Abstract
Obesity and its comorbidities are taking an increasing toll on human health. Key pathways that were identified with single gene variants in humans and model organisms have led to improved understanding and treatment of rare cases of human obesity. However, similar progress remains elusive for the more common multifactorial cases of metabolic dysfunction and disease. A survey of mouse chromosome substitution strains (CSSs) provided insight into the complex genetic control of diet-induced obesity and related conditions. We now report a survey of 60 traits related to obesity and metabolic syndrome in mice with a single substituted chromosome as well as selected traits measured in congenic strains derived from the substituted strain. We found that each strain that was resistant to diet-induced obesity had a distinct phenotype that uniquely modeled different combinations of traits related to metabolic disease. For example, the chromosome 6 CSS remained insulin resistant in the absence of obesity, demonstrating an atypical relationship between body weight and insulin resistance. These results provide insights into the genetic control of constant components of this mouse model of diet-induced metabolic disease as well as phenotypes that vary depending on genetic background. A better understanding of these genotype-phenotype relationships may enable a more individualized diagnosis and treatment of obesity and the metabolic syndrome.
Keywords: C57BL/6J, A/J, metabolic syndrome, quantitative trait locus, congenic strain
the impact of obesity on human health is considerable given the comorbidities such as cardiovascular disease and type 2 diabetes. Heritable factors account for 45–75% of variation in body mass index (BMI), the most common measure of obesity (8). Genetic studies have revealed a great deal about the molecular basis of obesity, with substantial progress coming from studies of mouse models (4). Genes such as leptin and its receptor are mutated in rare cases of human obesity and mouse models of monogenic obesity (7). However, most cases of human obesity are thought to be polygenic, and the identity of the genes and pathways involved has largely eluded discovery in both humans and mice (2).
The metabolic basis of human obesity is similarly complex. Various findings support important contributions of feeding behavior, nonexercise activity thermogenesis, in utero environment, microbial content in the gut, and resting energy expenditure, among others (10, 15–17, 21, 25, 29). At the individual level, the metabolic basis of obesity is probably attributable to variable combinations of these and other factors. To study the many genetic and metabolic factors at work within an individual, a complete panel of mouse chromosome substitution strains (CSSs) was analyzed for a series of complex traits including diet-induced obesity and its associated phenotypes (Ref. 26; Shao H and Nadeau JH, unpublished observations). These surveys found that large-effect quantitative trait loci (QTLs) and epistasis are common features of polygenic traits in mice. The genetic and metabolic bases of these QTLs may therefore have considerable clinical interest because of their potential to further our understanding of the relationship between genotype and phenotype as well as suggest novel targets for pharmaceutical treatments.
The CSS panel is comprised of 22 strains of mice, each with a single A/J-derived chromosome, with the remainder of the genome derived from strain C57BL/6J (B6). B6 and A/J mice respond differently to an obesogenic diet (5, 28). B6 mice become obese and develop many characteristics of metabolic syndrome, whereas A/J mice remain relatively lean and physiologically normal. Our laboratory has identified 17 CSSs that are resistant to diet-induced obesity including the chromosome 6 substitution strain C57BL/6J-Chr 6A/J (B6.A6) (26). In this report, results are presented characterizing the metabolic basis for obesity resistance in A/J, B6.A6, and the congenic strains that define an obesity resistance QTL on chromosome 6, Obrq2, which alone accounts for >50% of the body weight difference between the parental strains B6 and A/J. Analysis of the metabolic profiles sheds light on the genetic complexity associated with obesity and the potentially unique etiologies underlying obesity resistance in each strain.
MATERIALS AND METHODS
Husbandry.
Mice were obtained from the Jackson Laboratory (B6 and A/J) or from breeding colonies at Case Western Reserve University (B6, A/J, B6.A6, 6C1, and 6C2). Mice were housed in ventilated racks and maintained at 21°C on a 12:12-h light-dark cycle. Litters were weaned at 3–4 wk of age. Mice had access to food and water ad libitum unless otherwise indicated. Breeding colonies were fed LabDiets 5010 chow (PMI Nutrition International). The Institutional Animal Care and Use Committee approved all procedures.
Generation of congenic strains 6C1 and 6C2.
(B6 × B6.A6) F2 mice were genotyped with 13 microsatellite markers spanning chromosome 6 with an average marker distance of 10.7 Mb (range 3–25.2 Mb). The markers used for genotyping were D6Mit138, D6Mit159, D6Mit223, D6Mit274, D6Mit384, D6Mit188, D6Mit391, D6Mit284, D6Mit36, D6Mit287, D6Mit254, D6Mit59, and D6Mit15. Selected F2 mice that inherited a recombinant chromosome were backcrossed to B6. Offspring that were heterozygous for the recombinant chromosome were intercrossed to homozygose the selected segment. The congenic strains were subsequently maintained by brother-sister mating. Strain 6C1 was genotyped as homozygous for the A/J-derived allele at marker D6Mit138 and homozygous for the B6-derived allele at the remaining 12 markers. Strain 6C2 was genotyped as homozygous for the A/J-derived allele at markers D6Mit138 and D6Mit159 and homozygous for the B6-derived allele at the remaining 11 markers.
