Abstract
An enhancement of peripheral chemoreflex sensitivity contributes to sympathetic hyperactivity in chronic heart failure (CHF) rabbits. The enhanced chemoreflex function in CHF involves augmented carotid body (CB) chemoreceptor activity via upregulation of the angiotensin II (ANG II) type 1 (AT1)-receptor pathway and downregulation of the neuronal nitric oxide synthase (nNOS)-nitric oxide (NO) pathway in the CB. Here we investigated whether exercise training (EXT) normalizes the enhanced peripheral chemoreflex function in CHF rabbits and possible mechanisms mediating this effect. EXT partially, but not fully, normalized the exaggerated baseline renal sympathetic nerve activity (RSNA) and the response of RSNA to hypoxia in CHF rabbits. EXT also decreased the baseline CB nerve single-fiber discharge (4.9 ± 0.4 vs. 7.7 ± 0.4 imp/s at Po2 = 103 ± 2.3 Torr) and the response to hypoxia (20.6 ± 1.1 vs. 36.3 ± 1.3 imp/s at Po2 = 41 ± 2.2 Torr) from CB chemoreceptors in CHF rabbits, which could be reversed by treatment of the CB with ANG II or a nNOS inhibitor. Our results also showed that NO concentration and protein expression of nNOS were increased in the CBs from EXT + CHF rabbits, compared with that in CHF rabbits. On the other hand, elevated ANG II concentration and AT1-receptor overexpression of the CBs in CHF state were blunted by EXT. These results indicate that EXT normalizes the CB chemoreflex in CHF by preventing an increase in afferent CB chemoreceptor activity. EXT reverses the alterations in the nNOS-NO and ANG II-AT1-receptor pathways in the CB responsible for chemoreceptor sensitization in CHF.
Keywords: angiotensin, nitric oxide, chemoreceptor, carotid body, heart failure
profound sympathohumoral activation is characteristic of all types of chronic heart failure (CHF) (10, 13, 46). Initially, activation of the sympathetic nerve system is beneficial as a protective mechanism to maintain arterial blood pressure when cardiac output falls due to the ventricular dysfunction. However, the sympathetic nervous system can be a two-edged sword. The progressive and sustained increase of sympathetic nerve activation contributes to deterioration of cardiac function and eventual mortality of CHF (10). The peripheral chemoreflex is an excitatory reflex that increases sympathetic outflow and blood pressure (27). Studies from our laboratory have shown that peripheral chemoreflex sensitivity is enhanced in rabbits with pacing-induced CHF (21, 22, 25, 43). An augmented afferent input from carotid body (CB) chemoreceptors is involved in the enhancement of peripheral chemoreflex function in CHF rabbits (22, 25, 44). In addition, our laboratory's previous studies have shown that elevation of angiotensin II (ANG II) with upregulation of ANG II type 1 (AT1) receptors and suppression of neuronal nitric oxide synthase (nNOS) with nitric oxide (NO) depletion in the CB contribute to the enhanced CB chemoreceptor sensitivity in the CHF state (22, 25, 44).
Exercise training or “conditioning” (EXT) is increasingly recognized as a beneficial treatment to enhance functional capacity and quality of life in patients with CHF, in addition to the standard pharmacological interventions, such as β-adrenergic blockers and angiotensin-converting enzyme inhibitors/receptor blockers (1, 8, 29, 36). Although the mechanisms by which EXT is beneficial to CHF patients are still unclear, EXT can increase cardiac vagal tone and reduce sympathetic outflow in CHF patients (9, 19). A recent study has reported that EXT reduces renal sympathetic nerve activity (RSNA) in rabbits with CHF (26), and our laboratory has shown that EXT normalizes peripheral chemoreflex function in CHF rabbits (39). Here, we investigated whether EXT normalizes peripheral chemoreflex sensitivity and CB chemoreceptor function in CHF rabbits. We further examined CB ANG II and NO concentrations, expression of AT1 receptor and nNOS in the CB, and the role of nNOS and ANG II on EXT effects on CB chemoreceptor activity.
METHODS
Pacemaker implant and production of CHF.
All experiments were carried out on male New Zealand White rabbits weighing 2.5–3.5 kg. Experiments were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health (NIH Publication no. 85-23, revised 1996) and the American Physiological Society's Guide for the Care and Use of Laboratory Animals. Rabbits were assigned to sham-operated and CHF groups. They were housed in individual cages under controlled temperature and humidity and a 12:12-h dark-light cycle, and fed standard rabbit chow with water available ad libitum.
Rabbits were anesthetized with a cocktail consisting of 1.2 mg acepromazine, 5.9 mg xylazine, and 58.8 mg ketamine, given as an intramuscular injection. Using sterile technique, a left thoracotomy was performed, as previously described (43). Briefly, a pin electrode was attached to the left ventricle, and a ground electrode was secured to the left atrium for pacing. All wires were tunneled beneath the skin and exited in the midscapular area. The chest was closed. Rabbits were placed on an antibiotic regimen consisting of 5 mg/kg im Baytril for 5 days. After 2 wk, the pacing was started at 340 beats/min, held for 7 days, and then the rate was gradually increased to 380 beats/min, with an increment of 20 beats/min each week. The progression of CHF was monitored by weekly echocardiograms (Acuson Sequoia 512C with a 4 MHz probe) with the pacemaker turned off for at least 30 min before the recordings were started. Sham-operated animals underwent a similar period of echocardiographic measurements. CHF was characterized by a >40% reduction in ejection fraction and fraction of shortening (FS), and dilation of the left ventricle in both systole and diastole.
EXT protocol.
Rabbits were exercised on a motor-driven treadmill for a total of 30–40 min/day, 6 days/wk, for a total period of 4 or 5 wk. A warm-up period of 5 min at 5 m/min was followed by peak EXT (15–18 m/min) for 30 min, which was followed by a cool down of an additional 5 min at 5 m/min, whenever possible. Rabbits were started on their EXT protocol 1 wk before initiation of pacing to acquaint them with the treadmill and the EXT protocol. The pacemaker remained on during the EXT. Sedentary (SED) rabbits were placed on the treadmill with the same protocol, but not exercised.
