Abstract
Reduced uteroplacental blood flow is hypothesized to play a key role in altitude-associated fetal growth restriction. It is unknown whether reduced blood flow is a cause or consequence of reduced fetal size. We asked whether determinants of uteroplacental blood flow were altered prior to reduced fetal growth and whether vasoactive and/or angiogenic factors were involved. Women residing at low (LA; 1600 m, n = 18) or high altitude (HA; 3100 m, n = 25) were studied during pregnancy (20, 30, and 36 wk) and 4 mo postpartum (PP) using Doppler ultrasound. In each study, endothelin (ET-1), nitric oxide metabolites (NOx), soluble fms-like tyrosine kinase (sFlt-1) and placental growth factor (PlGF) levels were quantified. At HA, birth weights were lower (P < 0.01) and small-for-gestational age was more common (P < 0.05) compared with LA. HA was associated with lower uterine artery (UA) diameter (P < 0.01) and blood flow (P < 0.05). Altitude did not affect ET-1, sFlt-1 or PlGF; however, ET-1/NOx was greater and NOx lower during pregnancy and PP at HA vs. LA. ET-1/NOx was negatively associated with birth weight (20 wk, P < 0.01; 36 wk, P = 0.05) at LA and HA combined. At HA, UA blood flow (30 wk) was positively associated with birth weight (†). UA blood flow and ET-1/NOx levels accounted for 45% (20 wk) and 32% (30 wk) of birth weight variation at LA and HA combined, primarily attributed to effects at HA. We concluded that elevated ET-1/NOx and altered determinants of uteroplacental blood flow occur prior to altitude-associated reductions in fetal growth, and therefore, they are likely a cause rather than a consequence of smaller fetal size.
Keywords: fetal growth restriction, hypoxia, small-for-gestational age, uteroplacental oxygen delivery, pregnancy
small-for-gestational age (SGA) is a common complication of pregnancy that raises the risk of morbidity and mortality during the perinatal period, as well as in later life (2, 11). Among the many determinants of SGA is the chronic hypoxia of residence at high altitude (≥2,500 m; 8,200 ft). Birth weight declines progressively, and potently, with increasing altitude such that infants born to women permanently residing at elevations ≥2,500 m are at a greater risk of low birth weight than infants born at lower altitudes (13–15, 34). Altitudinal differences in gestational age, socioeconomic status, parity, maternal height, or the frequency of hypertensive complications do not explain the degree to which birth weight decreases with altitude, indicating that it is likely chronic hypoxia per se that is decreasing fetal growth (12, 13, 15). Despite the pervasive effect of high altitude on birth weight, the mechanisms by which hypoxia acts to reduce fetal growth are not well understood.
A considerable literature supports the hypothesis that reduced oxygen delivery to the uteroplacental circulation increases the risk of SGA at high altitude. Previously we have shown that uterine artery (UA) diameter and blood flow are lower near term in women residing at high (3,100 m; 10,170 ft, Leadville) compared with low altitude (1,600 m; 5,280 ft, Denver) in Colorado (36). Greater maternal arterial oxygen content and/or UA blood flow, both of which are determinants of uteroplacental oxygen delivery, are associated with protection from SGA at high altitude (20, 28, 32, 35, 36). The frequency of preeclampsia, another condition in which UA blood flow is reduced, also increases with altitude and is responsible for approximately one-half of the altitude-associated reduction in birth weight, further demonstrating the importance of adequate UA blood flow to fetal growth at high altitude (13, 15). It is unknown whether the hypoxia-associated reduction in UA blood flow contributes to or is a result of smaller fetal size since prior studies comparing the effect of altitude on UA blood flow used a single time point late in pregnancy (36, 37). Further, the mechanisms by which chronic hypoxia acts to reduce the pregnancy-associated rise in UA diameter and blood flow remain unknown.
We hypothesized that the pregnancy-associated rise in UA diameter and blood flow was diminished at high relative to low altitude by midpregnancy, prior to the reduction in fetal growth and thus likely contributes to reduced birth weight at high altitude. We also hypothesized that high-altitude pregnancy was associated with greater levels of vasoconstrictors relative to vasodilators, and antiangiogenic relative to proangiogenic factors, in the maternal circulation compared with low-altitude pregnancy. Specifically, we asked whether levels of the vasoconstrictor endothelin (ET-1) relative to the vasodilator nitric oxide metabolite (NOx) levels and/or levels of the antiangiogenic factor soluble fms-like tyrosine kinase 1 (sFlt-1) relative to the proangiogenic placental-like growth factor (PLGF) were greater at high than low altitude and, if so, whether variation in these factors contributed to altitudinal differences in UA blood flow parameters or birth weight. To test these hypotheses, we measured UA blood flow, fetal biometry, and circulating levels of ET-1, NOx, sFlt-1, and PLGF serially during pregnancy in 43 healthy residents of low (1,600 m, Denver) or high (3,100 m, Leadville) altitude in Colorado. Birth weight and other newborn information were obtained from labor and delivery records.
MATERIALS AND METHODS
Subject population.
Subjects were recruited through their prenatal care providers or by notices posted in local clinics and hospitals. Inclusion criteria were that the woman be of good health and normal nutritional status, ≤20 wk pregnant, having a singleton pregnancy, receiving prenatal care, and willing to participate. Exclusion criteria were factors known to increase the risk of preeclampsia and/or alter fetal growth such as obesity, multiple gestations, gestational diabetes, diabetes, or chronic hypertension. Forty-three women residing at 1,600 m (n = 18, Denver) or 3,100 m (n = 25, Leadville) in Colorado were enrolled for study; all women included for study met these criteria and gave their informed consent to study procedures previously approved by the Colorado Multiple Institutional Review Board of the University of Colorado Denver. None of the women developed gestational diabetes, and only one woman developed late-onset preeclampsia (36 wk); her study results were retained for the present analyses, as her data did not differ from those of normotensive subjects for any of the primary outcome variables.
Study protocol.
