Abstract
Factors controlling cardiac sympathetic nerve activity (CSNA) in the normal state and those causing the large increase in activity in heart failure (HF) remain unclear. We hypothesized from previous clinical findings that activation of cardiac mechanoreceptors by the increased blood volume in HF may stimulate sympathetic nerve activity (SNA), particularly to the heart via cardiocardiac reflexes. To investigate the effect of volume expansion and depletion on CSNA we have made multiunit recordings of CSNA in conscious normal sheep and sheep paced into HF. In HF sheep (n = 9) compared with normal sheep (n = 9), resting levels of CSNA were significantly higher (34 ± 5 vs. 93 ± 2 bursts/100 heart beats, P < 0.05), mean arterial pressure was lower (76 ± 3 vs. 87 ± 2 mmHg; P < 0.05), and central venous pressure (CVP) was greater (3.0 ± 1.0 vs. 0.0 ± 1.0 mmHg; P < 0.05). In normal sheep (n = 6), hemorrhage (400 ml over 30 min) was associated with a significant increase in CSNA (179 ± 16%) with a decrease in CVP (2.7 ± 0.7 mmHg). Volume expansion (400 ml Gelofusine over 30 min) significantly decreased CSNA (35 ± 12%) and increased CVP (4.7 ± 1.0 mmHg). In HF sheep (n = 6) the responses of CSNA to both volume expansion and hemorrhage were severely blunted with no significant changes in CSNA or heart rate with either stimulus. In summary, these studies in a large conscious mammal demonstrate that in the normal state directly recorded CSNA increased with volume depletion and decreased with volume loading. In contrast, both of these responses were severely blunted in HF with no significant changes in CSNA during either hemorrhage or volume expansion.
Keywords: heart failure, sympathetic nervous system, hemorrhage, cardiac volume
cardiac sympathetic nerve activity (CSNA) plays an important role in the control of normal cardiac function and also in cardiovascular pathology where, for example, increased levels in heart failure (HF) can promote the progression of the disease and lead to development of arrhythmias and sudden death (23). Despite these critical roles, the factors controlling CSNA in the normal state and those leading to the increased CSNA in HF remain relatively poorly understood. In recent studies, in which we directly recorded CSNA, we found that the arterial baroreflex control of CSNA was not desensitized in a pacing model of HF in conscious sheep (36). This raised the possibility that cardiac neural reflexes, specifically the cardiac sympathoexcitatory mechanoreflex driven by the fluid retention and increased cardiac filling pressures, contribute to the increased CSNA in HF.
Previous studies have demonstrated that cardiac reflexes responding to changes in cardiac filling pressures can selectively alter CSNA. In anesthetized normal dogs, left atrial stretch increased CSNA but decreased renal SNA and had no effect on other sympathetic outflows (19). These data suggested that an increase in cardiac filling pressures may selectively stimulate CSNA via the Bainbridge reflex, which is mediated by left and right atrial stretch receptors (4). In healthy subjects, changes in cardiac filling pressures resulted in reciprocal changes in muscle SNA (7, 33), but the effect on CSNA is unclear. Studies in normal subjects indicate that reductions in cardiac filling pressures resulted in either no change (3) or an increase in cardiac norepinephrine spillover (29).
In HF patients, there are a number of lines of evidence indicating that the cardiovascular and SNA responses to activation of cardiopulmonary mechanoreceptors are abnormal. In HF, upright tilt (20) and lower body negative pressure (LBNP) (13), which lower cardiac filling pressures, are associated with forearm vasodilatation or attenuated vasoconstriction compared with the vasoconstrictor response in normal subjects (1). Reductions in cardiac filling pressures, induced by sodium nitroprusside or LBNP, increased total norepinephrine spillover but caused a paradoxical decrease or no change in cardiac norepinephrine spillover in patients with HF (3, 21, 29, 31).
These findings raise the possibility that the CSNA responses to changes in cardiac filling pressures are impaired in HF compared with the healthy state, but CSNA has never been directly recorded in response to blood volume changes. We have, therefore, examined the effect of changes in blood volume on directly recorded CSNA in conscious normal sheep and in sheep with pacing-induced HF. Studies were performed in conscious animals to avoid the depressant effect of anesthesia, which is more potent on CSNA than on other sympathetic outflows (26). We hypothesized that increasing cardiac filling pressures in normal animals would increase CSNA and that this response would be attenuated in the HF group. We also expected reducing filling pressures would decrease CSNA in HF animals to a greater extent than normal animals.
