Abstract
Skeletal muscle atrophy is evident after muscle disuse, unloading, or spaceflight and results from decreased protein content as a consequence of decreased protein synthesis, increased protein breakdown or both. At this time, there are essentially no human data describing proteolysis in skeletal muscle undergoing atrophy on Earth or in space, primarily due to lack of valid and accurate methodology. This particular study aimed at assessing the effects of short-term unloading on the muscle contractile proteolysis rate. Eight men were subjected to 72-h unilateral lower limb suspension (ULLS) and intramuscular interstitial levels of the naturally occurring proteolytic tracer 3-methylhistidine (3MH) were measured by means of microdialysis before and on completion of this intervention. The 3MH concentration following 72-h ULLS (2.01 ± 0.22 nmol/ml) was 44% higher (P < 0.05) than before ULLS (1.56 ± 0.20 nmol/ml). The present experimental model and the employed method determining 3MH in microdialysates present a promising tool for monitoring skeletal muscle proteolysis or metabolism of specific muscles during conditions resulting in atrophy caused by, e.g., disuse and real or simulated microgravity. This study provides evidence that the atrophic processes are evoked rapidly and within 72 h of unloading and suggests that countermeasures should be employed in the early stages of space missions to offset or prevent muscle loss during the period when the rate of muscle atrophy is the highest.
Keywords: contractile protein breakdown, microdialysis, muscle atrophy, spaceflight simulation, 3-methylhistidine
spaceflight and simulation models of no-weight-bearing activity provoke lower limb skeletal muscle atrophy (1, 3, 4, 21, 33, 34) as a result of altered protein metabolism leading to decreased muscle contractile protein content (14, 30, 31, 35, 42). The net effect on the skeletal muscle protein pool is governed by the two opposing processes working in concert, i.e., protein synthesis and breakdown. Although it is has been shown that atrophy induced by disuse is accompanied by decreased protein synthesis (6, 7, 10), based on human studies, there is less substantial evidence to support that muscle unloading provokes increased proteolytic activity.
Unfortunately, until now, feasible and viable methods that measure protein degradation in vivo in humans have not been at hand. Albeit 3-methylhistidine (3MH), through urinary excretion, has been used as a marker of muscle protein degradation following bouts of acute heavy resistance exercise (18, 25), the amount of 3MH disposed in urine reflects only changes in the rate of contractile protein breakdown at the global level (22, 23). The arteriovenous difference across limbs has been successfully measured (26, 41) to minimize the potential interference from body regions with a high turnover rate, e.g., the gastrointestinal system (43). However, such an invasive and complex approach is not attractive for experiments involving otherwise healthy individuals subjected to interventions such as unloading or microgravity. Recently, Trappe et al. (41) adopted the “semi-invasive” microdialysis technique (16, 17, 24) for in vivo intramuscular 3MH measurements and allowing for quantitative determination of proteolysis of the contractile elements actin and myosin of individual muscles. This approach circumvents the aforementioned limitations of whole body and nonskeletal muscle contributions to the contractile proteolysis measurements, while taking advantage of the natural tracer characteristics of 3MH (i.e., 3MH cannot be reused for protein synthesis). In addition, the measurements obtained with microdialysis have been confirmed in parallel experiments using the arteriovenous balance technique (41). With the aid of microdialysis, altered skeletal myofibrillar proteolysis has been noted with aging (41) and following strenuous artificial (i.e., electrically stimulated) contractile activity (13). Hence, this methodology presents a lenient and appealing tool to be employed in healthy populations for both mechanistic and applied studies. This particular study was prompted by our long-term interest in exploring the fate of skeletal muscle in response to spaceflight.
Given the high rate of global lower limb muscle atrophy during the initial week(s) of spaceflight, unloading, or disuse (8, 19, 20, 28, 33), altered contractile protein metabolism should also be evident early on in response to such challenges. In case changes in both protein synthesis and breakdown occur, short-term unloading protocols could readily be employed for mechanistic studies of human skeletal muscle to explore the events leading to atrophy, reducing the need for costly and demanding long-term unloading or bed rest studies.