Women were scheduled for study at weeks 20, 30, and 36 of pregnancy and 4 mo postpartum for a measurement in the nonpregnant state, since it was not possible to recruit women prior to their pregnancy. Actual times of study were 21.6 ± 0.3, 30.7 ± 0.3, 36.0 ± 0.2 wk of pregnancy and 4.4 ± 0.4 mo postpartum. Reproductive history and demographic characteristics were obtained by questionnaire on the first visit. Each study consisted of a general clinical exam, which was followed by a blood draw and ultrasound study. The general clinical exam included assessments of maternal body weight using a balance scale, height by stadiometer, blood pressure by arm cuff sphygmomanometer (expressed as an average of right and left sides), heart rate, arterial oxygen saturation (SaO2) by transdermal pulse oximetry and qualitative determinations of proteinuria and edema. Women were classified as preeclamptic when both hypertension (2+ blood pressure measurements >140 mmHg systolic and/or >90 mmHg diastolic at least 6 hrs apart) and proteinuria (qualitative reading of >1+ proteinuria or 300 mg/l in a 24-h collection) were noted during physical examination and/or in medical records. Women with a single elevated blood pressure with or without proteinuria were classified as normal.
Assessment of angiogenic and vasoactive factors.
Venous blood samples were collected with minimal use of a tourniquet from either the antecubital or dorsal hand vein. Samples were processed for measurement of hematocrit in duplicate using the microcentrifuge technique and of hemoglobin in triplicate using the cyanmethemoglobin method. Plasma samples were obtained from venous blood withdrawn into collection tubes containing EDTA, centrifuged, and stored in aliquots at −80°C for the measurement of sFlt-1 (specificity of 3.5 pg/ml; 5.5% and 3.2% for interassay and intra-assay precision, respectively) and PLGF (specificity of 7 pg/ml; 10.9% and 5.6% inter- and intra-assay precision, respectively) by immunoassay (R&D Systems, Minneapolis, MN) following manufacturer's specifications. Serum samples were prepared promptly and stored in aliquots at −80°C for subsequent assays of circulating vasoactive factors; namely, ET-1 and nitric oxide metabolites (nitrates, nitrites, and nitrosothiols [NOx]). ET-1 levels were quantified by immunoassay (specificity of 0.064 pg/ml; 4.6% and 2.6% inter-assay and intra-assay precision, respectively; R&D Systems, Minneapolis, MN) following manufacturer specifications. NOx levels were measured in triplicate using a NO chemiluminescence analyzer (model no. 280; Sievers, Boulder, CO), which measures combined NO, NO2-, NO3-, and nitrosothiol levels. This assay uses serum samples withdrawn into N2-flushed syringes and injected into the analyzer which contains 2 ml of 0.1 M vanadium chloride in 1 N HCl and heated to 90°C to convert the NO2-, NO3, and nitrosothiols back to NO. We generated linear calibration curves by measuring the NO produced by 10 pM sodium nitrate solution (minimum detection limit = 5 mM, repeatability for NOx quantities in nitrate standards in serum = 10%). Values were averaged from results of duplicate or triplicate tests at each time point.
Ultrasound studies.
Vessel diameters, velocities, and fetal biometry were measured pericutaneously using an ATL3000 ultrasound unit with a 4 MHz curved linear array probe that had been configured for obstetrical use with color imaging and Doppler. Maternal vessels were examined bilaterally, and results were reported as a single value—the average from the right and left sides—for diameter, velocities, and volumetric flow. The same operator performed all measurements for studies carried out in Denver, as well as in Leadville, so as to minimize interoperator variability. For each study, the common iliac and external iliac arteries were visualized 2–3 cm anterior and posterior, respectively, to the bifurcation of the external and internal iliac arteries. The UA was measured at its crossover with the external. Vessel diameters were obtained first, with mean luminal diameter calculated from the images obtained in the common and external iliac arteries without color at midsystole and middiastole as {[2 × (diastolic + systolic diameter)]/3}. Images without color were used since color imaging exaggerates luminal diameter. Because it was difficult to visualize the UA without color and to discriminate between systole and diastole in all subjects, values were obtained from the best image, irrespective of cardiac phase and adjusted to remove the effect of color imaging by using the linear relationship between the with- (x) and without- (y) color values measured in the common iliac artery at an equivalent anatomical depth to that used for visualization of the UA. Once the diameter measurements were completed, the probe was rotated using anatomical landmarks to ensure that the same portion of the vessel was being insonated, a series of 5–7 cardiac beats selected with the angle of insonation and size of the sampling frame being adjusted to obtain optimal velocity signals. After selecting the High Q-Automatic Doppler Measurement mode, time-averaged mean flow velocity (TAM) was calculated for the same consecutive beats, the mean vessel diameter entered, and volumetric flow calculated as (r2 × TAM × 60) where r is the vessel radius in centimeters squared, TAM expressed in centimeters per second, and flow in milliliters per minute. Because high angles of insonation introduce considerable error into the measurement of velocity, the angle was required to be ≤45° for all velocity and volumetric flow calculations. Peak systolic velocity, either minimum or end-diastolic velocity, and the TAM values were calculated using a minimum of three and usually at least five consecutive cardiac cycles.
Uteroplacental vascular resistance was calculated as mean arterial blood pressure (mmHg)/UA volumetric flow (ml/min). UA vascular resistance indices were noted as well; namely, the UA pulsatility index [PI; (peak systolic velocity − peak end-diastolic velocity)/mean peak flow velocity], resistance index [RI; (peak systolic velocity − peak end-diastolic velocity)/peak systolic velocity] and the systolic to diastolic ratio (S/D, peak systolic velocity/peak end-diastolic velocity). The ratios of external to common iliac flow (EI/CI), UA to common iliac flow (UA/CI), and uterine to external iliac artery flow (UA/EI) were calculated as indices of the distribution of common iliac flow to the leg (external iliac) vs. the uteroplacental circulation (UA).
