Abstract
Central and intraperitoneal C75, an inhibitor of fatty acid synthase and stimulator of carnitine palmitoyl-transferase-1, inhibits eating in mice and rats. Mechanisms involved in feeding inhibition after central C75 have been identified, but little is yet known about how systemic C75 might inhibit eating. One issue is whether intraperitoneal C75 reduces food intake in rats by influencing normal physiological controls of food intake or acts nonselectively, for example by eliciting illness or aversion. Another issue relates to whether intraperitoneal C75 acts centrally or, similar to some other peripheral metabolic controls of eating, activates abdominal vagal afferents to inhibit eating. To further address these questions, we investigated the effects of intraperitoneal C75 on spontaneous meal patterns and the formation of conditioned taste aversion (CTA). We also tested whether the eating inhibitory effect of intraperitoneal C75 is vagally mediated by testing rats after either total subdiaphragmatic vagotomy (TVX) or selective subdiaphragmatic vagal deafferentations (SDA). Intraperitoneal injection of 3.2 and 7.5 mg/kg of C75 significantly reduced food intake 3, 12, and 24 h after injection by reducing the number of meals without affecting meal size, whereas 15 mg/kg of C75 reduced both meal number and meal size. The two smaller doses of C75 failed to induce a CTA, but 15 mg/kg C75 did. The eating inhibitory effect of C75 was not diminished in either TVX or SDA rats. We conclude that intraperitoneal injections of low doses of C75 inhibit eating in a behaviorally specific manner and that this effect does not require abdominal vagal afferents.
Keywords: food intake, fatty acid oxidation, carnitine palmitoyl-transferase-1, fatty acid synthase, vagus, conditioned taste aversion
intraperitoneal injections of milligram doses of C75, an inhibitor of fatty acid synthase (FAS) that was originally developed for the treatment of cancer (21), dose-dependently decreased food intake in both mice and rats (10, 13, 20, 25, 26, 39). Intracerebroventricular injections of C75 doses ∼3 log units smaller had similar effects (1, 2, 25), suggesting that C75 can act centrally to inhibit eating.
In addition to its effect on FAS, C75 has other peripheral and central metabolic effects that might contribute to its eating inhibitory potency (2, 20, 22, 39, 43). One of these is the stimulation of carnitine palmitoyl-transferase-1 (CPT-1) (2, 29, 39). Increased CPT-1 activity stimulates fatty acid oxidation (FAO; 19), and several findings indicate that inhibition of peripheral FAO stimulates eating (11, 24, 36). There are some reports that administration of CPT-1 inhibitors into the central nervous system decreases food intake or body weight (31, 33). Recent findings, however, indicate that selective stimulation of CPT-1 activity in the brain is sufficient to inhibit eating and decrease body weight (2). First, the CPT-1-stimulatory action of C75 appears to contribute to the eating inhibitory effect of intracerebroventricular C75 because intracerebroventricular pretreatment with the CPT-1 inhibitor etomoxir attenuated the eating inhibitory effect of subsequent intracerebroventricular C75 injections (2). Second, intracerebroventricular injection of C89b, a novel compound that stimulates CPT-1 activity without affecting FAS activity, alone was sufficient to inhibit eating (2).
The data reviewed above clearly indicate that C75 acting centrally can inhibit eating. Other evidence suggests that C75 also contributes to body weight loss by increasing peripheral FAO and overall energy expenditure (38–40). It is unknown, however, whether C75 also acts at peripheral sites to inhibit eating. In the present study, we further investigated the effects of intraperitoneal C75 on eating in rats. We had two main goals. The first was to further investigate the behavioral specificity of the eating inhibitory effect of intraperitoneal C75. Clegg et al. (10) reported that injection of 15 mg/kg C75 ip induced a conditioned taste aversion (CTA) in rats. Here, we investigated the effect of injections of 3.2, 7.5, and 15 mg/kg C75 ip on spontaneous meal patterns and on the formation of CTA. The second goal was to investigate the role of the vagus nerve in mediating the eating inhibitory action of intraperitoneal C75. The vagus is a major bidirectional communication conduit between abdominal organs and the brain. It relays both information about ingested nutrients and metabolic status in the liver and other abdominal organs to the brain and metabolic control signals from the brain to the periphery (4, 15, 44). Furthermore, abdominal vagal afferents mediate the eating stimulatory effect of peripherally administered FAO inhibitors, such as mercaptoacetate and methylpalmoxyrate in rats (5, 17, 23, 35). If intraperitoneal C75 reduces food intake in part by stimulating peripheral FAO, this effect might depend on intact abdominal vagal afferents. To test this hypothesis, we investigated the effects of intraperitoneal C75 in rats with total subdiaphragmatic vagotomy (TVX) and in rats with subdiaphragmatic vagal deafferentation (SDA).
MATERIALS AND METHODS
Experiments 1–3 were conducted in the Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine and experiments 4 and 5 in the Institute of Animal Sciences, ETH Zurich. The procedures used were approved by the Johns Hopkins University Animal Care and Use Committee and the Veterinary Office of the Canton of Zurich, respectively.
Animals and housing.