More specifically, the main objective of the present study was to determine skeletal muscle contractile protein degradation, by means of the microdialysis technique, consequent to short-term simulated spaceflight. We hypothesized that 72-h lower limb unloading would provoke increased intramuscular interstitial levels of 3MH as a result of increased myofibrillar proteolysis.
MATERIALS AND METHODS
Subjects.
Eight men (mean ± SD; 25 ± 5 yr, 183 ± 3 cm, 76 ± 8 kg) were recruited from the greater Stockholm area. Following a medical examination, screening for any history of lower limb pathology, neuromuscular disorders, or cardiovascular disease, subjects were informed of the procedures, risks, and benefits associated with the experiments. Although some of these men were sedentary, a few took part in recreational or competitive sports programs. Written informed consent was obtained from each subject to comply with the current guidelines of the Institutional Review Board (IRB) at the Karolinska Institutet. After IRB approval, this study was conducted in accordance with the Declaration of Helsinki.
General procedures.
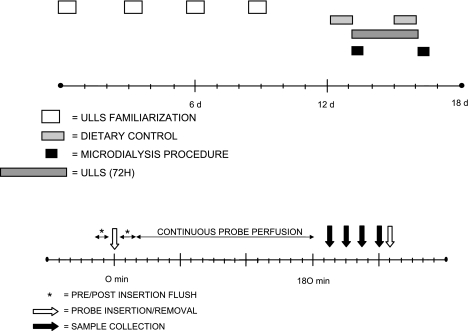
Two weeks before the intervention, all subjects underwent four sessions of orientation and familiarization to become accustomed to the different elements of the unilateral lower limb suspension (ULLS) technique (4, 34). Three days before the onset of the ULLS regime, the subjects were required to refrain from any strenuous physical activity, yet they followed their normal dietary routines. However, beginning 24 h before the scheduled pre- and post-ULLS measurements, menus were restricted to two standardized vegetarian meals, which both consisted of pasta, tomato sauce, tomatoes, and green salad (∼1,400 kcal). Subjects were in a fasted state from 10 h before and throughout the sample collections. Water was provided ad libitum. The subjects reported to the laboratory facility in the evening, where they slept and remained through the following day's measurements. Before and at completion of the 72-h intervention, 3MH concentration, as a marker of skeletal muscle protein breakdown, was measured from the vastus lateralis muscle in vivo using the microdialysis technique. While pre ULLS samples were acquired from the right leg, post ULLS measurements were performed on the left leg (Fig. 1).
Fig. 1.
General study design showing probe insertion, proteolysis measurements, and unilateral lower limb suspension (ULLS) intervention (top) and myofibrillar proteolysis measurements via microdialysis sampling (bottom). d, Day; 72H, 72 hour; Pre, before ULLS; Post, after ULLS.
ULLS.
To avoid weight bearing of the left leg at all times, the volunteers were subjected to a unilateral unloading protocol used by us in the past (34). Briefly, in any upright or ambulatory activity, short-length crutches, aided by handgrip and forearm support distal to the elbow (Swereco Rehab, Sollentuna, Sweden), were used. The right foot was equipped with a shoe having a 10-cm-thick sole. This removed weight bearing from the left unloaded limb yet allowed for movement about the hip joint. Subjects lived at home throughout the unloading period and were encouraged to maintain normal daily routines. To ensure compliance, all subjects were interviewed daily via telephone or in person. For extended periods in the erect position subjects were provided and wore medical-graded (class 2) stockings (BSN-jobst, Rutherford College, NC).
Microdialysis.
Microdialysis was performed on the vastus lateralis muscle to acquire pre- and post-ULLS data on muscle contractile protein breakdown. Between 6:00 and 7:00 AM, the CMA 60 microdialysis probes (30 mm, 20-kDa cutoff, CMA Microdialysis, Solna, Sweden) were inserted following a period of 5 min preperfusion at 10 μl/min with a sterile Ringer solution using a calibrated microinfusion pump (CMA Microdialysis, Solna, Sweden). Immediately following insertion, the probes were flushed at a perfusion rate of 10 μl/min for at least 10 min. To ensure no influence by the probe insertion on pre- and postunloading data, the catheters were continuously perfused for 3 h before collection while subjects remained at rest in the supine position (11). All dialysate samples were collected in 15-min aliquots in sealed microvials and weighed on a precision microbalance (Sartorius, Goettingen, Germany) before and after each period to determine actual dialysate volume. The microvials for each 15 min collection were immediately stored at 4°C, until completion of all acquisitions (maximum 2–3 h), and then they were weighed and stored at −80°C until assayed for 3MH.