Blood and plasma measurements.
Mice that were ∼135 days of age (ranging from 127 to 136 days) were fasted overnight, weighed, and anesthetized with an intraperitoneal injection of 0.8 mg/g Avertin. Insulin and glucose were measured in mice that were 147–151 days of age that had been anesthetized with isoflurane. Once anesthetized, nose-to-anus length and retroorbital blood glucose (OneTouch Ultra, LifeSpan) were measured. Insulin was measured with a mouse ultrasensitive insulin ELISA (Mercodia) in combination with a Wallac Victor3 1420 Multilabel Counter (Perkin Elmer). The homeostasis model assessment-insulin resistance (HOMA-IR) is presented as fasting glucose (mmol/l) × fasting insulin (mU/l)/22.5.
For studies involving B6, A/J, and B6.A6, mice were fasted for ∼24 h. Blood was collected by cardiac puncture into a 1-ml syringe (Becton Dickinson) and transferred to a heparinized Microtainer plasma separator tube (Becton Dickinson). Samples were centrifuged for 5 min at ∼2,000 g. Cholesterol, triglycerides, blood urea nitrogen (BUN), and β-hydroxybutyrate (BHB) were measured at Marshfield Laboratories (Marshfield, WI).
Plasma neutral sterols were measured with gas chromatography-mass spectrometry (Hewlett Packard 6890 GC with a 5973 mass spectrometer) using selected ion monitoring mode. Plasma amino acids were quantified by cation exchange chromatography on a high-performance liquid chromatography system (Dionix) using postcolumn derivitization with ninhydrin reagent (Pickering). Acylcarnitine profiles were measured with tandem mass spectroscopy (Micromass Quatro) (30).
For studies involving 6C1 and 6C2, mice were fasted for ∼16 h. Blood was collected from the retroorbital sinus with a microcapillary tube and placed in a Microtainer tube with EDTA (Becton Dickinson) for measurement of triglycerides and free fatty acids or with heparin (Becton Dickinson) for all remaining studies. Samples were centrifuged for 5 min at ∼2,000 g. Cholesterol, triglycerides, and BHB were measured according to manufacturer's protocols (Pointe Scientific) with a split-beam spectrophotometer (Genesys 5, Spectronic). Free fatty acids were measured according to the acyl-CoA synthetase (ACS)-acyl-CoA oxidase (ACOD) method with the NEFA C test kit (Wako Chemicals).
Liver triglycerides.
After blood collection, liver samples were collected, immediately frozen on dry ice, and stored at −80°C. A piece of liver (100–200 mg) was saponified in an equal volume (μl) by weight (mg) of 3 M KOH, 65% ethanol (24). The sample was incubated at 70°C for 1 h and then at room temperature for 24 h. The sample volume was adjusted to 500 μl of 50 mM Tris per 100 mg of tissue used. Each sample was then diluted 10-fold with 50 mM Tris (pH 7.5). Triglycerides were measured with commercially available reagents and standards (Pointe Scientific).
Histology.
Liver tissue was fixed in 10% formalin, sectioned at 5 μm, and stained with hematoxylin and eosin at the tissue procurement and histology core facility at Case Western Reserve University.
Diet studies.
Five-week-old male mice were placed on a high-fat simple carbohydrate diet (HFSC) or a low-fat complex carbohydrate diet (LFCC) or remained on the 5010 diet. The HFSC diet (Research Diets D12331) derives 58% of its kilocalories from fat (soybean and coconut oil), 25.5% of its kilocalories from carbohydrate (sucrose and maltodextrin), and 16.4% of its kilocalories from protein (casein). The LFCC diet (Research Diets D12328) derives 10.5% of its kilocalories from fat (soybean and coconut oil), 73.1% of its kilocalories from carbohydrate (corn starch and maltodextrin), and 16.4% of its kilocalories from protein (casein). The 5010 diet derives 12.7% of its kilocalories from fat, 58.5% of its kilocalories from carbohydrate, and 28.7% of its kilocalories from protein. Mice were weighed every 2 wk for ∼100 days (ranging from 90 to 106 days). BMI was calculated by dividing body weight (g) by the square of the anal-nasal length (cm).
Food intake.
Twenty-four-hour food consumption was measured over 4 consecutive days. During each 4-day period, a thin layer of bedding and a known quantity of the HFSC diet were provided. Food remaining after 24 h was weighed, and the procedure was repeated for 3 additional days. The average quantity of food consumed per mouse was calculated for group-housed mice (2–4 6C1 and 6C2 mice were housed per cage; B6, A/J, and B6.A6 were singly housed).
Statistics.