Male Sprague-Dawley rats (Charles River, Kingston, NY, and Sulzfeld, Germany) were housed individually in grated-floor stainless steel cages in rooms with controlled temperature 22 ± 2°C and humidity of ∼60%. The rats were maintained on 12:12-h light-dark cycle and had ad libitum access to tap water, standard rodent chow (cat. no. 2018; Harlan-Teklad, Madison, WI, or cat. no. 3430; Provimi Kliba, Kaiseraugst, Switzerland, respectively), and other diets as described below. Rats adapted for several days to the specific experimental procedures before testing began.
Experiment 1: spontaneous meal patterns.
The effects of intraperitoneal C75 on nocturnal spontaneous meal patterns were tested in eight rats that were housed in cages equipped with computer-controlled pellet (45 mg/pellet; Bioserv, Frenchtown NJ) dispensers (Med Associates, Georgia, VT). An infrared beam detected pellet removal from the feed trough and triggered pellet replacement. The times of pellet removals were recorded to the nearest 100 ms. Intraperitoneal injections of 0, 3.2, 7.5, and 15 mg/kg C75 were tested in random order. Meal patterns were generated with TongueTwister (version 1.46a) software (18) using a minimum meal size criterion of five pellets and a minimum intermeal interval criterion of 10 min. The latency to initiate the first meal after dark onset, mean nocturnal meal size, mean nocturnal number of meals, and nocturnal satiety ratios (subsequent intermeal interval/meal size) were analyzed by one-way ANOVA (StatView version 5.0.1 for Windows, SAS Institute, Cary, NC), followed up by Bonferroni-Holm tests (16).
Experiment 2: CTA with 15 mg/kg C75.
Fifteen experimentally naïve rats were maintained on chow and adapted to a 22-h daily water-deprivation schedule, with 2-h water access at the end of the light phase. Food was available ad libitum. Water intake was measured after 30 min and 2 h to verify that baseline intakes were stable. Rats were divided into two groups with roughly equal water intakes. On the conditioning day, rats were offered only a novel 0.125% saccharin solution for the first 30 min of fluid access. Saccharin was then removed, the rats were injected with 0 or 15 mg/kg C75 ip, as above, and water was offered for 90 min and again the next day for 2 h. The test day was the following day. Both 0.125% saccharin solution and water were offered, and 30-min intakes recorded. The percent of total 30-min fluid intake taken as saccharin was calculated, and the data from two groups were compared with t-tests.
Experiment 3: TVX.
Rats were maintained on Ensure liquid diet (Abbott Nutrition, Columbus, OH) to minimize the nonspecific effects of TVX on gastrointestinal motility and food intake (30). After adaptation to Ensure, rats were anesthetized with an intramuscular injection of a mixture of 8.6 mg/kg xylazine and 57.1 mg/kg ketamine HCl. A 3-cm midline laparotomy was made, the stomach was gently retracted, the ventral and dorsal esophageal vagal trunks were exposed by gently teasing them apart from the esophagus, and the hepatic, celiac, accessory celiac, and gastric branches were identified. Two 4-0 silk ligatures were placed ∼1 cm apart from each other around the ventral (right) vagal truck proximal to the hepatic and accessory celiac branches and two ligatures around the dorsal (left) trunk proximal to the celiac branch. The segments of nerve between the ligatures on each trunk were cut and removed, and the esophagus was inspected to ensure the absence of neural fibers. The abdominal muscles and skin were then sutured in separate layers. For sham operations, rats were laparotomized and the vagus was visualized but not further manipulated. Rats were intramuscularly injected with 60,000 units penicillin for antibacterial prophylaxis and 1 mg/kg flunixin meglumine for postoperative analgesia.
After recovery from TVX, as indicated by stable food intakes and increasing body weight trajectories, rats were adapted to a schedule of a daily 22-h period of food access beginning at onset of dark. This daily schedule permitted 2 h at the end of the light for experimental preparations and maintenance. Each week, a single intraperitoneal injection of C75 (FASgen, Baltimore, MD), freshly dissolved in 1 ml/kg glucose-free RPMI 1640 cell culture medium (11879, Invitrogen, Carlsbad, CA), or the RPMI vehicle alone was administered 5 min before dark onset, and food intake was measured 2, 4, and 22 h later. These measurements continued for at least 3 days following each injection. Doses of 0, 3.2, and 7.5 mg/kg C75 were tested in random order.
Completeness of total vagotomies was verified at death 1) by visual inspection of the surgical site and 2) by measurement of stomach contents remaining after 18-h access to chow, as previously described (32). Visual examination failed to reveal vagal fibers in any of the rats. The stomachs were then removed and rinsed clear of contents, which were air dried and weighed. One TVX rat's stomach contents weighed less than the largest value in the sham-operated group, suggesting an incomplete vagotomy. This rat's data were discarded, leaving five TVX rats and six sham-operated rats. Food intakes in these rats at 2-, 4-, and 22-h were analyzed separately. First, to determine whether C75 produced a dose-related inhibition of eating, data in each surgical group were analyzed by one-way ANOVA followed up by Bonferroni-Holm tests (16). Second, the effects of each C75 dose were compared directly between the two surgical groups by planned comparisons of the (RPMI-C75) difference in TVX vs. in sham-operated rats, using two-way ANOVA (C75 dose × surgical group) to generate the error term and Bonferroni-Holm tests (16) for the comparisons.