Assessment of fetal growth and birth weight.
Fetal biometry (abdominal and head circumference, biparietal diameter, and femur length) was conducted by Doppler ultrasound at 20, 30, and 36 wk of gestation. Peak systolic/end-diastolic (S/D) resistance indices for the fetal umbilical and middle cerebral arteries were also recorded. Birth weight, gestational age, sex, and length were obtained from medical records completed by hospital personnel at the time of delivery. Gestational age was calculated as that estimated at the week 20 ultrasound exam and, in all cases, was within 2 wk of that that calculated using weeks from the last menstrual period. Babies were classified as SGA when the gestational age- and sex-specific birth weight was less than the 10th percentile of published sea-level values (31). Babies born prior to 37 wk of gestational age were considered to be preterm. Ponderal index was calculated as kilograms per cubic meter.
Statistics.
Data are expressed as the means ± SE or percentages and 95% confidence intervals (CI) for proportions in the text, tables, and figures. All maternal blood flow and resistance values were averaged from measurements obtained on the woman's right and left sides. Altitude effects at single time points were evaluated using Student's t-tests for continuous variables and χ2-tests for nominal or ordinal variables. The effect of pregnancy or altitude (temporal comparisons) was assessed using one- or two-way ANOVA or analysis of covariance with Tukey's multiple comparisons test. Multiple linear regression models were used to identify maternal and infant characteristics related to birth weight among our study subjects, with the criterion for inclusion or exclusion set at P ≤ 0.10. All analyses were conducted using Statview (SAS, Santa Cruz, CA) or SPSS (Chicago, IL). Comparisons or relationships were considered significant when the two-tailed P < 0.05 and as trends when 0.05 < P < 0.10.
RESULTS
Maternal characteristics.
Maternal age, height, body mass index and body weight before pregnancy were similar between women residing at low (1,600 m) and high (3,100 m) altitude (Table 1). Gravidity and the proportion of women who were primigravid were also equivalent, although women residing at 3,100 m had slightly greater parity than those at 1,600 m. The week at which prenatal care began, the number of prenatal visits, maternal weight near term, pregnancy weight gain, and blood pressure at term were also similar between altitudes (Table 1).
Table 1.
Maternal characteristics
| Variable | Low Altitude | High Altitude | P Value |
|---|---|---|---|
| Altitude, m | 1600 | 3100 | |
| Age, yr | 28.2±1.0 (18) | 25.6±1.3 (25) | NS |
| Height, cm | 167.2±1.9 (16) | 162.5±2.3 (21) | NS |
| BMI, prepregnant, kg/m2 | 23.1±0.7 (16) | 24.1±1.1 (20) | NS |
| Body weight, | |||
| Prepregnant, kg | 66.0±2.3 (18) | 62.4±2.6 (22) | NS |
| Week 36, kg | 77.8±3.5 (11) | 76.6±2.6 (22) | NS |
| Gainwk 20–36, kg | 13.0 1.0 (11) | 13.9 1.6 (19) | NS |
| Gravidity, no. pregnancies | 2.2±0.3 (18) | 3.0±0.4 (26) | NS |
| Parity, no. livebirths | 0.5±0.2 (18) | 1.4±0.3 (26) | <0.05 |
| Primigravida, 1st pregnancy, % | 44 [24, 67] | 27 [13, 46] | NS |
| Prenatal visits, # | 11.3±0.7 (15) | 12.0±0.9 (21) | NS |
| 1st visit, wk | 8.9±0.8 (15) | 11.2±1.0 (21) | NS |
| Time since last pregnancy, mo | 27.1±13.3 (9) | 25.9±5.7 (15) | NS |
| BP (sys/dias), term, mmHg | 129±3/75±4 (10) | 122±3/75±4 (16) | NS |
Values are expressed as means ± SE with sample sizes in parentheses. BMI, body mass index; BP, blood pressure; sys, systolic; dias, diastolic.
Maternal blood flow characteristics.
Common iliac artery diameter increased progressively with pregnancy at low but not high altitude (Table 2). Neither common iliac flow velocity nor volumetric flow changed in either group across time (Table 2). External iliac artery diameter, flow velocity, and volumetric flow remained constant across time at each altitude. Across all time points considered together or at weeks 30 and 36, external iliac flow velocity and volumetric flow were greater at high than low altitude (Table 2).
Table 2.
