Abstract
N-methyl-d-aspartate (NMDA) receptor antagonism in the phrenic motonucleus area eliminates phrenic long-term facilitation (pLTF; a persistent augmentation of phrenic nerve activity after episodic hypoxia) in anesthetized rats. However, whether NMDA antagonism can eliminate ventilatory LTF (vLTF) in awake rats is unclear. The role of non-NMDA receptors in LTF is also unknown. Serotonin receptor antagonism before, but not after, episodic hypoxia eliminates pLTF, suggesting that serotonin receptors are required for induction, but not maintenance, of pLTF. However, because NMDA and non-NMDA ionotropic glutamate receptors are directly involved in mediating the inspiratory drive to phrenic, hypoglossal, and intercostal motoneurons, we hypothesized that these receptors are required for both formation and maintenance of vLTF. vLTF, induced by five episodes of 5-min poikilocapnic hypoxia (10% O2) with 5-min normoxia intervals, was measured with plethysmography in conscious adult male Sprague-Dawley rats. Either (±)-2-amino-5-phosphonovaleric acid (APV; NMDA antagonist, 1.5 mg/kg) or 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; non-NMDA antagonist, 10 mg/kg) was systemically (ip) injected ∼30 min before hypoxia. APV was also injected immediately after or 20 min after episodic hypoxia in additional groups. As control, vehicle was similarly injected in each rat 1–2 days before. Regardless of being injected before or after episodic hypoxia, vehicle did not alter vLTF (∼23%), whereas APV eliminated vLTF while having little effect on baseline ventilation or hypoxic ventilatory response. In contrast, CNQX enhanced vLTF (∼34%) while decreasing baseline ventilation. Collectively, these results suggest that activation of NMDA but not non-NMDA receptors is necessary for formation and maintenance of vLTF in awake rats.
Keywords: respiratory control; plasticity; intermittent hypoxia; (±)-2-amino-5-phosphonovaleric acid; 6-cyano-7-nitroquinoxaline-2,3-dione; N-methyl-d-aspartate
exposure to acute intermittent hypoxia (AIH; 3–10 episodes of short-term hypoxia) or repeated carotid afferent stimulation induces respiratory long-term facilitation (LTF; an augmentation of respiratory activity that lasts for many minutes to hours after the stimulation has ended). LTF has been identified in several animal species, including LTF of phrenic (pLTF), hypoglossal, and intercostal nerve activity in anesthetized animals (3, 16, 20, 28, 30, 38) and LTF of ventilation (vLTF) in awake animals (8, 34, 45, 49), expressed as an integrative function involving many muscles, such as diaphragm, genioglossus, and inspiratory intercostals. LTF of genioglossal muscle activity was also identified in cats (30) and neonatal rats (37). Although LTF of ventilation or genioglossal muscle activity was not elicited in normal humans during wakefulness (21), it was evident in the presence of elevated CO2 levels (19) or during non-rapid eye movement (NREM) sleep (11). LTF was also elicited during NREM sleep in individuals with inspiratory flow limitation (e.g., snorers and obstructive sleep apnea patients), mainly as a persistent decrease in upper airway resistance, an indication of upper airway dilatation due to dilator muscles LTF (1, 2). LTF has thus been postulated to be an adaptive mechanism (2, 3, 7, 26, 32) that may help stabilize upper airway patency in obstructive sleep apnea patients after repeated exposure to apneas and/or hypopneas.
The AIH-induced pLTF, hypoglossal LTF, and vLTF are all completely blocked by pretreatment of the broad-spectrum serotonin (5-hydroxytryptamine, 5-HT) receptor antagonist methysergide or the specific 5-HT2 receptor antagonist ketanserin (3, 18, 24, 26, 36), suggesting that the motor output LTF of the major inspiratory muscles requires 5-HT2 serotonin receptors. It has also been reported that systemic injection of ketanserin before AIH eliminated pLTF, whereas the same drug injection immediately after or 45 min after AIH had little effect on pLTF (i.e., LTF was similar), suggesting that activation of serotonin receptors is required for induction, but not maintenance, of pLTF in anesthetized rats (18).
Ionotropic glutamate receptors of NMDA (N-methyl-d-aspartate) and non-NMDA subtypes play key roles in synaptic plasticity, such as long-term potentiation (LTP), a putative cellular mechanism for learning and memory (22, 29). For example, LTP of the Schaffer collateral pathways in the hippocampus or many other synapses can no longer be elicited after NMDA receptor antagonism (22, 29). Numerous forms of learning and memory are also abolished or impaired by blockade or alteration of NMDA receptors (9, 48). In the respiratory control system, the synaptic transmissions of inspiratory drive from premotor neurons to phrenic, hypoglossal, and intercostal motoneurons all use glutamate as a neurotransmitter, and they are mediated through both NMDA and non-NMDA receptors (10, 31, 46). These glutamate receptors may be modified to produce plasticity in these synapses, as they do in the induction and maintenance of LTP (29, 47). Indeed, in our laboratory's recent studies using anesthetized rats, both systemic injection and microinjection (into the phrenic motonucleus region) of the NMDA receptor antagonist MK-801 eliminated the AIH-induced pLTF, suggesting that activation of NMDA receptors in the phrenic motonucleus region (most likely on the phrenic motoneuron) is required for pLTF (35). Despite these findings, the role of NMDA receptors in vLTF, whose expression involves other muscles besides diaphragm, has not been evaluated in awake animals. Whether those NMDA receptors are required for induction or maintenance of LTF is also unknown. In addition, as far as we are aware, the role of non-NMDA receptors in any form of LTF has not been explored previously.
The present study was thus designed to examine the effects of NMDA or non-NMDA receptor antagonism on the formation and/or maintenance of vLTF in awake rats. Because NMDA receptors are involved in mediating inspiratory drive to motoneurons of the major ventilation-related muscles, we speculated that NMDA receptors, like serotonin receptors, are another common mechanism shared by those major inspiratory muscles, and thus we hypothesized that the formation of vLTF would be blocked by NMDA receptor antagonism. Furthermore, we hypothesized that unlike serotonin receptors, NMDA receptors are required for the maintenance of vLTF as well, because NMDA receptors, if modified, could directly enhance ventilation via amplifying the descending inspiratory drives. Because non-NMDA receptors are also involved in many forms of synaptic plasticity, we hypothesized that non-NMDA receptors would also play a role in vLTF.