Experiment 4: CTA with 2.5 and 5 mg/kg C75.
The procedure of experiment 2 was repeated using 48 new rats. On the conditioning day, groups of 12 rats each with roughly equal water intakes were offered only a novel 0.125% saccharin solution for the first 30 min of fluid access and then were intraperitoneally injected with 0, 2.5, or 5 mg/kg C75 in 1 ml/kg RPMI, or 60 mg/kg body wt LiCl in 9.4 ml/kg H2O as a positive control. Food intakes were measured 1, 2, 4, and 24 h after injection. Again, as previously, a 30-min two-bottle preference test was done 2 days later. Data were analyzed by one-way ANOVA followed up by Bonferroni-Holm tests (16).
Experiment 5: SDA.
Rats were offered sweetened milk (35% sweetened condensed milk in water; Migros, Zurich, Switzerland) in addition to ground chow for 2 days, injected with 100 μl antibiotic sc (Borgal 24%, Intervet; Veterinaria, Zurich, Switzerland) on the day prior to surgery, and preoperatively injected with 80 μg/kg acepromazin ip (Prequillan, Arovet, Zollikon, Switzerland), 50 μg/kg atropine sulfate sc (Sintetica, Mendrisio, Switzerland), 4 mg/kg xylazine ip (Rompun; Bayer, Leverkusen, Germany), and 80 mg/kg ketamine ip (Narketan 10; Vetoquinol, Bern, Switzerland). The left dorsal (afferent) vagal rootlets at the brain stem and the dorsal (left) esophageal trunk of the vagus in the abdomen were visualized and sectioned as previously described (3, 30, 41). For sham surgeries the skull and abdomen were opened, and the vagal rootlets and the abdominal vagus were exposed but not manipulated further. Carprofen (5 mg/kg sc; Rimadyl; E. Gräub, Bern, Switzerland) was injected once daily for 3 days for postsurgical analgesia. Food was withheld for 12 h after surgery, and only sweetened milk and moistened sweet chow mash (sweetened milk + powdered chow, 2:3) were offered for 2 to 3 days before reinstating access to ground chow.
SDA rats recovered from surgery in ∼10 days, as indicated by stable food intakes and increasing body weight trajectories. Beginning 2 wk after surgery, independent crossover tests of dark-onset intraperitoneal injections of 2.5 and 5 mg/kg C75 vs. RPMI were done, with 3 days between trials. Saline injections were given on the intervening days to minimize possible carryover effects. Food intake was measured 2, 4, and 24 h after injection.
SDA was verified functionally and histologically, as previously published (3, 30). Loss of vagally mediated satiation in response to CCK (27) was used as a functional verification of the SDA. After a 4-h fast, the rats were intraperitoneally injected with 4 μg/kg of CCK-8 (Bachem, Budendorf, Switzerland) or saline at dark onset in a cross-over design, chow was offered, and 30-min food intake was measured. Sham-operated rats ate 41 ± 6% less after CCK than after saline. Two SDA rats that decreased 30-min food intake after intraperitoneal CCK > 30% were considered incomplete, and their data were not included in the analysis. The histological verification was based on loss of retrograde labeling of vagal motor neurons in the dorsal motor nucleus of the vagus (DMX) after injection of 2 mg/ml fluorogold ip (Fluorochrome, Denver, CO) (30, 34). The numbers of fluorogold-labeled neurons in the left and right DMX in all sections that included the area postrema were counted by an observer blind to the rat's surgery. The criterion for a complete SDA was that the number of labeled cells in the right DMX be < 3% of the number in the left DMX; this was the case in all SDA rats, resulting in a total of eight each SDA and sham rats. Food intakes in these rats at 2, 4, and 24 h were analyzed as described in Experiment 3.
RESULTS
Experiment 1: spontaneous meal patterns.
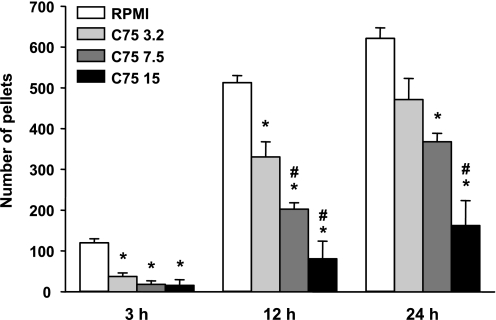
C75 potently reduced 3-, 12-, and 24-h food intakes (F3,21 = 22.83, 35.33, and 22.37, respectively, all P values <0.0001; Fig. 1). Doses of 3.2, 7.5, and 15 mg/kg C75 each reduced 3-h food intake, with no significant dose relation, perhaps because of a floor effect. All three doses also reduced total 12-h nocturnal food intake, and at this time point their potencies were significantly dose related. The effects on 24-h food intake were similar, except the effect of the 3.2 mg/kg C75 dose was no longer significant.
Fig. 1.
Effect of 3.2, 7.5, or 15 mg/kg C75 ip on 3-, 12-, and 24-h cumulative intakes of 45-mg food pellets. C75 or vehicle (RPMI) were tested in a within-subject crossover design, with 1 wk between trials. Each bar represents the mean ± SE of 8 rats. *Less than after RPMI, P < 0.05; #C75 effect larger than that of the next lower dose P < 0.05.