Citrate synthase activity assay.
Citrate synthase (CS) activity in the soleus muscle was measured as a biochemical marker of the extent of EXT (3). Rabbits were euthanized at the end of the study, and both soleus muscles were taken, frozen at −80°C, and stored until processed. CS activity was measured spectrophotometrically at 412 nm from whole muscle homogenates, as previously described (40). The activity was expressed as micromoles of substrate used per minute per gram wet weight.
RSNA.
RSNA recording electrodes were implanted as described previously (43). In all rabbits, 3 days before the experiments, a pair of electrodes was implanted on the left renal sympathetic nerves, and a ground electrode was sutured to the perirenal fat. At that time, arterial-venous catheters were inserted into the right carotid artery and jugular vein. RSNA recording was performed 3 days after surgery.
Changes of RSNA in response to the stimulation of peripheral chemoreceptors were measured in sham and CHF rabbits in the conscious state, as described previously (43). As in our previous studies, the pacemaker was turned off for at least 30–45 min before experimental procedures. RSNA were expressed as %maximum, and maximal RSNA was determined in each rabbit by an intravenous bolus injection of sodium nitroprusside (100 μg/kg) (26). Peripheral chemoreceptors were stimulated preferentially by allowing the rabbits to breathe graded mixtures of hypoxic gas (3–5 min) under isocapnic conditions. Because hypoxic stimulation of ventilation induces hyperventilatory hypocapnia, 2–4% CO2 was added to the hypoxic gases to maintain relatively constant arterial Pco2 (PaCO2) during hyperventilation (43). arterial Po2 (PaO2), PaCO2, and pH of arterial blood were measured (Supplemental Table 1; the online version of this article contains supplemental data) by a blood-gas analyzer (ABL5, Radiometer, Copenhagen, Denmark).
Table 1.
Body weight, ventricular weight, echo data, and soleus muscle citrate synthase activity from different groups
| Pretreatment | SED-Sham | EXT-Sham | SED-CHF | EXT-CHF | |
|---|---|---|---|---|---|
| n | 64 | 16 (4–5 wk) | 16 (4–5 wk) | 16 (4–5 wk) | 20 (4–5 wk) |
| BW, kg | 2.96±0.05 | 3.72±0.09 | 3.62±0.13 | 3.89±0.15 | 3.83±0.11 |
| LVW/BW, g/kg | 1.57±0.07 | 1.55±0.09 | 2.09±0.10* | 1.95±0.10* | |
| LVEDD, mm | 13.3±0.3 | 14.2±0.5 | 13.9±0.6 | 17.3±0.5* | 17.2±0.5* |
| LVESD, mm | 8.4±0.2 | 8.5±0.4 | 8.5±0.5 | 13.2±0.4* | 13.0±0.4* |
| FS, % | 40.8±1.2 | 41.8±1.5 | 41.4±1.6 | 20.3±1.9* | 22.5±1.8* |
| EF, % | 74.6±1.1 | 74.5±1.9 | 75.2±2.1 | 48.9±2.2* | 51.2±1.8* |
| CS activity, μmol·min−1·g−1 | 1.52±0.06 | 2.16±0.05* | 1.47±0.07 | 2.02±0.06*† |
Values are means ± SE; n, no. of animals. SED, sedentary; EXT, exercise training; CHF, chronic heart failure; LVW/BW, ratio of left ventricular weight to body weight; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; FS, fractional shortening; EF, ejection fraction; CS, citrate synthase.
P < 0.01 vs. SED-Sham;
P < 0.05 vs. SED-CHF.
Peripheral chemoreflex function curves were fitted to the equation: RSNA = a + b/(PaO2 − c) using SigmaPlot software (43). The parameter b is the slope coefficient and was used to compare the peripheral chemoreflex sensitivity among the groups.
CB chemoreceptor fiber activity.
Single-unit action potentials were recorded from CB chemoreceptor fibers in the carotid sinus nerve (CSN), as our laboratory has described previously (44). Briefly, the left or right carotid sinus region was vascularly isolated and perfused with Krebs-Henseleit solution (in mM: 120 NaCl, 4.8 KCl, 2.0 CaCl2, 2.5 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 5.5 glucose; 10 ml/min, temperature 37°C). Perfusate was bubbled with O2/CO2/N2 gas mixture to maintain Po2 at 100–110 Torr, Pco2 at 30–35 Torr, and pH at 7.35–7.45 as the normoxic condition. Po2, Pco2, and pH of the buffer solution perfusing the carotid sinuses were measured by gas- and ion-selective electrodes with 1201 chemical microsensor (Diamond General, Ann Arbor, MI). Flow through the isolated sinus was set at 10 ml/min at a perfusion pressure of 80 mmHg via a screw clamp on the effluent line, which was adjusted throughout the experiments to keep flow and pressure constant.
Autonomic innervation to the carotid sinus region was eliminated by stripping all visible neural connections among the carotid sinus, the superior cervical, and nodose ganglia. The CSN was exposed and transected near the petrosal ganglion to interrupt neural efferents to the CB.
The CSN was covered with mineral oil, and fine slips of nerve filaments were placed on a silver electrode. Impulses were amplified with a bandwidth of 100 Hz to 3 kHz (Grass P511, Grass Instrument, Quincy, MA), displayed on an oscilloscope (2120 Oscilloscope, BK Precision), and fed into a rate meter (FHC, Brunswick, ME) whose window discriminators were set to accept potentials of the particular amplitude. Impulses were counted by the rate meter in 1-s bins. The action potential and rate meter signals [discharge frequency (DF)] were fed into an analog-to-digital converter (ADInstruments, Colorado Springs, CO) attached to a computer. Bundles that had one, or at most two, easily distinguishable active fibers were used. Chemoreceptor afferents were identified by their sparse and irregular discharge at normoxia and by their response to hypoxia and NaCN.
