Abstract
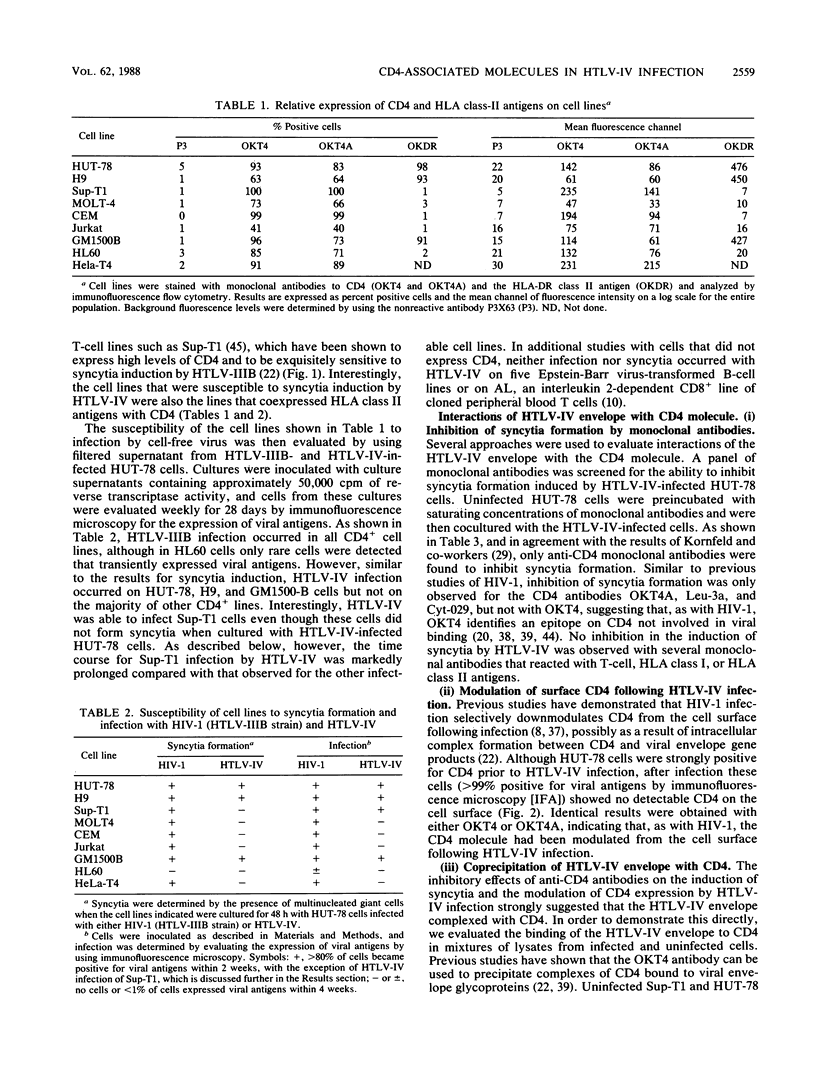
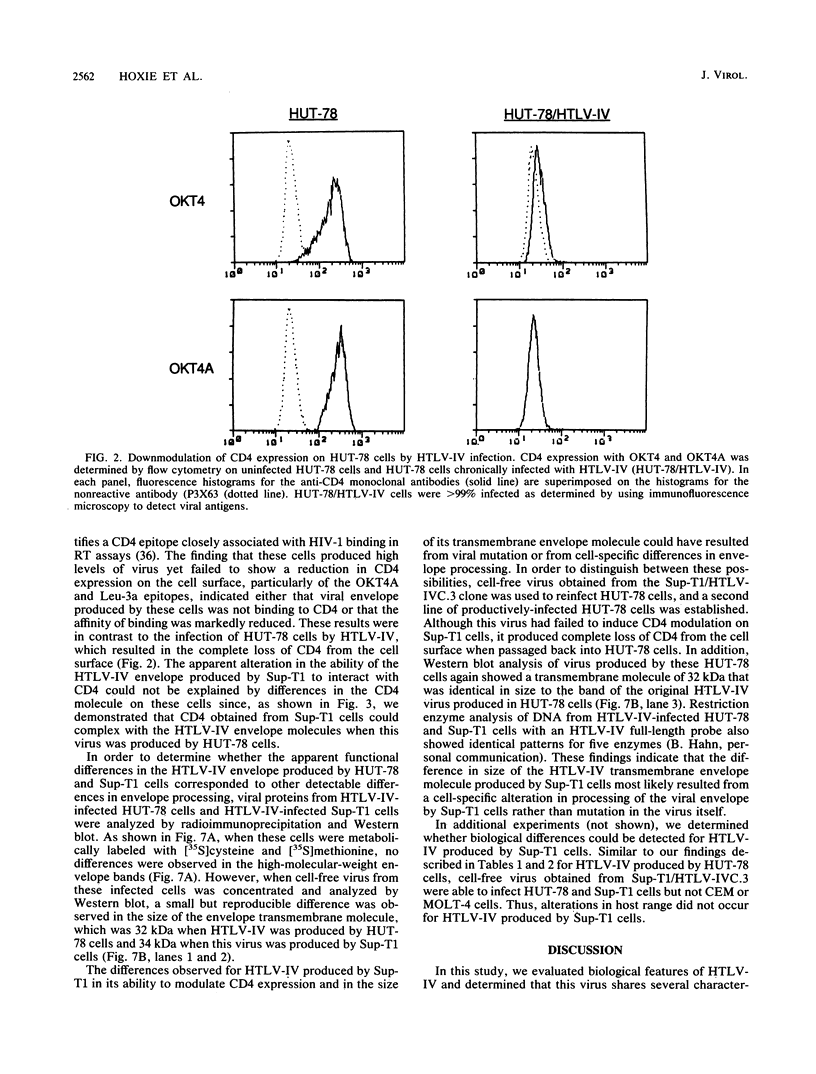
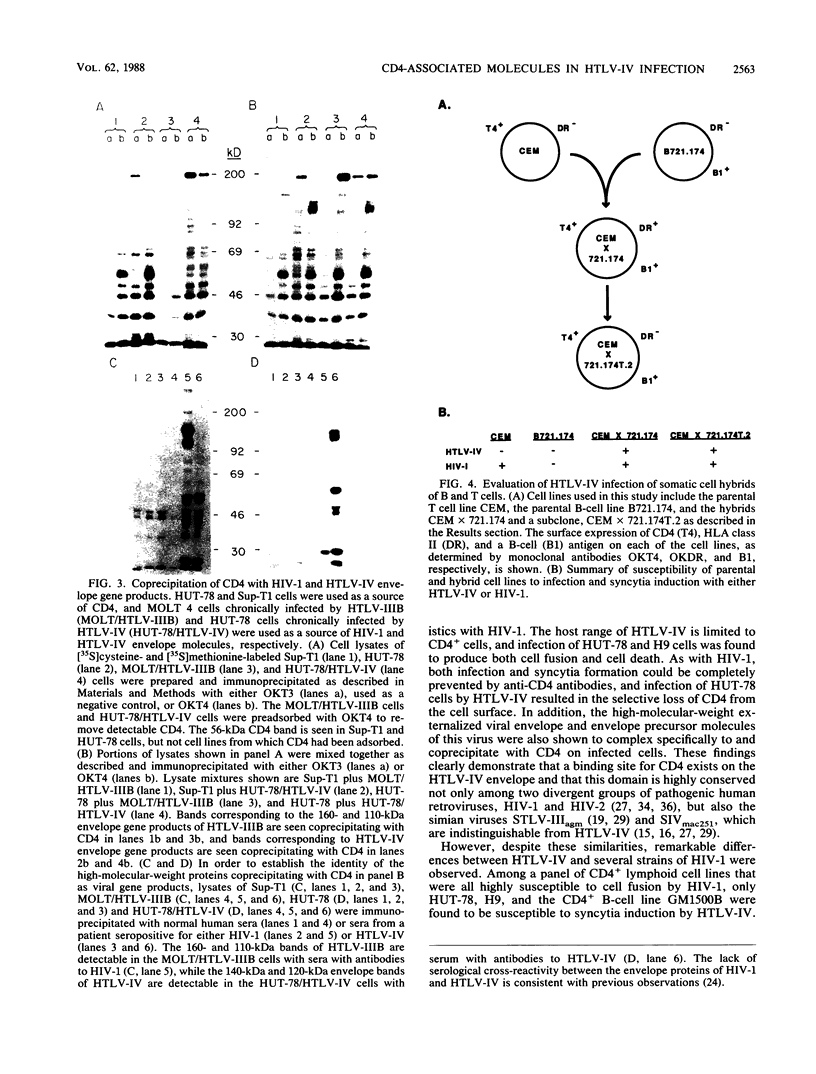
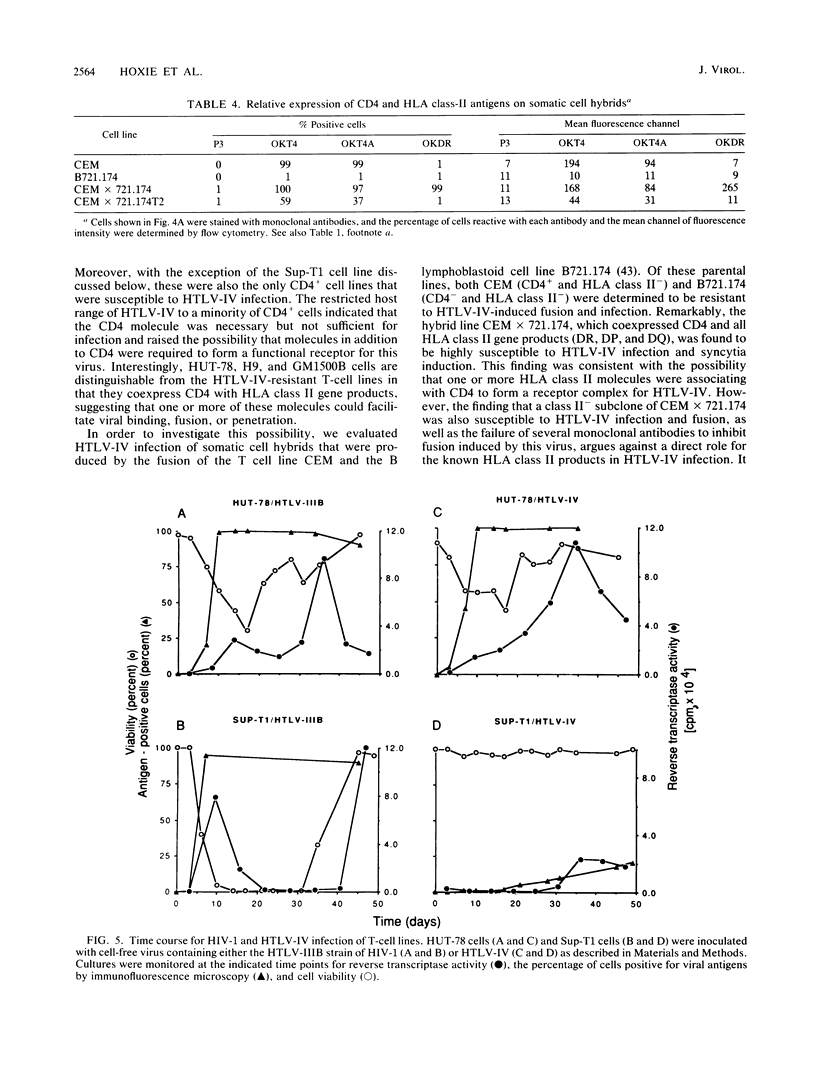
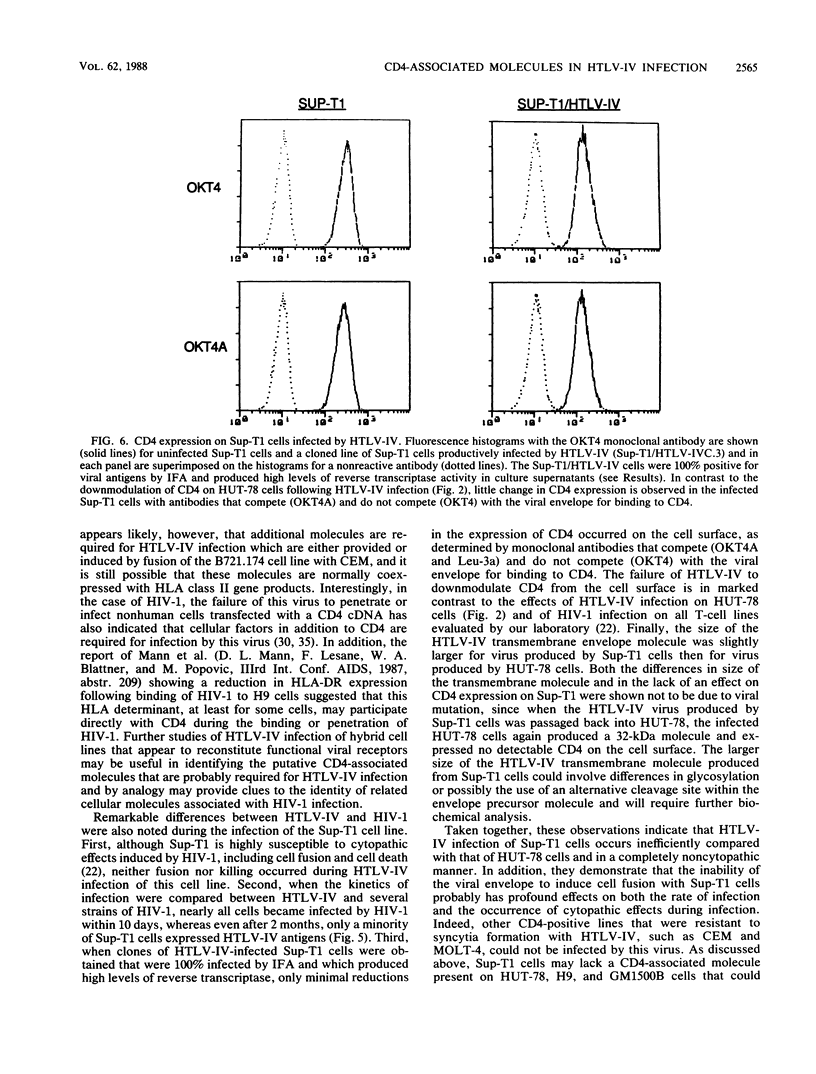
We have analyzed a number of biological features of HTLV-IV, a retrovirus indistinguishable from a macaque isolate of simian immunodeficiency virus (SIV), and compared this virus with several strains of human immunodeficiency virus type 1 (HIV-1). Although HTLV-IV was found to be similar to HIV-1 in its tropism for CD4+ lymphocytes, its effects on CD4 expression and the ability of its externalized envelope molecule to form a complex directly with the CD4 molecule, a number of striking differences were noted. Unlike with HIV-1, the range of cells susceptible to HTLV-IV infection and syncytia formation was restricted to a subset of CD4+ cell lines, particularly those that coexpressed CD4 with human leukocyte antigen (HLA) class II antigens. An analysis of the patterns of HTLV-IV infection with B x T somatic cell hybrids indicated that for this virus, molecules in addition to CD4 were probably required to facilitate infection and cell fusion. Additional studies of HTLV-IV infection of Sup-T1 cells, which are exquisitely sensitive to cytopathic effects induced by HIV-1, demonstrated that HTLV-IV infection could occur in the absence of cytopathic effects and, remarkably, with minimal or no downmodulation of the CD4 molecule from the cell surface. The failure of HTLV-IV infection to reduce the expression of several CD4 epitopes suggested that the HTLV-IV envelope produced by Sup-T1 cells was altered in its ability to interact with or bind to