METHODS
The Harvard Medical Area Standing Committee on Animals approved all experimental procedures used herein. Experiments were conducted on 30 adult male Sprague-Dawley rats (250–350 g, ∼3 mo old; colony CDIGS, Charles River, Wilmington, MA). These rats were housed in cages with stable room temperature (∼21°C), and they were supplied with food and water ad libitum.
Ventilatory Measurement
Ventilation was measured by use of a custom-made 3-liter whole body flow-through plethysmograph (Buxco Electronics, Sharon, CT). Individual, freely behaving, awake rats were placed in the precalibrated plethysmographic chamber connected via a controlled leak to a reference chamber. The atmosphere within the animal chamber was maintained with air flowing through the chamber at a rate of 3 l/min. A bias flow was connected to an aerosol port of the chamber to maintain the O2 concentration within the animal chamber. A custom-made computer software system (Biosystem XA, Buxco Electronics) monitored the output of a differential pressure transducer (model TRD5100) connected between the animal and reference chambers. This software system provided a breath-by-breath display of minute ventilation (V̇e), tidal volume (Vt), and respiratory frequency (fr) before (baseline), during, and after the AIH that was used to induce vLTF.
In selected rats (n = 10), the computer also continuously monitored the output of the CO2 analyzer (Servomex Transducers, Norwood, MA) that sensed alternately the gases entering and exiting the plethysmographic chamber. With the known flow rate, the measured CO2 gas concentration was used to calculate the CO2 production (V̇co2) that defines metabolic rate. In those rats, V̇co2 was measured shortly before (original baseline) and after drug injection (real baseline) to examine the drug effect on metabolic rate. In eight additional rats, body temperature was measured before and after drug injection with a rectal thermometer. A common temperature 37.5°C was designated to all other rats. V̇co2 and body temperature were not measured in all rats because turning the gases flow switch and inserting the rectal thermometer tended to increase rat's activities, especially in those with drug injection, thus interfering the ventilatory measurement (see strict rule below in Experimental Procedures). In the beginning of each experiment, the values of body weight, body temperature, and the barometric pressure reading were inputted to the software system. The temperature in the chamber was ∼21°C (which was similar to that in the housing facility) at all times and was also continuously monitored by the system.
NMDA and Non-NMDA Receptor Antagonists
The two drugs used in this study were (±)-2-amino-5-phosphonovaleric acid (APV; a competitive NMDA receptor antagonist) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; a competitive non-NMDA receptor antagonist). Both drugs are widely used ionotropic glutamate receptor antagonists (Sigma-Aldrich, St. Louis, MO), easily dissolved in saline and can cross the blood-brain barrier (23).
Experimental Procedures
Rats were placed in the plethysmographic chamber and allowed to adapt to the chamber for ∼1 h. LTF was elicited by five episodes of 5-min poikilocapnic hypoxia (10% O2), separated with 5-min intervals of normoxia. V̇e was measured before (over 10 min), during, and after this AIH protocol to determine resting V̇e (baseline), hypoxic ventilatory response (HVR), and vLTF, respectively (see Fig. 1). In the animal chamber, the shift from normoxia to the target hypoxia level (10% O2) took <1 min, and the shift from hypoxia to normoxia took <30 s. During these hypoxic and normoxic episodes, only the final 2 min of data were included in the analysis. Hypoxic V̇e was not significantly different among five episodes, and it was thus averaged. The HVR is defined as an increase from baseline to the averaged hypoxic V̇e, normalized as a percentage of the baseline. After the last hypoxia episode, V̇e was measured at 15-min intervals (i.e., 15, 30, 45, and 60 min) with each value representing a 5-min average (e.g., the 15-min posthypoxia value is an average of the data collected between 15 and 20 min). LTF at each time point is defined as an increase from baseline in posthypoxia V̇e at that specific time point, normalized as a percentage of the baseline. To facilitate analysis, the overall vLTF, represented by a set of absolute V̇e values (Fig. 2), was simplified to a single relative value, vLTF magnitude, by averaging the first three individual LTF values at 15, 30, and 45 min posthypoxia (Table 1). The vLTF magnitude was thus calculated by the equation: 100 × [(V̇e15 + V̇e30 + V̇e45)/3 − baseline]/baseline. This three-value-average method was also used in assessing the magnitude of both fr LTF and Vt LTF.
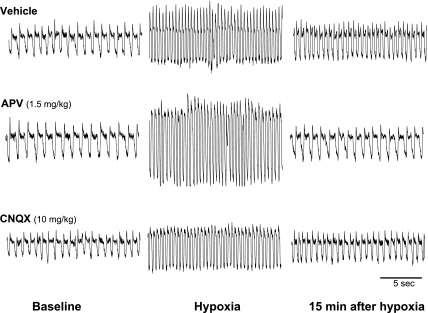
Fig. 1.
Representative traces of breathing pattern from 3 rats administered with vehicle, (±)-2-amino-5-phosphonovaleric acid (APV), or 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) before acute intermittent hypoxia (AIH; 5 episodes of 5-min 10% O2). These traces were recorded by plethysmography before (baseline), during (hypoxia), and 15 min after AIH. Inspiration is represented by the downward deflection of the recording.
Fig. 2.
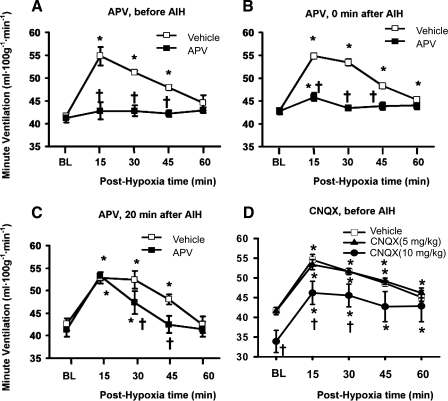
Effect of N-methyl-d-aspartate (NMDA) or non-NMDA receptor antagonism on ventilatory long-term facilitation in awake rats. Minute ventilation was measured before (baseline; BL), during, and after AIH (5 episodes of 5-min 10% O2 hypoxia). Data recorded during AIH are not included. Ventilatory long-term facilitation was measured twice in each rat in A (n = 6, no vehicle data in 1 rat), B (n = 6), and C (n = 4), with vehicle injection in the first measurement and APV injection in the second one. Ventilatory long-term facilitation was measured (after CNQX injection) only once in both 5 mg/kg (n = 6) and 10 mg/kg (n = 6) groups in D, with control group data being the combination of 5 vehicle rats in A and 1 vehicle rat in the 5 mg/kg CNQX group. Many error bars are totally masked by the relatively bigger symbols. Results are expressed as means ± SE. *Significant difference from BL, P < 0.05. †Significant difference from vehicle group, P < 0.05.