Unpaired t-tests were used to compare body weight, BMI, fat pad weight, blood chemistry, liver triglycerides, and food intake between pairs of strains. A two-way ANOVA was used to analyze the effect of strain, diet, and strain × diet interactions on weight gain. Variance was compared with the F-test. The P values presented have been corrected according to the Bonferroni method to account for the number of tests for each trait. Results are presented as means ± SE.
RESULTS
Genetic resistance to diet-induced obesity.
A/J mice and the B6.A6 CSS are resistant whereas B6 mice are susceptible to diet-induced obesity when fed a HFSC diet (26, 27). Additional independent body weight measurements of strains B6, A/J, and B6.A6 confirmed this finding (Table 1, Supplemental Fig. S1A).1 The data demonstrate a 9.7-g (22%) body weight difference between strains B6.A6 (34.6 g) and B6 (44.3 g), representing ∼75% of the 12.8-g difference between the parental strains A/J and B6 (Table 1).
Table 1.
Adiposity-related phenotypes of B6, A/J, and B6.A6 mice
| Strain | Weight, g (HFSC diet) | n | Weight, g (LFCC diet) | n | BMI, g/cm2 (HFSC diet) | n | Fat Pads, g (HFSC diet) | n |
|---|---|---|---|---|---|---|---|---|
| B6 | 44.29±0.81 | 49 | 31.01±0.32 | 28 | 0.39±0.01 | 17 | 2.60±0.14 | 17 |
| A/J | 31.46±0.47‡ | 54 | 26.35±0.40‡ | 29 | 0.31±0.01‡ | 10 | 1.70±0.21* | 10 |
| B6.A6 | 34.60±0.77†‡ | 36 | 28.24±0.51†‡ | 17 | 0.33±0.01‡ | 14 | 1.89±0.24* | 13 |
Values are means ± SE for n mice. HFSC, high-fat simple carbohydrate; LFCC, low-fat complex carbohydrate; BMI, body mass index.
P < 0.05 (relative to B6);
P < 0.05 (relative to A/J);
P < 0.0001 (relative to B6).
A gene-environment interaction in diet-induced obesity.
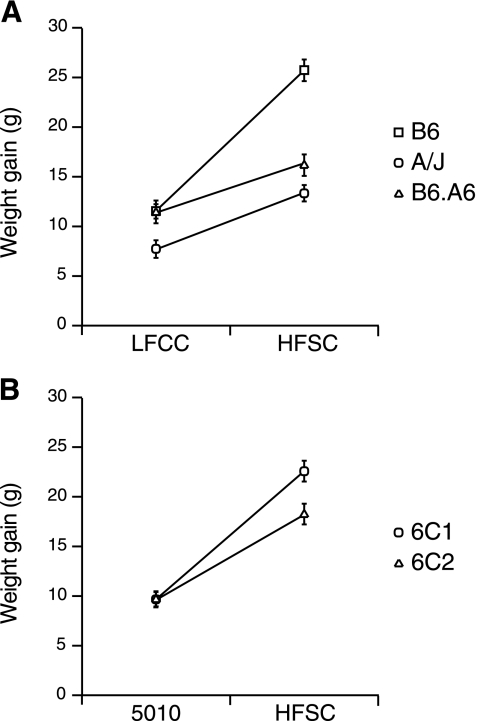
To determine whether the obesity resistance of B6.A6 was specific to the HFSC diet, body weight was also examined with the LFCC diet. B6.A6 was again leaner than B6, demonstrating that obesity resistance is not diet specific, although the weight difference was significantly larger on the HFSC diet (Table 1). Comparisons of weight gained on the HFSC and LFCC diet revealed significant effects of strain and diet and an interaction between strain and diet (Fig. 1A).
Fig. 1.
Gene-diet interaction affects weight gain. A: comparison of weight gained between 35 and 135 days of age while on the low-fat complex carbohydrate (LFCC) or high-fat simple carbohydrate (HFSC) diet in B6, A/J, and B6.A6; ANOVA revealed significant effects of strain (P < 0.0001) and diet (P < 0.0001) and an interaction between strain and diet (P < 0.0001). B: comparison of weight gain between 35 and 135 days of age on the 5010 or HFSC diet in 6C1 and 6C2 by ANOVA revealed significant effects of strain (P < 0.006) and diet (P < 0.00001) and an interaction between strain and diet (P < 0.0004).
Semidominant inheritance of obesity resistance.
To characterize the inheritance pattern of the obesity resistance phenotype of B6.A6, we analyzed the body weight of offspring from a cross between B6 and B6.A6 mice. These mice inherited one B6-derived and one A/J-derived chromosome 6 so that they were heterosomic for chromosome 6 but were otherwise homozygous for B6-derived chromosomes. The body weight of these mice was significantly less than that observed for B6 mice [43.89 ± 0.73 g (n = 39) vs. 46.56 ± 0.79 g (n = 55); P < 0.05] but greater than that observed for B6.A6 mice [36.42 ± 0.55 g (n = 63); P < 0.0001] and A/J mice [32.32 ± 0.77 g (n = 30); P < 0.0001]. The intermediate value suggests semidominant inheritance.
