Abstract
Hypothalamic neurons are regarded as essential for integrating thermal afferent information from skin and core and issuing commands to autonomic and behavioral effectors that maintain core temperature (Tc) during cold exposure and for the control of energy expenditure more generally. Caudal brain stem neurons are necessary elements of the hypothalamic effector pathway and also are directly driven by skin and brain cooling. To assess whether caudal brain stem processing of thermal afferent signals is sufficient to drive endemic effectors for thermogenesis, heart rate (HR), Tc, and activity responses of chronic decerebrate (CD) and control rats adapted to 23°C were compared during cold exposure (4, 8, or 12°C) for 6 h. Other CDs and controls were exposed to 4 or 23°C for 2 h, and tissues were processed for norepinephrine turnover (NETO), a neurochemical measure of sympathetic drive. Controls maintained Tc for all temperatures. CDs maintained Tc for the 8 and 12°C exposures, but Tc declined 2°C during the 4°C exposure. Cold exposure elevated HR in CDs and controls alike. Tachycardia magnitude correlated with decreases in environmental temperature for controls, but not CDs. Cold increased NETO in brown adipose tissue, heart, and some white adipose tissue pads in CDs and controls compared with their respective room temperature controls. These data demonstrate that, in neural isolation from the hypothalamus, cold exposure drives caudal brain stem neuronal activity and engages local effectors that trigger sympathetic energetic and cardiac responses that are comparable in many, but not in all, respects to those seen in neurologically intact rats.
Keywords: energy balance, thermoregulation, anterior hypothalamus, heart rate, body temperature, sympathetic drive, norepinephrine turnover, white adipose tissue, brown adipose tissue
there is broad support for localizing the neural control of thermoregulation (see, e.g., Refs. 7, 8, 13, 38, 53, 58) and energy expenditure to the hypothalamus (see, e.g., 20, 47, 57). Specifically, a variety of data support the view that neurons of the medial preoptic area (POa) respond to and integrate thermal afferent signals arising in skin, core, and brain and issue efferent commands to downstream autonomic and behavioral effectors that maintain core temperature (Tc) in response to environmental challenges (8, 13, 38, 47, 58). The dorsomedial hypothalamus (DMH) also is implicated in thermoregulatory control (18, 35). POa and DMH neurons project to the nucleus raphe pallidus (RPa) in the caudal brain stem. RPa neurons are a critical element in the hypothalamic efferent pathway that triggers sympathetic responses, including interscapular brown adipose tissue (IBAT) thermogenesis, increased heart rate (HR) and blood pressure, and tail vasoconstriction (18, 35, 38, 42). RPa-mediated sympathetic responses contribute to the maintenance of Tc when rodents are exposed to cold environments. When RPa neurons are inactivated, cold-induced regulatory responses are blocked (38, 42).
A variety of data suggest that, in addition to their contribution to efferent control, neurons of the caudal brain stem (midbrain and hindbrain) receive skin thermal afferent information. For example, neurons in the midbrain reticular formation respond to the cooling of the skin (40). Similarly, the neurophysiological response of neurons of the medullary raphe (raphe magnus and RPa) are highly sensitive to mild cooling of the skin (46; see also Refs. 32 and 41). Some of these neurons also respond to alterations in Tc (46). Skin cooling-driven neurophysiological responses also can be recorded from the medullary raphe of acutely decerebrated, anesthetized rats (17, 54), indicating that the caudal brain stem responses are not secondary to forebrain processing. Skin cooling also increases the neurophysiological activity of lateral parabrachial neurons (lPBN) (34, 39). Perhaps most interesting are findings that spinal projections from thermally responsive medullary raphe neurons contribute to brown adipose tissue (BAT) activation and other thermoregulatory defenses against environmental cold challenges (46). The thermal sensitivity of these caudal brain stem neurons and their participation in sympathetic outflow to the periphery suggest that they may be part of a “cold-defense” pathway (46).
Although the anterior hypothalamus is commonly described as the thermoregulatory center, there is precedent in the literature for the idea that neurons within more caudal levels of the neuraxis also play an integrative role in thermoregulatory control. These “caudal neurons” are activated by thermal afferents and appear to engage circuits that trigger sympathetic and behavioral responses, thereby participating in some degree of feedforward or feedback regulation of Tc. This view, that the thermoregulatory control system is neurally distributed with contributions arising from both hypothalamic and caudal, extrahypothalamic sites, is emphasized in seminal papers by Lipton (31), Chambers et al. (14), and Amini-Sereshki (1, 2) and in a synthetic review by Satinoff (50) published in the 1970s but has apparently disappeared from discussions of thermoregulatory control. Among the relevant findings, cooling the skin does not elicit shivering in spinal animals. However, with a transection higher than the medulla and lower pons, the shivering response to cooling of the extremities is restored. This suggests a role for the caudal brain stem in thermoregulatory control. Perhaps the lack of continued discussion of the findings from these reports is due to their conclusion that, when compared with the thermoregulatory competence achieved by hypothalamic circuits in the intact brain, the degree of regulatory control achieved by more caudal levels of the neuraxis is “incomplete” and “fragmentary.” It was asserted, for example, that the initiation of thermoregulatory responses in these transected neurological preparations requires more extreme thermal challenges than in intact preparations (50). These hypotheses are evaluated here through studies that examine the thermoregulatory competence of cold-exposed, freely moving, chronically maintained decerebrate rats (i.e., complete severing of the forebrain from the caudal brain stem at the mesodiencephalic junction). This was accomplished by measuring Tc, sympathetic (HR), and behavioral responses (locomotor activity) in awake, chronic supracollicular decerebrate rats and identically maintained neurologically intact control rats exposed to 4°C and to intermediate cold environmental temperatures (8 and 12°C). In addition, norepinephrine turnover (NETO), a neurochemical measure of sympathetic drive, was measured in the heart, IBAT, and several white adipose tissue (WAT) depots. In these experiments, we tested the hypothesis that, in the absence of hypothalamic neural processing and hypothalamic-caudal brain stem communication, caudal brain stem neurons responsive to thermal afferent signals will engage local neurons that activate sympathetic outflows and trigger energetic responses that result in a level of energetic and thermoregulatory control that is comparable in many, but perhaps not all, respects to that seen in neurologically intact controls.