Maternal blood flow characteristics
| Variable | Altitude | Nonpregnant # | Week 20 | Week 30 | Week 36 | P-Time | P-Altitude |
|---|---|---|---|---|---|---|---|
| CI | |||||||
| Dia, cm | Low | 0.72±0.05 (16) | 0.64±0.04 (15)d | 0.82±0.07 (16) | 0.91±0.04 (14)b | <0.01 | NS |
| High | 0.79±0.05 (18) | 0.74±0.05 (11) | 0.79±0.05 (18) | 0.83±0.03 (24) | NS | ||
| P-altitude | NS | NS | NS | NS | |||
| TAM, cm/s | Low | 7.7±2.1 (3) | 10.8±1.2 (11) | 10.7±1.2 (9) | 11.6±3.2 (9) | NS | NS |
| High | 9.9±1.8 (8) | 12.4±2.1 (10) | 12.4±1.6 (14) | 12.2±2.0 (14) | NS | ||
| P-altitude | NS | NS | NS | NS | |||
| Flow, ml/min | Low | 237±32 (3) | 252±56 (10) | 226±38 (9) | 436±127 (9) | NS | NS |
| High | 315±71 (8) | 310±41 (10) | 406±72 (13) | 376±54 (14) | |||
| P-altitude | NS | NS | NS | NS | NS | ||
| EI | |||||||
| Dia, cm | Low | 0.58±0.03 (16) | 0.60±0.02 (16) | 0.58±0.04 (16) | 0.62±0.03 (14) | NS | NS |
| High | 0.60±0.02 (18) | 0.60±0.03 (11) | 0.63±0.02 (18) | 0.64±0.03 (24) | NS | ||
| P-altitude | NS | NS | NS | NS | |||
| TAM, cm/s | Low | 8.1±1.2 (12) | 10.5±1.8 (12) | 9.0±1.2 (12) | 8.8±1.7 (9) | NS | <0.05 |
| High | 13.4±2.2 (7) | 9.5±1.6 (10) | 11.6±1.2 (18) | 10.7±0.8 (24) | NS | ||
| P-altitude | <0.05 | NS | NS | NS | |||
| Flow, ml/min | Low | 110±18 (12) | 164±21 (11) | 120±14 (12) | 134±23 (9) | NS | <0.05 |
| High | 214±38 (7) | 185±61 (10) | 227±30 (18) | 228±29 (24) | NS | ||
| P-altitude | <0.05 | NS | <0.05 | NS† | |||
| UA | |||||||
| Dia, cm | Low | 0.34±0.03 (16)b,c,d | 0.53±0.02 (17)a | 0.50±0.02 (17)a | 0.50±0.02 (14)a | <0.001 | <0.01 |
| High | 0.37±0.01 (18)b,c,d | 0.45±0.02 (11)a | 0.46±0.01 (18)a | 0.45±0.01 (24)a | <0.001 | ||
| P-altitude | NS | <0.01 | NS | <0.05 | |||
| TAM, cm/s | Low | 5.4±0.6 (16)b,c,d | 31.5±3.6 (16)a | 41.9±5.6 (17)a | 45.0±5.2 (14)a | <0.0001 | NS |
| High | 8.9±1.3 (18)b,c,d | 37.7±5.8 (11)a | 37.5±2.8 (17)a | 45.9±5.4 (22)a | <0.0001 | ||
| P-altitude | <0.05 | NS | NS | NS | |||
| Flow, ml/min | Low | 31±6 (16)b,c,d | 463±66 (16)a | 512±92 (17)a | 613±121 (14)a | <0.001 | <0.05 |
| High | 63±12 (18)b,c,d | 398±65 (11)a | 381±40 (17)a | 477± 61 (22)a | <0.0001 | ||
| P-altitude | <0.05 | NS | NS | NS | |||
| RI | Low | 0.93±0.03 (16)b,c,d | 0.55±0.02 (16)a | 0.46±0.02 (17)a | 0.46±0.05 (14)a | <0.0001 | NS |
| High | 0.85±0.03 (18)b,c,d | 0.55±0.03(11)a | 0.49±0.02 (17)a | 0.49±0.02 (22)a | <0.0001 | ||
| P-altitude | NS† | NS | NS | NS | |||
| PI | Low | 3.14±0.44 (15)b,c,d | 0.91±0.07 (14)a | 0.71±0.05 (16)a | 0.70±0.15 (13)a | <0.0001 | NS |
| High | 2.71±0.32 (18)b,c,d | 0.91±0.12 (10)a | 0.74±0.04 (17)a | 0.74±0.04 (21)a | <0.0001 | ||
| P-altitude | NS | NS | NS | NS | |||
| S/D | Low | 5.86±0.85 (11)b,c,d | 2.29±0.14 (17)a | 1.90±0.08 (17)a | 1.86±0.16 (14)a | <0.0001 | NS |
| High | 6.54±1.41 (16)b,c,d | 2.34±0.24 (11)a | 2.00±0.08 (17)a | 1.98±0.06 (22)a | <0.0001 | ||
| P-altitude | NS | NS | NS | NS | |||
| UA/CI | Low | 0.12±0.06 (3) | 1.85±0.49 (9) | 1.94±0.49 (9) | 3.49±1.47 (9) | NS | NS* |
| High | 0.18±0.06 (8) | 3.24±2.46 (10) | 1.40±0.65 (13) | 1.27±0.27 (12) | NS | ||
| P-altitude | NS | NS | NS | NS | |||
| UA/EI | Low | 0.27±0.06 (12)c | 2.02±0.51 (10) | 4.93±1.67 (12)a | 3.46±0.91 (9) | <0.05 | NS |
| High | 0.59±0.23 (7) | 2.66±0.77 (10) | 2.43±0.47 (17) | 2.15±0.45 (22) | NS† | ||
| P-altitude | NS | NS | NS | NS | |||
| EI/CI | Low | 0.41 (1) | 1.06±0.29 (8) | 0.82±0.28 (8) | 0.32±0.07 (6) | NS | NS |
| High | 1.71±0.75 (3) | 1.26±0.73 (10) | 1.02±0.39 (13) | 1.59±0.57 (14) | NS | ||
| P-altitude | NS | NS | NS |
Values are presented as means ± SE with sample sizes in parentheses;
, 0.10 > P > 0.05.
Interaction between time and altitude, P < 0.05;
superscripts denote differences (at P < 0.05) between time points within a given altitude group when nonpregnant,
at week 20,
at week 30,
and at week 36.
CI, common iliac artery; Dia, diameter; TAM, time-averaged mean flow velocity; EI, external iliac artery; UA, uterine artery; RI, resistance index; PI, pulsatility index; S/D, systolic/diastolic flow velocity ratio.
Nonpregnant values were obtained 4 mo postpartum.
As a result of increased UA vessel diameter and higher flow velocity, pregnancy increased UA blood flow dramatically at both altitudes (Table 2). Across all time points considered together, UA diameter and volumetric flow were greater at low altitude than high altitude, such that the pregnancy-associated rise in UA diameter at 3,100 m was half that seen at 1,600 m (Table 2). The altitudinal difference in UA diameter was achieved by 20 wk of pregnancy, after which point no further vessel diameter expansion occurred at either altitude (Table 2).
Pregnancy did not alter the proportion of common iliac blood flow directed toward the UA within either altitude group (Table 2). However, at high altitude, the UA blood flow relative to that of the common iliac artery tended to decrease across pregnancy compared with the pattern observed at low altitude (Table 2). At low altitude, blood flow in the UA relative to the external iliac increased across time (P < 0.05); a similar pattern was apparent at high altitude, although it did not reach significance.