Table 1.
Effects of NMDA and non-NMDA receptor antagonism on ventilatory parameters before, during, and after AIH
| APV Injection |
CNOX Injection | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before AIH |
0 min After AIH | 20 min After AIH | Before AIH | ||||||
| Vehicle (n = 5) | 1.5 mg/kg (n = 6) | Vehicle (n = 6) | 1.5 mg/kg (n = 6) | Vehicle (n = 4) | 1.5 mg/kg (n = 4) | Vehicle (n = 6) | 5 mg/kg (n = 6) | 10 mg/kg (n = 6) | |
| V̇e, ml·100 g−1·min−1 | |||||||||
| Baseline | 41.6±0.6 | 41.2±1.0 | 42.9±0.6 | 42.8±0.7 | 42.5±1.3 | 41.3±1.6 | 41.71±0.5 | 41.6±0.9 | 33.9±2.8† |
| HR | 160.3±11.9 | 148.7±10.9 | 156.2±7.9 | 160.6±12.2 | 163.2±13.5 | 156.1±20.5 | 166.3±11.4 | 155.8±10.1 | 151.7±11.9 |
| LTF | 23.4±2.6* | 3.9±2.0† | 21.9±1.1* | 3.9±1.4*† | 20.2±0.9* | 15.5±1.8* | 23.9±2.1* | 23.8±2.2* | 33.6±4.4*† |
| Vt, ml/100 g | |||||||||
| Baseline | 0.52±0.03 | 0.47±0.03 | 0.5±0.04 | 0.51±0.02 | 0.52±0.03 | 0.53±0.02 | 0.54±0.03 | 0.56±0.05 | 0.45±0.04 |
| HR | 28.8±3.5 | 37.9±6.8 | 53.7±5.0 | 49.5±6.1 | 30.6±4.4 | 25.3±7.7 | 28.4±3.1 | 30.4±4.8 | 39.2±7.9 |
| LTF | 8.1±2.1* | 4.7±3.2 | 13.6±5.1* | 10.1±3.2* | 2.0±5.4 | 1.0±3.8 | 8.0±1.9* | 8.9±3.5* | 16.4±4.2* |
| fR, breaths/min | |||||||||
| Baseline | 81.5±5.2 | 89.8±4.4 | 88.7±6.3 | 85.2±4.8 | 82.6±3.7 | 78.5±5.8 | 79.0±5.3 | 74.9±3.1 | 76.3±3.4 |
| HR | 102.8±10.1 | 80.7±5.7 | 67.0±5.0 | 73.9±5.4 | 99.4±11.6 | 104.7±17.4 | 108.2±10.5 | 95.6±3.4 | 84.1±6.7 |
| LTF | 14.2±3.9* | −0.2±3.2† | 7.9±4.1 | −5.1±3.5† | 19.0±7.3* | 15.5±5* | 14.6±7.4* | 14.3±5.4* | 14.4±2.7* |
Values are means ± SE; n, no. of rats. NMDA, N-methyl-d-aspartate; APV, (±)-2-amino-5-phosphonovaleric acid; CNQX, 6-cyano-7-nitrquinoxaline-2,3-dione; AIH, acute intermittent hypoxia (5 episodes of 5-min 10% O2); V̇e, minute ventilation; Vt, tidal volume; fR, respiratory frequency; HR, hypoxic response to 5-min 10% O2 in V̇e, Vt, and fR: % increase above baseline in the respective hypoxic value; LTF, long-term facilitation magnitude of V̇e, Vt, and fR: % increase above baseline in the average of posthypoxia 15–45 min respective values.
Significant difference from 0% increase above baseline, P < 0.05.
Significant difference from respective vehicle group, (P < 0.05).
Ventilatory parameters (V̇e, Vt, and fr) were measured continuously in 1-min time bins following a strict rule, as previously described (33, 34, 36). Briefly, these parameters were measured when rats were observed to be awake and in a quiet state. If rats moved or appeared to be asleep (e.g., eyes closed or heads curled under their bodies) during recording, the whole data collected in that 1-min bin would be rejected from the analysis. This rejection was done blindly; i.e., values were unknown when these 1-min-bin data were rejected. Our criteria for data acceptance are the combination of 1) open eyes, 2) no body movement, and 3) a normal breathing pattern displayed continuously on the computer screen (Fig. 1). An additional rejection algorithm was also included in the computer software breath-by-breath analysis that allows for further rejection of individual artificial breaths. These strict rejection criteria helped to generate more consistent results. Baseline V̇e in the present study was very consistent between and within experimental groups. All the following ventilatory measurements were made at ∼2:00 PM, and they continued from baseline to posthypoxia 60 min.
Effect of APV (injected before AIH) on LTF.
The entire ventilatory measurement (before, during, and after AIH) protocol was conducted twice in the same rats. The first measurement protocol (as control) was initiated 30–60 min after systemic (ip) injection of vehicle (1 ml saline, n = 5). One or 2 days later, the second measurement protocol was initiated 30–60 min after systemic injection of APV (1.5 mg/kg, 1 ml, n = 6; no first measurement in 1 rat). Thus the effect of NMDA receptor antagonism (before AIH) on vLTF was examined in the same rats.
Effect of APV (injected after AIH) on LTF.
The ventilatory measurement protocol was conducted twice in the same rats. Both vehicle (1 ml saline) in the first measurement (as control) and APV (1.5 mg/kg, 1 ml) in the second measurement were (ip) injected immediately after (n = 6) or 20 min after AIH (n = 4) in two additional groups. The two measurements were separated by 1 or 2 days. Thus the effect of NMDA receptor antagonism (after AIH) on vLTF was also examined in the same rats.
