Abstract
Perturbations in body weight have been shown to affect energy expenditure and efficiency during physical activity. The separate effects of weight loss and exercise training on exercise efficiency or the proportion of energy derived from fat oxidation during physical activity, however, are not known. The purpose of this study was to determine the separate and combined effects of exercise training and weight loss on metabolic efficiency, economy (EC), and fat oxidation during steady-state moderate submaximal exercise. Sixty-four sedentary older (67 ± 0.5 yr) overweight to obese (30.7 ± 0.4 kg/m2) volunteers completed 4 mo of either diet-induced weight loss (WL; n = 11), exercise training (EX; n = 36), or the combination of both interventions (WLEX; n = 17). Energy expenditure, gross efficiency (GE), EC, and proportion of energy expended from fat (EF) were determined during a 1-h submaximal (50% of peak aerobic capacity) cycle ergometry exercise before the intervention and at the same absolute work rate after the intervention. We found that EX increased GE by 4.7 ± 2.2%. EC was similarly increased by 4.2 ± 2.1% by EX. The addition of concomitant WL to EX (WLEX) resulted in greater increases in GE (9.0 ± 3.3%) compared with WL alone but not compared with EX alone. These effects remained after adjusting for changes in lean body mass. The proportion of energy derived from fat during the bout of moderate exercise increased with EX and WLEX but not with WL. From these findings, we conclude that exercise training, either alone or in combination with weight loss, increases both exercise efficiency and the utilization of fat during moderate physical activity in previously sedentary, obese older adults. Weight loss alone, however, significantly improves neither efficiency nor utilization of fat during exercise.
Keywords: gross efficiency, exercise economy, aging
obesity and aging have both been associated with alterations in resting energy metabolism (10, 33). A number of studies have also examined the effects of diet-induced weight loss (23, 32), exercise (15, 35), and the combination of diet and exercise (27, 37) on energy expenditure and substrate metabolism during resting postabsorptive, postprandial, or insulin-stimulated conditions. Far fewer studies have been conducted to examine energy metabolism during physical activity in obesity (16, 19) or aging (5). In particular, the effects of weight loss or exercise training on energy metabolism during physical activity have not been fully elucidated.
Skeletal muscle work efficiency may be expressed as gross efficiency (GE), net efficiency, or delta efficiency. The basic definition of GE during steady-state exercise is the ratio of work accomplished to the total energy expended during that specific activity and is expressed as a percentage (13). Net efficiency and delta efficiency use the change in work performed and the change in energy expanded either from baseline (13) or computed from the slope of a linear relationship between energy expended and work accomplished (8). Another common concept related to efficiency is the term of economy (EC), which is a measure of oxygen consumption (V̇o2) per unit of work (26).
The concept of exercise efficiency may be important to consider from two very different perspectives. A higher or improved efficiency may be beneficial to sports performance in athletes. Coyle et al. (8) have shown that competitive cyclists who had a higher proportion of type 1 muscle fibers with a higher oxidative capacity were more efficient during cycling. On the other hand, and perhaps counterintuitively from a sports performance perspective, requiring less energy for activity, i.e., greater efficiency, may be a disadvantage for obesity, weight loss, or maintenance of weight loss. Obese subjects have a lower efficiency than normal-weight subjects for cycling (22) as well as for walking and stepping (7). During and after weight loss, skeletal muscle work efficiency is increased (34); this is even most evident at lower levels of physical activity (29, 34). However, these studies did not examine the separate effects of increased physical activity and weight loss on exercise efficiency. Therefore, the purpose of this study was to determine the separate and combined effects of weight loss and exercise training on exercise efficiency and economy in older overweight and obese subjects. Moreover, another objective was to determine the distinct and combined effects of weight loss and exercise on substrate, i.e., fat and carbohydrate utilization during the same bout of moderate exercise. We hypothesized that chronic exercise would have more of an impact on exercise efficiency and fat oxidation compared with diet-induced weight loss.
METHODS
Study Design and Subjects
A total of 64 older (67 ± 0.5 yr old) overweight or obese (30.7 ± 0.4 kg/m2) volunteers (38 women and 26 men) were included in this study. Fifty-two of them participated in a 3 arms randomized clinical trial consisting in a 16-wk intervention of either diet-induced weight loss (WL) or exercise training (EX) or the combination of both interventions (WLEX). Twelve subjects were part of our pilot study and received without randomization the EX intervention.
None of the volunteers were engaged in regular physical exercise (>1 time/wk) and all were weight stable (±3 kg) for at least 6 mo before the study. Subjects were excluded if they had a history of type 2 diabetes, coronary heart disease, peripheral vascular disease, or uncontrolled hypertension or if they were taking chronic medications known to affect glucose homeostasis. We also excluded subjects who had among the screening testing an anemia (hematocrit <34%), clinical hypothyroidism (thyroid-stimulating hormone >8μIU/ml), or elevated liver enzymes (25% above the reference range). The protocol was approved by the University of Pittsburgh Institutional Review Board. All volunteers gave written informed consent.
