Abstract
Microinjection of the neuronal inhibitor muscimol into the dorsomedial hypothalamus (DMH) suppresses increases in heart rate (HR), mean arterial pressure (MAP), and circulating levels of adrenocorticotropic hormone (ACTH) evoked in air jet stress in conscious rats. Similar injection of muscimol into the caudal region of the lateral/dorsolateral periaqueductal gray (l/dlPAG) reduces autonomic responses evoked from the DMH, leading to the suggestion that neurons in the l/dlPAG may represent a descending relay for DMH-induced increases in HR and MAP. Here, we examined the role of neuronal activity in the caudal l/dlPAG on the increases in MAP, HR, and plasma ACTH seen in air jet stress in rats. Microinjection of muscimol into the caudal l/dlPAG reduced stress-induced increases in HR and MAP, while identical injections into sites just dorsal or into the rostral l/dlPAG had no effect. Microinjection of a combination of the glutamate receptor antagonists 2-amino-5-phosphonopentanoate (AP5) and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) into the caudal l/dlPAG decreased stress-induced increases in HR alone only at the end of the 20-min stress period but significantly accelerated return to baseline. Surprisingly, microinjection of muscimol into the caudal l/dlPAG also reduced the stress-induced increase in plasma ACTH by 51%. Compared with unstressed control rats, rats exposed to air jet stress exhibited ∼3 times the number of Fos-positive neurons in the l/dlPAG. These findings suggest that neurons in the l/dlPAG are activated in air jet stress and that this activity contributes to increases in HR, MAP, and plasma ACTH.
Keywords: heart rate, blood pressure, adrenocorticotropic hormone, glutamate receptors, c-fos
in response to environmental stress, the mammalian central nervous system generates a response consisting of an integrated pattern of endocrine and autonomic changes that are aimed at enhancing the probability of the organism's survival. These responses are characterized by increases in heart rate (HR), mean arterial pressure (MAP) and plasma concentration of ACTH, and changes in behavior. Although these peripheral changes have been well characterized, the central pathways and mechanisms involved in their generation have only been recently and incompletely elucidated. One of the paradigms that has been employed in studies of these central mechanisms is air jet stress, which has been shown to elicit all of the above changes in conscious rats (2, 29, 44, 46, 47, 54). Depending upon the exact model employed, air jet stress may involve elements of psychological, physical, and even cold stress. The increases in HR, MAP, and plasma ACTH seen in this paradigm appear to be dependent upon the activation of neurons in the dorsomedial hypothalamus (DMH) (44, 46, 47). Disinhibition or activation of neurons in the DMH can induce increases in HR, MAP, locomotor activity, and in plasma ACTH that resemble the responses observed during stress (14–16, 23, 37, 43, 45, 53). Although the increases in plasma ACTH evoked by disinhibition of the DMH are likely to be mediated through direct projections to the nearby paraventricular nucleus (PVN), the increase in HR evoked by disinhibition of the DMH, or by air jet stress can be suppressed by inhibiting neuronal activity in the rostral raphe pallidus (rRPa) (37, 54). This finding, together with correlative anatomical data, suggests that tachycardia evoked from the DMH and, accordingly, in air jet stress is mediated through a direct projection to neurons in the rRPa (36).
Interestingly, however, the increases in MAP and HR evoked by stimulation of the DMH are also reduced after microinjection of either muscimol, a GABAA receptor agonist and neuronal inhibitor, or ionotropic glutamate receptor antagonists into a specific region of the lateral/dorsolateral column of the periaqueductal gray (l/dlPAG) (14–16). The periaqueductal gray (PAG) is a mesencephalic region that has been proposed to play a role in specific cardiovascular changes associated with different emotional behaviors observed during the defense reaction (4, 5, 9, 11, 12). This region is divided into distinct columns, the stimulation of which result in different patterns of effects that are thought to be associated with active or with passive responses during threatening situations (4). Previous studies have shown that while stimulation of the ventrolateral column of the PAG (vlPAG) reduces HR, MAP, and locomotor activity (4, 5, 9, 10), stimulation of the l/dlPAG generates increases in HR and MAP (15) that resemble the responses observed during air jet stress or after disinhibition of the DMH. Therefore, neurons in the l/dl PAG have been suggested to represent a descending relay mediating autonomic responses evoked from the DMH (14–16).
Thus, because 1) the DMH has been shown to play a key role in the physiological changes seen in air jet stress, including increases in HR and MAP, and 2) increases in HR and MAP evoked from the DMH can be suppressed by inhibiting neurons in the l/dl PAG, it seems plausible that activity of neurons in the l/dl PAG plays a role in the generation of air jet stress-induced physiological changes. Support for this idea comes from recent studies employing phosphorylated cAMP response element binding protein (pCREB) phosphorylation and expression of fos protein as markers for neuronal activation, which suggest that neuronal activity in the PAG (including both l/dlPAG and vlPAG) is increased during experimental stress or anxiety (6, 28). However, Lam and colleagues (26) reported that bilateral excitotoxic lesions of the dorsal PAG using high doses of N-methyl-d-aspartate (NMDA), while reducing the reflex HR responses to fluctuations of MAP, failed to affect the increases in HR and MAP seen in this paradigm.
Therefore, we tested the hypothesis that neuronal activity in the PAG plays a role in the increases in HR and MAP seen in air jet stress in rats by examining the effect of acute microinjection of muscimol into the l/dl PAG on the physiological responses evoked during air jet stress. We also examined the role of glutamate receptors in this region employing microinjections of 2-amino-5-phosphonopentanoate (AP5) and 2,3- dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), antagonists of NMDA and AMPA subtypes, respectively. Surprisingly, we found that not only stress-induced increases in MAP and HR but also the accompanying increases in plasma ACTH were suppressed by inhibition of the l/dl PAG with muscimol.
METHODS
All experiments were performed on male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) weighing 290–220 g (n = 33). Animals were housed individually and maintained in a 12:12-h light-dark cycle. Free access to water and food were allowed. All procedures conform to the regulations set forth by National Institutes of Health and were approved by the Institutional Animal Care and Use Committee.