CD4. Additional differences were also noted in the size of the transmembrane envelope molecule of HTLV-IV produced by Sup-T1 cells, indicating that cell-specific alterations in processing of the HTLV-IV envelope occurred during the production of virus in this cell line. Understanding the basis for these biological differences between HTLV-IV and the HIV-1 viruses may help to elucidate more general mechanisms for pathogenesis of other members of the SIV and HIV families of retroviruses.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. S., Coligan J. E., Barin F., McLane M. F., Sodroski J. G., Rosen C. A., Haseltine W. A., Lee T. H., Essex M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985 May 31;228(4703):1091–1094. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- Barin F., M'Boup S., Denis F., Kanki P., Allan J. S., Lee T. H., Essex M. Serological evidence for virus related to simian T-lymphotropic retrovirus III in residents of west Africa. Lancet. 1985 Dec 21;2(8469-70):1387–1389. doi: 10.1016/s0140-6736(85)92556-5. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Arthur L. O., Tsai C. C., Sowder R., Copeland T. D., Henderson L. E., Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986 Nov;60(2):483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham P. R., Weiss R. A., Dalgleish A. G., Exley M., Whitby D., Hogg N. Human immunodeficiency virus infection of monocytic and T-lymphocytic cells: receptor modulation and differentiation induced by phorbol ester. Virology. 1987 May;158(1):44–51. doi: 10.1016/0042-6822(87)90236-4. [DOI] [PubMed] [Google Scholar]