Intervention Groups
WL.
To achieve the goal of 10% weight loss, subjects were prescribed a caloric deficit of 500–1,000 kcal/day based on recent food records combined with a low-fat diet (<30% of calories from fat). Subjects met weekly with a registered dietician for individual counseling, review of food records, and weight monitoring.
EX.
The exercise training protocol consisted of a 16-wk moderate-intensity supervised aerobic exercise regimen. Subjects were asked to engage in three to five sessions per week with at least three sessions supervised in our facility. The intensity and duration of the exercise sessions was progressively adapted to reach 45 min and 75% of their peak aerobic capacity (V̇o2peak). Subjects could walk, bike, or row, although walking was the primary mode of exercise. Exercise intensity was monitored by the use of heart rate (HR) monitors (Polar Electro Oy, Kempele, Finland). For the first 8 wk, the prescription was based on the subject's peak HR achieved during the baseline graded exercise test. For the second 8 wk, the prescription was adapted from a submaximal exercise test performed at the midpoint. Exercise logs and HR monitors were also used for the unsupervised sessions.
WLEX.
Subjects in this group received both of the interventions described above.
Outcome Measures and Testing
All of the outcome measures were assessed before and after the 16-wk intervention following a preintervention-postintervention research design. It is important to note that the two groups who underwent weight loss maintained stable weight for a period of 2 wk before the postintervention measurements.
Anthropometric measures included weight and height. Weight was measured on a calibrated medical digital scale (model BWB-800, Tanita, Tokyo, Japan) in undergarments. Height was measured at the same time with a wall-mounted stadiometer. Body mass index (BMI) was calculated as weight (kg) divided by square height (m2).
Blood analyses performed for the screening procedure were processed through standard hospital-certified laboratory protocols.
Lean body mass (LBM) and fat mass (FM) were assessed by dual-energy X-ray absorptiometry (GE Lunar Prodigy and Encore 2005 software version 9.30, Lunar). LBM was used to express measures and computations in relative units.
V̇o2peak was measured using a graded exercise protocol on an electronically braked cycle ergometer (Ergoline 800S, Sensormedics, Yorba Linda, CA) as described previously (31). HR, blood pressure, and electrocardiogram were recorded before, during and after the exercise test. V̇o2 was computed via indirect calorimetry (Moxus, AEI Technologies, Pittsburgh, PA). To account for a possible learning effect, 14 subjects repeated this test at both time points; thus they performed a total of 4 tests (2 before the intervention and 2 after the intervention).
Percutaneous muscle biopsies were obtained by a physician from the vastus lateralis muscle after an overnight visit. The proportions of type I and type II muscle fibers were determine by the use of histochemical analysis as previously described (31).
A steady-state submaximal exercise test was performed 1 wk apart of the overnight visit. At baseline, subjects exercised on the braked cycle ergometer (Ergoline 800S, Sensormedics) at 50% of their predetermined peak aerobic capacity. After the intervention, the participants repeated the test at the same absolute work rate compared with preintervention. Indirect calorimetry, used to measure V̇o2 and carbon dioxide production (V̇co2), was performed at 15, 30, 45, and 60 min of exercise. Cadence was maintained constant throughout the 1-h exercise bout and was similar in the pre- and postintervention measurements. Only data from the last three time points (minutes 28–30, 43–45, and 58–60) were used to ascertain steady-state levels. We did not use the first 15 min of data due to the fact that during the preintervention, workload adjustments were made during that time window to match exactly 50% of the V̇o2peak. Subjects were instructed to avoid strenuous physical activity for 2 days before and to eat at least 200 g of carbohydrates for 3 days before the submaximal exercise test to ensure adequate glycogen stores for the exercise bout. In addition, they were asked to record food intake in a diary for the 3 days before this test so that they could replicate their diet during the 3 days preceding the postintervention test. To assess the variation in the measurement tool and a possible learning effect, a subset of individuals (n = 14) performed two tests before and two tests after the intervention. For each of these time points, V̇o2 at the same work was not lower in the second test compared with the first test, indicating no learning effect.
Efficiency and Substrate Oxidation Computations
The mean values of V̇o2 and V̇co2 of the last five data points collected at each of the three steady-state time points from the submaximal exercise test were used to compute cycling efficiency and substrate oxidation values.