Po2 was altered by bubbling the perfusate with gas mixtures of different O2 concentrations (approximate 15, 8, and 4%) achieving a Po2 of 100–110, 55–65, and 35–40 Torr, respectively (Supplemental Table 2). The gas mixtures contained a constant fraction of ∼5% CO2 (30–35 mmHg) and a balance of N2.
The stimulus-response characteristics of the CB chemoreceptors were quantified by the equation: DF = a + b/(Po2 − c) (44). The slope coefficient b was used to compare the CB chemoreceptor sensitivity among the groups.
Western blot analysis for AT1 receptor and nNOS.
At the end of the study, CBs were rapidly removed and immediately frozen in dry ice and stored at −80°C for Western blot, ANG II, and NO measurements. The protein was extracted with the lysing buffer (10 mM Tris, 1 mM EDTA, 1% SDS, pH 7.4) plus protease inhibitor cocktail (100 μl/ml). Following a centrifugation at 12,000 g for 20 min at 4°C, the protein concentration in the supernatant was determined using a bicinchoninic acid (BCA) protein assay kit (Pierce Chemical, Rockford, IL). The protein sample was mixed with loading buffer containing β-mercaptoethanol and heated at 100°C for 5 min. Equal amounts of protein were loaded. Protein was fractionated in a 10% polyacrylamide gel along with molecular weight standards and transferred to polyvinylidene difluoride membrane. The membrane was probed with a mouse anti-AT1 receptor, nNOS antibodies (Santa Cruz), and a peroxidase-conjugated goat anti-mouse IgG (Pierce Chemical, Rockford, IL). The signal was detected using enhanced chemiluminescence substrate (Pierce Chemical), and the bands were analyzed using UVP BioImaging Systems. Protein loading was controlled by probing all Western blots with anti-β-tubulin mouse antibody (Santa Cruz) and normalizing AT1-receptor or nNOS protein intensity to that of β-tubulin.
ANG II concentration in the CB.
Tissue homogenate was prepared from CB samples. CB ANG II concentrations were measured by ANG II 125I radioimmunoassay kit (Buhlmann Laboratories). The final ANG II concentration was counted by 1470 Automatic Gamma Counter (Perkin Elmer, Shelton, CT) and calculated with a standard curve generated for each experiment.
NO concentration in the CB.
CB homogenate was prepared in the same manner as described above. NO concentration was determined by the Apollo 1000 NO sensor (WPI, Sarasota, FL). NO probe (ISO-NOPF100, WPI) was calibrated based on a solution of 0.1 M CuCl2 using the NO donor, S-nitroso-N-acetyl-l-penicillamine (SNAP) at concentrations of 5, 25, 50, 100, 200, and 400 nM. The NO concentration in the CB was calculated with this SNAP standard curve and normalized by total protein (BCA protein assay kit, Pierce Chemical, Rockford, IL).
Data analysis.
All data are presented as means ± SE. Statistical significance was determined by a two-way ANOVA, with a Bonferroni procedure for post hoc analysis for multiple comparisons. All data were confirmed by the Kolmogorov-Smirnov test to fit reasonably within normal distribution, and equal variance was confirmed by the Levene test. Statistical significance was accepted when P < 0.05. A power analysis was conducted to assess whether the sample size was sufficient to ensure P < 0.05.
RESULTS
Basal hemodynamics and CS activity in experimental groups.
Table 1 lists the resting hemodynamic parameters in SED-sham, EXT-sham, SED-CHF, and EXT-CHF groups. The data for each parameter were collated from all rabbits within each group. Measurements were made with the pacer turned off for at least 30 min. Rapid left ventricular pacing induced CHF by the 3rd or 4th week of pacing. CHF significantly enlarged left ventricular weight-to-body weight ratio, left ventricular end-diastolic diameter, and left ventricular end-systolic diameter, and reduced shortening fraction and ejection fraction by 51.3 ± 1.9 and 36.2 ± 1.6%, respectively, from pretreatment baselines in SED rabbits (P < 0.05, Table 1). Previous studies have shown that EXT at similar intensities in paced rabbits does not significantly alter cardiac hypertrophy and dysfunction (26, 30). In the present study, we observed similar results (Table 1).
CS activity in the soleus muscle was significantly higher in both EXT groups compared with the SED groups (Table 1), which indicated a marked effect of EXT. There was no significant difference between EXT-sham and EXT-CHF groups, demonstrating a similar EXT effect in the two groups.
Effect of EXT on renal sympathetic response to the CB chemoreflex in sham and CHF rabbits.
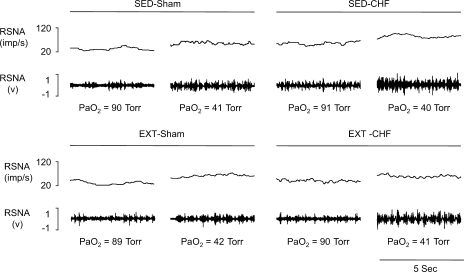
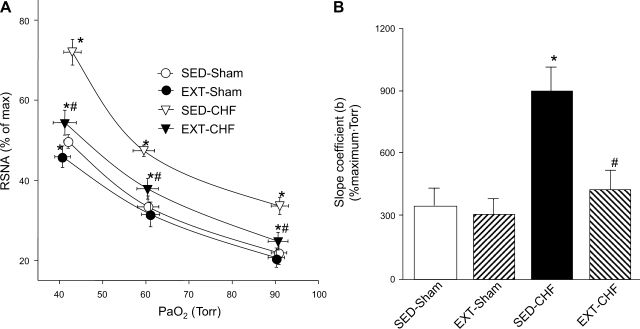
In SED rabbits, baseline RSNA at normoxia in the CHF group was elevated compared with that in shams (Figs. 1 and 2). Similarly, the isocapnic hypoxia-induced increases in RSNA and slope coefficient (b) were significantly augmented in the SED-CHF rabbits vs. SED-sham rabbits (Figs. 1 and 2).