- Clavel F., Guyader M., Guétard D., Sallé M., Montagnier L., Alizon M. Molecular cloning and polymorphism of the human immune deficiency virus type 2. Nature. 1986 Dec 18;324(6098):691–695. doi: 10.1038/324691a0. [DOI] [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- DeFreitas E. C., Dietzschold B., Koprowski H. Human T-lymphocyte response in vitro to synthetic peptides of herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1985 May;82(10):3425–3429. doi: 10.1073/pnas.82.10.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMars R., Chang C. C., Shaw S., Reitnauer P. J., Sondel P. M. Homozygous deletions that simultaneously eliminate expressions of class I and class II antigens of EBV-transformed B-lymphoblastoid cells. I. Reduced proliferative responses of autologous and allogeneic T cells to mutant cells that have decreased expression of class II antigens. Hum Immunol. 1984 Oct;11(2):77–97. doi: 10.1016/0198-8859(84)90047-8. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Swenson R. B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc Natl Acad Sci U S A. 1986 Jul;83(14):5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Gonda M. A., Shaw G. M., Popovic M., Hoxie J. A., Gallo R. C., Wong-Staal F. Genomic diversity of the acquired immune deficiency syndrome virus HTLV-III: different viruses exhibit greatest divergence in their envelope genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4813–4817. doi: 10.1073/pnas.82.14.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Kong L. I., Lee S. W., Kumar P., Taylor M. E., Arya S. K., Shaw G. M. Relation of HTLV-4 to simian and human immunodeficiency-associated viruses. Nature. 1987 Nov 12;330(6144):184–186. doi: 10.1038/330184a0. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Mullins J. I. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell. 1987 May 8;49(3):307–319. doi: 10.1016/0092-8674(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Alpers J. D., Rackowski J. L., Huebner K., Haggarty B. S., Cedarbaum A. J., Reed J. C. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science. 1986 Nov 28;234(4780):1123–1127. doi: 10.1126/science.3095925. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Flaherty L. E., Haggarty B. S., Rackowski J. L. Infection of T4 lymphocytes by HTLV-III does not require expression of the OKT4 epitope. J Immunol. 1986 Jan;136(2):361–363. [PubMed] [Google Scholar]
- Hoxie J. A., Haggarty B. S., Rackowski J. L., Pillsbury N., Levy J. A. Persistent noncytopathic infection of normal human T lymphocytes with AIDS-associated retrovirus. Science. 1985 Sep 27;229(4720):1400–1402. doi: 10.1126/science.2994222. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., Alroy J., Essex M. Isolation of T-lymphotropic retrovirus related to HTLV-III/LAV from wild-caught African green monkeys. Science. 1985 Nov 22;230(4728):951–954. doi: 10.1126/science.2997923. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., Barin F., M'Boup S., Allan J. S., Romet-Lemonne J. L., Marlink R., McLane M. F., Lee T. H., Arbeille B., Denis F. New human T-lymphotropic retrovirus related to simian T-lymphotropic virus type III (STLV-IIIAGM). Science. 1986 Apr 11;232(4747):238–243. doi: 10.1126/science.3006256. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., McLane M. F., King N. W., Jr, Letvin N. L., Hunt R. D., Sehgal P., Daniel M. D., Desrosiers R. C., Essex M. Serologic identification and characterization of a macaque T-lymphotropic retrovirus closely related to HTLV-III. Science. 1985 Jun 7;228(4704):1199–1201. doi: 10.1126/science.3873705. [DOI] [PubMed] [Google Scholar]