Maternal circulating vasoactive and growth-related factors.
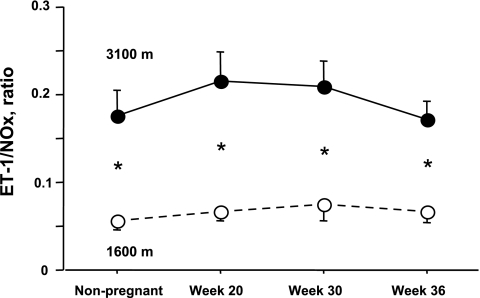
Neither pregnancy nor altitude significantly affected ET-1 levels (Table 3). Pregnancy raised sFlt-1, as well as PlGF levels by midpregnancy at both altitudes, and to a similar extent. In addition, at low altitude only, pregnancy reduced NOx (Table 3). However, no significant interaction effects between pregnancy and altitude were observed for NOx, s-Flt-1, and PlGF (Table 3). High-altitude residence was associated with 20% greater increase ET-1 levels near term relative to low altitude, a large but statistically nonsignificant difference, and reduced NOx values in the nonpregnant state, as well as during pregnancy (Table 3). As a result, the ratio of ET-1 to NOx was markedly greater at high vs. low altitudes at all time points (Fig. 1). There were no differences between altitudes in sFlt-1, PlGF, or the ratio between sFlt-1 and PlGF at any time.
Table 3.
Circulating maternal vasoactive and growth-related factors
| Variable | Altitude | Nonpregnant # | Week 20 | Week 30 | Week 36 | P-Time | P-Altitude |
|---|---|---|---|---|---|---|---|
| ET-1, pg/ml | Low | 1.18±0.12 (16) | 0.83±0.08 (14) | 1.01±0.14 (16) | 0.98±0.14 (13) | NS | |
| High | 1.32±0.12 (18) | 0.84±0.04 (10) | 1.07±0.10 (17) | 1.22±0.12 (21) | NS† | NS | |
| P-altitude | NS | NS | NS | NS | |||
| NOx, μM | Low | 29.8±4.6 (16) | 13.8±1.2 (14)a | 20.0±3.0 (16) | 16.5±1.3 (13)a | <0.01 | |
| High | 13.1±3.5 (17) | 5.6±1.5 (10) | 7.5±1.7 (17) | 12.8±3.9 (19) | NS | P < 0.0001 | |
| P-altitude | <0.01 | <0.001 | <0.01 | NS | |||
| sFlt-1, pg/ml | Low | 18±6 (16)b,c,d | 490±57 (14)a,d | 710±67 (16)a,d | 1072±69 (13)a,b,c | <0.0001 | |
| High | 7±4 (18)b,c,d | 568±107 (10)a,d | 816±113 (17)a | 1109±90 (22)a,b | <0.0001 | NS | |
| P-altitude | NS | NS | NS | NS | |||
| PlGF*, μM | Low | 335±40 (13)c | 661±85 (16)b,d | 352±73 (13)c | <0.01 | ||
| High | 269±44 (10) | 544±95 (16) | 356±52 (20) | <0.05 | NS | ||
| P-altitude | NS | NS | NS | ||||
| sFlt-1/PlGF* | Low | 1.6±0.2 (13) | 1.6±0.4 (16) | 11.6±6.7 (13) | NS | ||
| High | 2.9±0.9 (10) | 3.4±1.3 (16) | 4.9±0.9 (20) | NS | NS | ||
| P-altitude | NS | NS | NS |
Values are expressed as means ± SE with sample sizes in parentheses.
, 0.10 > P > 0.05;
superscripts denote differences (at P < 0.05) between time points within a given altitude group when nonpregnant,
at week 20,
at week 30,
and at week 36.
ET-1, endothelin-1; NOx, nitric oxide metabolites; sFlt-1, soluble Flt receptor-1; PlGF, placental-like growth factor.
PlGF is only present during pregnancy; therefore, no values are available for PlGF or sFlt-1/PlGF in the nonpregnant state.
Nonpregnant values were obtained 4 mo postpartum.
Fig. 1.
Level of endothelin (ET-1) relative to nitric oxide metabolites (NOx) in the maternal circulation were elevated in the nonpregnant state, as well as at all times of measure during pregnancy.
Fetal biometry and infant characteristics.
Fetal head circumference was smaller at high than low altitude at week 30 and tended to be so at weeks 20 and 36 (Table 4). However, after correcting for the actual gestational age at which studies were performed, the differences in head circumference at week 36 disappeared (32.2 ± 0.4 and 32.1 ± 0.3 cm at low vs. high altitude, respectively, P = NS). Femur length and abdominal circumference were similar at the two altitudes at all time points, and umbilical artery S/D resistance indices declined with increasing gestation at both altitudes. There were no differences between altitude groups at any time in the umbilical or the middle cerebral artery S/D ratios, whether or not values were adjusted for variation in actual gestational age.
Table 4.
Fetal biometry characteristics
| Variable | Altitude | Week 20 | Week 30 | Week 36 | P-altitude |
|---|---|---|---|---|---|
| Head circ, cm | Low | 20.1±0.6 (18) | 29.3±0.3 (17) | 32.8±0.4 (14) | |
| High | 18.6±0.7 (11) | 28.2±0.4 (18) | 31.7±0.4 (24) | NS | |
| P-altitude | NS† | <0.05 | NS† | ||
| Femur length, cm | Low | 3.7±0.1 (18) | 5.8±0.1 (17) | 6.9±0.1 (14) | NS |
| High | 3.4±0.1 (11) | 5.7±0.1 (18) | 6.6±0.1 (24) | ||
| P-altitude | NS | NS | <0.05 | ||
| Abdominal circ, cm | Low | 17.3±0.6 (18) | 27.0±0.5 (17) | 32.6±0.4 (14) | |
| High | 16.5±0.9 (11) | 26.3±0.5 (18) | 31.1±0.4 (24) | NS | |
| P-altitude | NS | NS | <0.05 | ||
| Umb S/D ratio | Low | 4.0±0.2 (18) | 3.2±0.2 (17) | 2.5±0.1 (13) | |
| High | 4.5±0.3 (10) | 3.2±0.2 (18) | 2.5±0.1 (24) | NS | |
| P-altitude | NS | NS | NS | ||
| MCA S/D ratio | Low | 4.6±0.3 (15) | 6.0±0.4 (17) | 5.4±0.6 (13) | |
| High | 4.8±0.5 (9) | 7.0±0.6 (16) | 6.4±0.8 (23) | NS | |
| P-altitude | NS | NS | NS |
Values are expressed as means ± SE, 95% confidence intervals in parentheses.