Fig. 1.
Chart recordings of renal sympathetic nerve activity (RSNA) under normoxic and hypoxic states from sedentary (SED)-sham, exercise-trained (EXT)-sham, SED-chronic heart failure (CHF), and EXT-CHF rabbits. PaO2, arterial oxygen partial pressure.
Fig. 2.
A: RSNA-PaO2 relationship in SED-sham, EXT-sham, SED-CHF, and EXT-CHF rabbits. B: slope coefficient (b) for RSNA-PaO2 relationship in SED-sham, EXT-sham, SED-CHF, and EXT-CHF rabbits. Values are means ± SE; n = 8 in each group. *P < 0.05 vs. SED-sham, #P < 0.05 vs. SED-CHF.
In EXT-sham rabbits, baseline RSNA, the response of RSNA to mild isocapnic hypoxia (PaO2 ∼60 Torr), and slope coefficient were not altered vs. SED-sham rabbits, but the response of RSNA to severe isocapnic hypoxia (PaO2 ∼40 Torr) was reduced compared with that in SED-sham rabbits (Figs. 1 and 2).
In EXT-CHF rabbits, baseline RSNA and the RSNA response to both mild and severe hypoxic states were significantly reduced compared with SED-CHF rabbits. Although the baseline RSNA and the reflex response to hypoxia were not reduced to the levels seen in either EXT-sham or SED-sham rabbits (Figs. 1 and 2), there was no significant difference in the slope coefficient (b) between EXT-CHF and SED-sham rabbits (Fig. 2B).
Effect of EXT on CB chemoreceptor activity in sham and CHF rabbits.
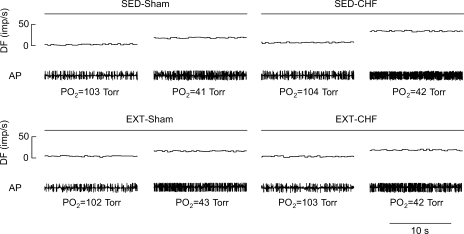
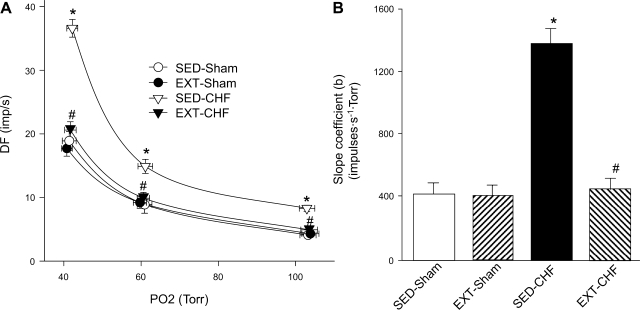
The baseline CB chemoreceptor activity at normoxia, the afferent response to isocapnic hypoxia, and the slope coefficient (b) were enhanced in SED-CHF rabbits vs. SED-sham rabbits (Figs. 3 and 4). In EXT-sham rabbits, the baseline afferent nerve discharge at normoxia, the CB chemoreceptor response to hypoxia, and the slope coefficient did not differ from those in SED-sham rabbits (Figs. 3 and 4). In EXT-CHF rabbits, however, the baseline CB chemoreceptor discharge, the afferent response to hypoxia, and the slope coefficient were markedly reduced compared with those in SED-CHF rabbits and did not differ from those in SED-sham or ETX-sham rabbits (Figs. 3 and 4).
Fig. 3.
Representative recording of afferent discharge in carotid body (CB) chemoreceptor fibers from SED-sham, EXT-sham, SED-CHF, and EXT-CHF rabbits. DF, discharge frequency; AP, action potential.
Fig. 4.
A: effect of EXT on CB chemoreceptor sensitivity in sham and CHF rabbits. B: slope coefficient (b) for the DF-Po2 relationship in SED-sham, EXT-sham, SED-CHF, and EXT-CHF rabbits. Values are means ± SE; n = 8 in each group. *P < 0.05 vs. SED-sham, #P < 0.05 vs. SED-CHF.
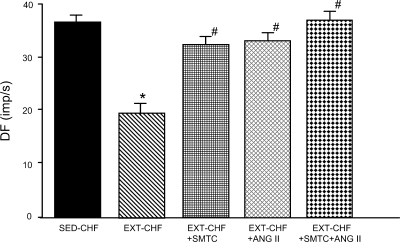
Effects of S-methyl-l-thiocitrulline and ANG II on CB chemoreceptor activity response to hypoxia in SED-CHF and EXT-CHF rabbits.
In previous studies, our laboratory demonstrated that either 1 μM S-methyl-l-thiocitrulline (SMTC) (a specific nNOS inhibitor) or 100 pM ANG II markedly enhanced CB afferent responsiveness to hypoxia in SED-sham rabbits, but are without effect on CB chemoreceptor responses to hypoxia in SED-CHF rabbits (22, 25). In the present study after EXT, both SMTC and ANG II significantly increased CB chemoreceptor responsiveness to hypoxia in EXT-CHF rabbits (Fig. 5), which is similar to that shown in SED-Sham rabbits (22, 25). Additionally, coperfusion of the CB with SMTC and ANG II in EXT-CHF rabbits further elevated the CB chemoreceptor response to hypoxia compared with that by SMTC or ANG II alone (P > 0.0.5, Fig. 5).
Fig. 5.
Effects of S-methyl-l-thiocitrulline (SMTC; 1 μM) and angiotensin II (ANG II; 100 pM) on CB chemoreceptor response to severe hypoxia (PaO2 = 40.5 ± 2.1 Torr) in EXT-CHF rabbits. Values are means ± SE; n = 4 in each group. *P < 0.05 vs. SED-CHF, #P < 0.05 vs. EXT-CHF.