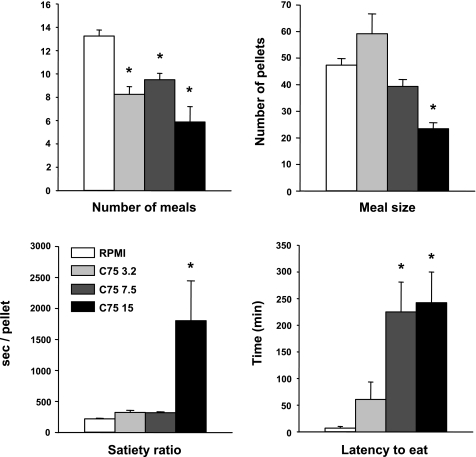
The eating inhibitory actions of 3.2 and 7.5 mg/kg C75 were expressed as decreases in the number of nocturnal spontaneous meals (F3,21 = 16.07, P < 0.0001) with no change in nocturnal meal size (Fig. 2). Only 15 mg/kg C75 decreased meal size (F3,21 = 13.45, P < 0.0001) as well as meal number (F3,21 = 16.07, P < 0.0001), with the result that this dose also markedly increased the satiety ratio (F3,21 = 4.97, P < 0.01). The latency to the first nocturnal meal was markedly, and similarly, increased by 7.5 and 15 mg/kg C75 (F3,21 = 6.42, P < 0.01).
Fig. 2.
Effect of C75 on nocturnal meal patterns. Intraperitoneal injections of 3.2, 7.5, or 15 mg/kg C75 or vehicle (RPMI) were tested in a within-subject crossover design, with 1 wk between trials. Pellets weighed 45 mg. Each bar represents the mean ± SE of 8 rats. *Less than after RPMI, P < 0.05.
Experiments 2 and 4: CTA.
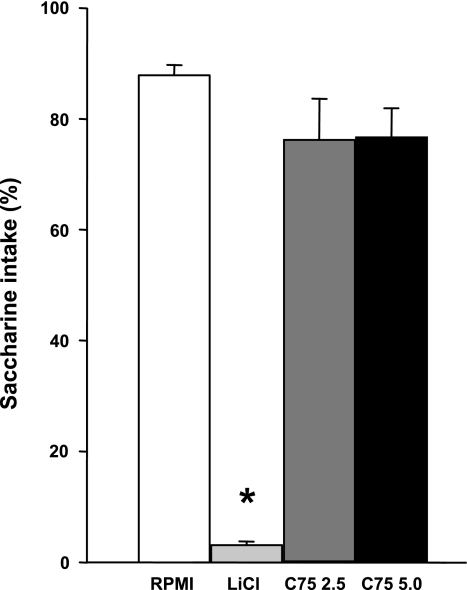
The potency of intraperitoneal C75 to support CTA learning appeared to be dose dependent. That is, in experiment 2, 15 mg/kg C75 elicited a marked reduction in saccharin preference (9.5 ± 4.1% and 87.0 ± 5.3%) in C75 and control rats, respectively, (t13 = 11.69, P < 0.001), whereas in experiment 4, neither 2.5 nor 5 mg/kg C75 detectably reduced saccharine preference, although LiCl, the positive control, did (F3,44 = 64.25, P < 0.0001; Fig. 3). In contrast, in experiment 4, 5 mg/kg C75 and LiCl both reduced food intake at 1 and 2 h after injection (1 h: F3,44 = 4.50, P < 0.01; 2 h: F3,44 = 3.36, P < 0.05; Table 1). At 4 h, 5 mg/kg C75, but not LiCl, still appeared to reduce food intake, but the overall ANOVA did not reveal statistical significance (4 h: F3,44 = 2.68, P = 0.06; 24 h: F3,44 = 1.27, P > 0.05; Table 1).
Fig. 3.
Effect of C75 or LiCl on 0.5-h 0.125% saccharin intake as %total fluid intake in a 2-bottle saccharin vs. water preference test. Intraperitoneal injections on the previous (conditioning) day were C75 (2.5 or 5.0 mg/kg), LiCl at 60 mg/kg, or vehicle (RPMI). Each bar represents the mean ± SE of 12 rats. *Less than after RPMI, P < 0.05.
Table 1.
Comparison of the eating inhibitory effects of LiCl and C75
|
Time |
||||
|---|---|---|---|---|
| 1 h | 2 h | 4 h | 24 h | |
| RPMI, 1 ml/kg | 3.1±0.4 | 6.2±0.6 | 6.9±0.7 | 23.5±0.7 |
| LiCl, 60 mg/kg | 1.6±0.4* | 3.9±0.6* | 5.7±0.8 | 21.7±0.7 |
| C75, 2.5 mg/kg | 2.7±0.6 | 4.8±0.7 | 6.8±0.8 | 21.8±1.1 |
| C75, 5.0 mg/kg | 1.1±0.4* | 3.3±0.7* | 4.2±0.6 | 21.1±1.0 |
Values are means ± SE.
Less than vehicle (RPMI) (P < 0.05, Bonferroni-Holm test after significant ANOVA).
Experiment 3: TVX.