METHODS
Maintenance and surgery.
Male Sprague-Dawley rats (Charles River Laboratories) weighing 275–325 g on arrival were housed individually in plastic bins in a room maintained at 23 ± 2°C and a 12:12-h light-dark cycle (lights on 8:00 a.m. to 8:00 p.m.). All animals were maintained in accordance with the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee. Pelleted food (Purina Rodent Chow 5001) and water were available ad libitum initially. All rats were then habituated to four, daily 9-ml gavage feedings of powered rodent diet suspended in water (L1001; Research Diets, New Brunswick, NJ). This feeding regime provided rats with 79 kcal/day and ample hydration; chow and water were no longer available. All procedures used were approved by the University of Pennsylvania animal care and use committee.
Control sham surgery or unilateral brain transection at the supracollicular level was performed with a blunt L-shaped surgical spatula (22) under ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) anesthesia and Meloxicam analgesia (3.0 mg/kg). After hemitransection (1 wk), full decerebration was completed, and control rats were anesthetized and subject to a second sham surgery. At the time of the second hemitransection, rats in the telemetry experiment were implanted with an ER-4000 transponder (Mini-Mitter, Bend, OR). The transponder core was positioned in the peritoneal cavity for activity and Tc measurement. The two attached wire leads were positioned subcutaneously in the upper right and lower left quadrants of the rat's chest and secured for HR measurement. A minimum of 2 wk was allowed for recovery before the start of thermal exposure experiments.
Procedure for transponder experiments.
Naive chronic decerebrate (CD; n = 10) and pair-fed intact (n = 5) rats were exposed to three thermal conditions of 4, 8, or 12°C in a counterbalanced order; in each case, rats also were evaluated under room temperature (23°C) conditions for a period preceding cold exposure. Four days intervened between each of the three test conditions. On the day of each condition, rats received two gavage feedings (at 1.5 and 4 h after lights-on) and were then transferred to an inactive chromatography refrigerator with open doors in a room maintained at 23°C. Rats were placed individually in plastic cages positioned on receivers on the shelves of the refrigerator unit. No bedding was provided to preclude nesting. For all conditions, rats remained in this environment for 2.5 h (at 23°C); this time is referred to as the precold baseline period. Two hours into the precold baseline period, rats were gavage fed a third meal. The 6-h period of cold thermal exposure commenced 30 min later. The doors were closed, and the refrigerator was activated to achieve one of the three targeted (4, 8, or 12°C) temperatures. Target ambient temperatures were achieved within 20 min. Following the 6-h exposure period, the doors were opened, and the refrigerator was shut off; temperature in the unit returned to 23°C within 20 min. Recording continued in the postcold period. During the entire period, rats were positioned in the refrigerator unit, and HR, Tc, and activity were measured telemetrically every 30 s, 5 min, and 5 min, respectively. Experimenters entered the room periodically to monitor posture, position, and behavior of the rats.
Following completion of all experimental conditions, CD rats were anesthetized with ketamine, xylazine, and acepromazine (as above) and then perfused transcardially with heparinized saline followed by 10% formalin. Brains were removed from the crania, washed in saline, and then submerged in 10% formalin. Brains were cyroprotected in a 20% sucrose solution before being embedded in albumin gelatin and cut with a freezing microtome in 50-μm sagittal sections. Sections stained with cresyl violet were examined to verify completeness of the brain transection.
Procedure for NETO experiments.
Additional naive CD (n = 18) and pair-fed intact (n = 18) rats were used for NETO measurements using the α-methyl-p-tyrosine (α-MPT) method. α-MPT is a competitive inhibitor of tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis. This allows the estimation of NETO by the rate of norepinephrine (NE) disappearance from the sympathetic nerve terminals in the tissues of interest. The α-MPT methyl ester hydrochloride (Sigma Aldrich, St. Louis, MO) was prepared by first adding ∼0.5 ml of glacial acetic acid (1 μl/mg α-MPT) and then diluting to the final concentration with 0.15 M NaCl. Basal levels of NETO were determined for one-third of the animals in the control and CD groups (n = 6 each) that were untreated and killed (time 0), and tissues were harvested for subsequent NE content measures. The remaining rats (n = 12/group) were injected intraperitoneally with α-MPT (250 mg α-MPT/kg; 25 mg/ml) between 0800 and 1000. One-half of these rats was immediately placed in the cold (4°C) unit, and the other one-half was placed in a room temperature (23°C) thermal environment. Later (2 h), rats were decapitated. Inguinal WAT (IWAT), retroperitoneal WAT (RWAT), epididymal WAT (EWAT), as well as IBAT and heart were rapidly removed, weighed, frozen in liquid nitrogen, and then stored at −80°C until assayed for NE content to determine NETO as described below. NETO was calculated on a per pad basis rather than per gram or per milligram protein because total NETO per pad reflects the physiological impact of the cold stimulus on the sympathetic drive to the tissue. This is the procedure and data expression we have used previously (9, 51, 60). The decline in NE content is linear using this procedure (9), a requirement for accurate NETO estimation (16).
HPLC analysis of NE tissue content.
NE tissue content was measured using reverse-phase with electrochemical detection following our modification (60) of the method of Mefford (33). Briefly, tissue was thawed and homogenized in a solution containing dihydroxybenzylamine (DHBA, internal standard) in 0.2 M perchloric acid with 1 mg/ml ascorbic acid (PCA/AA). The amount of tissue processed and DHBA added was varied to obtain NE values within the range of the standards (∼25–250 mg WAT were used with 10 ng of DHBA added; ∼200 mg of IBAT was used with 50 ng of DHBA added). Following centrifugation for 10 min at 7,500 g, catecholamines were extracted from the homogenate with alumina and eluted in PCA/AA. Catecholamines were assayed using an ESA (Bedford, MA) HPLC system with electrochemical detection (Cuolochem II). The mobile phase was Cat-A-Phase II, and the column was a C18 reverse-phase column. NE turnover was calculated in IWAT, RWAT, EWAT, IBAT, and heart by subtracting the NE content (ng NE/tissue) of the baseline NETO group (time 0) from the 2-h thermal exposure group according to the method of Brodie et al. (11). Briefly, calculations were made according to the following formulas: k = (log[NE]0 − log[NE]4)/(0.434 × 4) and K = k × [NE]0, where k is the rate constant of NE efflux, [NE]0 is the initial NE concentration, [NE]4 is the final NE concentration, and K = NETO.