Decreased adiposity in B6.A6 males.
To test whether the obesity resistance of B6.A6 males is associated with decreased adiposity, gonadal fat pad weight was measured in B6, A/J, and B6.A6 males fed the HFSC diet. Relative to the fat pads in B6, A/J and B6.A6 had fat pads that were 35% and 27% smaller, respectively (Table 1). Similarly, comparisons of BMI between strain B6 and both A/J and B6.A6 were consistent with comparisons of body weight and fat pad mass (Table 1). Therefore, a decrease in adiposity contributed to the lower body weight of strains A/J and B6.A6 relative to B6.
Feeding behavior did not differ between B6 and B6.A6 mice.
Consumption of HFSC chow in B6, A/J, and B6.A6 singly housed males was measured at 35 days of age (0 days on HFSC diet), 85 days of age (50 days on HFSC diet), and 135 days of age (100 days on HFSC diet) and was normalized to body weight. Relative to B6, food intake per gram of body weight did not differ in B6.A6 and was increased in A/J at each time point (Table 2). Thus a reduction in food intake did not account for the resistance to diet-induced obesity of strains A/J and B6.A6.
Table 2.
Food consumption on HFSC diet
| Time Point, days on diet | Strain | n | Food Intake, g/g body wt |
|---|---|---|---|
| 0 | B6 | 7 | 0.14±0.01 |
| A/J | 8 | 0.16±0.01* | |
| B6.A6 | 7 | 0.15±0.01 | |
| 50 | B6 | 7 | 0.09±0.01 |
| A/J | 6 | 0.12±0.01* | |
| B6.A6 | 7 | 0.08±0.01† | |
| 100 | B6 | 8 | 0.07±0.01 |
| A/J | 7 | 0.09±0.01* | |
| B6.A6 | 7 | 0.07±0.01† | |
| 80–90 | 6C1 | 8 | 0.07±0.01 |
| 6C2 | 7 | 0.07±0.01 |
Values are means ± SE for n mice.
P < 0.05 (relative to B6);
P < 0.001 (relative to A/J).
B6.A6 was resistant to metabolic disease.
Phenotypes related to metabolic disease were examined in strains B6, A/J, and B6.A6 after 50 and 100 days on the HFSC diet (Table 3). Relative to B6 mice, A/J mice had lower levels of liver triglycerides and higher levels of BHB after 50 days on the HFSC diet. Plasma triglycerides and total cholesterol did not differ significantly. Relative to B6, B6.A6 mice also had decreased liver triglycerides after 50 days on the HFSC diet; all other measurements remained unchanged. Strain differences at this early time point may more directly reflect primary metabolic differences rather than secondary effects attributable to consequences of significant differences in body weight later in the study.
Table 3.
Metabolic characteristics of B6, A/J, and B6.A6 mice on HFSC diet
| Trait | B6 | n | A/J | n | B6.A6 | n | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 85 days of age (50 days on HFSC diet) | ||||||||||||
| Body weight, g | 30.46±0.96 | 10 | 25.83±0.64a | 10 | 28.26±0.65 | 10 | ||||||
| Blood urea nitrogen, mg/dl | 25±2 | 10 | 17±1a | 10 | 22±1e | 10 | ||||||
| β-Hydroxybutyrate, mM | 1.2±0.1 | 10 | 1.8±0.1a | 10 | 1.3±0.1 | 10 | ||||||
| Total cholesterol, mg/dl | 113±7 | 10 | 103±3 | 10 | 107±3 | 10 | ||||||
| Plasma triglycerides, mg/dl | 97±23 | 10 | 69±5 | 10 | 68±8 | 10 | ||||||
| Liver triglycerides, mg/g tissue | 133±13 | 9 | 77±4a | 10 | 64±4b | 9 | ||||||
| 135 days of age (100 days on HFSC diet) | ||||||||||||
| Body weight, g | 43.05±1.32 | 10 | 30.77±1.12c | 10 | 36.27±1.87a | 9 | ||||||
| Blood urea nitrogen, mg/dl | 21±1 | 10 | 17±1b | 10 | 29±1b,e | 9 | ||||||
| β-Hydroxybutyrate, mM | 1.3±0.1 | 10 | 1.7±0.1a | 10 | 1.6±0.1 | 9 | ||||||
| Total cholesterol, mg/dl | 166±10 | 10 | 101±5c | 10 | 129±5a,d | 9 | ||||||
| Plasma triglycerides, mg/dl | 66±9 | 10 | 76±8 | 10 | 103±10a | 9 | ||||||
| Glucose, mg/dl | 175±7 | 37 | 149±5a | 22 | 169±5d | 25 | ||||||
| Insulin, μg/l | 0.61±0.07 | 37 | 0.30±0.05a | 22 | 0.56±0.13 | 25 | ||||||
| HOMA-IR | 0.23±0.03 | 37 | 0.09±0.02a | 22 | 0.20±0.05 | 25 | ||||||
| Liver triglycerides, mg/g tissue | 169±14 | 10 | 80±8b | 10 | 109±10a | 10 | ||||||
Values are means ± SE for n mice. HOMA-IR, homeostasis model assessment-insulin resistance.