The Dataquest telemetric system (Data Sciences, St. Paul, MN) was employed for measurement of HR and MAP. Animals were anesthetized with ketamine and xylazine (80 mg/kg ketamine and 11.5 mg/kg xylazine ip), and the flexible catheter of a telemetric probe (Data Sciences) was implanted into the aorta through the right femoral artery. The transmitter body was placed in the abdominal cavity and sutured to the abdominal wall.
Five days after the transmitter implantation, the rats were reanesthetized (80 mg/kg ketamine and 11.5 mg/kg xylazine ip), and guide cannulas (26 gauge, Plastics One, Roanoke, VA) were implanted bilaterally for microinjection of drugs into the PAG, as described previously (14–16). Briefly, rats were placed in a stereotaxic apparatus with the incisor bar positioned at 3.5 mm below the interaural line. For the l/dlPAG, target coordinates were 7.8 mm posterior, 0.7 mm lateral, 4.8 mm ventral relative to bregma. The guide cannulas were secured by two screws, Vetbond glue, and dental acrylic. Dummy cannulas were inserted into the guides, and animals were returned to their home cages for recovery. At least 5 days were allowed for recovery before beginning one of the experimental protocols described below.
All experiments were performed as follows in a room in which the temperature was maintained at 24–25°C. On the day of the experiment, animals were brought to the experimentation room and positioned on a telemetry plate receiver in their home cage 2 h prior to the beginning of the protocol. The experiment commenced only after stabilization of physiological parameters (HR, MAP, and locomotor activity) for at least 15 min. Microinjections were performed with a bilateral microinjector (33 gauge, 1 mm longer than the guide cannula; Plastics One, Roanoke, VA) connected to a 10 μl Hamilton syringe with Teflon tubing (ID 0.12 mm; OD 0.65 mm; Bioanalytical Systems, West Lafayette, IN). The syringe was mounted in an infusion pump (KD Scientific, Holliston, MA) that was used to deliver 100 nl of injectate over 30 s. The microinjection was considered successful if, immediately after removal of the microinjector, flow appeared within 3 s after the pump was reactivated, indicating that the injector was not clogged. After the microinjection, all rats were subjected to a 20-min period of air jet stress, in which the rat was placed in an acrylic restraining tube and subjected to a stream of air directed at the animal's head at a rate of 40 l/min, as described previously (46, 47, 54).
The first series of experiments examined the effect of microinjection of muscimol (300 pmol/100 nl) into the caudal or rostral l/dlPAG bilaterally (n = 10 and n = 4, respectively) on the increases in HR and MAP evoked by air jet stress. Each rat was subjected to two different trials 2 days apart and in random order in which either saline vehicle (100 nl) or muscimol (300 pmol; Sigma, St. Louis, MO) was microinjected bilaterally into the l/dlPAG followed 5 min later by air jet stress for 20 min. This dose of muscimol (300 pmol/100 nl) was chosen based on the study of de Menezes and colleagues (16), which demonstrated that microinjection of this dose in the l/dlPAG was effective in reducing the physiological responses evoked by disinhibition of DMH.
The second series of experiments examined the effect of microinjection of a combination of glutamate receptor antagonists into the caudal l/dlPAG bilaterally (n = 6) on cardiovascular changes produced by air jet stress. Each rat was subjected to two different trials 2 days apart and in random order in which either saline vehicle (100 nl) or a combination of the glutamate receptor antagonists AP5 (200 pmol; Sigma) and NBQX (100 pmol; Sigma) was microinjected into the l/dlPAG in a volume of 100 nl followed 5 min later by air jet stress for 20 min. This dose of AP5 was selected because microinjection of 100 pmol of AP5 into the DMH was shown previously to produce a selective blockade of local NMDA receptors and to reduce the increases in HR and MAP seen during air jet stress (44). The dose of NBQX employed was demonstrated in a similar microinjection study in the amygdala to reduce the increases in HR and MAP produced by microinjection of bicuculline methiodide (BMI) in the same region (41).
The third series of experiments examined the effect of microinjection of muscimol into the caudal l/dlPAG on increases in plasma ACTH levels evoked by air stress (n = 6). For these experiments, no telemetric probe was employed, but a femoral arterial catheter was implanted 5 days after implantation of guide cannulas in the caudal l/dlPAG. Animals were reanesthetized, and the femoral arterial catheter (1 cm of a 6-cm length of Teflon leader tubing inserted into a 20-cm length of Tygon tubing; Small Parts, Miami Lakes, FL) was implanted, routed subcutaneously, and exteriorized in the area between the shoulder blades, fixed to a harness (Kent Scientific, Torrington CT), and flushed with saline. Each rat was subjected to two different trials 2 days apart and in random order, in which either saline vehicle (100 nl) or muscimol (Sigma; 300 pmol) was microinjected into the l/dlPAG followed 5 min later by the air jet stress for 20-min duration. A blood sample (0.35 ml) was collected at t = −5 min (5 min before the beginning of stress) and again at the end of the stress procedure (i.e., at t = 20 min) using a tuberculin syringe containing 0.06 ml of a mixture (1:1) of 20 mg/ml EDTA and aprotinin (Sigma). After withdrawal, the blood sample was immediately transferred to chilled Eppendorf tubes and centrifuged to obtain plasma that was stored at −80°C until analysis. ACTH was measured by radioimmunoassay, as described previously (27).
In all microinjection experiments, the injectate was mixed with fluorescent microspheres (FluoSpheres, 0.04 μm, 5% of solids) to mark the site of injection. The solution of microspheres represented 10% of the total drug solution. At the completion of experiments, rats were deeply anesthetized with pentobarbital sodium (80 mg/kg ip) and subjected to transcardial perfusion with 60 ml saline followed by 120 ml 4% buffered paraformaldehyde in 0.1 M phosphate-buffered saline. The brain was then removed, stored in 4% buffered paraformaldehyde overnight, and then transferred to a 30% sucrose solution until saturation. Coronal sections (40 μm) at the level of the PAG column were cut on a cryostat, mounted on slides with Vectashield HardSet Mounting Medium (Vector Laboratories, Burlingame, CA), and coverslipped. Slides were analyzed using a microscope Leica DM LB with a fluorescent digital camera (Diagnostic Instruments, Starling Heights, MI). Sites of injections were approximated using the atlas of Paxinos and Watson (35).