Data analysis.
Statistical analysis of Tc, HR, and activity data was performed using the Statistica software package (Tulsa, OK). Data, presented as means ± SE, were analyzed using two-way ANOVA, with neurological condition and thermal condition as the main variables. Comparisons between treatment means were analyzed by Tukey's honest significant difference test. In addition, variation within each thermal condition was analyzed by two-way ANOVAs and Tukey's tests to assess differences between the 6-h thermal period and 2.5-h room temperature baseline period, with neurological preparation and experimental thermal condition as main variables. In addition, within-group comparisons were made between the three, 2-h segments of the 6-h cold exposure period and for the initial 2-h pre- and the immediate 2-h postcold exposure periods via one-way ANOVAs. Comparisons between treatment means were analyzed by Tukey's honest significant difference test. For analysis of NETO data, a two-way ANOVA with neurological condition and time of death postinjection as the main variables was used. Duncan's New Multiple Range post hoc tests were conducted when appropriate for pairwise comparisons (NCSS version 2000 software, Kaysville, UT). Differences between the means for all tests were considered statistically significant if P < 0.05. The exact probabilities and other test values are omitted for simplicity and clarity of the presentation of the results.
RESULTS
Histology.
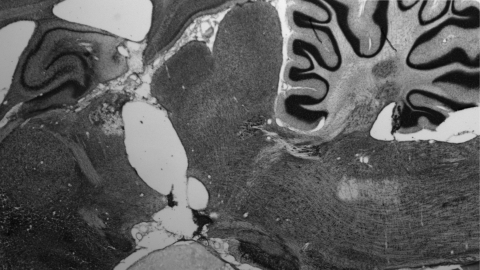
Consecutive cell-stained, 50-μm sagittal sections from the brains of decerebrated rats were carefully analyzed at various medial-lateral levels to determine the completeness of the intended supracollicular transection. The brains of each of the decerebrate rats included in the analysis were found to be completely transected in the supracollicular plane. Figure 1 shows the transection plane in a representative decerebrate rat.
Fig. 1.
Representative sagittal section from the brain of a chronic supracollicular decerebrate rat.
Tc.
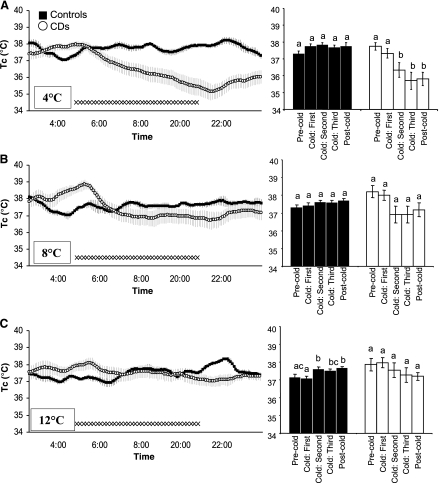
Pair-fed intact control rats maintained their 37.1 ± 0.27°C baseline Tc (observed during the 2.5-h baseline period of room temperature exposure that preceded the cold exposure) throughout the 6-h exposure to 4°C. By contrast, Tc of CD rats decreased on average 1.97 ± 0.40°C compared with their precold exposure baseline temperatures by the end of the 4°C cold exposure (Fig. 2A, left). Analysis of the effects of 4°C exposure as a function of time (comparisons between: the initial 2-h precold baseline; the three 2-h segments of the 6-h cold exposure period; and the immediate 2-h postcold exposure period) revealed that significant decreases in the Tc of CD rats were first observed after 2 h of exposure, in the 2- to 4-h interval. There was no further statistical decrease in Tc from baseline during the interval between 4 and 6 h into cold exposure (Fig. 2A, right).
Fig. 2.
Left: successive 5-min, group average core temperature (Tc) values of chronic decerebrate (CD; ○) and pair-fed intact (▪) rats during the 2.5-h precold exposure room temperature baseline period, the 6-h cold exposure, and the postcold period. SEs are denoted with light gray vertical lines. Right: group average temperature for 2-h intervals (one interval in the precold exposure room temperature baseline period, three successive intervals during cold exposure period, and one postcold return to room temperature interval). A: 4°C condition; B: 8°C condition; C: 12°C condition. Different lowercase letters above the histograms on right indicate instances of statistically significant differences (e.g., b denotes significant differences from a). Common letters indicate no significant difference (e.g., bc is not different from b but differs from a).
In response to 8°C cold exposure, the Tc of CD and control rats did not differ significantly from that observed during their respective room temperature baseline period that preceded cold temperature onset (Fig. 2B, left and right). Similarly, in response to the 12°C exposure, Tc of CD rats did not differ significantly from the Tc observed during the baseline period (Fig. 2C, left and right). Tc of 12°C exposed control rats was comparable to that for the room temperature baseline period except for the 2- to 4-h period of cold exposure and postcold period where Tc was slightly elevated significantly.
HR.