Energy expended (EE) during steady-state exercise (expressed in kcal/min) was calculated adapting the formula of Brouwer (6):
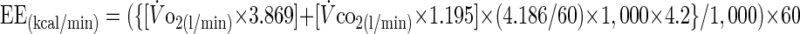 |
GE during steady-state exercise was calculated as the ratio of the work accomplished per minute (W converted into kcal/min) to the EE per minute (in kcal/min) (8):
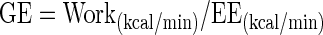 |
Exercise economy (EC) was computed as the ratio of the work accomplished per minute (W converted to kcal/min) by the mean V̇o2 (l/min) (26):
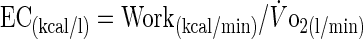 |
Systemic carbohydrate (Cho-ox) and fat oxidation (Fat-ox) rates were calculated using the stoichiometric equations of Frayn (11):
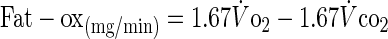 |
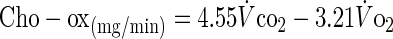 |
The Fat-ox value were then transformed into kilocalories per minute and expressed as a proportion of energy derived from fat (EF):
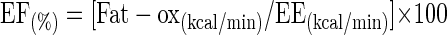 |
Protein oxidation rates were not included based on our laboratory's prior work demonstrating that rates of urinary nitrogen excretion were similar in lean and obese subjects during resting conditions (16) and on the assumptions that the amount of proteins oxidized and other metabolic processes (such as gluconeogenesis from proteins, ketone body formation, and lipogenesis) during exercise are quantitatively negligible compared with glucose and fatty acid oxidation (28).
Statistical Analysis
Data are presented in terms of means ± SE. After exploring the data for outliers and checking the assumptions, one-way ANOVAs were performed to compare baseline characteristics between groups. When needed, post hoc tests were used with the Tukey-Kramer honestly significant adjustment. If the assumptions of normality (Shapiro-Wilk test) and of equal variances (Levene test) were not met, baseline comparisons between groups for these specific variables were performed using the nonparametric Kruskal Wallis test. Baseline sex differences were explored with an independent t-test or the nonparametric Mann-Whitney test.
Simple linear regressions were performed to look at the linear relationship between efficiency measures and BMI, V̇o2peak and proportion of muscle fiber type I.
A 3 × 2 repeated-measures ANOVA was performed on the dependent variables as a function of intervention (3 levels: WL, EX, WLEX) and time (2 levels: pre- and postintervention). Pairwise comparisons using the Bonferroni adjustment for multiple comparisons were conducted (on the between and/or the within subject factors) to discriminate between means when ANOVA yielded significant results. The assumptions of compound symmetry were checked with the Box's M and the Mauchly's test. To examine the additive effect between the WL and the EX interventions, a priori pairwise comparisons were performed on the effects of intervention on the efficiency measures (GE and EC). The pairs (WLEX vs. WL) and (WLEX vs. EX) were compared using post hoc (pairwise comparisons on the between subject variable) within the 3 × 2 repeated-measures ANOVA model.
A multivariate regression analysis was used to look at the predictors of the change in efficiency.
For all analyses, the alpha level was set a priori at 0.05. All statistics were performed using JMP version 5.0.1.2 and SPSS 16.0 for Macintosh.
RESULTS
Baseline Characteristics
Baseline characteristics in the three groups are presented in Table 1. BMI was lower (P < 0.05) in the EX group compared with the WL and WLEX group. All of the submaximal exercise measures (EE, GE, EC, and EF) at baseline were similar among the three groups whether the data were expressed in absolute terms (Table 1) or relative to LBM (data not shown). All of the variables were normally distributed with the exception of EE and EF. Equality of variances was assumed for all the variables except for LBM or EF. However, a nonparametric test (Kruskal Wallis) revealed that EE, LBM, and EF were also similar among groups.
Table 1.
Baseline characteristics
| WL | EX | WLEX | |
|---|---|---|---|
| n | 11 | 36 | 17 |
| Sex, male/female | 5/6 | 13/23 | 8/9 |
| Age, yrs | 68.2±1.5 | 67.0±0.6 | 66.2±0.9 |
| BMI, kg/m2 | 31.6±1.0A | 29.7±0.6B | 32.2±0.8A |
| LBM, kg | 47.3±2.5 | 47.8±1.5 | 51.6±2.9 |
| FM, kg | 38.4±2.0A | 31.4±1.5B | 36.3±1.9A |
| BF, % | 43.6±1.8 | 38.4±1.6 | 40.3±1.8 |
| Type I fibers, % | 51.9±3.9 | 44.2±3.1 | 50.7±2.9 |
| V̇o2peak, ml·kgLBM−1·min−1 | 29.3±1.6 | 33.5±1.0 | 33.3±1.2 |
| EE, kcal/min | 4.2±0.28 | 4.6±0.21 | 5.0±0.33 |
| GE | 0.10±0.01 | 0.12±0.01 | 0.12±0.00 |
| EC, kcal/l | 0.49±0.03 | 0.58±0.02 | 0.58±0.02 |
| EF, % | 38.4±2.0 | 44.2±2.5 | 41.1±4.6 |
Values are means ± SE; n, no. of subjects. WL, diet-induced weight loss; EX, exercise training; WLEX, combination of both interventions; BMI, body mass index; LBM, lean body mass; FM, fat mass; BF, percent body fat; V̇o2peak, peak oxygen uptake; EE, energy expenditure during submaximal exercise; GE, gross efficiency; EC, economy; EF, proportion of energy derived from fat. Values with different letters are significantly different (1-way ANOVA), P < 0.05.