METHODS
Adult merino ewes (35–49 kg body wt) were housed in individual metabolism cages in association with other sheep. Experiments began when sheep were accustomed to laboratory conditions and human contact. Sheep were fed a diet of oaten chaff (800 g/day), and water was offered ad libitum. All experiments were approved by the Animal Experimentation Ethics Committee of the Howard Florey Institute.
Surgical procedures.
Prior to the studies, sheep underwent 2 or 3 aseptic surgical procedures, each separated by 2 wk recovery. Anesthesia was induced with intravenous sodium thiopental (15 mg/kg) and, following intubation, was maintained with 1.5–2.0% isoflurane/O2. In the first stage, sheep were prepared with a carotid arterial loop. In the sheep to be induced into HF, a pacemaker lead (Medtronic, Minneapolis, MN) was inserted under fluoroscopic guidance via the right jugular vein into the right ventricle. The lead was exteriorized on the neck and connected to an external pacemaker. In a separate further operation, intrafascicular electrodes were implanted in the left cardiothoracic nerves as previously described (37). The electrodes were implanted in nine normal animals and nine HF animals. Experiments were conducted on standing, conscious sheep, and to minimize any effect of surgical stress, experiments were not started until the 4th day after implantation of the electrodes.
In all operations, animals were treated with antibiotics (Ilium Propen, procaine penicillin; Troy Laboratories, Smithfield, Australia or Mavlab, Australia) at the start of surgery and then for 2 days postoperatively. Postsurgical analgesia was maintained with intramuscular injection of flunixin meglumine (1 mg/kg; Troy Laboratories or Mavlab) at the start of surgery and then 4 and 16 h postsurgery.
On the day before implantation of recording electrodes, arterial and venous cannulae were inserted into the carotid artery and jugular vein as described previously (36, 37). The cannulae for measurement of arterial pressure (AP) and central venous pressure (CVP) were connected to separate pressure transducers (model TDXIII; Cobe) tied to the wool on the sheep's back. The pressures were corrected to compensate for the height of the transducers above the level of the heart.
Experimental protocols.
The development of HF was assessed by measurement of ejection fraction and fractional shortening using short-axis M-wave echocardiography on conscious sheep lying on the right side. Following placement of ventricular pacing leads, a basal measurement was made before the start of ventricular pacing, at 200–220 beats/min. Echocardiography was then performed weekly with the pacing switched off. Sheep were considered to be in HF when ejection fraction had fallen to <40%. All experiments were conducted with the pacing switched off.
CSNA was recorded differentially between the pair of electrodes with the best signal-to-noise ratio. The signal was amplified 100,000 times, filtered (bandpass 300–1,000 Hz), displayed on an oscilloscope, and passed through an audio amplifier and loud speaker. Sympathetic nerve activity (5,000 Hz), AP (100 Hz), and CVP (100 Hz) were recorded on a computer using a CED micro 1401 interface and Spike 2 software (Cambridge Electronic Design).
Four days after surgical implantation of cardiac sympathetic nerve electrodes, a 5-min recording of resting CSNA, AP, and CVP was made in conscious sheep in the normal state and in HF. The animals were then subjected to either hemorrhage (n = 6 in each group) or volume expansion (n = 6 in each group) on a randomly allocated basis. Following a recovery period of at least 24 h, the second protocol was performed. Both protocols could not be performed on all sheep due to deterioration of the CSNA signal in some animals. For the volume expansion series, Gelofusine (Braun Australia, New South Wales, Australia) was infused until the total volume infused reached 400 ml. The rate was titrated to between 350–500 ml/30 min to try to keep AP constant, although this was not always possible toward the end of the volume expansion. For the hemorrhage protocol, the venous cannula was connected to a blood infusion bag and blood was removed under gravity at a rate of 20 ml/min, until 450 ml of blood was removed. Following the end of the hemorrhage, the blood was reinfused back into the animal.
Data analysis.
Data were analyzed on a beat-to-beat basis using custom-written routines in the Spike 2 program. For each heartbeat the program determined diastolic, systolic, and mean arterial blood pressures and the number of discriminated spikes above threshold between the following diastolic pressures. The threshold was set at a level so that spikes from small bursts were counted. The threshold for each animal was checked over the 60-s control recording to ensure that each burst crossed the threshold and that spikes from the smallest bursts were counted. These data were used to calculate CSNA both as total nerve activity (spikes/s), and as burst incidence (bursts/100 heart beats). The control level of CSNA, determined as spikes/s during 60 s of control, was taken as 100% and changes in CSNA are reported as percentage change from control. Cardiovascular variables were calculated over 60-s periods at the end of the 5-min control and when 100, 200, 300, and 400 ml of Gelofusine had been infused or blood removed.