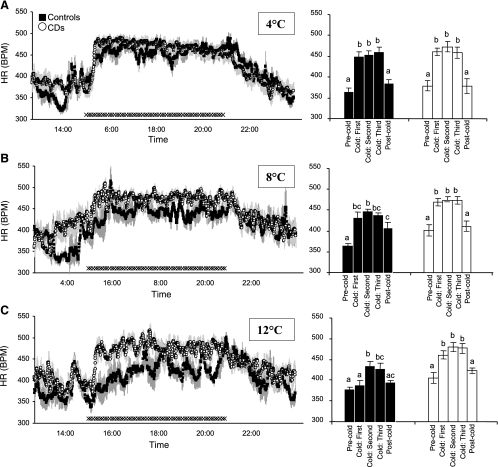
Regardless of neurological condition, the HR of all rats increased by an average of 87 ± 6 beats/min in response to the 4°C cold exposure (Fig. 3A, left and right) when compared with the HR observed during the room temperature baseline period (374 ± 8 beats/min; P < 0.05). Similar to results with the 4°C exposure, all rats showed an increase in HR in response to the 8°C compared with the room temperature baseline exposure (P < 0.05); CD and control rats did not differ in their HR response to the 8°C cold exposure condition (Fig. 3B, left and right); HR was elevated in both surgical groups in response to 12°C exposure (Fig. 3C; P < 0.05). The HR of CD rats rose to 471 ± 8 beats/min compared with their room temperature baseline HR of 406 ± 14 beats/min (P < 0.05). Neurologically intact pair-fed control rats elevated HR (415 ± 12 beats/min) compared with their HR in the room temperature baseline period (376 ± 8 beats/min), but the magnitude of the tachycardia for controls was significantly lower than that observed for CD rats (P < 0.05), and the latency for the elevated HR was longer than that observed for CD rats, appearing 2 h after cold onset (Fig. 3C, right).
Fig. 3.
Left: successive 30-s, group average heart rate (HR, beats/min) values of CD (○) and pair-fed intact (▪) rats during the 2.5-h precold exposure room temperature baseline period, the 6-h cold exposure, and the postcold period. SEs are denoted with light gray vertical lines. Right: group average HR for 2-h intervals (one interval in the precold exposure room temperature baseline period, three successive intervals during cold exposure period, and one postcold return to room temperature interval). A: 4°C condition; B: 8°C condition; C: 12°C condition. Different lowercase letters above the histograms on right indicate instances of statistically significant differences (b denotes significant differences). Common letters indicate no significant difference.
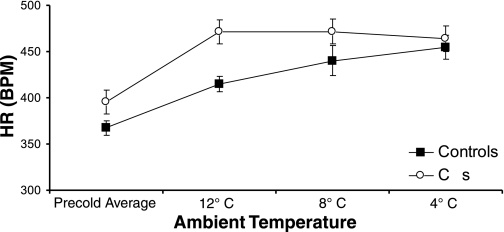
The 6-h HR averages for the three cold temperature conditions and average temperatures for the precold baseline period shown in Fig. 4 reveal that, for intact pair-fed rats, the elevation in HR during cold exposure was correlated with the reduction in ambient temperature (r2 = 0.994, P < 0.003). As discussed above, decerebrate rats also elevated their HR in response to cold exposure. Unlike the pattern observed in controls, however, the HR elevation seen in CDs was not correlated with decreasing temperature (r2 = 0.790, not significant). Rather, for CD rats, the increase in HR seen for the lowest cold temperature (4°C) was equivalent in magnitude to that of the intermediate cold temperatures (8 and 12°C).
Fig. 4.
HR averages for cold conditions and precold room temperature baseline periods show that, for intact pair-fed rats, the HR elevation was indirectly correlated with the reduction in ambient temperature relative to 23°C room temperature (r2 = 0.994, P < 0.003). Unlike controls, however, the HR elevation seen in CDs was not graded as a function of decreasing temperature (r2 = 0.790, not significant).
Spontaneous activity.
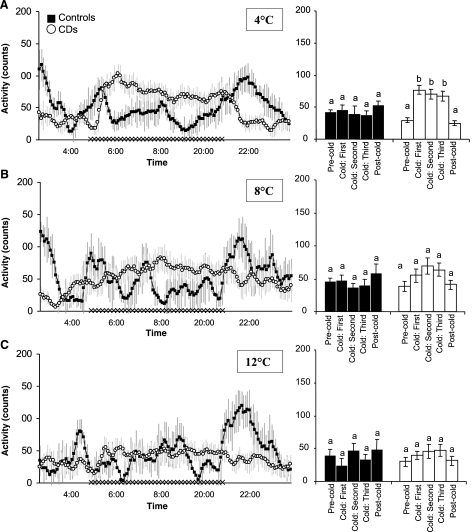
The transition from room temperature to the 4°C thermal environment was marked by increases in spontaneous activity counts for CD rats (Fig. 5A, left and right). Average activity counts increased from 30.1 ± 6.1 in the room temperature control condition to 65.0 ± 8.2 counts/15 min for 4°C (P < 0.05). By contrast, cold exposure did not affect activity counts of control rats; average activity counts in the room temperature baseline period of 35.3 ± 5.0 counts/15 min were unchanged by 4°C exposure (Fig. 5A). Neither group of rats altered their activity level when exposed to 8 or 12°C (Fig. 5, B and C).
Fig. 5.
Left: successive 5-min, group average activity counts of CD (○) and pair-fed intact (▪) rats during the 2.5-h precold exposure room temperature baseline period, the 6-h cold exposure, and the postcold period. SEs are denoted with light gray vertical lines. Right: group average activity counts for 2-h intervals (one interval in the precold exposure room temperature baseline period, three successive intervals during cold exposure period, and one postcold return to room temperature interval). A: 4°C condition; B: 8°C condition; C: 12°C condition. Different lowercase letters above the histograms on right indicate instances of statistically significant differences; common letters indicate no significant difference.
Four degree cold exposure NETO and tissue masses.
Decerebration triggered significant increases in the mass of IWAT, RWAT, and IBAT, but not the heart, compared with the sham-operated controls (P < 0.05; Table 1), indicating an overall increase in total body fat triggered by supracollicular transection, as we have reported previously (24). Heart NETO, often indicative of general sympathetic activity (e.g., Ref. 59), was significantly increased by cold exposure in both neurological groups to the same extent (P < 0.05; Table 2). Cold exposure significantly increased IBAT NETO in control rats as expected, and notably in CDs as well (P < 0.05; Table 2); indeed, CD IBAT NETO was increased approximately fourfold more than in their respective neurologically intact controls. There also were differential increases in NETO across WAT pads (Table 2). Specifically, cold exposure did not increase NETO in all WAT pads but did increase IWAT NETO in cold vs. warm CDs (P < 0.05), with a suggestive CD cold vs. warm increase in RWAT. Decerebration itself increased IWAT and EWAT NETO in the warm environment compared with their respective controls (Ps < 0.05). Mathematically, there were some NETO values that were negative (Table 2); although this is a physiological impossibility, it can occur when basal levels of NETO are unusually low as they are in WAT and nonstimulated BAT. Conservatively, therefore, the calculated negative values were considered to represent low-to-absent physiologically significant sympathetic drives.
