Abstract
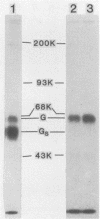
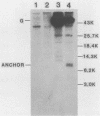
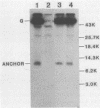
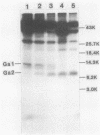
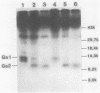
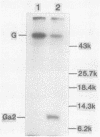
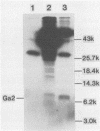
Wild-type vesicular stomatitis virus-infected cells contained multiple carboxy-terminal fragments of the envelope glycoprotein G. They migrated in 16% polyacrylamide gels with two dominant apparent molecular weights, 14,000 and 9,000. Both fragments were immunoprecipitated by two antibodies, anti-G(COOH) and anti-G(stem), made against the last 15 amino acids at the carboxy terminus and against the first 22 amino acids of the ectodomain adjacent to the transmembrane region of G, respectively. Pulse-chase experiments in the presence and absence of tunicamycin indicated that the higher-molecular-weight fragment, Gal, was generated first, presumably in the rough endoplasmic reticulum, and then apparently chased into the faster-migrating, stable fragment, Ga2. Exposure of infected cells to radioactive palmitic acid labeled Ga2. Ga2 was detected in purified virions. These results show that a polypeptide approximately 71 amino acids long is transported and incorporated into budding virions. What signals are operative and whether this C-terminal fragment of G protein is transported as a complex with other viral or host cell proteins are presently unknown.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. A., Rose J. K. Incorporation of a charged amino acid into the membrane-spanning domain blocks cell surface transport but not membrane anchoring of a viral glycoprotein. Mol Cell Biol. 1985 Jun;5(6):1442–1448. doi: 10.1128/mcb.5.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams G. A., Rose J. K. Structural requirements of a membrane-spanning domain for protein anchoring and cell surface transport. Cell. 1985 Jul;41(3):1007–1015. doi: 10.1016/s0092-8674(85)80081-7. [DOI] [PubMed] [Google Scholar]
- Bergmann J. E., Tokuyasu K. T., Singer S. J. Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1746–1750. doi: 10.1073/pnas.78.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Huang A. S. Conserved peptides in the proteins of vesicular stomatitis virus. Virology. 1979 Jun;95(2):445–453. doi: 10.1016/0042-6822(79)90499-9. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Morrison T. G. Characterization of the soluble glycoprotein released from vesicular stomatitis virus-infected cells. J Virol. 1983 Jan;45(1):80–90. doi: 10.1128/jvi.45.1.80-90.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. S., Doherty R., O'Rourke E. J., Ariel N., Huang A. S. Effects of transport inhibitors on the generation and transport of a soluble viral glycoprotein. Virology. 1987 Oct;160(2):482–484. doi: 10.1016/0042-6822(87)90021-3. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Huang A. S. Further characterization of the vesicular stomatitis virus temperature-sensitive O45 mutant: intracellular conversion of the glycoprotein to a soluble form. J Virol. 1986 Aug;59(2):210–215. doi: 10.1128/jvi.59.2.210-215.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch V., Berkaloff A. Analyse d'un mutant thermolabile du virus de la stomatite vésiculaire (VSV. Ann Inst Pasteur (Paris) 1971 Jul;121(1):101–106. [PubMed] [Google Scholar]
- Garreis-Wabnitz C., Kruppa J. Intracellular appearance of a glycoprotein in VSV-infected BHK cells lacking the membrane-anchoring oligopeptide of the viral G-protein. EMBO J. 1984 Jul;3(7):1469–1476. doi: 10.1002/j.1460-2075.1984.tb01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A., Rizzolo L., Rindler M., Adesnik M., Sabatini D. D., Gottlieb T. Nonpolarized secretion of truncated forms of the influenza hemagglutinin and the vesicular stomatitus virus G protein from MDCK cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3738–3742. doi: 10.1073/pnas.84.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeve L., Garreis-Wabnitz C., Zauke M., Breindl M., Kruppa J. The soluble glycoprotein of vesicular stomatitis virus is formed during or shortly after the translation process. J Virol. 1986 Mar;57(3):968–975. doi: 10.1128/jvi.57.3.968-975.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Machamer C. E., Rose J. K. Glycosylation allows cell-surface transport of an anchored secretory protein. Cell. 1985 Sep;42(2):489–496. doi: 10.1016/0092-8674(85)90106-0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Greenawalt J. W., Wagner R. R. Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology. 1966 Oct;30(2):161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- Irving R. A., Ghosh H. P. Shedding of vesicular stomatitis virus soluble glycoprotein by removal of carboxy-terminal peptide. J Virol. 1982 Apr;42(1):322–325. doi: 10.1128/jvi.42.1.322-325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. 3. Intracellular synthesis and extracellular appearance of virus-specific proteins. Virology. 1971 Dec;46(3):678–690. doi: 10.1016/0042-6822(71)90070-5. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S. P., Huang A. S. Shedding of the glycoprotein from vesicular stomatitis virus-infected cells. J Virol. 1978 Aug;27(2):330–339. doi: 10.1128/jvi.27.2.330-339.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Florkiewicz R. Z., Rose J. K. A single N-linked oligosaccharide at either of the two normal sites is sufficient for transport of vesicular stomatitis virus G protein to the cell surface. Mol Cell Biol. 1985 Nov;5(11):3074–3083. doi: 10.1128/mcb.5.11.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsikkö K., Simons K. The budding mechanism of spikeless vesicular stomatitis virus particles. EMBO J. 1986 Aug;5(8):1913–1920. doi: 10.1002/j.1460-2075.1986.tb04444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddington L., Machamer C. E., Rose J. K. Cytoplasmic domains of cellular and viral integral membrane proteins substitute for the cytoplasmic domain of the vesicular stomatitis virus glycoprotein in transport to the plasma membrane. J Cell Biol. 1986 Jun;102(6):2147–2157. doi: 10.1083/jcb.102.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddington L., Woodgett C., Rose J. K. Replacement of the cytoplasmic domain alters sorting of a viral glycoprotein in polarized cells. Proc Natl Acad Sci U S A. 1987 May;84(9):2756–2760. doi: 10.1073/pnas.84.9.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Wade-Glass M., Rabson M. S., Yang Y. C. The E5 transforming gene of bovine papillomavirus encodes a small, hydrophobic polypeptide. Science. 1986 Jul 25;233(4762):464–467. doi: 10.1126/science.3014660. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Proteolipids. Annu Rev Biochem. 1981;50:193–206. doi: 10.1146/annurev.bi.50.070181.001205. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staufenbiel M., Lazarides E. Ankyrin is fatty acid acylated in erythrocytes. Proc Natl Acad Sci U S A. 1986 Jan;83(2):318–322. doi: 10.1073/pnas.83.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]