In addition to analyzing the data using spike counts, the data were analyzed using full-wave rectification and integration of the CSNA signal as described previously (30). The same 60 s of control in each animal was used to calculate the control levels of CSNA (arbitrary values), which was taken as 100%, and changes in CSNA were calculated as percentage changes from control. There were no differences in the conclusions drawn using either integration (data not shown) or spike counts.
Data are expressed as means ± SE and were analyzed using two-way repeated-measures ANOVA (SigmaStat, Access Softek, version 2.03). If significant, further appropriate post hoc tests were carried out, and multiple comparisons were corrected for using the Bonferroni adjustment. A P < 0.05 was considered statistically significant. To examine the effect of CVP changes on CSNA, linear regression lines showing the relationship between changes in CVP and CSNA were drawn for individual animals using data at selected time points. The average of the slopes and intercepts were used to draw an average regression line. The slopes of the regression lines in the HF and normal groups, during hemorrhage and volume expansion, were compared using an unpaired t-test.
RESULTS
Resting hemodynamics and CSNA in normal and HF sheep.
Left ventricular ejection fraction and fractional shortening, measured in conscious sheep by echocardiography, gradually decreased over 6–8 wk of rapid ventricular pacing at 200–220 beats/min. In HF animals, 1–2 days before implantation of cardiac sympathetic recording electrodes, ejection fraction had decreased from 81 ± 2% to 37 ± 2% (P < 0.001) and fractional shortening had decreased from 48 ± 2% to 17 ± 1% (P < 0.001).
Resting levels of CSNA and AP were calculated on a beat-to-beat basis from data collected during the control periods. In HF animals (n = 9), mean AP (MAP) was significantly lower (P < 0.05), CVP was significantly higher (P < 0.05), and heart rate (HR) tended to be higher than in normal animals (n = 9) (Table 1). The resting level of CSNA measured as burst incidence was significantly higher in the HF group compared with the normal group (P < 0.05) (Table 1).
Table 1.
Mean resting levels in normal animals and animals in heart failure from 5 min of control data
| Normals | Heart Failure | |
|---|---|---|
| Central venous pressure, mmHg | 0.0±1.0 | 3.0±1.0* |
| Mean arterial pressure, mmHg | 87±2 | 76±3* |
| Heart rate, beats/min | 73±4 | 81±3 |
| CSNA, spikes/s | 11±2 | 21±1* |
| CSNA burst incidence, % | 34±5 | 93±2* |
Values are means ± SE; n = 9 per group. CSNA, cardiac sympathetic nerve activity.
P < 0.05.
CSNA responses in normal and HF sheep during hemorrhage.
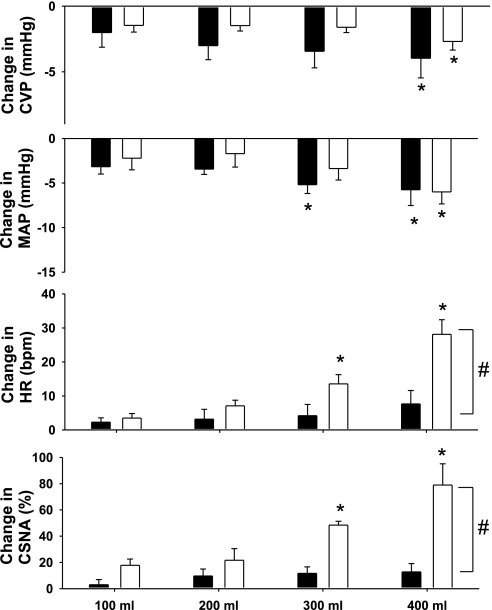
Hemorrhage was conducted in six animals in each group and was associated with a gradual decrease in CVP and a small fall in MAP, with the magnitude of the changes being similar in the normal and HF sheep (Figs. 1 and 2). There was a tendency for pulse pressure to decrease over time in the normal group, but this did not reach statistical significance (P = 0.12). There was no change in pulse pressure during hemorrhage in the HF group. During hemorrhage, there were progressive, significant increases in HR and CSNA in normal sheep but no significant changes in HF sheep. After 400 ml of blood had been withdrawn in the normal and HF groups, there were respective decreases in CVP of 2.7 ± 0.7 and 4.0 ± 1.5 mmHg, and in MAP of 6 ± 1 and 6 ± 2 mmHg. At this time, in the normal group, HR had increased by 29 ± 4 beats/min and CSNA had increased by 179 ± 16%, whereas there were no significant changes in HR or CSNA in the HF group (Fig. 2). The responses of HR and CSNA over time were significantly different between the two groups (significant interaction between group and time, P < 0.05).
Fig. 1.