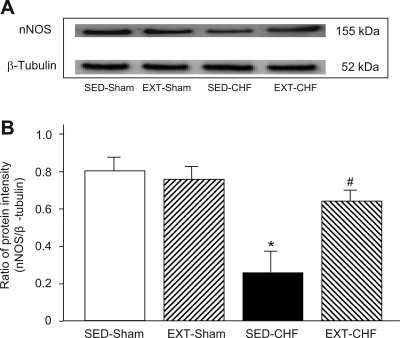
Expression of nNOS protein and NO level in CB.
Western blot data revealed that the expression of nNOS protein was lower in the CB from SED-CHF rabbits than that from SED-sham rabbits (Fig. 6). The nNOS protein expression in the CB was not affected by EXT in sham rabbits. In the EXT-CHF rabbits, however, nNOS protein was significantly higher compared with that in the CB from SED-CHF rabbits (Fig. 6).
Fig. 6.
Expression of neuronal nitric oxide synthase (nNOS) protein in the CB from SED-sham, EX-sham, SED-CHF, and EXT-CHF rabbits. The representative (A) and summary (B) results for protein expression of nNOS are given. Values are means ± SE; n = 6 in each group. *P < 0.05 vs. SED-sham, #P < 0.05 vs. SED-CHF.
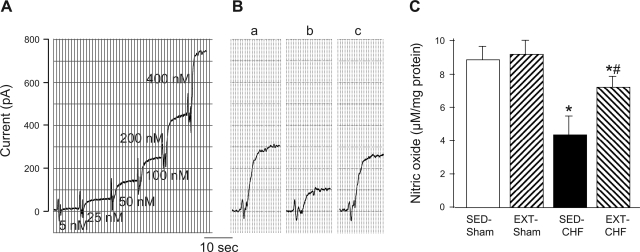
The NO concentration in CBs from SED-CHF rabbits was remarkably lower than that from SED-sham rabbits (Fig. 7), consistent with data from our laboratory's previous study (22). NO production in the CBs from EXT-CHF rabbits was significantly enhanced compared with that from SED-CHF rabbits but was less than the normal level seen in SED-sham rabbits. NO level was not affected by EXT in sham rabbits (Fig. 7).
Fig. 7.
Nitric oxide (NO) level in the CBs from SED-sham, EXT-sham, SED-CHF, and EXT-CHF rabbits. A: dose-response standard curve of S-nitroso-N-acetyl-l-penicillamine (SNAP) (a NO donor, see details in text). B: representative measurement of NO level in the CB homogenate from SED-sham (Ba), SED-CHF (Bb), and EXT-CHF (Bc) rabbits. C: NO concentration in the CBs from SED-sham, EXT-sham, SED-CHF, and EXT-CHF rabbits. Values are means ± SE; n = 5 in each group. *P < 0.05 vs. SED-sham, #P < 0.05 vs. SED-CHF.
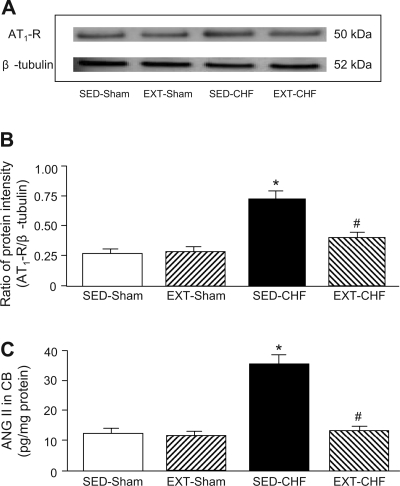
Expression of AT1-receptor protein and ANG II concentration in CB.
The expression of AT1-receptor protein in CBs from SED-CHF rabbits was significantly increased compared with that from SED-sham rabbits (Fig. 8). In sham rabbits, EXT did not affect AT1-receptor protein in CB. However, the enhancement of AT1-receptor protein in the CB from SED-CHF was significantly blunted in EXT-CHF rabbits (Fig. 8).
Fig. 8.
Expression of ANG II type 1 (AT1) receptor (AT1R) protein and ANG II concentration in the CB from SED-sham, EXT-sham, SED-CHF, and EXT-CHF rabbits. The representative (A) and summary (B) results for protein expression of AT1R are given. *P < 0.05 vs. SED-sham, #P < 0.05 vs. SED-CHF. C: ANG II concentration in the CBs from SED-sham, EXT-sham, SED-CHF, and EXT-CHF rabbits. Values are means ± SE; n = 6 (A and B) and n = 5 (C) in each group. *P < 0.05 vs. SED-sham, #P < 0.05 vs. SED-CHF.
The tissue ANG II concentration in the CBs from SED-CHF rabbits was significantly elevated compared with that in SED-sham rabbits (Fig. 8). Similar to AT1 protein, EXT blunted the enhanced ANG II concentration in the CB from CHF rabbits but did not affect the ANG II concentration in CBs from sham rabbits (Fig. 8).
DISCUSSION
The major findings of this study are that EXT normalizes the exaggerated peripheral chemoreflex function and CB chemoreceptor sensitivity in CHF rabbits. The ability of EXT to prevent alterations in the ANG II/AT1-receptor and nNOS/NO signaling pathways in the CB in CHF, confirmed from afferent recordings and protein analyses in the CB after EXT, provides a logical explanation for these results based on our laboratory's previous studies (21, 22, 25, 43, 44).
EXT can serve as an effective treatment to improve the quality of life and survival rate in patients with CHF (1, 8, 29, 36). However, the mechanisms for these beneficial effects of EXT are not understood. Recent studies have shown that EXT increases cardiac vagal tone and reduces sympathetic outflow in the CHF patients (9, 19) and in CHF rabbits (26, 30) without significant improvement in cardiac function. The ability of EXT to reduce sympathetic outflow is likely a key point toward improving prognosis in CHF. Previously, our laboratory has shown that an enhanced sensitivity of the peripheral chemoreflex contributes to sympathetic activation in CHF, because inhibition of peripheral chemoreceptor activity decreases RSNA in CHF, but not in sham rabbits (43). In the present study, EXT significantly attenuated the enhanced peripheral chemoreflex function and resting RSNA induced by CHF (Figs. 1 and 2). Thus normalization of peripheral chemoreflex function is an important component to the beneficial effects of EXT on sympathetic tone in CHF.