Table 1.
Mean Tissue Mass (g) ±SE
| Tissue | T = 0 | Control Room Temp | Cold | T = 0 | CD Room Temp | Cold |
|---|---|---|---|---|---|---|
| IWAT | 3.01±0.39 | 3.57±0.65 | 3.46±0.66 | 4.77a±0.62 | 5.55a±0.51 | 4.77a±0.60 |
| EWAT | 3.36±0.52 | 3.41±0.23 | 3.45±0.37 | 3.97±0.31 | 4.04±0.37 | 3.88±0.42 |
| RWAT | 3.04±0.43 | 3.18±0.26 | 3.13±0.35 | 3.63a±0.29 | 3.92a±0.34 | 3.92a±0.34 |
| IBAT | 0.20±0.03 | 0.28±0.03 | 0.23±0.03 | 0.35a±0.07 | 0.30a±0.30 | 0.36a±0.04 |
| Heart | 1.10±0.05 | 1.17±0.05 | 1.22±0.12 | 1.04±0.03 | 1.13±0.07 | 1.10±0.07 |
=P < 0.05 versus same tissue/temperature condition in control rats.
Table 2.
Mean Total Tissue NETO ±SE
| Tissue | Control Room Temp | Cold | CD Room Temp | Cold |
|---|---|---|---|---|
| IWAT | −8.10±1.58 | −3.61±0.64 | 12.27b±1.99 | 27.02a,c,d±4.39 |
| EWAT | −0.25±0.0.07 | 0.33±0.09 | 5.81b,c±1.89 | 6.71b,c±2.19 |
| RWAT | 4.58±0.89 | 3.54±0.69 | 3.85±0.77 | 5.76±1.15 |
| IBAT | −15.36±7.35 | 9.29a±1.95 | −1.14±0.22 | 35.19a,b,c,d±6.99 |
| Heart | 35.75±5.41 | 68.66a±10.40 | 46.43±5.06 | 63.32a±6.9 |
=P < 0.05 versus room temp. of same surgery type.
=P < 0.05 versus Control room temp.
=P < 0.05 versus Control Cold.
=P < 0.05 versus CD room temp.
DISCUSSION
To test the competence of the control of energy expenditure by the caudal brain stem as well as its control of sympathetic efferent activity, Tc, HR and behavioral responses were recorded from awake, freely moving CD and pair-fed neurologically intact control rats following exposure to three different levels of cold ambient temperatures for 6-h periods. Both CD and pair-fed intact rats maintained Tc during the exposures to 8 and 12°C. Increases in HR rapidly followed cold onset in both groups. These and the NETO data suggest that the contributors to heat production and the stability of Tc by CD rats included increased sympathetic drives to heart and IBAT and elevated HR, likely resulting in increased IBAT blood flow that would facilitate the distribution of heat resulting from IBAT thermogenesis. Collectively, the present data reveal a range of energetic response competencies and local caudal brain stem control of sympathetic outflows in chronically maintained decerebrate rats not anticipated by previous studies (e.g., Refs. 7, 38, and 45). Furthermore, the range of response similarities, including Tc defense and increased HR, observed between CD and intact rats at intermediate temperatures of 8 and 12°C is not consistent with the notion that extreme temperatures are required to drive a set of intact-like thermoregulatory responses in the absence of hypothalamic/forebrain-to-caudal brain stem neurological connection (50).
Although a wide range of similarities in the compensatory responses of CD and control rats was observed, some significant differences are noteworthy. CD rats did not maintain Tc over the 6-h exposure to 4°C, the most extreme cold environment. In spite of increased HR and locomotor activity, and from the extrapolated findings of the NETO experiment suggesting increased IBAT thermogenesis, CD rats lost ∼2°C in Tc during the 4°C exposure. This result is consistent with the view that the thermoregulatory competence of the neurally isolated caudal brain stem is, to some extent, incomplete (50). Another difference between intact and CD rats that suggests a contribution from hypothalamic/forebrain processing to the energetic response and thermoregulatory control of intact rats was in the precision of HR adjustments in response to changes in skin thermal input with increasing ambient cold temperature. The HR of control rats was highly correlated with cold temperature magnitude, with the greatest increase of HR observed at 4°C (see Fig. 4). By contrast, the elevation of CD HR was not incremental over the 4 to 12°C range; rather, the HR elevation for 12°C was as large as that observed with the 4°C exposure.
In considering which aspects of thermoregulatory control are not recruited in CD rats potentially contributing to the loss of Tc in the 4°C condition, it is useful to explore the contribution of thermogenesis as well as that of heat loss (43). The thermogenic response of intact controls includes shivering and nonshivering processes (12). From our measures here and other reports, it appears that IBAT NETO (nonshivering thermogenesis) in CD rats is similar in both magnitude and latency to that of controls exposed to 4°C. CDs also increase nonshivering thermogenesis in a manner comparable to controls in response to fourth ventricular injections of the melanocortin 3/4 receptor agonist melanotan II, at least in terms of uncoupling protein-1 mRNA, a relatively reliable indicator of IBAT thermogenesis. Similarly, the latency and magnitude of the tachycardia induced by 4°C exposures in CD rats were indistinguishable from that of controls. By contrast, differences in shivering thermogenesis may contribute to the observed differences in Tc maintenance in 4°C exposed CD rats. This assertion is based on the marked reduction in lean body mass (∼15–20%) of the CDs shown in our previous studies (23, 24), but not assessed here. Rats were periodically observed while in the cold environment for the occurrence of shivering; however, visual assessment of shivering proved unreliable; its measurement is best quantified using electromyographic techniques, and this approach will be applied in future studies. Another potential contributor to the loss of Tc during 4°C exposures in CD rats is the effectiveness of mechanisms designed to limit heat loss. In rats, tail vasoconstriction (not measured in the current experiments) is important to the maintenance of Tc during cold exposure, and, importantly, the neurochemical control of IBAT thermogenesis and that for tail vasoconstriction is separable (43).