, 0.10 > P > 0.05. Umb, umbilical artery; S/D, systolic/diastolic flow velocity ratio; MCA, middle cerebral artery; SGA, small-for-gestational age.
Babies born at high altitude weighed 420 g less than their lower-altitude counterparts (Table 5) or 391 g less if variation in gestational age was taken into account (3,062 ± 39 and 3,453 ± 41 g at low vs. high altitude P < 0.01). There were no altitude differences in ponderal index. Whereas nearly one-quarter of all infants were SGA at high altitude, none were SGA at low altitude. There were no differences between altitudes in gestational age, the frequency of preterm delivery, the length of time between the current and previous pregnancy, Apgar scores, or baby sex. Delivery by cesarean section was relatively common but equivalent between altitudes (Table 5).
Table 5.
Newborn characteristics
| Variable | Low Altitude | High Altitude | P-Altitude |
|---|---|---|---|
| Birth weight, g | 3455±112 (15) | 3035±71 (21) | <0.01 |
| Length, cm | 51.0±0.7 (15) | 49.3±0.6 (21) | NS† |
| Ponderal index, kg/m3 | 26.2±0.8 (15) | 25.5±0.7 (21) | NS |
| Gestational age, wk | 39.5±0.3 (16) | 39.4±0.3(24) | NS |
| SGA, % | 0.0 [0.0, 20.4] (15) | 22.7 [10.1, 43.4] (22) | <0.05 |
| Preterm, % | 6.2 [0.6, 23.2] (18) | 8.3 [1.6, 22.5] (26) | NS |
| C-section, % | 31.2 [13.1, 55.6] (16) | 22.7 [9.7, 44.6] (21) | NS |
| Apgar, 1 min | 8 [4, 9] (16) | 8 [4, 9] (21) | NS |
| Apgar, 5 min | 9 [7, 9] (16) | 9 [8, 10] (16) | NS |
| Baby sex, % male | 42.8 [22.2, 67.4] (16) | 57.1 [38.6, 76.2] (24) | NS |
Values are mean ± SE with sample sizes in parentheses, 95% confidence intervals in parentheses and mode with range in brackets.
, 0.10>P > 0.05.
Relationship between maternal and infant characteristics.
Since multiple variables affect birth weight, we used linear regression analysis to identify the influences of such variables in our data set. Gestational age was strongly correlated with birth weight (r values = 0.49 to 0.59 for each group, all P < 0.05), as was maternal body weight at week 36 (r = 0.50 at high altitude, P < 0.05; r = 0.35 for the two groups combined, P = 0.07). None of the other maternal characteristics in Table 1 were so related.
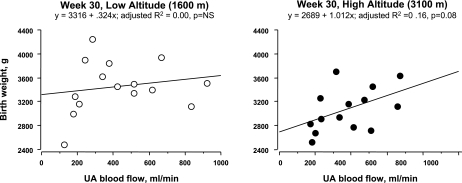
UA blood flow was unrelated to birth weight at low altitude but was positively associated at high altitude, both at week 30 (Fig. 2) and at week 36 (Table 6). Using multiple regression analysis for the two altitudes combined, variation in week 30 UA blood flow, gestational age, and maternal body weight could account for 25% of the variation in birth weight (Table 6). There were no such relationships at low altitude alone, but at both weeks 30 and 36 at high altitude, 40% of the variation in birth weight was attributable to these variables, with UA blood flow exerting the strongest effect.
Fig. 2.
Uterine artery (UA) blood flow at 30 wk of pregnancy was unassociated with birth weight at low altitude (left). In contrast, at high altitude, women with greater UA blood flow at 30 wk tended to deliver infants of heavier birth weight (right).
Table 6.
Results of multiple regression analyses: Relationship between birth weight (y), gestational age (x1), maternal body weight (x2), and uterine artery blood flow (x3)
| Time and Altitude Group | R2 Adjusted | Intercept | β1 GesAge | β2 MatWt | β3 UA Flow |
|---|---|---|---|---|---|
| Week 20, low and high | NS | NS | NS | NS | NS |
| Low | NS | NS | NS | NS | NS |
| High | NS | NS | NS | NS | NS |
| Week 30, low and high | 0.25 (P < 0.05) | −2355 (NS) | 117 (NS) | 9.1 (NS) | 0.65 (P = 0.07) |
| Low | NS | NS | NS | NS | NS |
| High | 0.40 (P = 0.05) | −1823 (NS) | 97 (NS) | 9.2 (NS) | 1.01 (P = 0.08) |
| Week 36, low and high | NS | NS | NS | NS | NS |
| Low | NS | NS | NS | NS | NS |
| High | 0.40 (P < 0.05) | −1696 (NS) | 85 (NS) | 14.2 (P<0.05) | 0.55 (P < 0.05) |
GesAge, infant's gestational age at delivery (wk); MatWt, maternal body weight (kg); UA flow, uterine artery blood flow (ml/min); ET-1/NOx, ratio of circulating levels of endothelin-1 (pg/ml) to nitric oxide metabolite.