Microdialysis probe recovery.
Microdialysis probe recovery for 3MH was calculated from our laboratory's previously obtained recovery data from 3MH determinations in the vastus lateralis muscle of young men during resting conditions (41). Probe recovery in this approach was determined in vivo by the internal reference technique (29). This technique measures the loss of an internal tracer placed in the perfusate and assumes the loss of the tracer to the interstitial fluid is equivalent to the recovery of the compound of interest from the interstitial fluid into the dialysate. In this case a radioactive tracer was used, and the in vivo recovery was calculated using the following formula: (perfusatedpm − dialysatedpm)/perfusatedpm, where dpm is disintegrations per minute. Given that conditions known to influence probe recovery e.g., probe length, pore size, the compound of interest, pump-probe alignment and vial orientation, were identical in the present and the above study by Trappe and coworkers, we are confident recovery was the same in the two studies.
3MH determination.
The procedure for 3MH determination has been described in detail elsewhere (9, 15, 41, 44). In brief, in any dialysate sample 3MH concentration was determined by high-performance liquid chromatography (250 mm × 4.6 mm C-18 reverse-phase column, flow rate 1.5 ml/min) and fluorometric detection (1100 Series, Agilent Technologies, Wilmington, DE). Derivatization was completed by placing 25 μl of dialysate or standard (M19930, Pfaltz and Bauer, Waterbury, CN), 65 μl of borate buffer (0.4 M boric acid, adjusted to pH 12.2 with NaOH), and 65 μl of fluorescamine reagent (160 mg fluorescamine in 100 ml acetonitrile) in a 0.6-ml microcentrifuge tube, which was then vortexed and allowed to stand at room temperature for 5 min. Ten microliters of concentrated perchloric acid was added to the tube and subsequently capped and heated at 80°C for 1 h. After cooling to room temperature, samples were neutralized with 25 μl of 0.05 M MOPS in 3 M NaOH. Samples were injected with an autosampler (1100 Series, Agilent Technologies, Palo Alto, CA) maintained at 4°C. The mobile phase was 25% acetonitrile and 75% 10 mM Na2HPO4 adjusted to pH 7.5 with phosphoric acid. Peaks were monitored at 365 nm (excitation) and 460 nm (emission) and integrated with chromatography software (Chemstation, Agilent Technologies, Wilmington, DE). Dialysate concentration was determined from a standard curve of 3MH. Interstitial 3MH concentration for each sample was determined by accounting for probe recovery (see Microdialysis probe recovery). Calculated interstitial 3MH concentrations from all four 15-min aliquots collected (when available) were used to average each time point (pre- and post-ULLS).
Statistics.
Paired t-test was used to compare interstitial 3MH levels and microdialysis vial weights. Significance was accepted at P < 0.05. Data are presented as means ± SE, unless noted.
RESULTS
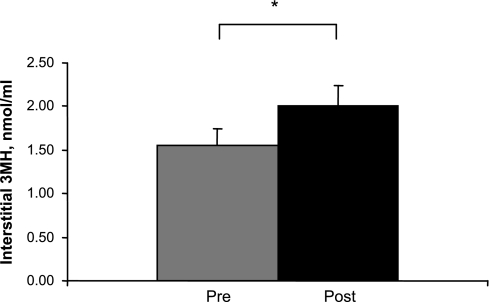
The concentration of interstitial 3MH following 72-h ULLS (2.01 ± 0.22 nmol/ml) was 44% higher (P < 0.05) than before ULLS (1.56 ± 0.20 nmol/ml) (Fig. 2). The coefficient of variance for the four 15-min aliquots that were used for 3MH analysis and sampled pre- and post-ULLS were 8.9 ± 1.4% and 6.9 ± 0.9%, respectively. Dialysate weights were similar (P > 0.05) to the expected weight of 30 mg (perfusate density 1 g/ml, dialysate volume 30 μl) across collection periods. Dialysate sample weights averaged 29.3 ± 0.3 and 29.5 ± 0.2 mg, respectively pre and post ULLS.