A final series of experiments examined the expression of Fos protein in the l/dlPAG after air jet stress. In these experiments, naive rats were brought to the experimental room 5 h before the commencement of the experiment to allow acclimatization. Animals were then divided in two groups: one group (n = 3) was subjected to 20 min of air jet stress as described above, while the other (n = 4) remained in their home cage as controls. Ninety minutes later, the animals were deeply anesthetized with pentobarbital sodium (60 mg/kg body wt ip), and then perfused transcardially with 40 ml of saline (0.9%) containing 15 000 U/I heparin sulfate for 30 = 40 s followed by 120 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 10–15 min. After perfusion, the brains were removed and postfixed by immersion in paraformaldehyde 4% for 2 h at room temperature. Brains were then transferred to sucrose 20% in 0.01 M PBS overnight at 4°C for cryoprotection, frozen on dry ice, and stored at −80 °C. The brain was blocked and a series of 25-μm coronal sections was cut on a cryostat from each brain starting anterior to the rostral PAG and extending caudally to the most posterior part of the PAG, collected in 0.01 M PBS (pH 7.4) and stored at −20°C in a cryoprotectant solution (mixture of 25% glycerol, 30% ethylene glycol in 0.2 M PBS) until subsequent analysis.
Immunocytochemistry was used to visualize fos protein in every fifth section from the l/dlPAG in all rats (52). Briefly, sections were washed in 0.01 M PBS three times followed by treatment with 0.5% H2O2 to block the endogenous peroxide activity from the tissue. The sections were again washed in PBS three times and then incubated in 0.5% Triton-X-100 in 0.01 M PBS for 30 min to facilitate antibody penetration. The sections were then transferred to 10% normal horse serum in 0.01 M PBS for 20 min to minimize nonspecific binding of the primary antibody (Sigma). After this period, the sections were incubated in polyclonal rabbit antisera against c-Fos (cat. # PC38; Calbiochem, San Diego, CA) at 1:10,000 dilution for 2 days at 4°C on a shaker. After this period, the sections were washed in 0.01 M PBS three times and then were incubated in biotinylated goat anti-rabbit IgG (1:200; Jackson ImmunoResearch, West Grove, PA) for 3 h at room temperature. After rinsing in PBS, sections were incubated in avidin-biotin-peroxidase complex (ABC Elite kit, Vector Laboratories) for 60 min. The sections were washed in PBS followed by a quick rinsing in 0.05 M Tris-buffer (pH 7.8). Staining was developed in freshly prepared Tris-buffer solution containing 0.025% diaminobenzidine tetrachloride, 0.06% nickel-ammonium sulfate, and 0.0027% H2O2 for ∼2 min to yield dark purple color in reacting nuclei, while minimizing nonspecific background staining. The color reaction was stopped by rinsing the sections in Tris buffer.
The sections were mounted on charged slides and left to air dry for 24–48 h. After dehydration in increasing concentrations of ethanol, the sections were washed four times in Histosol (National Diagnostics, Atlanta, GA). The slides were coverslipped in DPX-mounting solution (Fluka) and allowed to dry at least overnight before analysis. Fos-positive neurons were counted manually in the l/dlPAG and ventrolateral PAG in five sections from each rat at the level corresponding to the focus of the experiments above by an observer blinded as to treatment.
Data analysis.
Telemetric data for HR and MAP were acquired continually during the baseline and experimental periods and were averaged over 4-min intervals for presentation and analysis. All data are expressed as means ± SE. Data for HR and MAP for the entire 70-min observation period (i.e., from 10 min prior to a 20-min period of air jet stress to 40 min after its termination) were analyzed by two-way repeated-measures ANOVA with time and treatment as the two repeated factors using STATISTICA for Windows v 5.1 (StatSoft, Tulsa, OK), and Fisher's LSD test was employed for post hoc analysis. Plasma levels of ACTH were compared by Student's paired t-test, and numbers of Fos-positive neurons were analyzed by group t-test. For all statistical analyses, the critical P value was P < 0.05.