Effect of CNQX (injected before AIH) on LTF.
The measurement protocol was conducted only once in these rats, not only because we knew from the NMDA study that vehicle injection had no effect on LTF but also because non-NMDA receptor antagonism did not appear to impair LTF in a pilot study. The measurement was initiated ∼30 min after systemic (ip) injection of CNQX at 5 mg/kg (1 ml, n = 6) or 10 mg/kg (1 ml, n = 6). Thus the effect of non-NMDA receptor antagonism (before AIH) on vLTF was examined in different rats.
Data Analysis
The between-group differences in baseline (V̇e, Vt, and fr) and the individual posthypoxia (V̇e, Vt, and fr) values were statistically analyzed by use of a two-way ANOVA with repeated measures (SigmaStat version 3.0, Jandel, San Rafael, CA), followed by Student-Newman-Keuls post hoc tests. Only baseline and posthypoxia data were included in this analysis (data recorded during AIH not included). The between-group differences in (V̇e, Vt, and fr) LTF magnitude (calculated by the 3-value-average method) and the between- or within-group differences in HVR were analyzed by use of a one-way ANOVA. The paired t-test was also used in some cases (specified in each case). All values are expressed as means ± SE. P < 0.05 was considered significant.
RESULTS
Baseline and HVR
The resting V̇e, Vt, and fr were not significantly changed (all P > 0.153; Table 1; Fig. 2) following systemic (ip) injection of APV (1.5 mg/kg) or CNQX (5 mg/kg). However, the resting V̇e was significantly decreased after injection of CNQX at a 10 mg/kg dose (45.2 ± 1.4 vs. 33.9 ± 2.8 ml·100 g−1·min−1, n = 6; P = 0.003, paired t-test), and it was significantly different between the vehicle and CNQX (10 mg/kg) groups (P < 0.05; Table 1; Fig. 2D). The HVR was not significantly changed after APV (1.5 mg/kg) or CNQX (5 or 10 mg/kg) injection (all P > 0.396; Table 1). Hypoxic responses in Vt and fr were also not significantly changed after APV or CNQX (all P > 0.086; Table 1). In five APV rats and five CNQX (5 mg/kg) rats, metabolic rate (V̇co2) was not significantly different immediately before and after the APV (2.5 ± 0.4 vs. 2.4 ± 0.4 ml·100 g−1·min−1, n = 5; P = 0.33, paired t-test) or CNQX (2.2 ± 0.2 vs. 2.2 ± 0.3 ml·100 g−1·min−1, n = 5; P = 0.98, paired t-test) injection. In five APV rats and three CNQX (5 mg/kg) rats, body temperature was also unchanged after the APV (37.4 ± 0.1 vs. 37.4 ± 0.07°C, n = 5; P = 0.8, paired t-test) or CNQX (37.2 ± 0.1 vs. 37.2 ± 0.1°C, n = 3) injection. These results suggest that baseline V̇e, Vt, fr, body temperature, and metabolic rate as well as HVR were not significantly changed following systemic (ip) injection of APV (1.5 mg/kg) or CNQX (5 or 10 mg/kg), with one exception that 10 mg/kg CNQX injection greatly reduced baseline Ve.
Effect of APV (Injected at Different Time) on LTF
Before AIH.
The AIH stimulus protocol (5 episodes of 10% O2 hypoxia) induced vLTF in vehicle rats but not in the rats pretreated with APV (1.5 mg/kg; Figs. 1 and 2A). The two-way ANOVA revealed a significant interaction effect [F(4,10) = 10.0; P < 0.002] between the (within subject) drug factor (2 levels: pre- and post-APV) and (within subject) time factor (5 levels: baseline, posthypoxia 15, 30, 45, and 60 min) in V̇e data (absolute values). The posthypoxia V̇e value in APV rats was significantly lower than that in vehicle rats at 15-, 30-, and 45-min time points (all P < 0.05), but it was not different from baseline at any time point (all P > 0.833; Fig. 2A). The LTF magnitude (relative values calculated by the 3-value-average method), which was 23.4 ± 2.6% above baseline in vehicle rats, was eliminated in APV rats (Table 1). These results suggest that vLTF was eliminated by the NMDA receptor antagonism with APV (1.5 mg/kg) injected before AIH.
0 min after AIH.
The AIH induced vLTF in vehicle rats but not in the APV rats, in which vehicle or APV was injected immediately after the AIH (Fig. 2B). The two-way ANOVA revealed a significant interaction effect [F(4,18) = 69.5; P < 10−6] between drug and time factors in V̇e data. The posthypoxia V̇e value in APV rats was significantly lower than that in vehicle rats at 15-, 30-, and 45-min points (all P < 0.05), and it was significantly higher than baseline at 15 min (P = 0.0024) but not other points (all P > 0.228; Fig. 2B). The LTF magnitude, which was 21.9 ± 1.1% in vehicle rats, was eliminated (<5%) in APV rats (Table 1). These results suggest that vLTF could not be induced by AIH if NMDA receptors were blocked by APV (1.5 mg/kg) immediately after AIH.
20 min after AIH.
The AIH induced a normal vLTF in vehicle rats when vehicle was injected 20 min after AIH (Fig. 2C). The AIH also induced a normal vLTF at posthypoxia 15 min time point when APV was injected 20 min after the AIH (vLTF difference between vehicle and APV rats at 15-min point is not significant, P = 0.834), but vLTF at 30- and 45-min points were significantly reduced and eliminated, respectively (both P < 0.05; Fig. 2C). The two-way ANOVA revealed a significant interaction effect [F(4, 12) = 9.9; P < 0.0009] between drug and time factors in V̇e data. The posthypoxia V̇e value was not significantly different between APV and vehicle rats at 15-min point (P = 0.76), but was significantly lower in APV vs. vehicle rats at 30- and 45-min points (both P < 0.05; Fig. 2C). The overall vLTF magnitude also appeared to be reduced (vehicle: 20.2 ± 0.9 vs. APV: 15.5 ± 1.8%), although the difference was just short of statistical significance (P = 0.057; Table 1). These results suggest that APV (1.5 mg/kg) injection at 20 min after AIH can substantially decrease or eliminate a fully developed vLTF at subsequent 30- and 45-min time points.