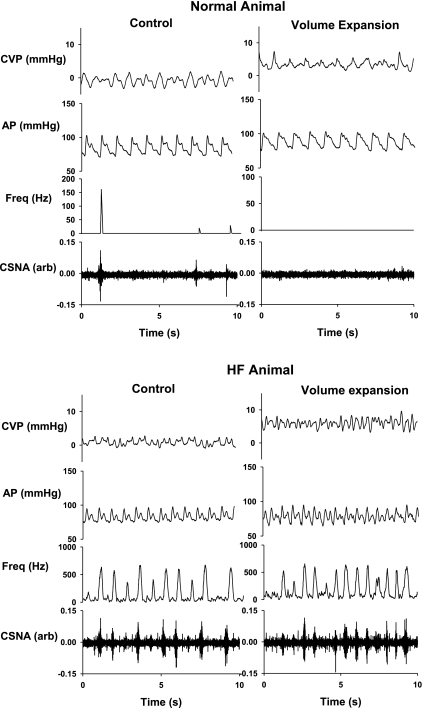
Raw figure from 1 normal (top) and 1 heart failure (HF) animal (bottom) showing the changes in central venous pressure (CVP), arterial pressure (AP), and cardiac sympathetic nerve activity (CSNA) during control and when 400 ml of blood was removed. arb, arbitrary units; freq, frequency.
Fig. 2.
Change in mean AP (MAP), CVP, heart rate (HR), and CSNA during hemorrhage in HF (black bars) and normal (white bars) animals (n = 6 in each group). *P < 0.05 from respective control; #P < 0.05 between groups over time.
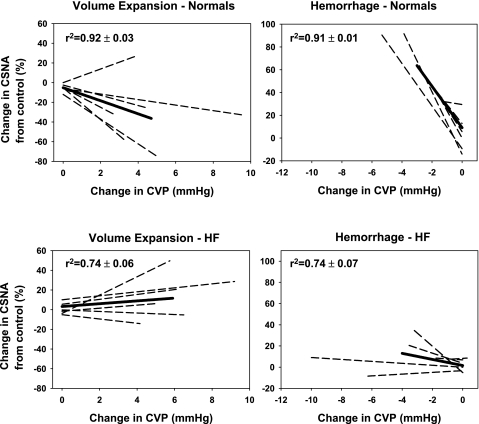
Linear regression lines relating the change in CSNA for a given change in CVP were drawn for individual sheep in both groups. For a given change in CVP, the change in CSNA was significantly less in HF animals than in normal animals (Fig. 3). This was reflected in the slopes of the average regression lines which were significantly lower in the HF group (−2.87 ± 2.1; r2 = 0.74 ± 0.07) than in the normal group (−18.2 ± 3.6; r2 = 0.91 ± 0.01) (P < 0.05).
Fig. 3.
Average and individual regression lines showing the relation between changes in CSNA as a function of changes in CVP for normal (top) and HF (bottom) groups. The panels indicate the regression lines during volume expansion (left) and hemorrhage (right) in normal and HF groups. The dotted lines are individual animals and the straight line is the mean regression line. Comparisons are between the slopes of the regression lines in the normal and HF groups. The r2 values represent the goodness of fit for the individual animals.
CSNA responses in normal and HF sheep during volume expansion.
Volume expansion with 400 ml Gelofusine was associated with an increase in CVP but no significant change in MAP or HR in normal (n = 6) or HF (n = 6) sheep (Figs. 4 and 5). There was no change in pulse pressure in either of the groups over time. These changes were associated with a significant decrease in CSNA in the normal animals but no change in CSNA in the HF group (Figs. 4 and 5). After 400 ml of Gelofusine had been infused into the normal and HF groups there were respective increases in CVP of 4.7 ± 1.0 and 5.9 ± 1.0 mmHg. At this time, in the normal group CSNA had significantly decreased by 35 ± 12%, whereas there was no significant change in CSNA in the HF group (Fig. 5). The changes in CSNA over time were significantly different between the two groups (P < 0.05). The slopes of the average regression lines relating the change in CSNA for a given change in CVP were lower in the HF animals than the normal animals, but this did not reach statistical significance (1.4 ± 1.8 for the HF group vs. −6.6 ± 3.4 for the normal group; P = 0.06) (r2 = 0.74 ± 0.06 for HF animals and 0.92 ± 0.03 for normal animals (Fig. 3).
Fig. 4.
Raw figure from 1 normal (top) and 1 HF (bottom) animal showing the changes in CVP, AP, and CSNA during control and when 400 ml of Gelofusine was infused.
Fig. 5.
Change in CVP, MAP, HR, and CSNA during volume expansion in HF (black bars) and normal (white bars) animals (n = 6 in both groups). *P < 0.05 from respective control; #P < 0.05 between groups over time.