The CBs are a pair of small arterial chemoreceptor organs, which sense blood PaO2, PaCO2, and pH, and reflexly influence cardiopulmonary function via primary afferent fibers in the CSN (11, 12). Because rabbits lack functional aortic chemoreceptors (2, 45), the peripheral chemoreflex is attributable mainly to the CBs in this species. Our present study reveals that EXT nearly completely normalizes the exaggerated CB chemoreceptor sensitivity observed in CHF rabbits, in addition to blunting the chemoreflex sympathetic activation. These results confirm our laboratory's previous studies showing an augmented afferent input from CB chemoreceptors is involved in the enhancement of peripheral chemoreflex function in CHF rabbits (22, 25, 44). Furthermore, these results suggest that EXT protects the CHF rabbits against peripheral chemoreflex dysfunction via maintenance of normal CB chemoreceptor sensitivity.
nNOS/NO is involved in the enhanced CB chemoreceptor sensitivity in CHF rabbits. Our laboratory's previous study found that NOS inhibition (NG-nitro-l-arginine) in the CB increased the baseline discharge of CB chemoreceptors in the normoxic state and the chemoreceptor response to isocapnic hypoxia in sham rabbits, but had very little effect on afferent activity in either condition in CHF rabbits (44). Conversely, the NO donor, SNAP, inhibited CB chemoreceptor activity to a much greater extent in CHF than in sham rabbits (44). Furthermore, gene transfer of nNOS (Ad.nNOS) to the CB in CHF rabbits enhanced nNOS protein expression and NO level in the CB and reversed the enhanced CB chemoreceptor activity seen in the CHF state (22). The specific nNOS inhibitor, SMTC, abolished the effect of Ad.nNOS on CB chemoreceptor activity (22). Like gene transfer of nNOS to the CB, EXT in the present study also increased nNOS expression and NO level in the CB (Figs. 6 and 7) and normalized the enhancement of the CB chemoreceptor sensitivity in CHF rabbits, which could be reversed by perfusion of the CB with nNOS inhibitor SMTC (Fig. 5). These results demonstrate that a marked downregulation of endogenous nNOS and NO level in the CB is involved in the enhanced CB chemoreceptor activity in CHF rabbits and that EXT maintains normal CB function in CHF in part by preventing downregulation of the nNOS/NO signaling pathway in the CB.
We have shown that elevated tissue ANG II and functional AT1 receptors in the CB also contribute to the augmented chemoreceptor afferent activity in CHF rabbits (25). Blockade of AT1 receptors diminished the CB chemoreceptor responses to hypoxia in the isolated CB from CHF rabbits but not from sham rabbits (25). This effect was associated with elevated CB ANG II concentration and expression of AT1 receptors in the CB from CHF rabbits (25). In the present study, EXT decreased ANG II concentration and expression of AT1 receptors in the CBs from CHF rabbits to the levels found in sham rabbits. Perfusion of the CB with ANG II reversed the effect of EXT to normalize enhanced CB chemoreceptor sensitivity in CHF rabbits. These results, taken together, suggest that EXT prevents the enhanced CB chemoreceptor activity of CHF via preventing upregulation of the ANG II/AT1-receptor signaling pathway, as well as preventing downregulation of the NOS/NO pathway in the CB.
Both ANG II/AT1 receptor and nNOS/NO modulate CB chemoreceptor sensitivity. Our present study suggests that an interaction exists between ANG II and nNOS, because the effects of SMTC and ANG II on afferent responsiveness to hypoxia, although similar, were not summative (Fig. 5), but it is not clear how these two signaling pathways interact with each other. Our previous observations have shown that NADPH oxidase-derived superoxide anion mediates the ANG II-enhanced CB chemoreceptor activity in CHF rabbits (21). ANG II may contribute to depressed bioavailable NO in the CB by suppressing nNOS gene expression (18) and increased scavenging of NO through superoxide anion production (34). Conversely, the downregulation of NO level in the CB in CHF may act to enhance the effects of ANG II by a reduced inhibitory effect of NO on the NADPH oxidase and superoxide formation (31, 32), while limiting peroxynitrite formation. It is clear that upregulation of ANG II/superoxide pathways and downregulation of NOS/NO pathways in the CB in CHF have complementary effects on CB chemoreceptor sensitization and that these effects are reversed by EXT in a similar complementary fashion. The relationship among EXT, ANG II/AT1 receptor-NADPH oxidase-superoxide, and nNOS/NO on CB chemoreceptor function needs further study.
Although the present study demonstrates an important contribution of elevated CB chemoreceptor input to the enhancement of peripheral chemoreflex function in CHF and the contribution of nNOS/NO downregulation and ANG II/AT1 receptor upregulation in the CB to this effect (22, 25), previous work from our group has shown a number of other cardiovascular reflex and central neural alterations also contribute to elevated peripheral chemoreflex sensitivity in CHF (38). Enhanced cardiac sympathetic afferent input augments the peripheral chemoreceptor reflex in CHF, and central ANG II, specifically located in the nucleus tractus solitarius, plays a major role in this reflex interaction (15). In addition, impairment of NO function within the paraventricular nucleus in CHF rats contributes to an augmented peripheral chemoreflex and subsequent elevation of sympathetic activity in CHF (37). Results from the present study indicate that EXT did not completely prevent enhanced peripheral chemoreflex function in CHF rabbits (Fig. 2), even though EXT maintained normal CB chemoreceptor activity (Fig. 4). This residual enhancement of CB chemoreflex responsiveness with EXT in CHF is likely to be central in origin and may reflect an interaction with somatic reflexes (41).