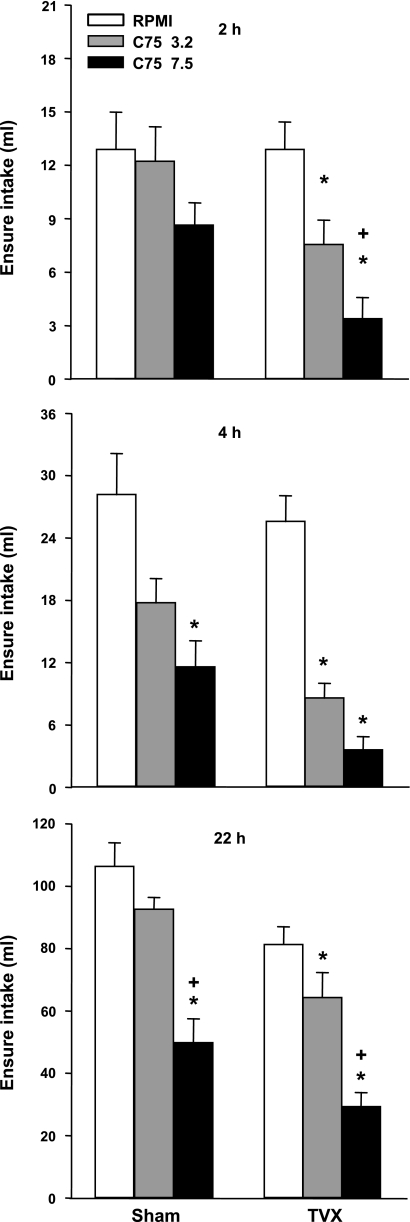
TVX did not reduce the eating inhibitory effect of C75. Rather, C75 significantly reduced cumulative food intake at each measurement in TVX rats and less statistically reliably in sham-operated rats (Fig. 4). The ANOVA results for cumulative food intakes in the sham and TVX rats, respectively, were: F2,10 = 2.01, not significant, and 25.02, P < 0.0001, after 2 h; 6.61, P < 0.05, and 78.36, P < 0.001, after 4 h; and F2,10 = 15.45 and 19.07, P values < 0.001, after 22 h. However, direct comparisons between the surgical groups produced no statistical evidence that either C75 dose affected eating differently in TVX and sham rats.
Fig. 4.
Effect of intraperitoneal injections of 3.2 or 7.5 mg/kg C75 or vehicle (RPMI) on cumulative 2-, 4-, and 22-h Ensure intakes in rats with total subdiaphragmatic vagotomy (TVX) or sham operations. The injections were done at dark onset in a crossover design, with 1 wk between trials. Each bar represents the mean ± SE of 6 TVX and 6 sham rats. *Less than RPMI in same surgical group, P < 0.05 ; +less than 3.2 mg/kg C75 in the same surgical group, P < 0.05.
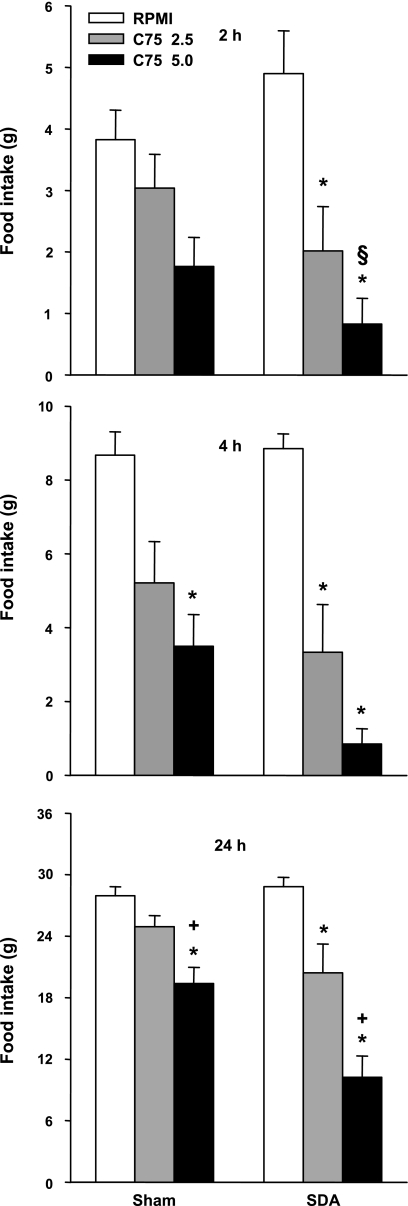
Experiment 5: SDA.
SDA did not reduce the eating inhibitory effect of C75. Again, C75 significantly reduced cumulative food intake at each measurement in SDA rats and less statistically reliably in sham-operated rats (Fig. 5). The ANOVA results for cumulative food intakes in the sham and SDA rats, respectively, were: F2,14 = 3.46, P = 0.06, and 9.03, P < 0.005, after 2 h; 6.44, P < 0.01, and 22.32, P < 0.001, after 4 h; and 16.26 and 25.53, P values < 0.001 after 24 h. Planned comparisons between the surgical groups indicated that the effect of 5.0 mg/kg C75 at 2 h was greater in SDA than in sham rats, perhaps in part because the SDA rats tended to eat more. No other significant differences were detected between the eating inhibitory effects of C75 in SDA and sham rats.
Fig. 5.