DISCUSSION
This is the first study to directly record CSNA during volume changes in conscious animals in the normal healthy state and in HF. The main findings were that reductions in cardiac filling pressures during hemorrhage were accompanied by increases in CSNA in normal animals, while volume expansion was associated with a significant decrease in CSNA. In contrast, both of these responses were severely blunted in HF with no significant changes in CSNA during either hemorrhage or volume expansion.
Decreases in cardiac filling pressures in normal animals.
The present finding that hemorrhage caused robust, parallel increases in CSNA and HR, associated with a reduction in cardiac filling pressures and a small fall in AP, is similar to the increase in cardiac norepinephrine spillover found in normal subjects during hypotensive LBNP (3). It is likely that these changes in CSNA and HR are an integrated response to unloading of arterial and cardiopulmonary baroreceptors. This is supported by the observation that the tachycardia during hemorrhage is mediated largely by changes in baroreceptor afferent activity (34, 35) and the finding that the sympathetic response to LBNP is reduced in cardiac transplant patients with denervated ventricles but innervated atria and pulmonary veins, indicating an important role for ventricular mechanoreceptors (27).
Increases in cardiac filling pressures in normal animals.
Studies on the effect of increasing cardiac filling pressures on HR date back to experiments by Bainbridge in 1915 who demonstrated that intravenous infusion of saline or blood increased HR (4). In human patients, the Bainbridge reflex, in terms of a HR response, has been observed in some but not all studies (5, 16). It is important to note that the Bainbridge reflex can be counteracted by changes in baroreceptor afferent activity (5), which may account for the different results observed. A role for CSNA in this reflex is suggested by the demonstration that distension of the left atrium or the pulmonary veins results in an increase in HR (11, 14) that is sympathetically mediated (18) and that activation of atrial A-type receptors by volume expansion increases efferent sympathetic nerve activity in the cat (15). Furthermore, in anesthetized dogs, activation of left atrial receptors by balloon distension increased CSNA, decreased renal SNA, and had no effect on splenic or lumbar SNA (19), although in another study, distension of the left atrium resulted in an initial period of inhibition followed by an excitation of CSNA (24).
A criticism of the above studies is that due to difficulties with recording CSNA in conscious animals they were completed in anesthetized animals and, in most cases, using an open-chest preparation. Considering the potent effect of anesthesia on cardiovascular reflexes, and on CSNA in particular (26), it is vital to confirm any findings in conscious, unstressed animals. It is also important to note that experiments that observed sympathoexcitation studied the specific responses to atrial stretch induced by balloon inflation. Volume expansion is a more physiological stimulus that activates the ventricular as well as the atrial receptors, which is important given the evidence that ventricular mechanoreceptors play a role in the responses to volume changes (27).
In our study, there was no cardiac sympathoexcitation under conditions of volume expansion in normal conscious sheep. While there was no change in MAP or pulse pressure, it is possible that the small nonsignificant increase in MAP may have offset any sympathoexcitatory response mediated by the cardiopulmonary afferents. This study cannot therefore exclude the possibility that selective activation of atrial receptors may activate the Bainbridge reflex and induce cardiac sympathoexcitation. Our finding that volume expansion did not cause cardiac sympathoexcitation indicates that the Bainbridge reflex is absent or relatively weak with its effects being overridden by the effect of arterial baroreceptors. The finding that there was no change in HR, although CSNA decreased, suggests a decrease in cardiac vagal activity in response to the volume loading.
Decreases in cardiac filling pressures in HF animals.
In patients with HF, there is evidence of a positive correlation between pulmonary artery pressures and cardiac norepinephrine spillover levels (22). These data support the concept that in HF a reflex responding to increases in cardiac filling pressures may contribute to the increased CSNA. This possibility has been explored further in HF patients by decreasing cardiac filling pressures via upright tilt, LBNP, and infusions of vasodilators (20, 21). Sodium nitroprusside infusion caused no change in cardiac norepinephrine spillover in patients with moderately severe HF, but an increase in normal subjects (29, 31). Similarly, Kaye et al. (21) observed a reduction in cardiac norepinephrine spillover with infusion of sodium nitroprusside in HF patients. These studies support the hypothesis that in HF increased cardiac filling pressures stimulate CSNA, although the fall in MAP during nitroprusside, which would have activated arterial baroreceptors, complicates the interpretation of these observations. Using a LBNP stimulus, which reduced filling pressures with no change in AP, a reduction in cardiac norepinephrine spillover was observed in HF patients but not normal subjects (3). Although this study supported the hypothesis that increased cardiac filling pressures in HF contribute to the increased CSNA in these patients, LBNP has been shown to causes changes in firing rates of baroreceptor afferents even in the absence of changes in MAP (17).