RESULTS
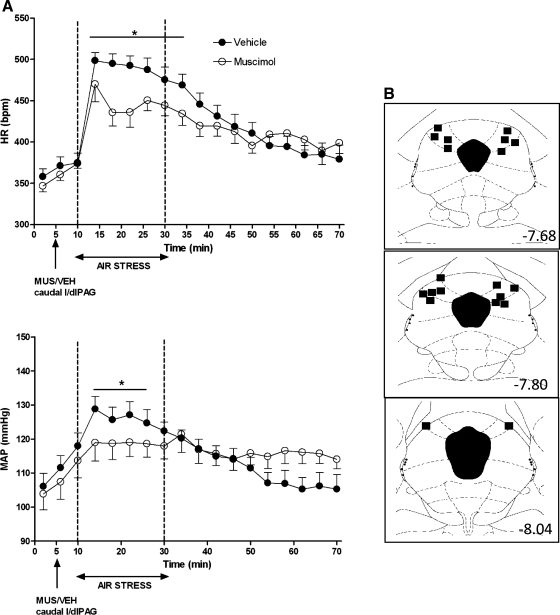
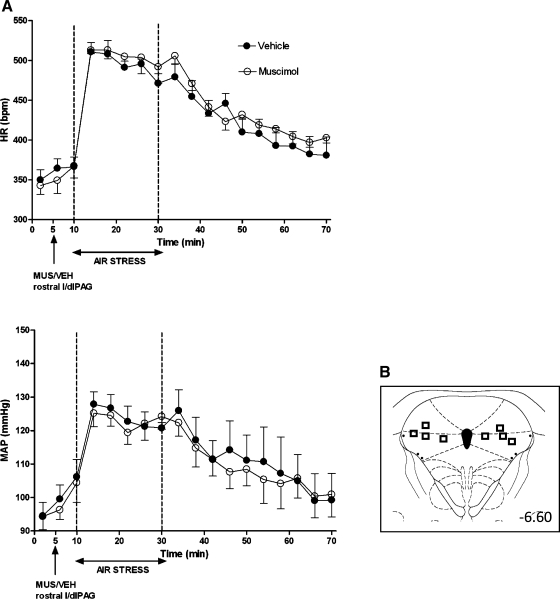
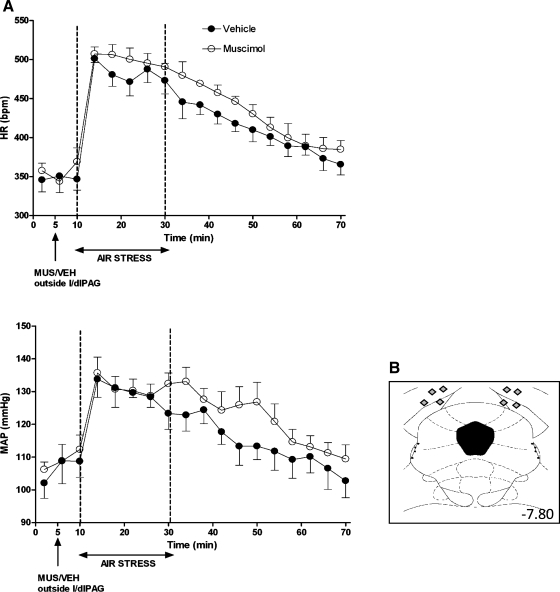
Baseline HR and MAP were not significantly different in any treatment group (Figs. 1–4). After microinjection of vehicle, air jet stress from t = 10–30 min typically evoked marked increases in HR (mean maximal increase over baseline = 124 ± 3 beats/min for the period between 16 and 22 min), and more moderate increases in MAP (mean maximal increase over baseline = +19 ± 1 mmHg; Figs. 1–4). After bilateral microinjection of muscimol (300 pmol) into the caudal l/dlPAG, air jet stress evoked increases in HR and MAP that were significantly reduced throughout the period of stress (i.e., for HR, significantly different at 4-min time intervals at t = 14, 18, 22, 26, 30, and 34 min) all or most (i.e., for MAP, significantly different at t = 14, 18, 22, and 26 min) of the 20-min stress period (significant for interaction of treatment × time and Fisher's LSD test, P < 0.05; see legend, Fig. 1). In contrast, stress-induced increases in HR and MAP were unaffected by identical microinjection of muscimol either into the rostral l/dlPAG (Fig. 2) or at the same rostrocaudal level but just outside of the caudal l/dlPAG (Fig. 3).
Fig. 1.
A: effect of injection of muscimol into caudal lateral/dorsolateral periaqueductal gray (l/dlPAG) on cardiovascular response to air jet stress. Mean values for heart rate (HR) and mean arterial pressure (MAP) over time (min) after microinjection of vehicle (VEH; 100 nl; •) or muscimol (MUS; 300 pmol/100 nl; ○) into caudal l/dlPAG at t = 5 min followed 5 min later by 20 min of air jet stress (n = 10). *Significant difference between muscimol and saline treatment in PAG by two-factor repeated-measures ANOVA [treatment and time as repeated factors) for interaction of treatment × time − HR: F (17, 153) = 3.82, P < 0.0001; MAP: F (17, 153) = 5.24, P < 0.0001] and Fisher's least significant difference (LSD) post hoc test, P < 0.05. B: schematic coronal sections of rat brain adapted from the atlas of Paxinos and Watson (35), illustrating approximate sites of injections of muscimol into caudal l/dlPAG for which data are reported at left. Numbers indicate distance from bregma in millimeters.
Fig. 2.
A: effect of injection of muscimol into rostral l/dlPAG on cardiovascular response to air jet stress. Mean values for HR and MAP over time (min) after microinjection of VEH (100 nl; •) or MUS (300 pmol/100 nl; ○) into rostral l/dlPAG at t = 5 min followed 5 min later by 20 min of air jet stress (n = 5). Two-factor repeated-measures ANOVA (treatment and time as repeated factors) revealed no significant differences for interaction of treatment × time − HR: F (17, 51) = 1.00, P = 0.47; MAP: F (17, 51) = 0.64, P = 0.84. B: schematic coronal sections of the rat brain adapted from the atlas of Paxinos and Watson (35), illustrating approximate sites of injections of muscimol into rostral l/dlPAG in experiments, for which data are reported at left. Numbers indicate distance from bregma in millimeters.
Fig. 3.
A: effect of injection of muscimol into sites dorsal to the caudal l/dlPAG on cardiovascular response to air jet stress (n = 4). Mean values for HR and MAP over time (min) after microinjection of VEH (100 nl; •) or MUS (300 pmol/100 nl; ○) into sites dorsal to caudal l/dlPAG at t= 5 min followed 5 min later by 20 min of air jet stress. Two-factor repeated-measures ANOVA [treatment and time as repeated factors) revealed no significant differences for interaction of treatment × time − HR: F (17, 51) = 0.89, P = 0.59; MAP: F (17, 51) = 1.31, P = 0.22]. B: schematic coronal sections of the rat brain adapted from the atlas of Paxinos and Watson (35) illustrating approximate sites of injections dorsal to caudal l/dlPAG in experiments for which data are reported at left. Numbers indicate distance from bregma in millimeters.
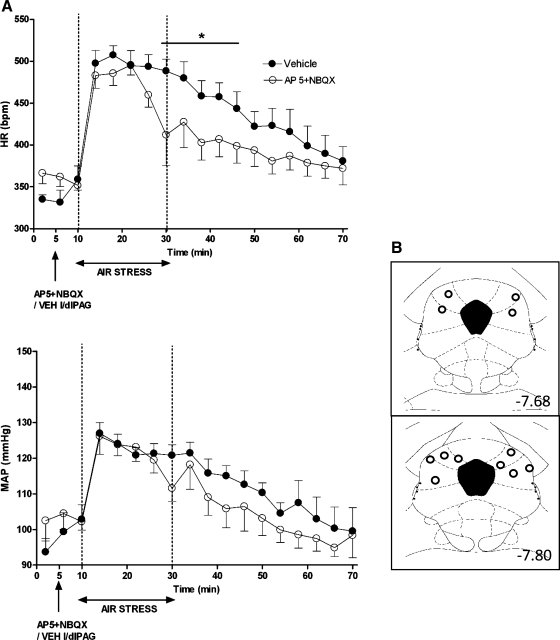
Fig. 4.
