AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol 47: 185–210, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Hardie DG Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144: 5179–5183, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Hardie DG New roles for the LKB1–>AMPK pathway. Curr Opin Cell Biol 17: 167–173, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase–development of the energy sensor concept. J Physiol 574: 7–15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 47: 1183–1188, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Hollingsworth JW, Chen BJ, Brass DM, Berman K, Gunn MD, Cook DN, Schwartz DA. The critical role of hematopoietic cells in lipopolysaccharide-induced airway inflammation. Am J Respir Crit Care Med 171: 806–813, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schonbeck U, Libby P. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol 26: 611–617, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Jhun B, Jin Q, Oh Y, Kim S, Kong Y, Cho J, Ha J, Bai H, Kang I. 5-Aminoimidazole-4-carboxamide riboside suppresses lipopolysaccharide-induced TNF-alpha production through inhibition of phosphatidylinositol 3-kinase/Akt activation in RAW 264.7 murine macrophages. Biochem Biophys Res Commun 318: 372–380, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, Liang MC, Cai D, Naumov GN, Bao L, Contreras CM, Li D, Chen L, Krishnamurthy J, Koivunen J, Chirieac LR, Padera RF, Bronson RT, Lindeman NI, Christiani DC, Lin X, Shapiro GI, Janne PA, Johnson BE, Meyerson M, Kwiatkowski DJ, Castrillon DH, Bardeesy N, Sharpless NE, Wong KK. LKB1 modulates lung cancer differentiation and metastasis. Nature 448: 807–810, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Jin Q, Feng L, Behrens C, Bekele BN, Wistuba II, Hong WK, Lee HY. Implication of AMP-activated protein kinase and Akt-regulated survivin in lung cancer chemopreventive activities of deguelin. Cancer Res 67: 11630–11639, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1: 15–25, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Kim TB, Kim SY, Moon KA, Park CS, Jang MK, Yun ES, Cho YS, Moon HB, Lee KY. Five-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside attenuates poly (I:C)-induced airway inflammation in a murine model of asthma. Clin Exp Allergy 37: 1709–1719, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Kuo CL, Ho FM, Chang MY, Prakash E, Lin WW. Inhibition of lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 gene expression by 5-aminoimidazole-4-carboxamide riboside is independent of AMP-activated protein kinase. J Cell Biochem 103: 931–940, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55: 2256–2264, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Lomas-Neira J, Chung CS, Perl M, Gregory S, Biffl W, Ayala A. Role of alveolar macrophage and migrating neutrophils in hemorrhage-induced priming for ALI subsequent to septic challenge. Am J Physiol Lung Cell Mol Physiol 290: L51–L58, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Lorne E, Zmijewski JW, Zhao X, Liu G, Tsuruta Y, Park YJ, Dupont H, Abraham E. Role of extracellular superoxide in neutrophil activation: interactions between xanthine oxidase and TLR4 induce proinflammatory cytokine production. Am J Physiol Cell Physiol 294: C985–C993, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Nath N, Giri S, Prasad R, Salem ML, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide ribonucleoside: a novel immunomodulator with therapeutic efficacy in experimental autoimmune encephalomyelitis. J Immunol 175: 566–574, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Pilon G, Dallaire P, Marette A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J Biol Chem 279: 20767–20774, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Rowe SJ, Allen L, Ridger VC, Hellewell PG, Whyte MK. Caspase-1-deficient mice have delayed neutrophil apoptosis and a prolonged inflammatory response to lipopolysaccharide-induced acute lung injury. J Immunol 169: 6401–6407, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 24: 1810–1820, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savov JD, Gavett SH, Brass DM, Costa DL, Schwartz DA. Neutrophils play a critical role in development of LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol 283: L952–L962, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 113: 274–284, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shenkar R, Schwartz MD, Terada LS, Repine JE, McCord J, Abraham E. Hemorrhage activates NF-κB in murine lung mononuclear cells in vivo. Am J Physiol Lung Cell Mol Physiol 270: L729–L735, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation: a question of life or death. J Biochem Mol Biol 35: 28–40, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Smith JA Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol 56: 672–686, 1994. [DOI] [PubMed] [Google Scholar]
- 48.Solaz-Fuster MC, Gimeno-Alcaniz JV, Casado M, Sanz P. TRIP6 transcriptional co-activator is a novel substrate of AMP-activated protein kinase. Cell Signal 18: 1702–1712, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Strassheim D, Asehnoune K, Park JS, Kim JY, He Q, Richter D, Kuhn K, Mitra S, Abraham E. Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J Immunol 172: 5727–5733, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Su RY, Chao Y, Chen TY, Huang DY, Lin WW. 5-Aminoimidazole-4-carboxamide riboside sensitizes TRAIL- and TNFα-induced cytotoxicity in colon cancer cells through AMP-activated protein kinase signaling. Mol Cancer Ther 6: 1562–1571, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Togbe D, Schnyder-Candrian S, Schnyder B, Doz E, Noulin N, Janot L, Secher T, Gasse P, Lima C, Coelho FR, Vasseur V, Erard F, Ryffel B, Couillin I, Moser R. Toll-like receptor and tumour necrosis factor dependent endotoxin-induced acute lung injury. Int J Exp Pathol 88: 387–391, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100: 328–341, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Tsuruta Y, Park YJ, Siegal GP, Liu G, Abraham E. Involvement of vitronectin in lipopolysaccaride-induced acute lung injury. J Immunol 179: 7079–7086, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, Hu LH, Li J, Ye JM. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 57: 1414–1418, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, Hue L, Andreelli F. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J Physiol 574: 41–53, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 88: 2219–2226, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Witters LA, Kemp BE. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J Biol Chem 267: 2864–2867, 1992. [PubMed] [Google Scholar]
- 58.Woollhead AM, Sivagnanasundaram J, Kalsi KK, Pucovsky V, Pellatt LJ, Scott JW, Mustard KJ, Hardie DG, Baines DL. Pharmacological activators of AMP-activated protein kinase have different effects on Na+ transport processes across human lung epithelial cells. Br J Pharmacol 151: 1204–1215, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol 574: 73–83, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang KY, Arcaroli JJ, Abraham E. Early alterations in neutrophil activation are associated with outcome in acute lung injury. Am J Respir Crit Care Med 167: 1567–1574, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Yun H, Lee M, Kim SS, Ha J. Glucose deprivation increases mRNA stability of vascular endothelial growth factor through activation of AMP-activated protein kinase in DU145 prostate carcinoma. J Biol Chem 280: 9963–9972, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zmijewski JW, Zhao X, Xu Z, Abraham E. Exposure to hydrogen peroxide diminishes NF-κB activation, IκB-α degradation, and proteasome activity in neutrophils. Am J Physiol Cell Physiol 293: C255–C266, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem 277: 32552–32557, 2002. [DOI] [PubMed] [Google Scholar]