While the results obtained from spillover techniques are important, cardiac norepinephrine spillover is an indirect steady state method and the inability of this technique to distinguish between direct changes in SNA and the reduced norepinephrine reuptake that occurs in HF (10), indicates that it is important to reexamine these responses using direct recordings of SNA. In addition, HF patients used in the studies of norepinephrine spillover were on medications that may have altered CSNA by a direct action or by actions secondary to the hemodynamic improvements that they induce.
Our study was conducted in conscious animals with stable pacing-induced HF, without the complication of medication. This animal model of HF has been used extensively and shows the symptoms of HF seen clinically, including decreased ventricular function and intense neurohumoral activation (8, 28, 32). Importantly, the level of neurohormonal activation in pacing models of HF closely parallels that in human HF patients (38).
We used hemorrhage as a stimulus to decrease cardiac filling pressures and observed no significant change in directly recorded CSNA in HF animals, in contrast to the large increase in CSNA for a similar reduction in CVP in normal animals. In HF animals, at the end of hemorrhage CVP had decreased to a level similar to that in normal sheep, but at this point there was no change in CSNA. As in the clinical studies, the accompanying decrease in MAP and unloading of arterial baroreceptors is a confounding factor that would stimulate CSNA. Thus, any reduction in the action of the Bainbridge reflex, due to the decreased filling pressures, may have been offset by an action of the arterial baroreceptors. However, supporting the notion that cardiac afferent reflexes are not a dominant factor causing sympathoexcitation in HF is the finding in dogs with pacing-induced HF that cardiac denervation did not inhibit the increase in plasma norepinephrine (25). Since the degree of sympathetic activation in HF shows marked regional heterogeneity, it was important to establish whether decreasing cardiac filling pressures had a selective effect to decrease the cardiac sympathoexcitation. Our findings, together with those of the cardiac denervation study (25), suggest that increased cardiac filling pressures are not an important factor driving the increased CSNA in HF, and if the Bainbridge reflex is evoked in HF its effects are offset by those of the arterial baroreceptors.
The effects of volume depletion on CSNA that we found are in general agreement with those from clinical studies of cardiac norepinephrine spillover (29, 31). In both cases the degree of cardiac sympathoexcitation in response to volume depletion in HF is strongly attenuated compared with the response in the healthy state. The impaired response of CSNA in HF was not because it was near maximal, since CSNA can be increased a further 40% when AP is decreased with sodium nitroprusside (36). Furthermore, the reduced CSNA response is unlikely to result from a smaller percentage of total blood volume being removed in the HF group, because the fall in CVP in the HF group tended to be greater than in normal group and the falls in MAP were similar. It would be expected that the fall in MAP during hemorrhage would stimulate CSNA via the arterial baroreflex, since in HF the CSNA arterial baroreflex sensitivity is normal (36). However, the resting level of CSNA is strikingly elevated and sits on a less steep part of the baroreflex curve so that a fall in MAP induces a smaller increase in CSNA in HF than in normal animals. An additional factor leading to the impaired response may be the lack of an increase in HR in the HF group, so that for a given firing frequency, activity will increase less than in the normal group in which there was a large increase in HR.
Increases in cardiac filling pressures in HF animals.
Analogous to the desensitization of the CSNA response to hemorrhage in HF, the inhibition of CSNA during volume loading seen in normal animals was completely absent in HF animals. The similar desensitization of the reflex inhibition of RSNA by cardiopulmonary reflexes (9, 41) suggests that this is a widespread effect on the sympathetic response to volume expansion. An explanation for a generalized desensitization is that it is due mainly to the reduced sensitivity of atrial vagal afferents, which were shown to become less sensitive in dogs with chronic high-output HF (40). Further evidence that the afferent pathway responding to volume is abnormal in HF was the lack of activation of neural pathways in the brain that were normally activated by volume expansion (2).
In conclusion, in normal sheep, volume depletion caused parallel increases in CSNA and HR, and volume loading decreased CSNA but had no effect on HR. In contrast, in a pacing model of HF in sheep these reflex responses were almost absent. The attenuated response of CSNA during changes in cardiac filling pressures in the HF animals suggests a striking impairment of cardiopulmonary mechanoreceptors.