- Kannagi M., Yetz J. M., Letvin N. L. In vitro growth characteristics of simian T-lymphotropic virus type III. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7053–7057. doi: 10.1073/pnas.82.20.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Li Y., Naidu Y. M., Butler C. V., Ochs M. F., Jaenel G., King N. W., Daniel M. D., Desrosiers R. C. Comparison of simian immunodeficiency virus isolates. Nature. 1988 Feb 18;331(6157):619–622. doi: 10.1038/331619a0. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kornfeld H., Riedel N., Viglianti G. A., Hirsch V., Mullins J. I. Cloning of HTLV-4 and its relation to simian and human immunodeficiency viruses. Nature. 1987 Apr 9;326(6113):610–613. doi: 10.1038/326610a0. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J., McHugh T., Casavant C., Stites D., Oshiro L. AIDS-associated retroviruses (ARV) can productively infect other cells besides human T helper cells. Virology. 1985 Dec;147(2):441–448. doi: 10.1016/0042-6822(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Reyes G. R., McGrath M. S., Stein B. S., Engleman E. G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986 May 30;232(4754):1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- Lowenstine L. J., Pedersen N. C., Higgins J., Pallis K. C., Uyeda A., Marx P., Lerche N. W., Munn R. J., Gardner M. B. Seroepidemiologic survey of captive Old-World primates for antibodies to human and simian retroviruses, and isolation of a lentivirus from sooty mangabeys (Cercocebus atys). Int J Cancer. 