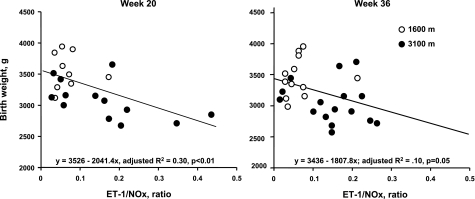
Lower ET-1/NOx levels were associated with heavier birth weights at weeks 20 and 36 for the two altitudes combined (Fig. 3). This relationship was primarily due to positive influences of NOx levels since it, but not ET-1, was related to birth weight alone. At week 20, UA blood flow and ET-1/NOx levels accounted for 45% of the variation in birth weight at the two altitudes combined (Table 7). At week 30, 32% of the variation in birth weight could be accounted for by trends toward positive associations with gestational age and UA blood flow and negative associations with ET-1/NOx. Such relationships were principally the result of associations present at high altitude, where 42% of the variation birth weight was attributable to higher UA blood flow and lower ET-1/NOx levels. Of note, although there was a strong relationship between ET-1/NOx and birth weight, no significant association was identified between ET-1 and UA blood flow within pregnancy or in the nonpregnant state. There were no associations between sFlt-1 or PlGF and UA blood flow or birth weight at any time or altitude (data not shown).
Fig. 3.
Women with higher ET-1 relative to nitric oxide metabolites (NOx) at 20 (left, P < 0.01) or 36 wk (right, P = 0.05) delivered infants of lower birth weight at both altitudes combined.
Table 7.
Results of multiple regression analyses: Relationship between birth weight (y), gestational age (x1), maternal body weight (x2), uterine artery blood flow (x3), and the ratio of ET-1 to NOx (x4)
| Time and Altitude Group | R2 Adjusted | Intercept | β1 GesAge | β2 MatWt | β3 UA Flow | β4 ET-1/NOx |
|---|---|---|---|---|---|---|
| Week 20, Low and high | 0.45 (P<0.05) | 9940 (P=0.06) | −170 (NS) | 9.3 (NS) | −0.65 (P=0.05) | −2344 (P<0.01) |
| Low | NS | NS | NS | NS | NS | NS |
| High | NS | NS | NS | NS | NS | NS |
| Week 30, Low and high | 0.32 (P<0.05) | −2051 (NS) | 112 (P=0.12) | 10.3 (NS) | 0.58 (P=0.10) | −1333 (P=0.12) |
| Low | NS | NS | NS | NS | NS | |
| High | 0.42 (P=0.10) | −1740 (NS) | 94 (NS) | 11.7 (NS) | 1.17 (P=0.08) | −1067 (NS) |
| Week 36, Low and high | NS | NS | NS | NS | NS | NS |
| Low | NS | NS | NS | NS | NS | NS |
| High | NS | NS | NS | NS | NS | NS |
DISCUSSION
Our study presents novel evidence concerning the mechanisms by which chronic hypoxia reduces fetal growth and the normal pregnancy-associated rise in UA blood flow. These are the first data to demonstrate that altitudinal differences in UA diameter occur by midpregnancy, prior to the reduction in fetal growth. Ours are also the first observations to show that maternal circulating vasoconstrictor (ET-1) relative vasodilator (NOx) levels are elevated at high compared with low altitude and are negatively associated with birth weight. These data supported our study's hypothesis that elevated levels of vasoconstrictor relative to vasodilator levels may contribute to altitude-associated reductions in birth weight by diminishing UA blood flow.
This study was limited by certain practical constraints. To control for other factors affecting UA blood flow and fetal growth, we recruited normal women at no known risk for pregnancy complications. However, because it was not possible to recruit women prior to pregnancy, postpartum rather than prepregnant studies were conducted. Although this permitted us to obtain measures of physiological parameters in the nonpregnant state, it may have reduced our ability to detect the effects of pregnancy relative to comparisons made with prepregnant measures. However, because UA values obtained when nonpregnant were similar at the two altitudes and there were no differences between altitudes in the proportion of women who were primiparous or the interval since prior pregnancy, we considered that the chronic hypoxia of residence at high altitude rather than some other factor (e.g., lingering effects of pregnancy) was responsible for the altitudinal differences in UA diameter and blood flow observed. It may be the case that our study, despite being adequately powered, did not detect altitudinal effects in some variables (e.g., UA blood flow at 36 wk) because of relatively high variability in flow values within our subject population. The fact that parity was slightly greater at high altitude may have introduced some bias in our interpretation of the effects of altitude on UA blood flow since the maternal vessels may undergo some permanent modification after a previous pregnancy that could influence blood flow and, in turn, fetal growth. For example, in one study, parity (≥1) was positively associated with UA resistance index but negatively associated with bilaternal UA protodiastolic notching (23). Intuitively, and because preeclampsia is more common in primiparous women, parity should have a positive effect on UA blood flow. Thus, because parity was slightly greater at high than low altitude, this could have reduced our ability to detect altitudinal differences in UA blood flow. However, the lack of altitudinal effect on UA blood flow at individual time points remained after including parity as a covariate.
As previously reported by Galan et al. (10), moderate altitude (Denver, Colorado, 1600 m) is associated with a 200 g reduction in birth weight relative to sea level (Milan, Italy) in healthy infants born to normotensive women (9, 10). This birth weight difference was attributed to demographic, socioeconomic, and/or maternal attributes, such as maternal age rather than hypoxia. Their conclusion was based on the fact that the ambient Po2 difference between these two altitudes is relatively small (160 mmHg vs. 130 mmHg) and that peripheral or central vascular resistance was similar in fetuses at each altitude, suggesting that no fetal hypoxia effect exists at moderate altitudes. If there is a true negative effect of moderate altitude on fetal growth, the difference in birth weight that we detected in our comparison of 1600 m to 3100 m would be amplified if we compared 3100 m to sea level. Our study's locations were in close proximity, a further advantage in terms of minimizing the effect of variations in maternal demographic characteristics on birth weight.