Perspectives and Significance
Previous studies by other groups have shown that in HF either sinoaortic or cardiac denervation did not prevent the increase in plasma norepinephrine (6, 25), suggesting that any defects in these inhibitory reflexes are not a major cause of the sympathoexcitation in HF. The findings of our present and previous study (36) have now demonstrated that neither the arterial nor the cardiopulmonary baroreflexes are a major factor determining the cardiac sympathoexcitation in HF. The reduced afferent inhibitory input from cardiopulmonary receptors that we observed in response to volume expansion in HF may, however, permit the increase in CSNA in spite of the increased blood volume. Further studies are required to determine whether other afferent neural reflexes, from the kidneys or skeletal muscle, play a role. Alternatively, the afferent signal may be humoral, possibly by actions of circulating peptide hormones, including angiotensin and endothelin acting on circumventricular organs (42), blood borne cytokines activating receptors on vascular cells of the blood brain barrier (12), or a direct central action of aldosterone, which can freely cross the blood brain barrier (39).
GRANTS
This work was supported by National Health and Medical Research Council of Australia Grant 232313 and the National Heart, Lung, and Blood Institute Grant 5-R01-HL-07 4932.
Acknowledgments
The authors are grateful to Tony Dornom, Alan McDonald, and Craig Thomson for their excellent technical assistance and to David Trevaks for Spike 2 programming.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abboud FM, Eckberg DL, Johannsen UJ, Mark AL. Carotid and cardiopulmonary baroreceptor control of splanchnic and forearm vascular resistance during venous pooling in man. J Physiol 286: 173–184, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akama H, McGrath BP, Badoer E. Volume expansion fails to normally activate neural pathways in the brain of conscious rabbits with heart failure. J Auton Nerv Syst 73: 54–62, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Azevedo ER, Newton GE, Floras JS, Parker JD. Reducing cardiac filling pressure lowers norepinephrine spillover in patients with chronic heart failure. Circulation 101: 2053–2059, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bainbridge FA The influence of venous filling upon the rate of the heart. J Physiol 50: 65–84, 1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbieri R, Triedman JK, Saul JP. Heart rate control and mechanical cardiopulmonary coupling to assess central volume: a systems analysis. Am J Physiol Regul Integr Comp Physiol 283: R1210–R1220, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Brandle M, Patel KP, Wang W, Zucker IH. Hemodynamic and norepinephrine responses to pacing-induced heart failure in conscious sinoaortic-denervated dogs. J Appl Physiol 81: 1855–1862, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Charkoudian N, Martin EA, Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Influence of increased central venous pressure on baroreflex control of sympathetic activity in humans. Am J Physiol Heart Circ Physiol 287: H1658–H1662, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Dibner-Dunlap ME, Thames MD. Baroreflex control of renal sympathetic nerve activity is preserved in heart failure despite reduced arterial baroreceptor sensitivity. Circ Res 65: 1526–1535, 1989. [DOI] [PubMed] [Google Scholar]
- 9.DiBona GF, Sawin LL. Reflex regulation of renal nerve activity in cardiac failure. Am J Physiol Regul Integr Comp Physiol 266: R27–R39, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, Kopin IJ, Goldstein DS, Esler MD. Cardiac sympathetic nerve function in congestive heart failure. Circulation 93: 1667–1676, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Fater DC, Schultz HD, Sundet WD, Mapes JS, Goetz KL. Effects of left atrial stretch in cardiac-denervated and intact conscious dogs. Am J Physiol Heart Circ Physiol 242: H1056–H1064, 1982. [DOI] [PubMed] [Google Scholar]
- 12.Felder RB, Francis J, Zhang ZH, Wei SG, Weiss RM, Johnson AK. Heart failure and the brain: new perspectives. Am J Physiol Regul Integr Comp Physiol 284: R259–R276, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson DW, Abboud FM, Mark AL. Selective impairment of baroreflex-mediated vasoconstrictor responses in patients with ventricular dysfunction. Circulation 69: 451–460, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Furnival CM, Linden RJ, Snow HM. Reflex effects on the heart of stimulating left atrial receptors. J Physiol 218: 447–463, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakumaki MO Function of the left atrial receptors. Acta Physiol Scand 344: 1–54, 1970. [PubMed] [Google Scholar]
- 16.Hakumaki MO Seventy years of the Bainbridge reflex. Acta Physiol Scand 130: 177–185, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Hartikainen J, Ahonen E, Nevalainen T, Sikanen A, Hakumaki M. Haemodynamic information encoded in the aortic baroreceptor discharge during haemorrhage. Acta Physiol Scand 140: 181–189, 1990. [DOI] [PubMed] [Google Scholar]