Moderate EXT used in the present study and in similar previous studies (26, 30) did not alter the magnitude or the rate of cardiac dysfunction during pacing. Thus, as often observed in humans (36), beneficial effects of EXT in CHF are not closely correlated with improved indexes of cardiac function. The peripheral signals that link EXT to restoration of normal CB proteomics and reflex function in CHF remain to be elucidated. Studies by Zucker and colleagues (26, 30) suggest the ability of EXT to suppress the renin-angiotensin system plays a key role.
EXT also has been shown to improve endothelial/vascular function in CHF (16, 20). We cannot discount the possibility that a reduction in CB blood flow or altered vascular function in the CB may contribute to the altered signaling pathways and enhanced afferent chemoreceptor activity of the CB in CHF. Nevertheless, we have observed changes in K+ channel function in isolated CB glomus cells from the CHF state that were directly influenced by pharmacological manipulation of both ANG II/AT1 receptor and nNOS/NO signaling pathways in the isolated cells (23, 24). Thus there is evidence to support an effect of ANG II and NO on glomus cell function, independent of the vasculature. An important objective of future studies should address the role of CB vascular function on chemoreceptor sensitivity in CHF.
Even though the present study supports a beneficial effect of EXT on cardiorespiratory control in CHF patients, the results cannot be directly extrapolated to the clinical setting. The constraints of our experimental design do not reflect that normally seen in the clinic. EXT was started at the same time that pacing was initiated to induce CHF, whereas, in the clinic, EXT often is initiated after overt CHF has been diagnosed. Nevertheless, the results from our study and others (26, 30) illustrate that intervention of EXT in early CHF has clear beneficial effects on cardiorespiratory reflexes and sympathetic function that do not rely on improvement in cardiac function per se.
In summary, the present study demonstrates a beneficial effect of EXT on chemoreflex function in CHF. EXT prevents the elevation in CB chemoreceptor discharge observed in CHF and prevents alterations in the ANG II/AT1 receptor and nNOS/NO signaling pathways in the CB that are responsible for this effect.
Perspectives.
EXT is recognized as an effective treatment to reduce neurohumoral activation (14) and enhance functional capacity and quality of life (1, 8, 29, 36) in patients with stable CHF. CHF patients typically exhibit a low tolerance for exercise (rapid fatigue) (33, 36), which is one of the primary symptoms that leads CHF patients to seek medical care. Without discounting other important contributing factors to exercise intolerance in CHF (17, 35), sensitization of the peripheral chemoreflex in CHF may aggravate intolerance to exercise by intensifying activation of sympathetic outflow to further constrain impaired blood flow to exercising muscle (41) and by intensifying dyspnea due to the exaggerated chemoreflex hyperventilation (4, 6, 7, 28, 33, 42). Indeed, suppression of the peripheral chemoreflex improves exercise tolerance in CHF patients (5). The beneficial effect of EXT to reverse exaggerated chemoreflex function, as we have shown here, is likely to be one of the important factors toward lowering neurohumoral activation and promoting higher exercise capacities in CHF patients with improved mobility and quality of life.
GRANTS
This study was supported by a Program Project Grant from the National Heart, Lung, and Blood Institute (PO1-HL62222).
Supplementary Material
Acknowledgments
The authors thank Kaye Talbitzer and Dr. Kurtis Cornish for surgical assistance and supervision of the heart failure animal core at University of Nebraska Medical Center.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 99: 1173–1182, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Chalmers JP, Korner PI, White SW. The relative roles of the aortic and carotid sinus nerves in the rabbit in the control of respiration and circulation during arterial hypoxia and hypercapnia. J Physiol 188: 435–450, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choate JK, Kato K, Mohan RM. Exercise training enhances relaxation of the isolated guinea-pig saphenous artery in response to acetylcholine. Exp Physiol 85: 103–108, 2000. [PubMed] [Google Scholar]
- 4.Chua TP, Clark AL, Amadi AA, Coats AJS. Relation between chemosensitivity and the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol 27: 650–657, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Chua TP, Ponikowski PP, Harrington D, Chambers J, Coats AJS. Contribution of peripheral chemoreceptors to ventilation and the effects of their suppression on exercise tolerance in chronic heart failure. Heart 76: 483–489, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chua TP, Ponikowski P, Webb-Peploe K, Harrington D, Anker SD, Piepoli M, Coats AJ. Clinical characteristics of chronic heart failure patients with an augmented peripheral chemoreflex. Eur Heart J 18: 480–486, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Ciarka A, Cuylits N, Vachiery JL, Lamotte M, Degaute JP, Naeije R, van de Bome P. Increased peripheral chemorecptors sensitivity and exercise ventilation in heart transplant recipients. Circulation 113: 252–257, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Demopoulos L, Bijou R, Fergus I, Jones M, Strom J, LeJemtel TH. Exercise training in patients with severe congestive heart failure: enhancing peak aerobic capacity while minimizing the increase in ventricular wall stress. J Am Coll Cardiol 29: 597–603, 1997. [DOI] [PubMed] [Google Scholar]
- 9.DiCarlo SE, Stahl LK, Bishop VS. Daily exercise attenuates the sympathetic nerve response to exercise by enhancing cardiac afferents. Am J Physiol Heart Circ Physiol 273: H1606–H1610, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Esler M, Kaye D, Lambert G, Esler D, Jennings G. Adrenergic nervous system in heart failure. Am J Cardiol 80: 7L–14L, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Eyzaguirre C, Fitzgerald RS, Lahiri S, Zapata P. Arterial chemoreceptors. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. Bethesda, MD: Am. Physiol. Soc., 1983, sect. 2, vol. III, pt. 2, chapt. 16, p. 557–621. [Google Scholar]
- 12.Fidone S, Gonzalez C. Initiation and control of chemoreceptor activity in the carotid body. In: Handbook of Physiology. The Respiratory System. Control of Breathing. Bethesda, MD: Am. Physiol. Soc., 1986, sect. 3, vol. II, pt. 1, p. 247–312. [Google Scholar]