P < 0.05 (relative to B6);
P < 0.001 (relative to B6);
P < 0.0001 (relative to B6);
P < 0.05 (relative to A/J);
P < 0.0001 (relative to A/J).
Significant differences between B6 and both A/J and B6.A6 were observed for body weight, cholesterol, and liver triglycerides after 100 days on the HFSC diet (Table 3). Relative to B6, A/J mice also had decreased fasting glucose levels, insulin levels, and HOMA-IR and increased BHB. Relative to B6, B6.A6 mice had increased plasma triglyceride levels. Overall, the significant decreases in body weight of A/J and B6.A6 were associated with improvements in certain key measures of metabolic disease relative to B6 rather than a general improvement in metabolic profile.
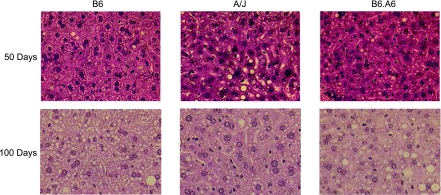
Liver triglyceride levels were reduced ∼50% in A/J and B6.A6 relative to strain B6 after 50 and 100 days on the HFSC diet (Table 3). Analysis of liver histology confirmed a decrease in both microvesicular and macrovesicular fat deposition (Fig. 2). Thus, as in humans, the degree of steatosis was correlated with body weight (1).
Fig. 2.
A/J and B6.A6 were resistant to fatty liver. Hematoxylin and eosin-stained liver sections (×400) after 50 or 100 days on the HFSC diet.
Differences in cholesterol synthesis or cholesterol absorption could account for the lower cholesterol levels in A/J and B6.A6 relative to B6. Markers for cholesterol biosynthesis (desmosterol, lathosterol, and 7-dehydrocholesterol) were significantly lower in strains A/J and B6.A6 relative to B6 (Supplemental Table S1). Among markers of cholesterol absorption (cholestanol, sitosterol, and campesterol), A/J mice did not differ from B6. Relative to B6, B6.A6 had decreased levels of campesterol, suggesting a decrease in cholesterol absorption (Supplemental Table S1). Despite this difference in absorption rates, it is likely that the reduction in cholesterol synthesis is a larger factor contributing to total cholesterol levels given the small amount of cholesterol in the HFSC diet.
Increased levels of BHB in A/J mice suggested differences in triglyceride catabolism in the fasted state. To investigate this question, the acylcarnitine profiles of B6, A/J, and B6.A6 were determined (Supplemental Table S2). No patterns were detected that would indicate differences in triglyceride catabolism.
Protein metabolism in B6.A6.
Relative to B6 mice, A/J mice had lower levels of BUN after both 50 and 100 days on the HFSC diet (Table 3). Relative to B6 mice, BUN levels in B6.A6 mice did not differ after 50 days on the HFSC diet but were significantly higher after 100 days (Table 3). Therefore, BUN levels differed between B6 and both A/J and B6.A6 mice; however, the direction of effect was not the same.
The altered BUN levels may reflect differences in protein catabolism, urea cycle flux, or renal function, among others. Protein catabolism rates were unchanged between B6 and A/J, as reflected by similar levels of the branched-chain amino acids leucine, isoleucine, and valine (Supplemental Table S3). Lower levels of citrulline in A/J mice suggest that variation in urea cycle flux may account for the reduced BUN levels, although the other urea cycle amino acids, arginine and ornithine, were unchanged. B6 and B6.A6 mice did not differ with respect to branched-chain or urea cycle amino acids, which is consistent with a decrease in renal function in B6.A6 mice relative to B6. Additional amino acid measurements revealed reduced levels of glutamine and taurine in A/J mice relative to B6 (Supplemental Table S3). B6.A6 mice also had reduced taurine levels relative to B6 (Supplemental Table S3).
The A/J-derived allele of Obrq2 conferred resistance to diet-induced obesity.
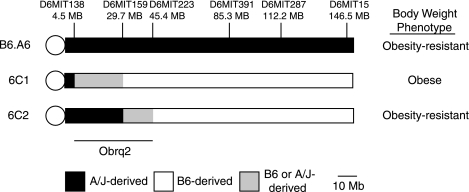
Chromosome 6 is 150 Mb and contains an estimated 1,195 protein-coding genes (Ensembl release 48). To localize obesity resistance QTLs on chromosome 6, a panel of congenic strains containing A/J-derived segments of chromosome 6 on an otherwise B6 background were fed the HFSC diet, and body weight was measured (Burrage and Nadeau, unpublished observation). Among four body weight QTLs identified, Obrq2, which had the largest effect, was defined by the congenic strains 6C1 (obese) and 6C2 (lean) and spans a 40.9-Mb interval between markers D6Mit138 and D6Mit223 (Fig. 3). The genomes of strains 6C1 and 6C2 differ only with respect to sequence within the Obrq2 interval; therefore any phenotypic differences between these two strains are due to the effects of Obrq2.