Effect of CNQX (Injected before AIH) on LTF
The AIH induced vLTF in the rats pretreated with CNQX (5 mg/kg, Fig. 2D). The combination of five vehicle rats in the APV (before AIH) group and one vehicle rat in this CNQX group was chosen as a vehicle control group (Table 1), because their body weight and injection timing were matched to this CNQX-treated group. There was no significant interaction effect [F(4,36) = 0.3; P = 0.869] in the mixed-factorial two-way ANOVA between the (between subject) drug factor and the (within-subject) time factor in V̇e data. There was also no difference in posthypoxia V̇e between CNQX and vehicle rats at any time point (all P > 0.393; Fig. 2D). The difference in vLTF magnitude was also insignificant (vehicle: 23.9 ± 2.1% vs. CNQX: 23.8 ± 2.2%; P = 0.974; Table 1). The CNQX (10 mg/kg) injection significantly decreased baseline V̇e (see Baseline and HVR above) and posthypoxia V̇e at 15 and 30 min time points (both P < 0.05 vs. vehicle group values), shifting the vLTF curve downward almost parallel (Fig. 2D). However, the AIH protocol induced an enhanced vLTF (magnitude: 33.6 ± 4.4%; P < 0.05 vs. the vehicle value; Table 1). These results suggest that non-NMDA receptor antagonism with CNQX (5–10 mg/kg ip) does not impair vLTF.
Vt LTF and fR LTF
There was significant Vt LTF in all vehicle groups except the vehicle (20 min after AIH) group (Table 1). This Vt LTF lost its statistical significance after APV injection before AIH, although it was not significantly altered by CNQX injection or any APV injection regardless of injection time (Table 1). There was also fR LTF in all vehicle groups (P < 0.05) except the vehicle (0 min after AIH) group (P = 0.083). This fR LTF was eliminated by APV injection before AIH and was significantly reduced by APV injection immediately after AIH. However, this fR LTF was only minimally changed by CNQX injection before AIH or APV injection 20 min after AIH (Table 1). These results suggest that vLTF results from both Vt and fR LTF in most rats and that APV (before AIH) but not CNQX injection eliminates fR (and Vt) LTF.
DISCUSSION
The present study demonstrated that systemic injection of the NMDA receptor antagonist APV (1.5 mg/kg ip), regardless of being injected before or immediately after AIH, eliminated the AIH-induced vLTF in conscious, freely behaving rats. APV injected at 20 min after AIH also substantially decreased or eliminated a fully developed vLTF at subsequent 30- and 45-min time points. Meanwhile, baseline V̇e and HVR were not significantly changed after the APV administration. In contrast, systemic injection of the non-NMDA receptor antagonist CNQX (5–10 mg/kg ip) did not impair vLTF even when baseline V̇e was substantially reduced (10 mg/kg). Taken together, these data suggest that activation of NMDA, but not non-NMDA, ionotropic glutamate receptors is required for both formation and maintenance of the AIH-induced vLTF in awake rats.
Methodological Consideration
Experiments on conscious, spontaneously breathing, freely moving rats provide a more physiologically relevant way to study the role of ionotropic glutamate receptors in the AIH-induced respiratory LTF, because possible confounding issues related to anesthesia, surgery, and restraint can be eliminated. In addition, measurements can be conducted following a within-subject experimental design; i.e., measurements of LTF can be conducted before and after drug administration in the same animal, thus reducing data variability and the number of animals used in the studies. We always injected vehicle first (i.e., vehicle and APV injections were not randomized) because we knew that vehicle injection does not have carryover effects on the second LTF measurement (see below) and we were not so sure about APV.
We conducted many time control experiments in our laboratory's previous study (33), and we found little difference in resting V̇e, HVR, or LTF between two measurements separated by 1 day (n = 6), or among four measurements separated by 1–4 days (n = 8). Our laboratory recently did another time control group (n = 3), in which LTF was measured four times in 5 days (at the 1st, 3rd, 4th, and 5th day), and there was little difference among them (unpublished observations). There were also seven vehicle or time control rats in several of our laboratory's previous studies, in which LTF was measured twice in 2 consecutive days (4 rats with both saline injection; 3 rats with only saline injection in the second measurement), and there was also little difference between measurements (vLTF magnitude: 19.0 ± 1.2% vs. 19.4 ± 1.5%, n = 4, P = 0.303; 18.2 ± 0.28% vs. 18.4 ± 0.25%, n = 3; P = 0.495). Collectively, these results unequivocally demonstrated that there are no vehicle injection or carryover effects on resting V̇e, HVR, or vLTF values between two measurements separated by 1–2 days.
We designated a common body temperature (37.5°C) to most rats. We argue, however, that the potential errors caused by this measure are minimal in our overall results and interpretations. First, rectal body temperature of most rats is very close to 37.5°C. Second, both LTF and HVR are relative values; i.e., they are both defined as increases above baseline, normalized as a percentage of the baseline value. Because the effects of the drugs on LTF and HVR were examined in the same rats, before and after the drug injection (i.e., a within-subject experimental design), these drug effects are also “relative.” Thus, even if there were errors in body temperature in some rats, these systematic errors would presumably be minimized by the double “normalizations” mentioned above.
Although APV injection (20 min after AIH) significantly decreased or eliminated a fully developed vLTF at subsequent 30- and 45-min time points, it did not significantly reduce the overall vLTF magnitude calculated by the three-value-average method (P = 0.057; Table 1), due likely to the limited rat number (n = 4) and the normal vLTF value at posthypoxia 15-min time point. The success rate of doing this kind of experiments was low because it was very difficult to measure V̇e at 30-min point shortly after APV injection. We tried eight rats but successfully collected data from only four rats.