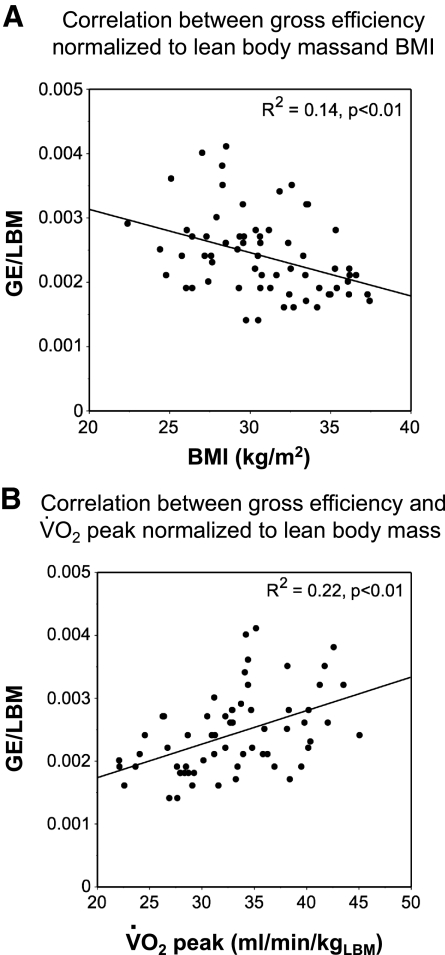
At baseline, BMI was negatively associated with GE (R2 = 0.09, P = 0.02). In contrast, V̇o2peak was positively associated with GE (R2 = 0.41, P < 0.01). Similar associations were found with GE normalized to LBM (Fig. 1). The same pattern was observed between BMI and EC (R2 = 0.15, P < 0.01) and between V̇o2peak and EC (R2 = 0.22, P < 0.01). No significant baseline association was found between the percentage of type I fibers and GE (R2= 0.01, P = 0.43) or EC (R2 = 0.01, P = 0.44).
Fig. 1.
Simple linear correlations at baseline between gross efficiency (GE), body mass index (BMI), and peak aerobic capacity (V̇o2peak). A: correlation between GE normalized to lean body mass and BMI. B: correlation between GE and V̇o2peak normalized to lean body mass (LBM).
At baseline, men (n = 26) weighed on average 11.5 kg more (P < 0.001) and had on average 16.8 kg more (P < 0.001) LBM than women (n = 38). The mean BMI was similar in men and women (30.6 and 30.8 kg/m2, respectively). Men had a higher cardiorespiratory fitness (V̇o2peak) than women when expressed in absolute terms (l/min) (52.3%; P < 0.001). This difference, however, was reduced when expressed in units relative to LBM (7.8%; P = 0.10). EE during the submaximal exercise bout was 47.9% higher (P < 0.001) in men compared with women. However, this difference disappeared (3.2%; P = 0.3) when expressed in relative units (kcal·min·kgLBM). The baseline values for GE ranged from 6.8 to 19.0% with a mean of 11.5 ± 0.3%. This is somewhat lower than that reported for trained athletes (19.8 ± 0.6%) (26) but within the range reported for healthy women (11.8 ± 0.3%) (4) and men (14.3 ± 0.1%) (14). In our study, GE was 14.8% higher (P = 0.008) in men than women when expressed in absolute units, but women had a 23.8% greater (P = 0.002) GE when expressed relative to LBM. EC had a similar pattern with a higher (16.3%; P = 0.005) absolute economy in men compared with women, but women had a 23.1% higher (P = 0.002) economy than men when EC was expressed relative to LBM. The proportion of type I fibers was not significantly different between the sexes (49.8 ± 2.6% in women and 44.6 ± 3.1% in men; P = 0.20). Women had a higher exercise Fat-ox compared with men (16.7 vs. 13.2 μmol·min−1·kgLBM−1; P = 0.01) with a higher proportion of EF (46.7% vs. 36.1%; P = 0.005). Conversely, men had a higher exercise Cho-ox compared with women (89.0 vs. 69.2 μmol·min−1·kgLBM−1; P = 0.005).