A: effect of injection of a combination of glutamate receptor antagonists (AP5 + NBQX) into caudal l/dlPAG on cardiovascular response to air jet stress (n = 6). Mean values for HR and MAP over time (min) after microinjection of VEH (100 nl; •) or glutamate receptor antagonist (AP5 300 pmol/100 nl + NBQX 100 pmol/00 nl; ○) into caudal l/dlPAG at t = 5 min followed 5 min later by 20 min of air jet stress. *Significant difference between antagonist and saline treatment in PAG by two-factor repeated-measures ANOVA (treatment and time as repeated factors) for interaction of treatment × time and Fisher's LSD post hoc test, P < 0.05, for HR but not MAP − HR: F (17, 85) = 2.48, P = 0.0032; MAP: F (17, 85) = 1.61, P = 0.08. B: schematic coronal sections of the rat brain adapted from the atlas of Paxinos and Watson (35), illustrating approximate sites of injections of muscimol into caudal l/dlPAG. Numbers indicate distance from bregma in millimeters.
In contrast to microinjection of muscimol, the microinjection of antagonists of glutamate receptors into the caudal l/dlPAG significantly affected the cardiovascular response to air jet stress only at the end of the stress period, that is, at the 4-min interval represented by the 30-min time point, and immediately following at the 34, 38, 42, and 46-min time points (significant for interaction of treatment × time by two-factor ANOVA for repeated measures; see legend of Fig. 4). Thus, microinjection of this combination of drugs shortened the time to recovery of baseline HR after termination of the stress.
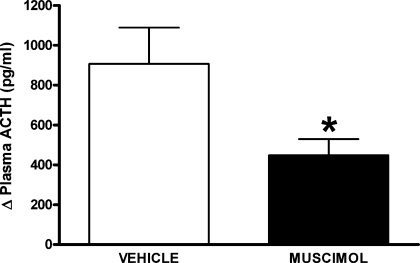
Air jet stress dramatically increased plasma ACTH concentration from baseline in all experiments after microinjection of vehicle (i.e., from 38 ± 9 pg/ml before to 986 ± 256 pg/ml after air jet stress). Prior microinjection of muscimol (300 pmol/100 nl) into caudal l/dlPAG reduced the increases in the plasma ACTH concentration from baseline observed after air jet stress by more than 51% (i.e., to 489 ± 91 pg/ml after air jet stress; Fig. 5).
Fig. 5.
Mean changes ± SE from baseline plasma ACTH levels after 20 min of air jet stress in rats (n = 6) after pretreatment with bilateral microinjections of either vehicle (100 nl saline, open bar) or muscimol (300 pmol, solid bar) into the caudal l/dlPAG (n = 6). *Significantly different from response after injection of saline by paired t-test, P < 0.05.
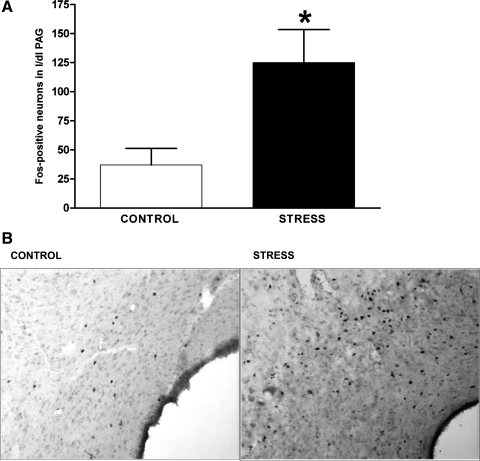
Compared with that seen in control rats, the number of Fos-positive neurons in the l/dlPAG region of rats exposed to air jet stress was significantly increased (mean number of Fos-positive neurons in l/dlPAG = 125 ± 28 in stressed rats vs. 37 ± 14 in unstressed controls; Fig. 6). Stressed rats also displayed a similar pattern of increased Fos expression in the ventrolateral PAG (81 ± 10 Fos-positive neurons in stressed rats vs. 26 ± 8 in controls).
Fig. 6.
A: mean number ± SE of Fos-expressing neurons in the caudal l/dlPAG in control rats (n = 4) and in rats subjected to 20 min of air jet stress (n = 3). Total for each rat represents the total number of fos-expressing neurons in five equivalent sections of l/dlPAG *Significant difference between air stress and control by unpaired t-test, P < 0.05 B: photomicrograph illustrating c-fos expression in the left caudal l/dlPAG of an unstressed control rat (left) and a rat subjected to 20 min of air jet stress.
Postmortem histology confirmed that injection sites in all microinjection experiments for which data were analyzed were located in the rostral, caudal l/dlPAG, or just outside the latter region in the anatomic control group (Figs. 1–4). In eight rats, one or both injection cannulas targeting the l/dlPAG were misplaced, and data from these animals were excluded from the above analysis. Injection sites in the rostral l/dlPAG were located ∼6.6 mm caudal from bregma (Fig. 2), while injection sites in the caudal l/dlPAG extended from 7.68 to 8.04 caudal to bregma (Figs. 1–4). Figure 7 illustrates an example of typical injection sites in the rostral l/dlPAG and in the caudal l/dlPAG.
Fig. 7.
Photomicrographs illustrating examples of sites of injection in the rostral and caudal l/dlPAG. All drugs were mixed with fluorescent microspheres to mark injection sites. Numbers represent distance from bregma in millimeters.