- 13.Floras JS Clinical aspects of sympathetic activation and parasympathetic withdrawal in heart failure. J Am Coll Cardiol 22: 72A–84A, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Gademan MG, Swenne CA, Verwey HF, van der Laarse A, Maan AC, van de Vooren H, van Pelt J, van Exel HJ, Lucas CM, Cleuren GV, Somer S, Schalij MJ, van der Wall EE. Effect of exercise training on autonomic derangement and neurohumoral activation. J Card Fail 13: 294–303, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Pan YX, Wang WZ, Li YL, Schultz HD, Zucker IH, Wang W. Cardiac sympathetic afferent stimulation augments the arterial chemoreceptor reflex in anesthetized rats. J Appl Physiol 102: 37–43, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation 108: 530–535, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, Coats AJ. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol 30: 1758–1764, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Kihara M, Umemura S, Kadota T, Yabana M, Tamura K, Nyuui N, Ogawa N, Murakami K, Fukamizu A, Ishii M. The neuronal isoform of constitutive nitric oxide synthase is up-regulated in the macula densa of angiotensinogen gene-knockout mice. Lab Invest 76: 285–294, 1997. [PubMed] [Google Scholar]
- 19.Kingwell BA, Dart AM, Jennings GL, Korner PI. Exercise training reduces the sympathetic component of the blood pressure-heart rate baroreflex in man. Clin Sci (Lond) 82: 357–362, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi N, Tsuruya Y, Iwasawa T, Ikeda N, Hashimoto S, Yasu T, Ueba H, Kubo N, Fujii M, Kawakami M, Saito M. Exercise training in patients with chronic heart failure improves endothelial function predominantly in the trained extremities. Circ J 67: 505–510, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Li YL, Gao L, Zucker IH, Schultz HD. NADPH oxidase-derived superoxide anion mediates angiotensin II-enhanced carotid body chemoreceptor sensitivity in heart failure rabbits. Cardiovasc Res 75: 546–554, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YL, Li YF, Liu D, Cornish KG, Patel KP, Zucker IH, Channon KM, Schultz HD. Gene transfer of neuronal nitric oxide synthase to carotid body reverses enhanced chemoreceptor function in heart failure rabbits. Circ Res 97: 260–267, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Li YL, Schultz HD. Enhanced sensitivity of Kv channels to hypoxia in the rabbit carotid body in heart failure: role of angiotensin II. J Physiol 575: 215–227, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li YL, Sun SY, Overholt JL, Prabhakar NR, Rozanski GJ, Zucker IH, Schultz HD. Attenuated outward potassium currents in carotid body glomus cells of heart failure rabbit: involvement of nitric oxide. J Physiol 555: 219–229, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, Patel KP, Wang W, Schultz HD. Angiotensin II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits. Cardiovasc Res 71: 129–138, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: a role for angiotensin II. Circulation 102: 1854–1862, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Marshall JM Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev 74: 543–594, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Meguro K, Adachi H, Oshima S, Taniguchi K, Nagai R. Exercise tolerance, exercise hyperpnea and central chemosensitivity to carbon dioxide in sleep apnea syndrome in heart failure patients. Circ J 69: 695–699, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Meyer K, Schwaibold M, Westbrook S, Beneke R, Hajric R, Görnandt L, Lehmann M, Roskamm H. Effect of short-term exercise training and activity restriction on functional capacity in patients with severe chronic congestive heart failure. Am J Cardiol 78: 1017–1022, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Mousa TM, Liu D, Cornish KG, Zucker IH. Exercise training enhances baroreflex sensitivity by an angiotensin II-dependent mechanism in chronic heart failure. J Appl Physiol 104: 616–624, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Muzaffar S, Jeremy JY, Angelini GD, Stuart-Smith K, Shukla N. Role of the endothelium and nitric oxide synthases in modulating superoxide formation induced by endotoxin and cytokines in porcine pulmonary arteries. Thorax 58: 598–604, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muzaffar S, Shukla N, Angelini G, Jeremy JY. Nitroaspirins and morpholinosydnonimine but not aspirin inhibit the formation of superoxide and the expression of gp91phox induced by endotoxin and cytokines in pig pulmonary artery vascular smooth muscle cells and endothelial cells. Circulation 110: 1140–1147, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Negrao CE, Middlekauff HR. Adaptations in autonomic function during exercise training in heart failure. Heart Fail Rev 13: 51–60, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A, Coats AJ. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am Heart J 137: 1050–1056, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ. Exercise and heart failure: a statement from the American heart association committee on exercise, rehabilitation, and prevention. Circulation 107: 1210–1225, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Reddy MK, Schultz HD, Zheng H, Patel KP. Altered nitric oxide mechanism within the paraventricular nucleus contributes to the augmented carotid body chemoreflex in heart failure. Am J Physiol Heart Circ Physiol 292: H149–H157, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Schultz HD, Li YL. Carotid body function in heart failure. Respir Physiol Neurobiol 157: 171–185, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz HD, Sun SY. Chemoreflex function in heart failure. Heart Fail Rev 5: 45–56, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Srere PA Citrate synthase. Methods Enzymol 13: 3–5, 1969. [Google Scholar]
- 41.Stickland MK, Miller JD, Smith CA, Dempsey JA. Carotid chemoreceptor modulation of regional blood flow distribution during exercise in health and chronic heart failure. Circ Res 100: 371–1378, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan MJ, Higginbotham MB, Cobb FR. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite hemodynamic and pulmonary abnormalities. Circulation 77: 552–559, 1988. [DOI] [PubMed] [Google Scholar]
- 43.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol 86: 1264–1272, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced activity of carotid body chemoreceptors in rabbits with heart failure: role of nitric oxide. J Appl Physiol 86: 1273–1282, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Verna A, Roumy M, Leitner LM. Loss of chemoreceptive properties of the rabbit carotid body after destruction of the glomus cells. Brain Res 100: 13–23, 1975. [DOI] [PubMed] [Google Scholar]
- 46.Zucker IH, Wang W, Brandle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis 37: 397–414, 1995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.