Effect of intraperitoneal injections of 2.5 or 5.0 mg/kg C75 or vehicle (RPMI) on 2-, 4-, and 24-h cumulative food intakes in rats with subdiaphragmatic vagal deafferentations (SDA) or sham operations. The injections were done at dark onset in a crossover design, with 3 days between trials. Each bar represents the mean ± SE of 8 SDA and 8 sham rats. *Less than RPMI in same surgical group P < 0.05; +less than 2.5 mg/kg C75 in the same surgical group, P < 0.05; §difference between effects of RPMI and 5.0 mg/kg C75 in SDA rats larger than in sham rats, P < 0.05.
DISCUSSION
The first aim of this study was to test whether low intraperitoneal doses of C75 inhibit eating in a behaviorally specific manner. This question arises from the report of Clegg et al. (10) that intraperitoneal injection of 15 mg/kg C75 induced CTA and pica in rats. To address this question, we assessed the effects of injections of 3.2, 7.5, and 15 mg/kg C75 ip on spontaneous meal patterns and on CTA formation. Our second aim was to determine whether the eating inhibitory effect of intraperitoneal C75 requires abdominal vagal afferents. Centrally administered C75 clearly has a direct central action that is sufficient to inhibit eating (1, 2, 10, 20), but this has not been shown yet for peripherally administered C75. Peripheral C75 could, for example, inhibit eating by activating vagal afferents, a hypothesis suggested by demonstrations that C75 can stimulate peripheral FAO (38, 39) and that abdominal vagal afferents are involved in the eating stimulatory effect of FAO inhibitors (5, 17, 23, 35). Our results indicate that intraperitoneal C75 can potently inhibit eating in the absence of a CTA and show that this occurs in the absence of abdominal vagal afferent signaling.
In our first series of tests, the threshold intraperitoneal C75 dose for a significant reduction in 22- or 24-h food intake was between 3.2 and 7.5 mg/kg, and in the second series, it was between 2.5 and 5.0 mg/kg. These are similar to the threshold of between 3 and 10 mg/kg C75 reported by Clegg et al. (10) who used a slightly different procedure. Neither 2.5 nor 5.0 mg/kg C75 elicited a CTA in a sensitive two-bottle test; although 5.0 mg/kg C75 reduced food intake as much or more than 60 mg/kg LiCl, which produced a marked CTA. As stated previously (10), 15 mg/kg C75 elicited a CTA. It is also interesting that 3.2 and 7.5 mg/kg C75 inhibited eating by selectively reducing meal frequency, whereas 15 mg/kg C75 reduced both meal frequency and meal size and increased the satiety ratio. The relative consistency of these data across experiments performed in two different laboratories under slightly different conditions (i.e., in rats fed chow or Ensure, in animals from different providers, etc.) suggests that low intraperitoneal doses (≈5–7.5 mg/kg) of C75 selectively influence controls of meal frequency in the absence of toxic or aversive effects and that such aversive effects appear with a threshold between 7.5 and 15 mg/kg C75 and cause reductions in meal size. To our knowledge, this is the first report of hypophagia in response to such low peripheral doses of C75 and the first dissociation of hypophagia and CTA in response to peripheral C75 in rats.
The doses of C75 that appeared to inhibit eating in a behaviorally specific fashion in intact rats also inhibited eating in rats with TVX or SDA, indicating that neither abdominal vagal afferents nor abdominal vagal efferents are necessary for the eating inhibitory effect of intraperitoneal C75. The most plausible explanation for these results is that peripherally administered C75 acts in the brain, not in the periphery, to inhibit eating. This is consistent with several other findings. First, as mentioned above, there is strong evidence that central administration of C75 is sufficient to inhibit eating (1, 2, 10, 20). Second, behaviorally specific intraperitoneal C75 treatment and central C75 treatment both produce the same alteration in meal pattern, i.e., a reduction in meal frequency (1). Third, radiolabeled C75 crosses the blood-brain barrier (25). Finally, in a recent study, mice lacking FAS specifically in the hypothalamus and β-cells of the pancreas were hypophagic but appeared to have normal insulin secretion (9).
Whether the central actions C75 also affect peripheral metabolism, such as those derived from peripheral FAO, requires further research. It is interesting to note in this context that intracerebroventricular administration of the α-adrenergic receptor antagonist phentolamine reversed the stimulatory effect of C75 on FAO in mice, suggesting that C75 acts in the brain to elicit a signal relayed via the splanchnic nerves to the periphery (8). Our data do not, however, prove that C75 acts only in the brain to inhibit eating. Rather, intraperitoneal C75 may also have peripheral endocrine or metabolic effects that indirectly affect the brain through a humoral signal, or may elicit a peripheral eating inhibitory signal that is relayed to the brain via spinal visceral afferents, which are unaffected by vagotomy.
Finally, it is interesting to note that the eating inhibitory effect of C75 tended to be increased in TVX and SDA rats and was significantly increased 2 h after 5.0 mg/kg C75 in SDA rats. This suggests that SDA may remove a dampening or compensatory effect on the central mechanism of C75-induced inhibition of feeding. We can only speculate on how this might happen. For example, there is evidence that vagal afferent signaling reduces the sensitivity of hindbrain neural circuits that control vagal efferent outflow in response to other neuropeptides (6). If recruitment of such circuits, perhaps through descending hypothalamic hindbrain projections (28), contributes to the effect of C75, C75 might cause a greater inhibition of food intake after SDA due to release from the tonic inhibition of these circuits by vagal afferent signaling.