NMDA Receptors and vLTF Formation
NMDA receptors are involved in several other mechanisms of breathing control, including the respiratory phase-switching mechanism (15, 27, 41); the central processing of carotid chemoafferent inputs in the nucleus tractus solitarius (40, 44); the inspiratory drive to phrenic, hypoglossal, and intercostal motoneurons (10, 31, 46); and the diaphragmatic neuromuscular transmission (25). MK-801 injection (3 mg/kg iv) has been reported to abolish HVR (44). Microinjection of MK-801 into the caudal nucleus tractus solitarius also reduced the HVR (40). Because these NMDA mechanisms all appear to be excitatory, we were surprised that APV did not impair HVR in the present study. We speculate that this difference might be caused by different doses; i.e., our doses (1.5 mg/kg) might be too low to impair the crucial synaptic transmission in the nucleus tractus solitarius. Consistent with this argument, MK-801 at 0.3–1.0 mg/kg also did not impair phrenic nerve response to short-term hypoxia (12). We noticed, however, that APV at 5 mg/kg greatly reduced HVR (APV: 97% vs. vehicle: 166%) in one rat in the present study.
We argue against the possibility that vLTF was impaired by NMDA receptor antagonism in the caudal nucleus tractus solitarius area or the diaphragmatic neuromuscular junction. This argument is based on two observations: 1) in the present study, APV did not reduce baseline V̇e or HVR; and 2) in our laboratory's previous study, both systemic injection and microinjection (into the phrenic motonucleus area) of MK-801 eliminated pLTF (35) in a preparation that bypasses the diaphragmatic neuromuscular junction and respiratory mechanics. In the present study, LTF was more sensitive to NMDA antagonism than baseline V̇e and HVR, due probably to the fact that the inspiratory drives to major inspiratory motoneurons are all mediated via non-NMDA as well as NMDA receptors, whereas vLTF is mediated primarily via NMDA receptors.
Whether NMDA receptors are required for hypoglossal or intercostal nerve LTF is currently unknown. However, because APV eliminated vLTF in the present study (Fig. 2), we postulate that activation of NMDA receptors is also required for both hypoglossal and intercostal LTF. vLTF is unlikely to be completely abolished, if NMDA receptor antagonism eliminates pLTF but not hypoglossal or intercostal LTF, because the remaining intercostal muscle LTF that increases ventilatory efforts and genioglossal LTF that presumably reduces upper airway resistance would still contribute to overall V̇e. This hypothesis, however, awaits more direct, experimental verification.
NMDA Receptors and vLTF Maintenance
It appears that respiratory LTF can be divided into at least two phases: induction and maintenance. For example, a recent study reported that pLTF was prevented by systemic injection of ketanserin before AIH, whereas pLTF was not affected when ketanserin was injected shortly after or 45 min after AIH, suggesting that activation of serotonin receptors is necessary to initiate (during AIH) but not maintain (following AIH) pLTF (18). In another study using an in vitro brain stem slice preparation from the neonatal rat, three episodes of 3-min bath application (and washout) of a 5-HT2 receptor agonist to activate 5-HT2 receptors on hypoglossal motoneurons, separated by 5-min intervals, induced both LTF of hypoglossal nerve activity and LTF of hypoglossal motoneuronal inspiratory-related drive current (7), suggesting that continuous presence of the agonist after the induction (3 bath applications) is not necessary for the expression of these two forms of LTF.
In contrast to these serotonin data, the present study demonstrated that LTF was not only prevented by systemic injection of APV before AIH but also eliminated by the same APV injection applied immediately after or 20 min after AIH. We chose 20 min instead of 45 min after AIH simply because vLTF only lasts for ∼45 min, whereas pLTF can last for hours (13). These data indicate that vLTF, even after it has been fully developed, can still be eliminated by subsequent NMDA receptor antagonism, thereby suggesting that activation of NMDA receptors is required for the maintenance of vLTF. The APV (before and 0 min after AIH) data (Fig. 2, A and B) also support the notion that NMDA receptors are required for LTF formation. However, we believe that those NMDA receptors are not required for LTF induction during AIH (see Potential Mechanism below) although the present study was not designed to directly test this hypothesis. To convincingly address this issue, one needs to block NMDA receptors only during but not after AIH. Because the effect of APV on NMDA receptors persists much longer than the AIH duration (45 min), this drug is not appropriate for this purpose.
Non-NMDA Receptors and vLTF Formation
Non-NMDA ionotropic glutamate receptors [e.g., -hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors] also play important roles in many forms of neural plasticity, e.g., long-term potentiation (LTP). The formation and maintenance of LTP are thought to involve an increase in the sensitivity of the postsynaptic AMPA receptors to glutamate (due to receptor subunit phosphorylation) as well as the overall number of AMPA receptors (22, 29). The present study, however, demonstrated that vLTF was even enhanced after systemic injection of CNQX (Table 1; Fig. 2D), thus disproving our non-NMDA part of the hypothesis and suggesting that non-NMDA receptors do not play an important role in the AIH-induced vLTF.
It is not surprising that only NMDA receptors appear to participate in the vLTF maintenance, whereas both NMDA and non-NMDA receptors are present on the associated motoneurons, because this is not unusual in the central nervous system. For example, in the Schaffer collateral LTP, both NMDA and non-NMDA receptors are present on the postsynaptic pyramidal neurons in the CA1 region. Nevertheless, NMDA receptors are required for the LTP induction but not maintenance, whereas non-NMDA receptors are almost certainly required for the LTP maintenance (29, 47).
We argue that these CNQX results cannot be explained by insufficient drug doses or unsuccessful drug delivery to relevant sites. CNQX is often used via systemic injection in neuropharmacological studies in both anesthetized and awake animals. The applied doses usually range from 0.7 to 5 mg/kg in studies with positive results (i.e., the drug successfully blocks a physiological event, and authors conclude the involvement of non-NMDA receptors in that event), and they range from 1 to 1.5 mg/kg in studies with negative results (i.e., the drug fails to block an event, and authors rule out the involvement of non-NMDA receptors). The doses (5 and 10 mg/kg) used in the present study are among the highest ones (we are aware that CNQX was sometimes used at very high doses in anti-convulsion- and anti-epilepsy-related studies). LTF can be easily abolished or partially impaired, e.g., vLTF magnitude was decreased to 8.8% by APV at 0.5 mg/kg in one rat in the present study. However, vLTF magnitude was not even slightly reduced after the high doses of CNQX (Table 1). In addition, although CNQX at 5 mg/kg had minimal effects on baseline V̇e, hypoxic V̇e and HVR, CNQX at 10 mg/kg substantially reduced baseline V̇e and (10% O2) hypoxic V̇e (vehicle: 111.2 ± 5.3 ml·100 g−1·min−1 vs. CNQX: 85.2 ± 8.0 ml·100 g−1·min−1; P = 0.022), suggesting that the drug had successfully reached the respiratory-related areas, including those synapses on the phrenic, hypoglossal, and intercostal motoneuron. In the meantime, HVR was minimally changed and vLTF magnitude was even enhanced (Table 1). Collectively, these data suggest that non-NMDA receptors are unlikely to play an essential role in vLTF.