Changes in Physical Characteristics
Changes in BMI, FM, and LBM are presented in Table 2. Although all groups lost a statistically significant amount of weight, BMI, and FM, the WLEX and WL groups lost significantly more weight in line with their weight-loss goal (8.5 ± 0.8 and 9.2 ± 1.4% for WLEX and WL, respectively). The WL group lost more LBM than the other groups (interaction effect P < 0.001). None of the intervention groups had a significant change in their fiber type proportion (Table 2).
Table 2.
Changes in body composition and V̇o2peak with intervention
| WL | EX | WLEX | |
|---|---|---|---|
| BMI | −9.3±1.4B* | −1.3±0.4A* | −8.6±0.8B* |
| FM | −16.4±2.6B* | −3.6±1.1A* | −18.7±2.1B* |
| LBM | −4.3±1.2C* | 0.1±0.4A | −1.5±0.5B* |
| Type I fibers | −1.2±8.7 | 16.7±8.1 | −1.6±7.1 |
| V̇o2peak | 0.4±4.5B | 10.4±2.1A* | 4.3±3.2A* |
Values are means ± SE given as percent change from baseline. Type I fibers, proportion of type I fibers. Values with different letters are significantly different, P < 0.05 (repeated-measures ANOVA).
P < 0.05 preintervention-postintervention comparisons (within-subject post hoc test).
Changes in Physical Fitness and Physical Activity
The EX and WLEX groups had an increase (P < 0.05) in V̇o2peak compared with WL (Table 2). Moreover, V̇o2peak increased (P < 0.05) within EX and within WLEX. The same pattern was found with unadjusted means. In the EX group, subjects exercised on average 3.5 ± 0.1 sessions/wk, expending 835 ± 67 kcal/wk over the course of the intervention. The WLEX group exercised on average 3.6 ± 0.2 sessions/wk, expending 912 ± 87 kcal/wk.
Changes in EE During Submaximal Cycling Exercise
Cadence was maintained constant throughout the submaximal test with an average of 61.0 ± 0.8 revolutions/min in the preintervention test and 63.0 ± 0.8 revolutions/min in the postintervention testing. To achieve 50% of their V̇o2peak during the submaximal test, subject's power output ranged from 20 to 75 W, with an average of 37.6 ± 1.9 W in the pre testing. The exact same wattage was maintained during the postintervention testing with an average of 37.6 ± 1.9 W. The mean V̇o2 for all subjects combined was 0.94 ± 0.03 l/min at baseline and 0.90 ± 0.03 l/min following intervention. Within each test, V̇o2 was constant across the last 45 min of submaximal exercise. V̇o2 values were also similar in two identical exercise bouts separated by 1 wk, both before and after the intervention (n = 14; P > 0.05).
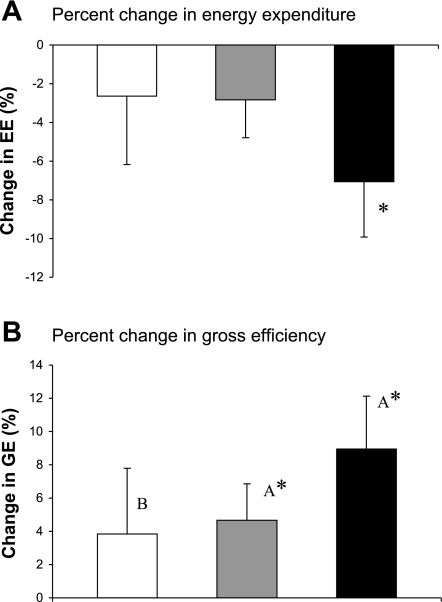
Changes in EE during submaximal exercise are presented in Fig. 2A. A main effect of time was found (P < 0.001). Post hoc analysis revealed that the decreased EE was only significant (P = 0.004) for the WLEX group.
Fig. 2.
Changes in energy expenditure (EE) and GE during submaximal exercise. A: percent change in EE. B: percent change in GE. Groups: diet-induced weight loss (open bar), exercise training (gray bar), combination of both interventions (black bar). Values are mean changes ± SE. Values with different letters (A and B) are significantly different, P < 0.05 (repeated-measures ANOVA). *P < 0.05 preintervention-postintervention comparison (within-subject post hoc test).
Changes in GE During Submaximal Cycling Exercise
Changes in GE during submaximal exercise are presented in Fig. 2B. Significant main effects of intervention (P = 0.04) and of time (P = 0.005) were found. The EX and the WLEX groups both had an increase in GE with intervention. The change in GE was not significant for the WL group. In the a priori pairwise comparisons, the WLEX group had a greater improvement in GE compared with the WL group (P = 0.02) but not to the EX group (P = 0.78).
Changes in EC During Submaximal Cycling Exercise
Significant main effect of intervention (P = 0.05) and of time (P = 0.007) were found. The EX and WLEX groups increased EC significantly more than the WL group. In the a priori pairwise comparisons, the WLEX group had a greater improvement in EC compared with the WL group (P = 0.03), but it was not greater than the improvement with EX (P = 0.74).