Fig. 3.
Map of chromosome 6 congenic strains. 6C1 and 6C2 were derived from B6.A6 and define Obrq2, a 40.9-Mb obesity resistance quantitative trait locus.
To test whether the effect on body weight of Obrq2 was specific to the HFSC diet, we measured weight gain on a standard rodent diet (LabDiets 5010) in addition to the HFSC diet. 6C2 mice were significantly leaner than 6C1 mice on both diets (Table 4). Comparison of weight gained between 6C2 and 6C1 on the 5010 and HFSC diets identified significant effects of strain, diet, and strain × diet interaction (Fig. 1B). Therefore, the effect of Obrq2 on body weight was not specific to a particular diet, although the effect was magnified with the HFSC diet. The growth curves of mice fed the HFSC diet are shown in Supplemental Fig. S1B. The difference in body weight was consistent with a decrease in adiposity, because the BMI of 6C2 was decreased relative to 6C1 (Table 4).
Table 4.
Adiposity-related phenotypes of 6C1 and 6C2 on 5010 and HFSC diets
| Strain | Weight, g (5010 diet) | n | Weight, g (HFSC diet) | n | BMI, g/cm2 (HFSC diet) | n |
|---|---|---|---|---|---|---|
| 6C1 | 29.83±0.24 | 17 | 41.63±0.57 | 67 | 0.37±0.01 | 21 |
| 6C2 | 28.68±0.36* | 17 | 36.86±0.56† | 58 | 0.35±0.01* | 29 |
Values are means ± SE for n mice.
P < 0.05 (relative to 6C1);
P < 0.0001 (relative to 6C1).
Obrq2 did not affect food consumption.
Food intake was measured in group-housed 6C1 and 6C2 mice after 80–90 days on the HFSC diet. At this time, the 6C1 mice were significantly heavier than the 6C2 mice [38.04 ± 1.41 g (n = 8) vs. 33.20 ± 1.08 g (n = 7); P < 0.03]. Food intake per gram of body weight did not differ between the two strains (Table 2), suggesting that differences in body weight were not attributable to differences in food consumption.
Inheritance pattern of Obrq2.
An imprinted region lies within the Obrq2 interval that contains the gene Mest (12, 14). Variation in Mest expression levels are correlated with weight gain among genetically identical B6 mice (13). To test for a parental effect, as would be expected if an imprinted gene was responsible for the Obrq2 phenotype, we analyzed F1 males derived from reciprocal crosses between B6 and 6C2. Body weight did not differ between offspring of the reciprocal crosses [40.73 ± 1.20 g (n = 28) vs. 39.50 ± 0.71 g (n = 27); P > 0.35], suggesting that there was no parental effect. Variance is another measure that can detect parental effects, and while the variance of the reciprocal crosses differed (P < 0.01), this was primarily due to a single cage of large mice. When the variance of weight gain rather than body weight was analyzed there was no longer a significant difference (P > 0.2). The mean values for body weight were therefore pooled and compared with the parental strains B6 and 6C2. The body weight of the (B6 × 6C2) F1 mice (40.13 ± 0.70 g, n = 55) was intermediate between the two parental strains, suggesting a semidominant inheritance pattern, as was also found for B6.A6.
Effects of Obrq2 on metabolic profile.
To characterize the phenotype controlled by Obrq2, we analyzed a series of metabolic traits in congenic strains 6C1 and 6C2 (Table 5). After 28 days on the HFSC diet, no differences between strains 6C1 and 6C2 were detected in cholesterol, triglycerides, or free fatty acid levels. In contrast, after 100 days on the HFSC diet, glucose, insulin, and HOMA-IR were reduced in strain 6C2 versus 6C1, whereas levels of free fatty acids were elevated. Thus, as was observed in B6.A6, the obesity resistance that is conferred by the A/J-derived allele of Obrq2 is associated with specific improvements in measures of metabolic disease as opposed to a more broad-based effect.
Table 5.