In summary, our data show for the first time that intraperitoneal injections of the FAS inhibitor C75 have a behavior-specific inhibitory effect on eating that is expressed as a decrease in spontaneous meal frequency and that does not require intact vagal afferents or efferents. These data suggest that this eating inhibitory effect is mainly due to a direct central action of C75.
Perspectives and Significance
The selective effect of C75 on spontaneous meal frequency rather than meal size is unusual and suggests that it does not act through the same central mechanism as most of the better-understood controls of eating, including both peripheral satiation signals, such as CCK, glucagon, and amylin, and adiposity signals, such as leptin and insulin, all of which selectively reduce spontaneous meal size in rats (see Refs. 14, 42). Intraperitoneal administration of the FAO inhibitor mercaptoacetate also stimulates eating by increasing meal frequency (24). This raises the possibility that modulation of FAO, either in the periphery or the brain, affects meal initiation, and thus meal frequency, rather than termination. Peripheral C75 and mercaptoacetate do not share identical mechanisms, however, because, as shown here, intraperitoneal C75 does not require vagal afferents to affect eating, whereas mercaptoacetate stimulates eating by activating vagal afferents (5). The effect of FAO on meal frequency appears to be consistent with the gradual increase in the contribution of FAO to the nutrient mix that is oxidized after fat-containing meals (37). Only a few other hypothesized physiological controls of eating, for example, transient declines in plasma glucose (7) and oleoethylanolamine (12), appear to have such selective actions on meal initiation. The functional and mechanistic interrelations of these controls of eating deserve research.
GRANTS
This work was funded by National Institutes of Health Grants DK-068054 (to S. Aja), DC-02979 and DK-064000 (to G. V. Ronnett), CA-087850 (to F. P. Kuhajda), DK-019302 (to T. H. Moran), and DK-060735 (to W. Langhans and N. Geary).
DISCLOSURES
FASgen provided C75 for the experiments. Under a licensing agreement between FASgen and the Johns Hopkins University, F. P. Kuhajda, and G. V. Ronnett are entitled to a share of royalties received by the University on sales of products described in this article. F. P. Kuhajda owns FASgen stock. G. V. Ronnett and T. H. Moran have an interest in FASgen stock that is subject to restrictions under University policy. The Johns Hopkins University manages the terms of these agreements in accordance with its policies on conflict of interest.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aja S, Bi S, Knipp SB, McFadden JM, Ronnett GV, Kuhajda FP, Moran TH. Intracerebroventricular C75 decreases meal frequency and reduces AgRP gene expression in rats. Am J Physiol Regul Integr Comp Physiol 291: R148–R154, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Aja S, Landree LE, Kleman AM, Medghalchi SM, Vadlamudi A, McFadden JM, Aplasca A, Hyun J, Plummer E, Daniels K, Kemm M, Townsend CA, Thupari JN, Kuhajda FP, Moran TH, Ronnett GV. Pharmacological stimulation of brain carnitine palmitoyl-transferase-1 decreases food intake and body weight. Am J Physiol Regul Integr Comp Physiol 294: R352–R361, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci 26: 11052–11060, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85: 1–17, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Brandt K, Arnold M, Geary N, Langhans W, Leonhardt M. Vagal afferents mediate the feeding response to mercaptoacetate but not to the β3-adrenergic receptor agonist CL 316,243. Neurosci Lett 411: 104–107, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Browning KN, Zheng Z, Gettys TW, Travagli RA. Vagal afferent control of opioidergic effects in rat brainstem circuits. J Physiol 575: 761–776, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campfield LA, Smith FJ. Blood glucose dynamics and control of meal initiation: a pattern detection and recognition theory. Physiol Rev 83: 25–58, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Cha SH, Hu Z, Chohnan S, Lane MD. Inhibition of hypothalamic fatty acid synthase triggers rapid activation of fatty acid oxidation in skeletal muscle. Proc Natl Acad Sci USA 102: 14557–14562, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarthy MV, Zhu Y, Lopez M, Yin L, Wozniak DF, Coleman T, Hu Z, Wolfgang M, Vidal-Puig A, Lane MD, Semenkovich CF. Brain fatty acid synthase activates PPARα to maintain energy homeostasis. J Clin Invest 117: 2539–2552, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clegg DJ, Wortman MD, Benoit SC, McOsker CC, Seeley RJ. Comparison of central and peripheral administration of C75 on food intake, body weight, and conditioned taste aversion. Diabetes 51: 3196–3201, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Friedman MI, Tordoff MG. Fatty acid oxidation and glucose utilization interact to control food intake in rats. Am J Physiol Regul Integr Comp Physiol 251: R840–R845, 1986. [DOI] [PubMed] [Google Scholar]
- 12.Fu J, Kim J, Oveisi F, Astarita G, Piomelli D. Targeted enhancement of oleoylethanolamide production in proximal small intestine induces across-meal satiety in rats. Am J Physiol Regul Integr Comp Physiol 295: R45–R50, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao S, Lane MD. Effect of the anorectic fatty acid synthase inhibitor C75 on neuronal activity in the hypothalamus and brainstem. Proc Natl Acad Sci USA 100: 5628–5633, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geary N Endocrine controls of eating: CCK, leptin, and ghrelin. Physiol Behav 81: 719–733, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Grundy D Signalling the state of the digestive tract. Auton Neurosci 125: 76–80, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Holm S A simple sequentially rejective multiple test procedure. Scand J Stat 6: 65–70, 1979. [Google Scholar]