Predictors of Improved Efficiency
To examine predictors of change in GE, change in weight, change in V̇o2peak, change in LBM, change in FM, sex, and intervention group were put into a stepwise multivariable regression. When examining all groups together, having less of a change in LBM (R2= 0.19, P = 0.02) and an improved V̇o2peak (R2 = 0.11, P = 0.008) were the only significant predictors of the improvement in GE.
Changes in Fat Oxidation During Submaximal Cycling Exercise
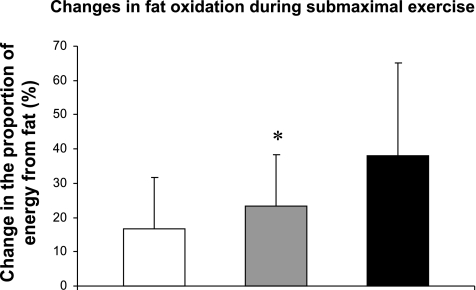
The changes in the proportion of energy derived from fat are presented in Fig. 3. A main effect of time was found (P = 0.04). Post hoc analysis revealed that EF increased (P = 0.048) within the EX group. When we combined all subjects that exercised (EX and WLEX), we also found a significant improvement in EF with intervention (P = 0.04). The WL group did not have an increase in fat oxidation.
Fig. 3.
Changes in fat oxidation during submaximal exercise. Groups: diet-induced weight loss (open bar), exercise training (gray bar), combination of both interventions (black bar). Values are mean changes ± SE. *P < 0.05 preintervention-postintervention comparison (within-subject post hoc test).
DISCUSSION
The effects of body weight changes and physical activity on exercise efficiency have received relatively little attention. In particular, the separate or distinct effects of intentional, energy restriction-induced weight loss and exercise training have not until now been examined. The key findings of this study were that exercise training with or without weight loss increased exercise efficiency and the amount of energy derived from fat during a bout of moderate exercise. However, weight loss itself does not appear to significantly enhance either exercise efficiency or fat oxidation during exercise in older overweight to obese men and women.
These increases in exercise efficiency and EC are consistent with previous work by Rosenbaum and colleagues (34), who found that a program of combined weight loss and increased physical activity enhanced efficiency at very low levels of muscular work in overweight subjects. The present study builds on this previous observation by delineating the separate effects of exercise and weight loss. The increase in efficiency specifically due to exercise training in these overweight to obese older subjects is consistent with previous studies conducted in younger normal-weight subjects (14, 18). Moreover, our findings indicate that at least part of the decreased efficiency that may occur with age (38) is explained by age-associated decreases in physical activity. This is in accord with earlier studies suggesting that exercise efficiency and EC may not change with advancing age in well-trained older subjects (1, 3, 36), or that lower exercise efficiency was reversed in older subjects following training (30).
The changes in exercise efficiency and V̇o2 following exercise training could be influenced by both peripheral and central effects. Exercise training, but not weight loss, increased the physical fitness (V̇o2peak). We did not detect significant changes in fiber type with exercise in the present study, which is in apparent contrast to two of our laboratory's previous studies in similar subjects (9, 31). However, this could be explained by the more rigorous three-group analysis in this study, particularly given that the magnitude of these changes were similar across these three studies. The possibility remains that alterations in energetics within muscle, including the proportion of type I muscle fibers, may play a role in altered efficiency. At the cellular level, our laboratory has shown that with a similar exercise intervention and subject population, mitochondrial activity and density were greatly enhanced by exercise (25). The increased efficiency in the present study was related to the improvement in physical fitness but not to the increase in the proportion of type I muscle fibers. Moreover, we did not observe an association between efficiency and fiber type at baseline. Rosenbaum et al. (34) found that the increases in efficiency were paralleled by increases in the capacity for oxidative phosphorylation determined by nuclear magnetic resonance spectroscopy. In addition, in young highly trained cyclists, a higher percentage of type I muscle fibers has been associated with higher efficiency (8). Thus the possibility remains that muscle fiber type, mitochondria activity, or oxidative capacity can account for efficiency at higher levels of work. It is also possible that changes in other characteristics within skeletal muscle, for example, capillarization or muscle blood flow, may contribute to the increased efficiency with exercise training. This requires further investigation.
Energy restriction-induced weight loss without an increase in physical activity in our study did not significantly increase efficiency. Moreover, the effects of weight loss and exercise were not additive. Body mass has been suggested to be an important factor in relation to efficiency (4). The changes in efficiency with weight loss could be attributed to the work of the moving legs (2) and the extra weight carried in the legs (20). Although these factors might explain the weight loss effects on improved efficiency, it is highly unlikely that they accounted for the changes in efficiency observed following exercise training because this intervention did not promote substantial weight loss.