Fig. 2.
Interstitial 3MH concentration before and after 72 h ULLS. Means are average of four 15-min acquisitions for all subjects (n = 8). *Difference pre- vs. post-ULLS, P < 0.05.
DISCUSSION
This particular study was initiated as a result of our overarching long-term goal to explore how skeletal muscle and other organ systems adapt to spaceflight and to define specific countermeasures to these effects (2, 3, 12, 14, 33, 34, 36–40, 42). The main novel finding of this study was that 72-h unloading induced increased skeletal muscle proteolysis in otherwise healthy men, as reflected in elevated interstitial 3MH concentrations. Thus it appears that both increased proteolysis and decreased protein synthesis (6, 7, 10) occur in the early phase of muscle unloading, eventually resulting in atrophy. Furthermore and not less importantly, the present 3-day unloading protocol presents a feasible and valid model for future in vivo studies exploring the atrophy process in human skeletal muscle.
Skeletal muscle atrophy at the whole muscle and single fiber level are evident with different human unloading models or spaceflight analogs within week(s) (8, 20, 33, 45), and loss of muscle seems to continue at a rather constant rate over the proceeding 3–4 wk (5, 34). Such changes are generally attributed to a net decrease in contractile protein content, resulting from an imbalance in protein metabolism (27, 31). Although studies have reported decreased skeletal muscle protein synthesis in unloaded volunteers subjected to lower limb unloading or bed rest, there are less conclusive human data showing increased contractile protein breakdown following such interventions. In the present study we showed that no more than 72-h unloading facilitates increased contractile protein breakdown. Although these results concert with the previous finding of increased 3MH plasma concentrations following short-term spaceflight, the investigators of that particular study attributed this effect to the stress response seen early on in the flight, not to the unloading per se (32). Because the present model mimics the unloading component of spaceflight with no apparent confounding factors of increased stress, we believe that the increased myosin and actin proteolysis shown in the present study must have originated from lack of weight bearing. Given that previous studies, employing the ULLS model over many weeks, showed no atrophy of the weight-bearing limb (4, 34), it is very unlikely it occurred in the present study or that our finding of increased 3MH was caused by a systemic effect.
The 3% decrease in lower limb muscle cross-sectional area, assessed by means of magnetic resonance imaging following 7 days of bed rest (8, 20), infers that molecular signs of atrophy should be evident early on after withdrawal of weight bearing. Indeed, the finding of a robust increase in 3MH concentration within the muscle in the present study implies muscle proteolysis is initiated within a 72-h period of unloading. This is in contrast to the inability of acute high intensity resistance exercise or 60 min of strenuous aerobic exercise to induce an increase in muscle proteolysis measured by interstitial 3MH concentration (13, 15, 41). These findings highlight the magnitude of the proteolytic signals evoked by unloading skeletal muscle.
In view of these findings, we believe that the experimental model introduced here could serve as a useful tool in research aimed at studying mechanisms regulating skeletal muscle size during conditions (e.g., disease, trauma, muscle disuse, or unloading), which may have implications for procedures (e.g., rehabilitation and nutritional interventions and treatment of the critically ill) employing other techniques (e.g., muscle biopsy sampling) as well.
The novel finding of the present study was the demonstration that, in otherwise healthy men, 72-h ULLS provoked increased proteolytic activity of contractile proteins, evidenced by a marked increase in interstitial 3MH concentration. Thus the microdialysis technique offers a promising tool for in vivo monitoring of protein breakdown of selected muscles in humans in response to reduced muscle use, e.g., real or simulated microgravity. Furthermore, the present findings are encouraging in that they support use of short-term unloading protocols to address questions exploring mechanisms involved in the skeletal muscle atrophy process in humans.