At room temperature, the CD rats had increased NETO in both IWAT and EWAT, but not RWAT, compared with similarly exposed control rats. At first blush, this result seems inconsistent with the elevated WAT pad masses and of total body fat in the CDs in the present experiment and our recent studies (24). Given that increased sympathetic drive to WAT is the principal initiator of lipid mobilization (for reviews, see Refs. 3 and 4), such increases in WAT NETO might be presumed to trigger increased lipolysis and consequent decreases in WAT pad mass (decreased adiposity), as we have seen previously in Siberian hamsters exposed to short “winter-like” photoperiods (60). One possible reason for the lack of this relation between WAT NETO and WAT pad mass in the present experiment is that, even with increases in sympathetic drive, if this is accompanied by increases in the rate of reesterification of released free fatty acids back into triacylglycerol, as is seen in obese compared with lean humans (29, 30), then there will not be a decrease in fat pad mass. Alternatively, there may be an insensitivity to the released NE by the white adipocytes of the CDs such that lipolysis is not triggered. Two in vivo studies in obese vs. lean humans suggest decreased sensitivity to β-adrenoceptor stimulation (27) and to electrical stimulation of sympathetic nerves innervating WAT (19). The specific mechanisms underlying such insensitivity are unclear, although across several genetic- and diet-induced models, the number of white adipocyte β-adrenoceptors is decreased in the obese vs. their lean counterparts (15), although changes in receptor affinity, second messenger cascades, and key intracellular lipolytic factors such as hormone-sensitive lipase and perilipin A also could be defective. Finally, NETO was not increased in CD RWAT, IBAT, or heart at room temperature, showing that rats with an isolated caudal brain stem are capable of differential trafficking of sympathetic outflow, as seen in neurally intact animals (for reviews, see Refs. 5 and 36).
In the present experiment, we did not observe a cold-induced increase in WAT NETO in the pair-fed sham-operated controls. This is inconsistent with another report where acute cold exposure to similar temperatures increased rat WAT NETO (21). This lack of response by control animals here may be due to the increase in adiposity resulting from the tube-feeding maintenance. That is, tube feeding is well established as a means of increasing efficiency of energy utilization (e.g., Ref. 25), likely through diminished thermogenesis (49), thereby promoting increases in adiposity. Although we did not have ad libitum-fed control rats in the present experiment, in another experiment where the tube feeding was identical to that in the present experiment and ad libitum-fed rats also were included, the tube-fed controls had significantly increased carcass lipid and fat pad masses compared with the ad libitum-fed controls. Thus, with both an increase in lipid fuels in their expanded WAT depots and increased insulation provided by the subcutaneous WAT pads (e.g., Ref. 48), the acute cold exposure here may not have provided enough of an energetic challenge to trigger the typical cold-induced increase in sympathetic drive to WAT. Despite the lack of a significant cold-induced increase in NETO across the WAT pads of the controls, acute cold exposure doubled CD IWAT NETO compared with their room temperature CD counterparts. One possible reason for the increased IWAT NETO of the CDs may be a greater need for lipid to fuel cold-induced increased thermogenesis, thereby helping maintain their Tc, which the CDs accomplished comparably to the neurally intact control rats during the first 2 h of cold expose. Indirect support of this notion was the marked increase in IBAT NETO by the cold-exposed CDs (∼4-fold that of cold-exposed controls). What is somewhat unclear is why there was such a marked increase in IWAT NETO compared with other WAT pads, since this would result in increased lipolysis from an adipose depot that may have a role in thermal insulation (e.g., see Ref. 48). What is clear, however, is that, unlike other WAT depots, IWAT NETO uniformly increases in response to a wide variety of lipid-promoting stimuli in Siberian hamsters [e.g., cold, food deprivation, cold + food deprivation, glucoprivation (10)], suggesting an important role as a provider of lipid to energetic challenges such as cold here.
A report by Nakamura and Morrison (38) considers the relation between three neural elements in the control of cold-induced IBAT thermogenesis and tachycardia. They show that these responses are significantly diminished by local injections of γ-aminobutyric acid (GABA)-a agonists in either the DMH or RPa or antagonist injections in the POa. The authors describe a serial relation among these three neural elements; POa projects to DMH that in turn projects to RPa. Perhaps more relevant to the discussion of our current data is how their model represents the projection path of central skin thermal afferent processing to the central effector path from RPa. In their model, skin thermal afferent information is viewed as directly affecting only the most rostral element in this serial pathway, the POa. A recent report from the same authors (39) adds to their model by showing that lPBN neurons are responsive to skin cooling and that these neurons project to POA. Collectively, the current data suggest and previous neurophysiological data in intact and also in decerebrated rats show that RPa neurons themselves are activated by cold exposure (17, 32, 46, 54), thereby suggesting a revision of that aspect of their model. We hypothesize that the potent effects of cold exposure on HR, IBAT, and heart NETO in CD rats, as well as the defense of Tc by CD rats at 8 and 12°C exposures, are the result of skin afferent processing in RPa neurons (not directly measured in our studies) that drive sympathetic outflows in the absence of descending neural projections from POa or from DMH, as well as in the absence of ascending projections from lPBN to POA. As noted earlier, the data of Rathner and colleagues (46) provide direct support for a cold skin thermal input that affects the neurophysiological activity of neurons in the caudal raphe at the level of the caudal part of the facial nucleus that, in turn, engage sympathetic outflows to IBAT and heart via raphe-spinal projections. Furthermore, these authors cite the data of Dickenson (17) and Taylor (54) who show that skin cooling also activates medullary raphe neurons in rats whose caudal brain stem was isolated from interconnection with forebrain elements via midcollicular level acute transection. Recent data of Osaka (45) showing that skin cooling of anesthetized acutely decerebrated rats (level of transaction not indicated) does not trigger increased energy expenditure are inconsistent with those of the aforementioned neurophysiological studies and with the data presented here. Overall, these neurophysiological data from anesthetized intact and decerebrated rats and our results from awake, behaving decerebrate rats make the case for a caudal brain stem control circuit linking the processing of skin cooling afferent input with sympathetic outflow and thermogenic response production.