An increase in efficiency may have significant implications for older men and women, who are at greater risk for the development of functional limitations. An increased efficiency during moderate levels of physical activity examined in this study would imply that activities of daily living would require less energy. This could be clinically relevant for older men and women, who typically have low capacity for physical work, that is, they would require proportionately less energy of their maximal functional capacity. Another perspective is that a decrease in energy required for physical activity (increased efficiency) may theoretically hinder efforts to lose weight or to maintain weight loss in obesity. In our study, the mean increase in efficiency due to the combination of weight loss and exercise would correspond to a decrease of 0.4 kcal/min expended during the 1 h of moderate exercise. At 3 h of exercise per week, this increased efficiency in our subjects would require them expend an additional 67 kcal/wk, or an additional ∼16 min of weekly leisure walking. Therefore, this should not be a practical concern.
Another key finding was that exercise training increased the relative reliance on fat oxidation during a moderate-intensity bout of exercise. This response was observed in subjects who exercised with or without parallel weight loss, but it was not observed with weight loss alone. These data indicate that older-aged, overweight to moderately obese men and women can increase their reliance on fat with just moderate increases in physical activity. This is in agreement with our laboratory's previous study in a similar group of subjects who had an increased fat oxidation during submaximal exercise in conjunction with an increase in the proportion of type I muscle fibers (31). These observations are supported by several previous studies demonstrating an increase in fat oxidation due to exercise training (12, 24) but not weight loss (17, 21). It is not clear whether the mechanisms responsible for the shift in substrate oxidation during a bout of physical activity also underlie the increased efficiency. Nevertheless, this study provides novel evidence that exercise training, but not energy restriction-induced weight loss, increases in vivo fatty acid oxidation during exercise.
Our study was not without limitations. We did not use efficiency measures taking into account resting values (net efficiency). Although we performed a test-retest paradigm to exclude a possible learning effect and test the variability in the measurements, we did not have a true control group to control for the variability in the tests. To exclude the confounding effect of a cardiovascular drift, we plotted heart rate and V̇o2 along time; neither HR nor V̇o2 increased during the submaximal exercise test. Measuring muscular efficiency during cycle ergometry may not apply to typical activities of daily living such as walking. This is especially true in situations where individuals alter their gait, i.e., specific morphotypes of obesity. Thus these results may not be generalized to all forms of exercise. In addition, we cannot discount the possibility that improvements in efficiency occurred due to biomechanical changes in movement. However, this is far less likely to occur during cycling, particularly because walking was the primary mode of exercise during training. We were also careful to account for any potential learning effect and did not observe a sex effect in the responses to intervention.
In summary, moderate increases in physical activity levels, either with or without concomitant weight loss, enhanced exercise efficiency in previously sedentary, overweight to obese men and women. In contrast, weight loss alone did not significantly enhance efficiency in these subjects. Moreover, exercise training, but not weight loss, increased the reliance on fatty acids during moderate exercise. Further research needs to be performed to determine whether or not these changes in energy metabolism due to exercise or weight loss play a role in modulating cardiometabolic risk or improving functional performance in old age or obesity.
GRANTS
This work was supported by American Diabetes Association clinical Research Award (to B. H. Goodpaster), National Institute on Aging Grant R01 AG-20128, General Clinical Research Center Grant 5M01 RR-00056, and Obesity Nutrition Research Center Grant 1P30 DK-46204.
Acknowledgments
We thank the volunteers for the participation in this study. We also acknowledge the valuable contributions of Krista Clark for directing the diet-induced weight loss programs.