HFSC diet-induced metabolic profile of Obrq2
| Trait | 6C1 | n | 6C2 | n | ||
|---|---|---|---|---|---|---|
| 28 days on HFSC diet | ||||||
| Body weight, g | 27.60±0.28 | 16 | 24.82±0.52† | 16 | ||
| Total cholesterol, mg/dl | 111±4 | 16 | 110±4 | 16 | ||
| Plasma triglycerides, mg/dl | 69±4 | 16 | 57±5 | 15 | ||
| Free fatty acids, mEq/l | 0.45±0.02 | 16 | 0.40±0.04 | 12 | ||
| 100 days on HFSC diet | ||||||
| Body weight, g | 41.79±0.69 | 31 | 38.02±1.16* | 21 | ||
| Glucose mg/dl | 151±6 | 28 | 119±6† | 27 | ||
| Insulin, μg/l | 0.49±0.06 | 28 | 0.33±0.03* | 27 | ||
| HOMA-IR | 0.16±0.03 | 28 | 0.09±0.01* | 27 | ||
| Total cholesterol, mg/dl | 155±4 | 27 | 152±5 | 19 | ||
| Plasma triglycerides, mg/dl | 60±3 | 29 | 65±3 | 21 | ||
| Free fatty acids, mEq/l | 0.42±0.02 | 28 | 0.50±0.02* | 20 | ||
| β-Hydroxybutyrate, mM | 0.6±0.1 | 21 | 0.7±0.1 | 18 | ||
Values are means ± SE for n mice.
P < 0.05 (relative to 6C1);
P < 0.0001 (relative to 6C1).
DISCUSSION
Mouse models of diet-induced obesity.
We characterized a series of metabolic traits related to obesity and metabolic syndrome in strains B6, A/J, B6.A6, 6C1, and 6C2. B6.A6 was associated with a 22% reduction in body weight on a HFSC diet relative to B6, corresponding to ∼75% of the difference between the parental strains A/J and B6 (Table 1; Ref. 26). The A/J-derived allele of Obrq2 conferred an 11% reduction in body weight relative to strain 6C1 (Table 4). Food intake was not decreased in A/J, B6.A6, or 6C2, suggesting that these obesity-resistant strains differ in efficiency of energy extraction or energy expenditure rather than energy intake.
The weight gain of each strain was examined in the context of diets that differed greatly with respect to the type and amount of fat and carbohydrate. A/J, B6.A6, and 6C2 gained significantly less weight regardless of the composition of the diet, although a gene × diet interaction was identified, whereas B6 mice were particularly susceptible to weight gain when fed a HFSC diet. Similar observations made in humans are perhaps best illustrated by the Pima Indians, whose switch from a physically demanding and calorie-restricted environment to an obesogenic environment revealed an inherited predisposition toward obesity and diabetes (20). These genetic susceptibility loci are thought to partially explain the obesity epidemic over the past 30 years, a time period too short for sweeping genetic changes (9). Further study of B6.A6 and 6C2 may provide insight into the mechanisms underlying this phenomenon that remains largely unexplained at the genetic and molecular levels.
Genetic background effects.
The search for the genetic basis of obesity resistance began with the A/J genome, then focused on a single chromosome, and finally progressed to a single segment of that chromosome. Characterization of a series of traits in each strain revealed no consistent profile of metabolic phenotypes that was associated with obesity resistance. Rather than simply narrowing the search for an obesity resistance gene with a fixed phenotype, we found evidence suggesting that the A/J genome harbors many QTLs whose actions depend on genetic background. For example, both A/J and 6C2 had improved insulin sensitivity relative to their respective controls, suggesting the presence of a gene(s) underlying this phenotype within the Obrq2 interval. However, B6.A6 contains the Obrq2 genomic interval but remained insulin resistant despite a similar body weight, suggesting another QTL with compensating effects. Additionally, although levels of plasma triglycerides did not differ between strains B6 and A/J, B6.A6 had increased plasma triglyceride levels. These context-dependent phenotypes highlight the utility of the CSS panel and the congenic strains derived from them even for the study of traits that do not differ between the parental strains B6 and A/J. The actions of these QTLs presumably depend on epistatic or counterbalancing alleles.
Epidemiologic evidence in humans suggests that genetic variants with large, nonadditive effects control predisposition to obesity (23). This is consistent with the genetic architecture of obesity that was described in mice based on analysis of the complete panel of CSSs and additional congenic strains (26). However, linkage and association studies in humans have been largely unsuccessful at uncovering these types of genetic interactions (3). The difficulty may lie in part in the dynamic effects of genetic background as evidenced above, a property that the CSSs and derived congenic strains are well-suited for studying (19).
Genotype-phenotype correlations within metabolic syndrome.