- 17.Horn CC, Tordoff MG, Friedman MI. Role of vagal afferent innervation in feeding and brain Fos expression produced by metabolic inhibitors. Brain Res 919: 198–206, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Houpt TA, Frankmann SP. TongueTwister: an integrated program for analyzing lickometer data. Physiol Behav 60: 1277–1283, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Jambor de Sousa UL, Koss MD, Fillies M, Gahl A, Scheeder MR, Cardoso MC, Leonhardt H, Geary N, Langhans W, Leonhardt M. CPT1-α over-expression increases long-chain fatty acid oxidation and reduces cell viability with incremental palmitic acid concentration in 293T cells. Biochem Biophys Res Commun 338: 757–761, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem 279: 19970–19976, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Kuhajda FP Fatty acid synthase and cancer: new application of an old pathway. Cancer Res 66: 5977–5980, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Landree LE, Hanlon AL, Strong DW, Rumbaugh G, Miller IM, Thupari JN, Connolly EC, Huganir RL, Richardson C, Witters LA, Kuhajda FP, Ronnett GV. C75, a fatty acid synthase inhibitor, modulates AMP-activated protein kinase to alter neuronal energy metabolism. J Biol Chem 279: 3817–3827, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Langhans W, Scharrer E. Evidence for a vagally mediated satiety signal derived from hepatic fatty acid oxidation. J Auton Nerv Syst 18: 13–18, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Leonhardt M, Langhans W. Fatty acid oxidation and control of food intake. Physiol Behav 83: 645–651, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 288: 2379–2381, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Miller I, Ronnett GV, Moran TH, Aja S. Anorexigenic C75 alters c-Fos in mouse hypothalamic and hindbrain subnuclei. Neuroreport 15: 925–929, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol Regul Integr Comp Physiol 272: R1245–R1251, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 443: 289–295, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Nicot C, Napal L, Relat J, Gonzalez S, Llebaria A, Woldegiorgis G, Marrero PF, Haro D. C75 activates malonyl-CoA sensitive and insensitive components of the CPT system. Biochem Biophys Res Commun 325: 660–664, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Norgren R, Smith GP. A method for selective section of vagal afferent or efferent axons in the rat. Am J Physiol Regul Integr Comp Physiol 267: R1136–R1141, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 9: 756–761, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Opsahl CA, Powley TL. Body weight and gastric acid secretion in rats with subdiaphragmatic vagotomy and lateral hypothalamic lesions. J Comp Physiol Psychol 91: 1284–1296, 1977. [DOI] [PubMed] [Google Scholar]
- 33.Pocai A, Lam TK, Obici S, Gutierrez-Juarez R, Muse ED, Arduini A, Rossetti L. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest 116: 1081–1091, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol Regul Integr Comp Physiol 253: R361–R370, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Ritter S, Taylor JS. Vagal sensory neurons are required for lipoprivic but not glucoprivic feeding in rats. Am J Physiol Regul Integr Comp Physiol 258: R1395–R1401, 1990. [DOI] [PubMed] [Google Scholar]
- 36.Scharrer E, Langhans W. Control of food intake by fatty acid oxidation. Am J Physiol Regul Integr Comp Physiol 250: R1003–R1006, 1986. [DOI] [PubMed] [Google Scholar]
- 37.Surina DM, Langhans W, Pauli R, Wenk C. Meal composition affects postprandial fatty acid oxidation. Am J Physiol Regul Integr Comp Physiol 264: R1065–R1070, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Thupari JN, Kim EK, Moran TH, Ronnett GV, Kuhajda FP. Chronic C75 treatment of diet-induced obese mice increases fat oxidation and reduces food intake to reduce adipose mass. Am J Physiol Endocrinol Metab 287: E97–E104, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Thupari JN, Landree LE, Ronnett GV, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc Natl Acad Sci USA 99: 9498–9502, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tu Y, Thupari JN, Kim EK, Pinn ML, Moran TH, Ronnett GV, Kuhajda FP. C75 alters central and peripheral gene expression to reduce food intake and increase energy expenditure. Endocrinology 146: 486–493, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Walls EK, Wang FB, Holst MC, Phillips RJ, Voreis JS, Perkins AR, Pollard LE, Powley TL. Selective vagal rhizotomies: a new dorsal surgical approach used for intestinal deafferentations. Am J Physiol Regul Integr Comp Physiol 269: R1279–R1288, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci 361: 1219–1235, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wortman MD, Clegg DJ, D'Alessio D, Woods SC, Seeley RJ. C75 inhibits food intake by increasing CNS glucose metabolism. Nat Med 9: 483–485, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Yamada T, Katagiri H. Avenues of communication between the brain and tissues/organs involved in energy homeostasis. Endocr J 54: 497–505, 2007. [DOI] [PubMed] [Google Scholar]