GRANTS
This investigation was supported by a grant from the National Aeronautics and Space Administration (to R. M. Linnehan). Partial funding was provided by the Swedish Council of Sports (to P. A. Tesch and T. Gustafsson), the Centre of Gender Medicine (to P. A. Tesch), the Swedish National Space Board (to P. A. Tesch), the Loo and Hans Osterman Foundation (to T. Gustafsson), and National Institute on Aging Grant AG-020532 (to T. A. Trappe).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akima H, Kawakami Y, Kubo K, Sekiguchi C, Ohshima H, Miyamoto A, Fukunaga T. Effect of short-duration spaceflight on thigh and leg muscle volume. Med Sci Sports Exerc 32: 1743–1747, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Alkner BA, Berg HE, Kozlovskaya I, Sayenko D, Tesch PA. Effects of strength training, using a gravity-independent exercise system, performed during 110 days of simulated space station confinement. Eur J Appl Physiol 90: 44–49, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Alkner BA, Tesch PA. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur J Appl Physiol 93: 294–305, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Berg HE, Dudley GA, Haggmark T, Ohlsen H, Tesch PA. Effects of lower limb unloading on skeletal muscle mass and function in humans. J Appl Physiol 70: 1882–1885, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Berg HE, Larsson L, Tesch PA. Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol 82: 182–188, 1997. [DOI] [PubMed] [Google Scholar]
- 6.de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol 585: 241–251, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270: E627–E633, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Ferrando AA, Stuart CA, Brunder DG, Hillman GR. Magnetic resonance imaging quantitation of changes in muscle volume during 7 days of strict bed rest. Aviat Space Environ Med 66: 976–981, 1995. [PubMed] [Google Scholar]
- 9.Fetterer RH, Allen PC. Eimeria acervulina infection elevates plasma and muscle 3-methylhistidine levels in chickens. J Parasitol 86: 783–791, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Gamrin L, Berg HE, Essen P, Tesch PA, Hultman E, Garlick PJ, McNurlan MA, Wernerman J. The effect of unloading on protein synthesis in human skeletal muscle. Acta Physiol Scand 163: 369–377, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez A, Anderstam B, Alvestrand A. Amino acid concentration in the interstitium of human skeletal muscle: a microdialysis study. Eur J Clin Invest 29: 947–952, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Haddad F, Baldwin KM, Tesch PA. Pretranslational markers of contractile protein expression in human skeletal muscle: effect of limb unloading plus resistance exercise. J Appl Physiol 98: 46–52, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hansen M, Trappe T, Crameri RM, Qvortrup K, Kjaer M, Langberg H. Myofibrillar proteolysis in response to voluntary or electrically stimulated muscle contractions in humans. Scand J Med Sci Sports: February 4, 2008. [DOI] [PubMed]
- 14.Haus JM, Carrithers JA, Carroll CC, Tesch PA, Trappe TA. Contractile and connective tissue protein content of human skeletal muscle: effects of 35 and 90 days of simulated microgravity and exercise countermeasures. Am J Physiol Regul Integr Comp Physiol 293: R1722–R1727, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Haus JM, Miller BF, Carroll CC, Weinheimer EM, Trappe TA. The effect of strenuous aerobic exercise on skeletal muscle myofibrillar proteolysis in humans. Scand J Med Sci Sports 17: 260–266, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Henriksson J Microdialysis of skeletal muscle at rest. Proc Nutr Soc 58: 919–923, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Hickner RC Applications of microdialysis in studies of exercise. Exerc Sport Sci Rev 28: 117–122, 2000. [PubMed] [Google Scholar]
- 18.Hickson JF, Wolinsky I, Rodriguez GP, Pivarnik JM, Kent MC, Shier NW. Failure of weight training to affect urinary indices of protein metabolism in men. Med Sci Sports Exerc 18: 563–567, 1986. [PubMed] [Google Scholar]
- 19.Jansson E, Sylven C, Arvidsson I, Eriksson E. Increase in myoglobin content and decrease in oxidative enzyme activities by leg muscle immobilization in man. Acta Physiol Scand 132: 515–517, 1988. [DOI] [PubMed] [Google Scholar]
- 20.LeBlanc A, Rowe R, Schneider V, Evans H, Hedrick T. Regional muscle loss after short duration spaceflight. Aviat Space Environ Med 66: 1151–1154, 1995. [PubMed] [Google Scholar]