DISCUSSION
Our results show that microinjection of muscimol (300 pmol) into the caudal l/dlPAG reduces the increases in HR, MAP, and plasma ACTH seen in air jet stress. As an anatomical control, we examined the effect of similar microinjections of muscimol into sites neighboring sites dorsal to the l/dlPAG and also into sites 1.2 mm rostral in the same column of the PAG. In neither case did these injections alter responses seen during air jet stress. Thus, the anatomical resolution of this intervention is sufficient to indicate that the reduction in the air stress response after microinjection of muscimol into the caudal l/dlPAG is a consequence of specific inhibition of local neurons and not the result of spread of the drug to other areas. Various studies have shown that air stress elicits neuroendocrine and cardiovascular changes that resemble those observed in other types of acute stress (2, 29, 44, 46, 47, 54). Stimulation of neurons in the caudal l/dlPAG of conscious rats results in flight responses accompanied by increases in HR and MAP that are similar to those observed during air jet stress in our experiments (4, 5, 9). Our findings replicate the previous data showing that air jet stress produces increases in HR, MAP, and plasma ACTH in conscious rats and point to the involvement of neuronal activity in the l/dl PAG in the generation of these changes.
Because our physiological data suggested that neurons in the l/dlPAG are activated in this paradigm, we evaluated the effect of air jet stress on the expression of c-Fos, a marker for neuronal activation (19–22, 24, 34) in a parallel set of experiments. Previous studies have reported that various paradigms for acute stress or anxiety such as immobilization, plus the maze test, and predator exposure can increase c-Fos expression in the l/dlPAG (3, 8, 40). Accordingly, the number of fos-positive neurons in the caudal l/dlPAG of rats subjected to air jet stress in the present study was greater than that seen in conscious unstressed rats. In fact, the distribution of fos-positive neurons at this level of the PAG overall was similar in all three stressed rats, with increases also apparent in the ventrolateral PAG in every rat (control −26 ± 8; stressed −81 ± 10). This finding suggests that neuronal activity in the caudal l/dl PAG is increased during air jet stress and, therefore, supports our physiological data. Thus, our results indicate that activation of these same neurons may play a role in generating the cardiovascular responses evoked during emotional stress in rats.
Although our results clearly implicate neurons localized in the l/dlPAG in the increases in HR and MAP elicited by air jet stress, this conclusion is at odds with the finding of a previous report that studied the effect of excitotoxic lesions of this region. Lam and colleagues (26) found that NMDA-induced lesions of the dorsal PAG (including l/dlPAG) did not reduce the increases in HR or MAP evoked by air stress. However, it is important to note that the stress paradigm employed by Lam and colleagues was somewhat different from that studied here; the air jet was delivered for only 100 ms and directed at a point above the lumbosacral region of the rat rather than the face. This technique appears to have been much less effective in eliciting cardiovascular changes than the paradigm that was used in the present study. Indeed, the air stress employed by Lam and colleagues increased HR by only 27 beats/min above baseline, and this increase lasted for only 12 s. Interestingly, the inhibition of l/dlPAG neurons in our study failed to alter the HR or MAP response in the first minute of air jet stress. Thus, the short duration of the air stress paradigm and its effect on HR in the study of Lam and colleagues may have been insufficient to permit detection of the effect of their lesions of the l/dlPAG. Instead, their findings are in agreement with ours in suggesting that the pathways mediating the initial increase in HR produced by air jet stress may involve other mechanisms and central nervous regions.
To evaluate the role of ionotropic glutamate receptors in the l/dlPAG in the responses evoked by stress, we examined the effect of microinjecting a combination of an NMDA glutamate receptor antagonist (AP5) and a non-NMDA glutamate receptor antagonist (NBQX) into the caudal l/dlPAG. Many of the excitatory projections to the PAG are glutamate, and it is known that glutamate receptors are widely distributed throughout PAG (1, 50). A similar dose of AP5 was able to reduce the tachycardia observed during air stress when microinjected into the DMH (44). Also, Soltis et al. (42) showed that the microinjection of NBQX, at the same dose used in the present study, into the amygdala of conscious rats was able to reduce the increases in HR and MAP evoked by the microinjection of BMI in the same area. However, unlike muscimol, antagonists of these receptors microinjected into the l/dl PAG did not affect the increases in HR and MAP seen during air jet stress. Instead, this treatment did reduce the time to return to baseline HR after the end of the period of air jet stress. This more rapid recovery may reflect an anxiolytic effect of injecting these drugs into this area of the PAG. Molchanov and Guimaraes (33) have shown that microinjection of AP7, another antagonist of NMDA receptors, into the lateral PAG increases the exploration time in the open arm during an elevated plus maze test, and suggested that this reflected an anxiolytic effect (33). As discussed above, previous studies have shown that c-Fos expression is increased in the l/dlPAG in different paradigms for acute stress or anxiety, including immobilization, elevated plus maze test, and exposure to a predator (3, 8, 40). Thus, the stress-induced neuronal excitation in the l/dl PAG that is dependent upon activity at glutamate receptors may be specifically related to an “anxiogenic” component of this paradigm that contributes to the maintenance of the evoked cardiovascular changes by blocking accommodation to the sustained stressor.
Interestingly, microinjection of muscimol (300 pmol) into the caudal l/dlPAG reduced the increases in plasma ACTH after 10 min of air stress by 51%. Our air jet stress paradigm caused a marked increase in plasma ACTH (+906 ± 106 pg/ml), presumably resulting from activation of CRH-containing neurons in the PVN that project to the median eminence and are thought to represent the final common pathway for stimulation of ACTH secretion from the anterior pituitary (31, 32). However, Cameron et al. (7) have shown that none of the columns of the PAG send direct projections to the PVN. On the other hand, the l/dlPAG sends projections to the DMH (7, 38, 39), a region that has been implicated in diverse stress-induced effects, including activation of the hypothalamic-pituitary-adrenal axis. Stotz-Potter and colleagues have shown that microinjection of muscimol into the DMH nearly abolishes the increases in HR and MAP seen in this paradigm and also reduces the accompanying increases in plasma ACTH levels (46). Neurons in the DMH project heavily to CRH-containing cells in the PVN (30, 48, 49), and these PVN-projecting neurons are known to be activated in experimental stress (13). In conscious unstressed rats, blockade of GABA-mediated inhibitory tone in the DMH by local microinjection of the GABAA receptor antagonist bicuculline methiodide (BMI), evokes increases in HR, MAP, and plasma ACTH similar to those observed during air jet stress (14–16, 23, 45). Interestingly, Soltis and DiMicco (43) have shown that the cardiovascular responses produced by blockade of GABAA receptors in the DMH depend on activity at ionotropic glutamate receptors in this region. Thus, one possible explanation for the fact that inhibition of the l/dlPAG reduces the increases in ACTH levels seen in air jet stress is that neurons in this region represent a source of glutamatergic excitatory drive to neurons in the DMH that are ultimately responsible for this effect.