Part of this work has been presented as a poster at the annual scientific meeting of the North American Association for the Study of Obesity in October 2007 in New Orleans, LA.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams WC Influence of age, sex, and body weight on the energy expenditure of bicycle riding. J Appl Physiol 22: 539–545, 1967. [DOI] [PubMed] [Google Scholar]
- 2.Anton-Kuchly B, Roger P, Varene P. Determinants of increased energy cost of submaximal exercise in obese subjects. J Appl Physiol 56: 18–23, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Astrand I Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl 49: 1–92, 1960. [PubMed] [Google Scholar]
- 4.Berry MJ, Storsteen JA, Woodard CM. Effects of body mass on exercise efficiency and V̇o2 during steady-state cycling. Med Sci Sports Exerc 25: 1031–1037, 1993. [PubMed] [Google Scholar]
- 5.Boon H, Jonkers RA, Koopman R, Blaak EE, Saris WH, Wagenmakers AJ, Van Loon LJ. Substrate source use in older, trained males after decades of endurance training. Med Sci Sports Exerc 39: 2160–2170, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer E On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (oxygen intake and carbonic acid output) and urine-N. Acta Physiol Pharmacol Neerl 6: 795–802, 1957. [PubMed] [Google Scholar]
- 7.Chen KY, Acra SA, Donahue CL, Sun M, Buchowski MS. Efficiency of walking and stepping: relationship to body fatness. Obes Res 12: 982–989, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Coyle EF, Sidossis LS, Horowitz JF, Beltz JD. Cycling efficiency is related to the percentage of type I muscle fibers. Med Sci Sports Exerc 24: 782–788, 1992. [PubMed] [Google Scholar]
- 9.Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab 294: E882–E888, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elia M, Ritz P, Stubbs RJ. Total energy expenditure in the elderly. Eur J Clin Nutr 54, Suppl 3: S92–S103, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Frayn KN Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55: 628–634, 1983. [DOI] [PubMed] [Google Scholar]
- 12.Friedlander AL, Jacobs KA, Fattor JA, Horning MA, Hagobian TA, Bauer TA, Wolfel EE, Brooks GA. Contributions of working muscle to whole body lipid metabolism are altered by exercise intensity and training. Am J Physiol Endocrinol Metab 292: E107–E116, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Gaesser GA, Brooks GA. Muscular efficiency during steady-rate exercise: effects of speed and work rate. J Appl Physiol 38: 1132–1139, 1975. [DOI] [PubMed] [Google Scholar]
- 14.Gissane C, Corrigan DL, White JA. Gross efficiency responses to exercise conditioning in adult males of various ages. J Sports Sci 9: 383–391, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 52: 2191–2197, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Goodpaster BH, Wolfe RR, Kelley DE. Effects of obesity on substrate utilization during exercise. Obes Res 10: 575–584, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Guesbeck NR, Hickey MS, MacDonald KG, Pories WJ, Harper I, Ravussin E, Dohm GL, Houmard JA. Substrate utilization during exercise in formerly morbidly obese women. J Appl Physiol 90: 1007–1012, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Henriksson J Training induced adaptation of skeletal muscle and metabolism during submaximal exercise. J Physiol 270: 661–675, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr 72: 558S–563S, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Kamon E, Metz KF, Pandolf KB. Climbing and cycling with additional weights on the extremities. J Appl Physiol 35: 367–370, 1973. [DOI] [PubMed] [Google Scholar]
- 21.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277: E1130–E1141, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Lafortuna CL, Proietti M, Agosti F, Sartorio A. The energy cost of cycling in young obese women. Eur J Appl Physiol 97: 16–25, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Martin WH, Dalsky GP, Hurley BF, Matthews DE, Bier DM, Hagberg JM, Rogers MA, King DS, Holloszy JO. Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am J Physiol Endocrinol Metab 265: E708–E714, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 61: 534–540, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moseley L, Jeukendrup AE. The reliability of cycling efficiency. Med Sci Sports Exerc 33: 621–627, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Nicklas BJ, Rogus EM, Goldberg AP. Exercise blunts declines in lipolysis and fat oxidation after dietary-induced weight loss in obese older women. Am J Physiol Endocrinol Metab 273: E149–E155, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci 16: 23–29, 1991. [PubMed] [Google Scholar]
- 29.Poole DC, Henson LC. Effect of acute caloric restriction on work efficiency. Am J Clin Nutr 47: 15–18, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Poulin MJ, Paterson DH, Govindasamy D, Cunningham DA. Endurance training of older men: responses to submaximal exercise. J Appl Physiol 73: 452–457, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Pruchnic R, Katsiaras A, He J, Kelley DE, Winters C, Goodpaster BH. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Metab 287: E857–E862, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Ravussin E, Burnand B, Schutz Y, Jequier E. Energy expenditure before and during energy restriction in obese patients. Am J Clin Nutr 41: 753–759, 1985. [DOI] [PubMed] [Google Scholar]
- 33.Ravussin E, Burnand B, Schutz Y, Jequier E. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. Am J Clin Nutr 35: 566–573, 1982. [DOI] [PubMed] [Google Scholar]
- 34.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol 285: R183–R192, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Starling RD Energy expenditure and aging: effects of physical activity. Int J Sport Nutr Exerc Metab 11, Suppl: S208–S217, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Trappe SW, Costill DL, Vukovich MD, Jones J, Melham T. Aging among elite distance runners: a 22-yr longitudinal study. J Appl Physiol 80: 285–290, 1996. [DOI] [PubMed] [Google Scholar]
- 37.van Aggel-Leijssen DP, Saris WH, Hul GB, van Baak MA. Short-term effects of weight loss with or without low-intensity exercise training on fat metabolism in obese men. Am J Clin Nutr 73: 523–531, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Woo JS, Derleth C, Stratton JR, Levy WC. The influence of age, gender, and training on exercise efficiency. J Am Coll Cardiol 47: 1049–1057, 2006. [DOI] [PubMed] [Google Scholar]