Additional work will be required to determine which elements of caudal brain stem processing contribute to the control of sympathetic output and the thermoregulatory competence observed here. The potential contribution of RPa neurons here is, of course, of great relevance, and lPBN neurons provide another important focus. Electrical stimulation of lPBN increases oxygen consumption, IBAT temperature, and HR (28). Cold-exposed rats with bilateral lPBN lesions loose Tc, and their oxygen consumption is elevated to a lesser extent than that of sham lesion rats or rats with lesions outside lPBN. Nakamura and Morrison (39) provide evidence of lPBN projection to POa, but these neurons also are known to provide afferent input to RPa and raphe magnus (26). It remains to be determined whether projections from lPBN to RPa play a functional role in the responses observed in CD rats and more generally in the thermoregulatory response of intact rats. As noted earlier, RPa neuronal output is modulated by GABAa agonists (38). RPa neurons also express melanocortin 4 receptors (MC4R), and these MC4R-expressing neurons are labeled after virus injection in IBAT (56). We have recently shown that doses of MC4R agonist that are without affect when applied to the brain ventricles increased Tc, HR, and IBAT temperature when delivered directly to the RPa of intact and CD rats (52). Functional contribution to the effects observed here also may involve the participation of other caudal brain stem neurons previously implicated in response to skin cooling or thermal control such as those in periaqueductal gray, peritrigeminal nucleus, inferior olive, and nucleus tractus solitarius (e.g., Ref. 55).
Perspectives and Significance
Other results (24) support and complement the energy regulatory competence of the isolated caudal brain stem revealed here with cold exposure. Specifically, chronic supracollicular decerebrate rats subjected to a 48-h period of food deprivation displayed essentially the same energetic (reduced expenditure) and hormonal responses as that of intact control rats to compensate for the reduced energy intake. Taken together with the current results, support is provided for the perspective that inputs of relevance to energetic regulation, such as those from skin thermal afferents and blood-born correlates of energy status, are processed by caudal brain stem neurons that in turn engage local effector circuits whose activity results in adjustments in sympathetically mediated energetic responses. Some differences in the response of CD and intact control rats were found in the current study and in one of our previous reports (24), but the overall degree of intact-like responses observed in the CD rats in these studies suggest that integrations performed in, and efferent control issued from, the caudal brain stem mimic to a large degree those observed in intact rats and generally attributed to hypothalamic processing. The results appear to demonstrate that a remarkable, although not exactly normal, ability to maintain body temperature and energy balance is apparent in CD rats in which higher brain centers that have been of considerable interest of late are disconnected from the caudal brain stem thermoregulatory mechanisms. Collectively, this supports the hypothesis that energy expenditure control is distributed across the neuraxis rather than localized to a single structure or level of the brain, the more commonly expressed perspective (see also Refs. 6, 37, 42, and 44).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants SCRO DK-21397 (awarded to H. J. Grill, T. J. Bartness, and R. B. Harris), R01 DK-53903 (awarded to R. B. Harris), R01 DK-35254 (awarded to T. J. Bartness) and R01 DK-21397 (awarded to H. J. Grill).
Acknowledgments
We thank Dr. Matt Hayes for helpful editorial input and for fruitful discussions about these studies. We thank Lisa Maeng, Karolina Skibicka, and Raven Jackson for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Amini-Sereshki L Brainstem control of shivering in the cat. I. Inhibition. Am J Physiol Regul Integr Comp Physiol 232: R190–R197, 1977. [DOI] [PubMed] [Google Scholar]
- 2.Amini-Sereshki L Brainstem control of shivering in the cat. II Facilitation Am J Physiol Regul Integr Comp Physiol 232: R198–R202, 1977. [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol Regul Integr Comp Physiol 275: R1399–R1411, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bartness TJ, Kay Song C, Shi H, Bowers RR, Foster MT. Brain-adipose tissue cross talk. Proc Nutr Soc 64: 53–64, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bartness TJ, Song CK. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res 48: 1655–1672, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Blessing WW BAT control shows the way: medullary raphe/parapyramidal neurons and sympathetic regulation of brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 288: R557–R560, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Boulant JA Hypothalamic mechanisms in thermoregulation. Fed Proc 40: 2843–2850, 1981. [PubMed] [Google Scholar]
- 8.Bratincsak A, Palkovits M. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience 127: 385–397, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 148: 5339–5347, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure, or glucoprivation. Am J Physiol Regul Integr Comp Physiol 294: R1445–R1452, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Brodie BB, Costa E, Dlabac A, Neff NH, Smookler HH. Application of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J Pharmacol Exp Ther 154: 493–498, 1966. [PubMed] [Google Scholar]
- 12.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460: 303–326, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Chambers WW, Seigel MS, Liu JC, Liu CN. Thermoregulatory responses of decerebrate and spinal cats. Exp Neurol 42: 282–299, 1974. [DOI] [PubMed] [Google Scholar]
- 15.Collins S, Daniel KW, Rohlfs EM. Depressed expression of adipocyte beta-adrenergic receptors is a common feature of congenital and diet-induced obesity in rodents. Int J Obes Relat Metab Disord 23: 669–677, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Cooper JR, Bloom SR, Roth RH. The Biochemical Basis of Neuropharmacology. New York, NY: Oxford Univ Press, 1982.