In contrast to the neuroendocrine response, the findings of da Silva et al. (15) seem to point to a role for a descending projection from the DMH to the l/dlPAG in the cardiovascular changes seen in this paradigm. They showed that microinjection of glutamate receptor antagonists into the l/dlPAG markedly reduced the increases in MAP and HR evoked by blockade of GABAA receptors in the DMH in conscious unstressed rats. Their results prompted them to conclude that neurons in the l/dl PAG must represent a major downstream relay for cardiovascular effects evoked from the DMH, interposed between neurons in the latter region and brain stem autonomic centers. If so, our results point to a complex bidirectional interaction between the PAG and the DMH in the generation of physiological changes seen in experimental stress. Clearly, more study is warranted to clarify this issue.
Perspectives and Significance
The current study provides evidence that neurons in the caudal l/dlPAG are activated during air jet stress and play a role in the associated cardiovascular and neuroendocrine response. Our results suggest that ascending input from neurons in this region of the PAG is responsible, in part, for the powerful activation of the hypothalamic-pituitary-adrenal axis evoked in this paradigm. The air jet stress model employed in this study is complex, involving components that are both physical (restraint, and perhaps cold owing to the dissipation of body heat produced by a constant stream of air) and psychological. Our findings provide no insight as to whether neurons in the l/dlPAG contribute more to the response to the physiological or psychological components of the model, but previous reports suggest a dominant role for the former (17, 18, 25, 51). Nevertheless, this, as well as the exact placement and role of these neurons in the relevant stress-related forebrain circuitry, particularly with regard to the DMH, are yet to be fully elucidated.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant NS19883-19 and by Conselho Nacional de Desenvolvimento Científico e Tecnológico/Fundação de Amparo á Pesquisa do Estado de Minais Gerais. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR015481–01 from the National Center for Research Resources, NIH.
Acknowledgments
A preliminary report of this work has been published in abstract form and presented at the Annual Meeting of the Society for Neuroscience in October 2006.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Albin RL, Makowiec RL, Hollingsworth Z, Dure IV, Leon, S, Penney JB, Young AB. Excitatory amino acid binding sites in the periaqueductal gray of the rat. Neurosci Lett 118: 112–115, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JJ, DiMicco JA. Effect of local inhibition of gamma-aminobutyric acid uptake in the dorsomedial hypothalamus on extracellular levels of gamma-aminobutyric acid and on stress-induced tachycardia: a study using microdialysis. J Pharmacol Exp Ther 255: 1399–1407, 1990. [PubMed] [Google Scholar]
- 3.Arnold FJ, De Lucas Bueno M, Shiers H, Hancock DC, Evan GI, Herbert J. Expression of c-fos in regions of the basal limbic forebrain following intracerebroventricular corticotropin-releasing factor in unstressed or stressed male rats. Neuroscience 51: 377–390, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull 53: 95–104, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci 17: 379–389, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Blundell J, Adamec R. Elevated pCREB in the PAG after exposure to the elevated plus maze in rats previously exposed to a cat. Behav Brain Res 175: 285–295, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Cameron AA, Khan IA, Westlund KN, Cliffer KD, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. I. Ascending projections. J Comp Neurol 351: 568–584, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Canteras NS, Goto M. Fos-like immunoreactivity in the periaqueductal gray of rats exposed to a natural predator. Neuroreport 10: 413–418, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Carrive P The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res 58: 27–47, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Carrive P, Bandler R. Control of extracranial and hindlimb blood flow by the midbrain periaqueductal grey of the cat. Exp Brain Res 84: 599–606, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Carrive P, Bandler R. Viscerotopic organization of neurons subserving hypotensive reactions within the midbrain periaqueductal grey: a correlative functional and anatomical study. Brain Res 541: 206–215, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Carrive P, Dampney RA, Bandler R. Excitation of neurones in a restricted portion of the midbrain periaqueductal grey elicits both behavioural and cardiovascular components of the defence reaction in the unanaesthetised decerebrate cat. Neurosci Lett 81: 273–278, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Cullinan WE, Helmreich DL, Watson SJ. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J Comp Neurol 368: 88–99, 1996. [DOI] [PubMed] [Google Scholar]
- 14.da Silva LG, de Menezes RC, dos Santos RA, Campagnole-Santos MJ, Fontes MA. Role of periaqueductal gray on the cardiovascular response evoked by disinhibition of the dorsomedial hypothalamus. Brain Res 984: 206–214, 2003. [DOI] [PubMed] [Google Scholar]
- 15.da Silva LG, Menezes RC, Villela DC, and Fontes MA. Excitatory amino acid receptors in the periaqueductal gray mediate the cardiovascular response evoked by activation of dorsomedial hypothalamic neurons. Neuroscience 139: 1129–1139, 2006. [DOI] [PubMed] [Google Scholar]
- 16.de Menezes RC, Zaretsky DV, Fontes MA, DiMicco JA. Microinjection of muscimol into caudal periaqueductal gray lowers body temperature and attenuates increases in temperature and activity evoked from the dorsomedial hypothalamus. Brain Res 1092: 129–137, 2006. [DOI] [PubMed] [Google Scholar]
- 17.de Oca BM, Fanselow MS. Amygdala and periaqueductal gray lesions only partially attenuate unconditional defensive responses in rats exposed to a cat. Integr Physiol Behav Sci 39: 318–333, 2004. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira RW, Del Bel EA, Guimaraes FS. Behavioral and c-fos expression changes induced by nitric oxide donors microinjected into the dorsal periaqueductal gray. Brain Res Bull 51: 457–464, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 29: 261–265, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Dragunow M, Peterson MR, Robertson HA. Presence of c-fos-like immunoreactivity in the adult rat brain. Eur J Pharmacol 135: 113–114, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Dragunow M, Robertson HA. Generalized seizures induce c-fos protein(s) in mammalian neurons. Neurosci Lett 82: 157–161, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Dragunow M, Robertson HA. Kindling stimulation induces c-fos protein(s) in granule cells of the rat dentate gyrus. Nature 329: 441–442, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol 280: H2891–H2901, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 328: 632–634, 1987. [DOI] [PubMed] [Google Scholar]