- 21.LeBlanc AD, Schneider VS, Evans HJ, Pientok C, Rowe R, Spector E. Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol 73: 2172–2178, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Long CL, Dillard DR, Bodzin JH, Geiger JW, Blakemore WS. Validity of 3-methylhistidine excretion as an indicator of skeletal muscle protein breakdown in humans. Metabolism 37: 844–849, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Long CL, Haverberg LN, Young VR, Kinney JM, Munro HN, Geiger JW. Metabolism of 3-methylhistidine in man. Metabolism 24: 929–935, 1975. [DOI] [PubMed] [Google Scholar]
- 24.Lonnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol Endocrinol Metab 253: E228–E231, 1987. [DOI] [PubMed] [Google Scholar]
- 25.Pivarnik JM, Hickson JF Jr, Wolinsky I. Urinary 3-methylhistidine excretion increases with repeated weight training exercise. Med Sci Sports Exerc 21: 283–287, 1989. [PubMed] [Google Scholar]
- 26.Rennie MJ, Bennegard K, Eden E, Emery PW, Lundholm K. Urinary excretion and efflux from the leg of 3-methylhistidine before and after major surgical operation. Metabolism 33: 250–256, 1984. [DOI] [PubMed] [Google Scholar]
- 27.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol 66: 799–828, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Richter EA, Kiens B, Mizuno M, Strange S. Insulin action in human thighs after one-legged immobilization. J Appl Physiol 67: 19–23, 1989. [DOI] [PubMed] [Google Scholar]
- 29.Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Methods 40: 31–38, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Stein TP, Leskiw MJ, Schluter MD. Diet and nitrogen metabolism during spaceflight on the shuttle. J Appl Physiol 81: 82–97, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Stein TP, Leskiw MJ, Schluter MD, Donaldson MR, Larina I. Protein kinetics during and after long-duration spaceflight on MIR. Am J Physiol Endocrinol Metab 276: E1014–E1021, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Stein TP, Schluter MD. Human skeletal muscle protein breakdown during spaceflight. Am J Physiol Endocrinol Metab 272: E688–E695, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Tesch PA, Berg HE, Bring D, Evans HJ, LeBlanc AD. Effects of 17-day spaceflight on knee extensor muscle function and size. Eur J Appl Physiol 93: 463–468, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Tesch PA, Trieschmann JT, Ekberg A. Hypertrophy of chronically unloaded muscle subjected to resistance exercise. J Appl Physiol 96: 1451–1458, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol 68: 1–12, 1990. [DOI] [PubMed] [Google Scholar]
- 36.Trappe S, Creer A, Minchev K, Slivka D, Louis E, Luden N, Trappe T. Human soleus single muscle fiber function with exercise or nutrition countermeasures during 60 days of bed rest. Am J Physiol Regul Integr Comp Physiol 294: R939–R947, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol 557: 501–513, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trappe SW, Creer A, Slivka D, Minchev K, Trappe TA. Single muscle fiber function with concurrent exercise Or nutrition countermeasures during 60 days of bed rest in women. J Appl Physiol 103: 1242–1250, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Trappe SW, Trappe TA, Lee GA, Widrick JJ, Costill DL, Fitts RH. Comparison of a space shuttle flight (STS-78) and bed rest on human muscle function. J Appl Physiol 91: 57–64, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Trappe T, Trappe S, Lee G, Widrick J, Fitts R, Costill D. Cardiorespiratory responses to physical work during and following 17 days of bed rest and spaceflight. J Appl Physiol 100: 951–957, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Trappe T, Williams R, Carrithers J, Raue U, Esmarck B, Kjaer M, Hickner R. Influence of age and resistance exercise on human skeletal muscle proteolysis: a microdialysis approach. J Physiol 554: 803–813, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trappe TA, Burd NA, Louis ES, Lee GA, Trappe SW. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf) 191: 147–159, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Vesali RF, Klaude M, Thunblad L, Rooyackers OE, Wernerman J. Contractile protein breakdown in human leg skeletal muscle as estimated by [2H3]-3-methylhistidine: a new method. Metabolism 53: 1076–1080, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Wassner SJ, Schlitzer JL, Li JB. A rapid, sensitive method for the determination of 3-methylhistidine levels in urine and plasma using high-pressure liquid chromatography. Anal Biochem 104: 284–289, 1980. [DOI] [PubMed] [Google Scholar]
- 45.Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JLW, Riley DA, Trappe SW, Trappe TA, Costill DL, Fitts RH. Effects of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol 516: 915–930, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]