The phenotypic differences among the three obesity-resistant mouse strains reported here resemble the diversity encompassed within the diagnosis of metabolic syndrome. Definitions of metabolic syndrome vary, but all center around the clustering of obesity, insulin resistance, dysglycemia, dyslipidemia, and hypertension (6). Individuals with metabolic syndrome are at a greater risk of developing diabetes, cardiovascular disease, nonalcoholic steatohepatitis, and polycystic ovary syndrome, among others (6). There is considerable phenotypic variability among affected individuals, which has led to questions regarding the utility of the diagnosis (11, 22). A study of healthy individuals found that 70% of individuals within the most insulin-sensitive tertile had normal body weight (BMI < 25), whereas 84% of individuals within the most insulin-resistant tertile were either overweight or obese (BMI > 25) (18). This leaves 30% of the insulin-sensitive individuals who are overweight or obese and 16% of the insulin-resistant individuals who are of normal body weight. These individuals, for unknown reasons, do not conform to the classic metabolic syndrome phenotype. Comparisons between the obesity-resistant strains A/J and 6C2 (insulin sensitive) and B6.A6 and 6C1 (insulin resistant) may help elucidate the genetic and molecular basis for genotype-phenotype correlations among the components of metabolic syndrome and thereby refine and improve the existing diagnostic criteria as well as improving our understanding of the etiology of this increasingly common medical condition.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants RR-12305 (J. H. Nadeau), F32-HL-82213 (D. A. Buchner), and DK-075040 (C. M. Croniger), NIH Training Grant GM-08613 (L. C. Burrage), and a gift from the Charles B. Wang Foundation. L. C. Burrage was supported in part by NIH Grant T32-GM-07250 to the Case Medical Scientist Training Program.
Supplementary Material
Acknowledgments
We thank Drs. Shawn McCandless, David Sinasac, and Haifeng Shao for helpful discussions.
Address for reprint requests and other correspondence: J. H. Nadeau, Dept. of Genetics, School of Medicine, Case Western Reserve Univ., Biomedical Research Bldg. 731, 2109 Adelbert Rd., Cleveland, OH 44106-4955 (e-mail: joseph.nadeau@case.edu).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol 17: S186–S190, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Barsh GS, Farooqi IS, O'Rahilly S. Genetics of body-weight regulation. Nature 404: 644–651, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bell CG, Walley AJ, Froguel P. The genetics of human obesity. Nat Rev Genet 6: 221–234, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Brockmann GA, Bevova MR. Using mouse models to dissect the genetics of obesity. Trends Genet 18: 367–376, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav 81: 243–248, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 365: 1415–1428, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Farooqi IS, O'Rahilly S. Monogenic obesity in humans. Annu Rev Med 56: 443–458, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Farooqi IS, O'Rahilly S. Genetic factors in human obesity. Obes Rev 8, Suppl 1: 37–40, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science 280: 1371–1374, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Hovi P, Andersson S, Eriksson JG, Jarvenpaa AL, Strang-Karlsson S, Makitie O, Kajantie E. Glucose regulation in young adults with very low birth weight. N Engl J Med 356: 2053–2063, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Kahn R Metabolic syndrome: is it a syndrome? Does it matter? Circulation 115: 1806–1811, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko-Ishino T, Kuroiwa Y, Miyoshi N, Kohda T, Suzuki R, Yokoyama M, Viville S, Barton SC, Ishino F, Surani MA. Peg1/Mest imprinted gene on chromosome 6 identified by cDNA subtraction hybridization. Nat Genet 11: 52–59, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, Faulk C, Skaf J, Kozak LP. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet 2: e81, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YJ, Park CW, Hahn Y, Park J, Lee J, Yun JH, Hyun B, Chung JH. Mit1/Lb9 and Copg2, new members of mouse imprinted genes closely linked to Peg1/Mest. FEBS Lett 472: 230–234, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283: 212–214, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science 307: 584–586, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023, 2006. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin T, Allison G, Abbasi F, Lamendola C, Reaven G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism 53: 495–499, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet 24: 221–225, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Pratley RE Gene-environment interactions in the pathogenesis of type 2 diabetes mellitus: lessons learned from the Pima Indians. Proc Nutr Soc 57: 175–181, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WGH, Boyce V, Howard BV, Bogardus C. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med 318: 467–472, 1988. [DOI] [PubMed] [Google Scholar]
- 22.Reaven GM The metabolic syndrome: is this diagnosis necessary? Am J Clin Nutr 83: 1237–1247, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Rice T Commingling and segregation analysis. In: Obesity: Genomics and Postgenomics, edited by Clement K, Sorensen TI. New York: Informa Healthcare, 2007, p. 59–76.
- 24.Salmon DM, Flatt JP. Effect of dietary fat content on the incidence of obesity among ad libitum fed mice. Int J Obes 9: 443–449, 1985. [PubMed] [Google Scholar]
- 25.Schwartz MW, Porte D Jr. Diabetes, obesity, and the brain. Science 307: 375–379, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, Justice M, O'Brien WE, Conti DV, Witte JS, Lander ES, Nadeau JH. Genetic dissection of complex traits with chromosome substitution strains of mice. Science 304: 445–448, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 44: 645–651, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37: 1163–1167, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1131, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Vreken P, van Lint AEM, Bootsma AH, Overmars H, Wanders RJA, van Gennip AH. Quantitative plasma acylcarnitine analysis using electrospray tandem mass spectrometry for the diagnosis of organic acidaemias and fatty acid oxidation defects. J Inherit Metab Dis 22: 302–306, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.