- 25.Ishide T, Amer A, Maher TJ, Ally A. Nitric oxide within periaqueductal gray modulates glutamatergic neurotransmission and cardiovascular responses during mechanical and thermal stimuli. Neurosci Res 51: 93–103, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Lam W, Louis WJ, Verberne AJ. Effect of dorsal periaqueductal grey lesion on baroreflex and cardiovascular response to air-jet stress. J Auton Nerv Syst 53: 35–42, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Levy AD, Cabrera TM, Brownfield MS, Battaglia G, Van de Kar LD. Long-term fluoxetine, but not desipramine, inhibits the ACTH and oxytocin responses to the 5-HT1A agonist, 8-OH-DPAT, in male rats. Brain Res 630: 148–156, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Lino-de-Oliveira C, de Oliveira RM, Padua Carobrez A, de Lima TC, del Bel EA, and Guimaraes FS. Antidepressant treatment reduces Fos-like immunoreactivity induced by swim stress in different columns of the periaqueductal gray matter. Brain Res Bull 70: 414–421, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Lisa M, Filippelli A, Marmo E, Wible JH Jr, DiMicco JA. Microinjection of muscimol into posterior hypothalamus blocks cardiovascular response to experimental stress in rats. Pharmacol Res 21 Suppl 1: 9–10, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Luiten PG, ter Horst GJ, Steffens AB. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog Neurobiol 28: 1–54, 1987. [DOI] [PubMed] [Google Scholar]
- 31.Meister B, Hokfelt T, Geffard M, Oertel W. Glutamic acid decarboxylase- and gamma-aminobutyric acid-like immunoreactivities in corticotropin-releasing factor-containing parvocellular neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology 48: 516–526, 1988. [DOI] [PubMed] [Google Scholar]
- 32.Merchenthaler I, Hynes MA, Vigh S, Schally AV, Petrusz P. Corticotropin releasing factor (CRF): origin and course of afferent pathways to the median eminence (ME) of the rat hypothalamus. Neuroendocrinology 39: 296–306, 1984. [DOI] [PubMed] [Google Scholar]
- 33.Molchanov ML, Guimaraes FS. Anxiolytic-like effects of AP7 injected into the dorsolateral or ventrolateral columns of the periaqueductal gray of rats. Psychopharmacology 160: 30–38, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science 237: 192–197, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam: Academic Press, 2007.
- 36.Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol 287: R472–R478, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Samuels BC, Zaretsky DV, DiMicco JA. Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J Physiol 538: 941–946, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaikh MB, Barrett JA, Siegel A. The pathways mediating affective defense and quiet biting attack behavior from the midbrain central gray of the cat: an autoradiographic study. Brain Res 437: 9–25, 1987. [DOI] [PubMed] [Google Scholar]
- 39.Siegel A, Schubert KL, Shaikh MB. Neurotransmitters regulating defensive rage behavior in the cat. Neurosci Biobehav Rev 21: 733–742, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Silveira MC, Sandner G, Graeff FG. Induction of Fos immunoreactivity in the brain by exposure to the elevated plus-maze. Behav Brain Res 56: 115–118, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Soltis RP, Cook JC, Gregg AE, Sanders BJ. Interaction of GABA and excitatory amino acids in the basolateral amygdala: role in cardiovascular regulation. J Neurosci 17: 9367–9374, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soltis RP, Cook JC, Gregg AE, Stratton JM, Flickinger KA. EAA receptors in the dorsomedial hypothalamic area mediate the cardiovascular response to activation of the amygdala. Am J Physiol Regul Integr Comp Physiol 275: R624–R631, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Soltis RP, DiMicco JA. GABAA and excitatory amino acid receptors in dorsomedial hypothalamus and heart rate in rats. Am J Physiol Regul Integr Comp Physiol 260: R13–R20, 1991. [DOI] [PubMed] [Google Scholar]
- 44.Soltis RP, DiMicco JA. Hypothalamic excitatory amino acid receptors mediate stress-induced tachycardia in rats. Am J Physiol Regul Integr Comp Physiol 262: R689–R697, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Soltis RP, DiMicco JA. Interaction of hypothalamic GABAA and excitatory amino acid receptors controlling heart rate in rats. Am J Physiol Regul Integr Comp Physiol 261: R427–R433, 1991. [DOI] [PubMed] [Google Scholar]
- 46.Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res 742: 219–224, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J Neurosci 16: 1173–1179, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ter Horst GJ, Luiten PG. Phaseolus vulgaris leuco-agglutinin tracing of intrahypothalamic connections of the lateral, ventromedial, dorsomedial and paraventricular hypothalamic nuclei in the rat. Brain Res Bull 18: 191–203, 1987. [DOI] [PubMed] [Google Scholar]
- 49.ter Horst GJ, Luiten PG. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Res Bull 16: 231–248, 1986. [DOI] [PubMed] [Google Scholar]
- 50.Tolle TR, Berthele A, Zieglgansberger W, Seeburg PH, Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J Neurosci 13: 5009–5028, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vianna DM, Landeira-Fernandez J, Brandao ML. Dorsolateral and ventral regions of the periaqueductal gray matter are involved in distinct types of fear. Neurosci Biobehav Rev 25: 711–719, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Zaretskaia MV, Zaretsky DV, Sarkar S, Shekhar A, Dimicco JA. Induction of Fos-immunoreactivity in the rat brain following disinhibition of the dorsomedial hypothalamus. Brain Res 1200: 39–50, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res 928: 113–125, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Zaretsky DV, Zaretskaia MV, Samuels BC, Cluxton LK, DiMicco JA. Microinjection of muscimol into raphe pallidus suppresses tachycardia associated with air stress in conscious rats. J Physiol 546: 243–250, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]