There are caveats in this interpretation/conclusion. First, we believe that the unchanged HVR and the enhanced LTF after 10 mg/kg CNQX resulted, at least in part, from the baseline V̇e decrease because both the HVR and LTF were numerically calculated as a percent increase above baseline. Second, CNQX is a poor penetrator of the blood-brain barrier. Although we used high doses in the present study, basic respiratory motor output and normal HVR remained. CNQX even at the highest dose (10 mg/kg) reduced the hypoxic V̇e by only ∼23%, suggesting that a significant portion of non-NMDA receptors might still function on the medullary-spinal respiratory neurons, particularly those involved in the essential components of respiratory rhythm generation and pattern formation. Finally, this conclusion (non-NMDA receptors are not crucial for vLTF) does not necessarily rule out the possibility that LTF of some respiratory neurons, especially those that are not major contributors to overall V̇e, involves or relies on non-NMDA receptors. For example, in that in vitro brain stem slice preparation, episodic activation of 5-HT2 receptors on hypoglossal motoneurons also increased the AMPA-mediated current in synaptically isolated hypoglossal motoneurons, suggesting that LTF of some hypoglossal motoneurons (or nerve activity) may involve AMPA receptors (7).
Potential Mechanism
Respiratory LTF (in form of phrenic nerve activity) was first elicited by repeated carotid sinus nerve stimulation in anesthetized cats (38) and later in rats (20, 28) or in forms of intercostal nerve (16) and genioglossus muscle activities (30). The fact that pLTF can be elicited by carotid sinus nerve stimulation and not abolished by decerebration or the spinal transection at C7-T1 level suggests that pLTF requires neural mechanisms located in the brain stem and/or the cervical spinal cord and that the carotid body, respiratory mechanics, systemic hypoxia, forebrain, and the lower spinal cord are not necessary for its basic expression (13). Recent studies further showed that activation of serotonin receptors and synthesis of brain-derived neurotrophic factor in the cervical spinal cord and activation of NMDA receptors in the phrenic motonucleus area are necessary for pLTF (4, 5, 35).
We use phrenic motoneurons as an example to explain the potential NMDA mechanism underlying vLTF, because the phrenic nerve innervates the major ventilatory muscle diaphragm and pLTF is the most-studied LTF form, whose neuronal and cellular mechanisms have been well described in some hypothetical models (13, 14, 17, 35, 39). Briefly, the carotid chemoafferent inputs, activated by episodic hypoxia or repeated carotid sinus nerve stimulation, excite the raphe nuclei. Released serotonin from the raphe serotonergic neuron terminals activates 5-HT2 receptors on the phrenic motoneuron. This initiates a series of intracellular signaling events, including an increase in protein kinase(s) activity that phosphorylates the subunit(s) of the NMDA receptors and augments the inward NMDA current, thereby leading to an elevated motoneuronal response to the descending inspiratory drive (i.e., a pLTF). This model may also explain the LTF of hypoglossal and intercostal motoneurons.
However, this model cannot satisfactorily explain the fR portion of vLTF. In most rats, fR LTF is even bigger than Vt LTF in magnitude (Table 1). Actually, one could argue that LTF also occurs in respiratory-related neurons located in the brain stem, such as the raphe serotonergic neurons, dorsal (DRG) and ventral respiratory group (VRG) premotor neurons, and pre-Bötzinger pacemaker neurons (6, 43), which are the major determinants of respiratory rhythm. Numerous DRG/VRG neurons and pre-Bötzinger complex neurons (6, 42) express NMDA receptors and receive projection from the raphe serotonin neurons. The respiratory rhythm generated in the pre-Bötzinger complex can also be modulated by NMDA receptor antagonism (42). Therefore, it is conceivable that AIH can induce LTF in the medullary neural circuitry that generates respiratory rhythm, which in turn produces fR LTF at motoneuron levels.
Because serotonin receptor antagonism abolishes pLTF, hypoglossal LTF, and vLTF (3, 26, 36), and NMDA receptor antagonism abolishes both pLTF (35) and vLTF (Fig. 2), we postulate that hypoglossal and intercostal LTF may not only require NMDA receptors, but also share the same underlying cellular mechanisms with pLTF (see above). In addition, because serotonin receptor antagonism after AIH was reported to have minimal effect on pLTF (18), whereas NMDA receptor antagonism after AIH eliminated vLTF (Fig. 2), we think that activation of 5-HT2 receptors initiates the aforementioned intracellular process, whereas modification of NMDA receptors is the “end product” of this process. In other words, the formation and maintenance of a specific LTF result mainly from phosphorylation of NMDA receptors on the associated motoneurons, whereas the gradual reduction with time and eventual disappearance of the LTF result primarily from dephosphorylation of those NMDA receptors. On the other hand, based on the above discussion, we believe those NMDA receptors are probably not required for LTF induction. Although we have examined the role of NMDA and non-NMDA receptors as well as the timing of their activation in vLTF, the present experimental design could not reveal the exact locations of these receptors. This limitation, however, does not challenge our overall conclusions about the requirement and timing of ionotropic glutamate receptor activation in vLTF.