- 17.Dickenson AH Specific responses of rat raphe neurones to skin temperature. J Physiol 273: 277–293, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol 292: R47–R63, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Dodt C, Lonnroth P, Fehm HL, Elam M. The subcutaneous lipolytic response to regional neural stimulation is reduced in obese women. Diabetes 49: 1875–1879, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Elmquist JK Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol Behav 74: 703–708, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Garofalo MA, Kettelhut IC, Roselino JE, Migliorini RH. Effect of acute cold exposure on norepinephrine turnover rates in rat white adipose tissue. J Auton Nerv Syst 60: 206–208, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res 143: 281–297, 1978. [DOI] [PubMed] [Google Scholar]
- 23.Harris RB, Bartness TJ, Grill HJ. Leptin responsiveness in chronically decerebrate rats. Endocrinology 148: 4623–4633, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Harris RB, Kelso EW, Flatt WP, Bartness TJ, Grill HJ. Energy expenditure and body composition of chronically maintained decerebrate rats in the fed and fasted condition. Endocrinology 147: 1365–1376, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Harris RB, Martin RJ. Changes in lipogenesis and lipolysis associated with recovery from reversible obesity in mature female rats. Proc Soc Exp Biol Med 191: 82–89, 1989. [DOI] [PubMed] [Google Scholar]
- 26.Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b). J Chem Neuroanat 13: 1–21, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Jocken JW, Goossens GH, van Hees AM, Frayn KN, van Baak M, Stegen J, Pakbiers MT, Saris WH, Blaak EE. Effect of beta-adrenergic stimulation on whole-body and abdominal subcutaneous adipose tissue lipolysis in lean and obese men. Diabetologia 51: 320–327, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi A, Osaka T. Involvement of the parabrachial nucleus in thermogenesis induced by environmental cooling in the rat. Pflugers Arch 446: 760–765, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Leibel RL, Forse RA, Hirsch J. Effects of rapid glucose infusion on in vivo and in vitro free fatty acid re-esterification by adipose tissue of fasted obese subjects. Int J Obes 13: 661–671, 1989. [PubMed] [Google Scholar]
- 30.Leibel RL, Hirsch J, Berry EM, Gruen RK. Alterations in adipocyte free fatty acid re-esterification associated with obesity and weight reduction in man. Am J Clin Nutr 42: 198–206, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Lipton JM Thermosensitivity of medulla oblongata in control of body temperature. Am J Physiol 224: 890–897, 1973. [DOI] [PubMed] [Google Scholar]
- 32.Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience 98: 301–309, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Mefford IN Application of high performance liquid chromatography with electrochemical detection to neurochemical analysis: measurement of catecholamines, serotonin and metabolites in rat brain. J Neurosci Methods 3: 207–224, 1981. [DOI] [PubMed] [Google Scholar]
- 34.Menendez L, Bester H, Besson JM, Bernard JF. Parabrachial area: electrophysiological evidence for an involvement in cold nociception. J Neurophysiol 75: 2099–2116, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Morrison SF Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci 19: 67–74, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Morrison SF Differential regulation of sympathetic outflows to vasoconstrictor and thermoregulatory effectors. Ann NY Acad Sci 940: 286–298, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci 85: 18–25, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 292: R127–R136, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 11: 62–71, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakayama T, Hardy JD. Unit responses in the rabbit's brain stem to changes in brain and cutaneous temperature. J Appl Physiol 27: 848–857, 1969. [DOI] [PubMed] [Google Scholar]
- 41.Nason MW, Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. J Neurosci 26: 1190–1198, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ootsuka Y, Blessing WW, McAllen RM. Inhibition of rostral medullary raphe neurons prevents cold-induced activity in sympathetic nerves to rat tail and rabbit ear arteries. Neurosci Lett 357: 58–62, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Ootsuka Y, Heidbreder CA, Hagan JJ, Blessing WW. Dopamine D2 receptor stimulation inhibits cold-initiated thermogenesis in brown adipose tissue in conscious rats. Neuroscience 147: 127–135, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Ootsuka Y, McAllen RM. Comparison between two rat sympathetic pathways activated in cold defense. Am J Physiol Regul Integr Comp Physiol 291: R589–R595, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Osaka T Thermogenesis elicited by skin cooling in anaesthetized rats: lack of contribution of the cerebral cortex. J Physiol 555: 503–513, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rathner JA, Owens NC, McAllen RM. Cold-activated raphe-spinal neurons in rats. J Physiol 535: 841–854, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richard D Energy expenditure: a critical determinant of energy balance with key hypothalamic controls. Minerva Endocrinol 32: 173–183, 2007. [PubMed] [Google Scholar]
- 48.Saarela S, Hissa R. Metabolism, thermogenesis and daily rhythm of body temperature in the wood lemming, Myopus schisticolor. J Comp Physiol [B] 163: 546–555, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Saito M, Minokoshi Y, Shimazu T. Metabolic and sympathetic nerve activities of brown adipose tissue in tube-fed rats. Am J Physiol Endocrinol Metab 257: E374–E378, 1989. [DOI] [PubMed] [Google Scholar]
- 50.Satinoff E Neural organization and evolution of thermal regulation in mammals. Science 201: 16–22, 1978. [DOI] [PubMed] [Google Scholar]
- 51.Shi H, Bowers RR, Bartness TJ. Norepinephrine turnover in brown and white adipose tissue after partial lipectomy. Physiol Behav 81: 535–542, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Skibicka KP, Grill HJ. Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology 149: 3605–3616, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanaka M, McAllen RM. A subsidiary fever center in the medullary raphe? Am J Physiol Regul Integr Comp Physiol 289: R1592–R1598, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Taylor DC The effects of nucleus raphe magnus lesions on an ascending thermal pathway in the rat. J Physiol 326: 309–318, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uno T, Roth J, Shibata M. Influence of the hypothalamus on the midbrain tonic inhibitory mechanism on metabolic heat production in rats. Brain Res Bull 61: 129–138, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148: 1550–1560, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Wolfgang MJ, Lane MD. The role of hypothalamic malonyl-CoA in energy homeostasis. J Biol Chem 281: 37265–37269, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience 133: 1039–1046, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Young JB, Landsberg L. Suppression of sympathetic nervous system during fasting. Science 196: 1473–1475, 1977. [DOI] [PubMed] [Google Scholar]
- 60.Youngstrom TG, Bartness TJ. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 268: R744–R751, 1995. [DOI] [PubMed] [Google Scholar]