1986 Oct 15;38(4):563–574. doi: 10.1002/ijc.2910380417. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- McClure M. O., Sattentau Q. J., Beverley P. C., Hearn J. P., Fitzgerald A. K., Zuckerman A. J., Weiss R. A. HIV infection of primate lymphocytes and conservation of the CD4 receptor. Nature. 1987 Dec 3;330(6147):487–489. doi: 10.1038/330487a0. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- McDougal J. S., Nicholson J. K., Cross G. D., Cort S. P., Kennedy M. S., Mawle A. C. Binding of the human retrovirus HTLV-III/LAV/ARV/HIV to the CD4 (T4) molecule: conformation dependence, epitope mapping, antibody inhibition, and potential for idiotypic mimicry. J Immunol. 1986 Nov 1;137(9):2937–2944. [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Rangan S. R., Baskin G. B., Gormus B. J., Wolf R. H., Andes W. A., West M., Montelaro R. C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986 May 22;321(6068):435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Howell D. N., Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21(3):235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Dalgleish A. G., Weiss R. A., Beverley P. C. Epitopes of the CD4 antigen and HIV infection. Science. 1986 Nov 28;234(4780):1120–1123. doi: 10.1126/science.2430333. [DOI] [PubMed] [Google Scholar]
- Smith S. D., Shatsky M., Cohen P. S., Warnke R., Link M. P., Glader B. E. Monoclonal antibody and enzymatic profiles of human malignant T-lymphoid cells and derived cell lines. Cancer Res. 1984 Dec;44(12 Pt 1):5657–5660. [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]