In conclusion, we found that pretreatment with the NMDA receptor antagonist APV prevented the AIH-induced vLTF. APV also impaired or eliminated vLTF after vLTF had been fully established. In contrast, pretreatment with the non-NMDA receptor antagonist CNQX even at very high doses did not impair vLTF. Collectively, these data suggest that although both NMDA and non-NMDA receptors are present on the motoneuron of several major inspiratory muscles and mediate the descending respiratory drive, only NMDA receptor activation is necessary for the formation and maintenance of the AIH-induced vLTF.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-64912.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol 91: 2751–2757, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep 21: 709–716, 1998. [PubMed] [Google Scholar]
- 3.Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blitz DM, Ramirez JM. Long-term modulation of respiratory network activity following anoxia in vitro. J Neurophysiol 87: 2964–2971, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci USA 101: 4292–4295, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol 73: 2083–2088, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Castellano C, Cestari V, Ciamei A. NMDA receptors and learning and memory processes. Curr Drug Targets 2: 273–283, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Chitravanshi VC, Sapru HN. NMDA as well as non-NMDA receptors mediate the neurotransmission of inspiratory drive to phrenic motoneurons in the adult rat. Brain Res 715: 104–112, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhuri S, Pierchala L, Aboubakr SE, Shkoukani M, Badr MS. Long-term facilitation of genioglossus activity is present in normal humans during NREM sleep. Respir Physiol Neurobiol 160: 65–75, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coles SK, Ernsberger P, Dick TE. A role for NMDA receptors in posthypoxic frequency decline in the rat. Am J Physiol Regul Integr Comp Physiol 274: R1546–R1555, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Eldridge F, Millhorn D. Oscillation, gating and memory in the respiratory control system. In: Handbook of Physiology. The Respiratory System. Control of Breathing. Bethesda, MD: Am. Physiol. Soc, 1986, sect. 3, vol. II, pt. I, chapt. 3, p. 93–114.
- 14.Feldman JL, Mitchell GS, Nattie. Breathing: rhythmicity EE, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foutz AS, Champagnat J, Denavit-Saubie M. Respiratory effects of the N-methyl-d-aspartate (NMDA) antagonist, MK-801, in intact and vagotomized chronic cats. Eur J Pharmacol 154: 179–184, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477: 469–479, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol 121: 135–146, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol 90: 2001–2006, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol 291: R1111–R1119, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol Regul Integr Comp Physiol 265: R811–R819, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Jordan AS, Catcheside PG, O'Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. J Appl Physiol 93: 2129–2136, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Kandel ER Cellular mechanisms of learning and the biological basis of individuality. In: Principles of Neural Science. New York: McGraw-Hill, 2000, p. 1247–1279.
- 23.Kent S, Kernahan SD, Levine S. Effects of excitatory amino acids on the hypothalamic-pituitary-adrenal axis of the neonatal rat. Brain Res Dev Brain Res 94: 1–13, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci 18: 8436–8443, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koyuncuoglu H, Kara I, Gunel MA, Nurten A, Yamanturk P. N-methyl-d-aspartate antagonists, glutamate release inhibitors, 4-aminopyridine at neuromuscular transmission. Pharmacol Res 37: 485–491, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21: 5381–5388, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling L, Karius DR, Speck DF. Role of N-methyl-d-aspartate receptors in the pontine pneumotaxic mechanism in the cat. J Appl Physiol 76: 1138–1143, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Ling L, Olson EB Jr, Vidruk EH, Mitchell GS. Integrated phrenic responses to carotid afferent stimulation in adult rats following perinatal hyperoxia. J Physiol 500: 787–796, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress? Science 285: 1870–1874, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol 82: 419–425, 1997. [DOI] [PubMed] [Google Scholar]
- 31.McCrimmon DR, Smith JC, Feldman JL. Involvement of excitatory amino acids in neurotransmission of inspiratory drive to spinal respiratory motoneurons. J Neurosci 9: 1910–1921, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuire M, Ling L. Ventilatory long-term facilitation is greater in 1- vs. 2-mo-old awake rats. J Appl Physiol 98: 1195–1201, 2005. [DOI] [PubMed] [Google Scholar]
- 33.McGuire M, Zhang Y, White DP, Ling L. Chronic intermittent hypoxia enhances ventilatory long-term facilitation in awake rats. J Appl Physiol 95: 1499–1508, 2003. [DOI] [PubMed] [Google Scholar]
- 34.McGuire M, Zhang Y, White DP, Ling L. Effect of hypoxic episode number and severity on ventilatory long-term facilitation in awake rats. J Appl Physiol 93: 2155–2161, 2002. [DOI] [PubMed] [Google Scholar]
- 35.McGuire M, Zhang Y, White DP, Ling L. Phrenic long-term facilitation requires NMDA receptors in the phrenic motonucleus in rats. J Physiol 567: 599–611, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol 286: R334–R341, 2004. [DOI] [PubMed] [Google Scholar]
- 37.McKay LC, Janczewski WA, Feldman JL. Episodic hypoxia evokes long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol 557: 13–18, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol 41: 87–103, 1980. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB Jr. Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol 90: 2466–2475, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol 478: 55–66, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteau R, Gauthier P, Rega P, Hilaire G. Effects of N-methyl-d-aspartate (NMDA) antagonist MK-801 on breathing pattern in rats. Neurosci Lett 109: 134–139, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Morgado-Valle C, Feldman JL. NMDA receptors in preBotzinger complex neurons can drive respiratory rhythm independent of AMPA receptors. J Physiol 582: 359–368, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris KF, Shannon R, Lindsey BG. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J Physiol 532: 483–497, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohtake PJ, Torres JE, Gozal YM, Graff GR, Gozal D. NMDA receptors mediate peripheral chemoreceptor afferent input in the conscious rat. J Appl Physiol 84: 853–861, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Olson EB, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchel GS. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol 91: 709–716, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Steenland HW, Liu H, Sood S, Liu X, Horner RL. Respiratory activation of the genioglossus muscle involves both non-NMDA and NMDA glutamate receptors at the hypoglossal motor nucleus in vivo. Neuroscience 138: 1407–1424, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Swartzwelder HS, Ferrari C, Anderson WW, Wilson WA. The drug MK-801 attenuates the development, but not the expression, of long-term potentiation and stimulus train-induced bursting in hippocampal slices. Neuropharmacology 28: 441–445, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Tonegawa S Mammalian learning and memory studied by gene targeting. Ann NY Acad Sci 758: 213–217, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol 499: 543–550, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]