- 18.Kappagoda CT, Linden RJ, Snow HM. A reflex increase in heart rate from distension of the junction between the superior vena cava and the right atrium. J Physiol 220: 177–197, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karim F, Kidd C, Malpus CM, Penna PE. The effects of stimulation of the left atrial receptors on sympathetic efferent nerve activity. J Physiol 227: 243–260, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassis E Cardiovascular response to orthostatic tilt in patients with severe congestive heart failure. Cardiovasc Res 21: 362–368, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Kaye DM, Jennings GL, Dart AM, Esler MD. Differential effect of acute baroreceptor unloading on cardiac and systemic sympathetic tone in congestive heart failure. J Am Coll Cardiol 31: 583–587, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Kaye DM, Lambert GW, Lefkovits J, Morris M, Jennings G, Esler MD. Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. J Am Coll Cardiol 23: 570–578, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol 26: 1257–1263, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Kollai M, Koizumi K, Yamashita H, Brooks CM. Study of cardiac sympathetic and vagal efferent activity during reflex responses produced by stretch of the atria. Brain Res 150: 519–532, 1978. [DOI] [PubMed] [Google Scholar]
- 25.Levett JM, Marinelli CC, Lund DD, Pardini BJ, Nader S, Scott BD, Augelli NV, Kerber RE, Schmid PG Jr. Effects of beta-blockade on neurohumoral responses and neurochemical markers in pacing-induced heart failure. Am J Physiol Heart Circ Physiol 266: H468–H475, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Matsukawa K, Ninomiya I, Nishiura N. Effects of anesthesia on cardiac and renal sympathetic nerve activities and plasma catecholamines. Am J Physiol Regul Integr Comp Physiol 265: R792–R797, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Mohanty PK, Thames MD, Arrowood JA, Sowers JR, McNamara C, Szentpetery S. Impairment of cardiopulmonary baroreflex after cardiac transplantation in humans. Circulation 75: 914–921, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Murakami H, Liu JL, Zucker IH. Blockade of AT1 receptors enhances baroreflex control of heart rate in conscious rabbits with heart failure. Am J Physiol Regul Integr Comp Physiol 271: R303–R309, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Newton GE, Parker JD. Cardiac sympathetic responses to acute vasodilation. Normal ventricular function versus congestive heart failure. Circulation 94: 3161–3167, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Ramchandra R, Barrett CJ, Guild SJ, Malpas SC. Evidence of differential control of renal and lumbar sympathetic nerve activity in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 290: R701–R708, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Ros M, Azevedo ER, Newton GE, Parker JD. Effects of nitroprusside on cardiac norepinephrine spillover and isovolumic left ventricular relaxation in the normal and failing human left ventricle. Can J Cardiol 18: 1211–1216, 2002. [PubMed] [Google Scholar]
- 32.Spinale FG, Mukherjee R, Iannini JP, Whitebread S, Hebbar L, Clair MJ, Melton DM, Cox MH, Thomas PB, de Gasparo M. Modulation of the renin-angiotensin pathway through enzyme inhibition and specific receptor blockade in pacing-induced heart failure. II. Effects on myocyte contractile processes. Circulation 96: 2397–2406, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Sundlof G, Wallin BG. Effect of lower body negative pressure on human muscle nerve sympathetic activity. J Physiol 278: 525–532, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thrasher TN, Keil LC. Arterial baroreceptors control blood pressure and vasopressin responses to hemorrhage in conscious dogs. Am J Physiol Regul Integr Comp Physiol 275: R1843–R1857, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Thrasher TN, Shifflett C. Effect of carotid or aortic baroreceptor denervation on arterial pressure during hemorrhage in conscious dogs. Am J Physiol Regul Integr Comp Physiol 280: R1642–R1649, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Watson AM, Hood SG, Ramchandra R, McAllen RM, May CN. Increased cardiac sympathetic nerve activity in heart failure is not due to desensitization of the arterial baroreflex. Am J Physiol Heart Circ Physiol 293: H798–H804, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson AM, Mogulkoc R, McAllen RM, May CN. Stimulation of cardiac sympathetic nerve activity by central angiotensinergic mechanisms in conscious sheep. Am J Physiol Regul Integr Comp Physiol 286: R1051–R1056, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Yarbrough WM, Spinale FG. Large animal models of congestive heart failure: a critical step in translating basic observations into clinical applications. J Nucl Cardiol 10: 77–86, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension 51: 727–733, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zucker IH, Earle AM, Gilmore JP. The mechanism of adaptation of left atrial stretch receptors in dogs with chronic congestive heart failure. J Clin Invest 60: 323–331, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zucker IH, Gorman AJ, Cornish KG, Lang M. Imparied atrial receptor modulation or renal nerve activity in dogs with chronic volume overload. Cardiovasc Res 19: 411–418, 1985. [DOI] [PubMed] [Google Scholar]
- 42.Zucker IH, Pliquett RU. Novel mechanisms of sympatho-excitation in chronic heart failure. Heart Fail Monit 3: 2–7, 2002. [PubMed] [Google Scholar]