The present results are consistent with our and others' studies demonstrating lower birth weights and UA blood flow at high compared with lower altitudes. In this study, the high-altitude infants weighed 420 g less than their low-altitude counterparts, which is within the ∼100 g reduction in birth weight per 1000 m elevation gain reported previously (13). Like the previous Colorado report, we found an altitude-associated reduction in UA vessel diameter, but not slower flow velocity. The present study had the advantage that all physiological measurements were made at high altitude, whereas our previous investigation required that high-altitude women descend to Denver (1,600 m) due to the absence of ultrasound imaging equipment at the high-altitude site(36). Further, in the present study, the same instrument was used, and the same operator performed all studies, guarding against the possibility that procedural factors were responsible for the altitudinal differences observed. The high-altitude women had greater absolute external iliac blood flow; however, across pregnancy, there was no difference between altitudes in the ratio of UA to external iliac blood flow. There was no difference between altitudes in the proportion of common iliac blood flow distributed toward the UA distribution at individual time points, which is unlike our previous study; however, across pregnancy, the proportion of UA to common iliac blood flow increased to a lesser extent at high relative to low altitude. These data likely reflect redistribution of common iliac flow away from the UA and toward the external iliac artery at high altitude; however, we were unable to detect a significant altitudinal difference in external relative to common iliac blood flows. This may be attributable to a limitation posed by the small sample sizes for the uterine to common iliac artery flow index due to the common iliac artery's deep and relatively flat anatomical location and consequent difficulty in obtaining a sufficiently low angle of insonation to permit calculation of volumetric flow.
An important advantage for our study was that Doppler measurements were taken before the onset of altitude-associated reductions in fetal growth. Krampl et al. (16) demonstrated that significant altitudinal reductions in biparietal diameter, occipitofrontal diameter, head circumference, abdominal circumference, and femur length became apparent between 25 and 29 wk of gestation and persisted onward (16). Consistent with this time course were the observations of smaller femur length and abdominal circumference seen at week 36 at high compared with lower altitude. Because smaller UA diameters were present by week 20, prior to the point at which altitude-associated reductions in fetal growth become apparent, we concluded that the lower levels of UA flow observed were likely due to smaller vessel diameter rather than a consequence of smaller fetal size and oxygen demand. More likely, limited UA blood flow as a result, in part, of smaller UA diameter, thereby reducing the delivery of oxygen and other vital nutrients required for fetal growth. Some recently have questioned whether the magnitude by which altitude decreases UA oxygen delivery is large enough to decrease fetal growth, given that even at high altitude UA oxygen delivery exceeds that necessary for adequate fetal growth (3, 37). One issue that has not been considered in great detail to resolve this question is whether uteroplacental oxygen delivery, as determined by arterial oxygen content and UA blood flow, is truly representative of oxygen delivery to the fetus and what role variations in placental oxygen extraction may play in the process. While there is clearly variation among studies in the absolute levels of volumetric flow seen in pregnancy, likely, largely due to differences in technique and postural conditions (4, 32), ample evidence exists to indicate the importance of chronic reductions in uteroplacental blood flow and/or increased resistance to UA blood flow for the fetal growth restriction seen in cases of preeclampsia in humans as well as in a variety of animal models (22, 27). The temporal evidence reported here showing that the reduction in UA flow preceded the onset of fetal growth restriction together with prior observations at high altitude (32, 36) further support the importance of uteroplacental blood flow for fetal growth.
We relied on venous rather than arterial blood for measuring vasoactive and growth-related substances for ethical reasons. We chose to measure circulating levels of ET-1, NOx, sFlt-1, and PlGF given previous studies showing their importance in relation to the occurrence of fetal growth restriction and preeclampsia (1, 7, 8, 17, 18, 25). For example, ET-1 is a potent vasoconstrictor that is known to be elevated in pregnancies complicated by preeclampsia or SGA (7, 8), and when its vasoconstrictor actions are inhibited with the use of ET-1 antagonists, hypoxia-associated reductions in uteroplacental blood flow and fetal growth in rat models are completely abolished (27). In this study, ET-1 levels were not elevated at high relative to low altitude during pregnancy or in the nonpregnant state. Our ability to detect alterations in ET-1 levels at high altitude may have been limited by our use of venous samples, since its metabolism in the tissues lowers venous relative to arterial levels (6). Alternatively, because long-term arterial laminar shear stress down-regulates ET-1 production (21), it is also possible that greater shear stress, resulting from smaller vessel diameters observed during high-altitude pregnancy counteracted or masked any hypoxia-associated stimulation of ET-1 production.
To our knowledge, this is the first study to find a relationship between NOx and the altitude-associated reduction in birth weight in humans. NO-induced vasodilation is known to play a critical role in the normal maternal vasodilatory response to pregnancy, as demonstrated by the ability of chronic treatment with the nitric oxide synthase (NOS) inhibitor nitro-l-arginine methyl ester to produce a preeclampsia-like syndrome and SGA in rats (33). However, reports are mixed as to whether NOx levels are reduced in preeclampsia or other pregnancy complications, likely due, in part, to dietary influences on NOx metabolite levels (5). Physiological responses and the ability to defend fetal growth may also be involved. For instance, up-regulation of NOS and increased endothelium-dependent UA vasodilation appears to protect ewes from chronic hypoxia-associated reductions in fetal growth, whereas fetal growth is diminished in guinea pigs showing NOS down-regulation and reduced NO-dependent vasodilation (29, 30). In the present study, we found lower NOx levels at high compared with low altitude in both the nonpregnant and pregnant states, possibly the result of lower NO production and/or decreased bioavailability. In support of the latter suggestion, NO can be consumed by superoxide under conditions of hypoxia, such as that encountered at high altitudes, to form highly reactive oxidant species that, in turn, can damage the vascular endothelium and impair its function (26). While we were not able to distinguish whether NO production or bioavailability was reduced in the present study, either possibility or both possibilities are consistent with the smaller UA diameters found at high altitude and the strong, negative relationship seen between ET-1/NOx at 20 wk of pregnancy and birth weight. We, therefore, concluded that reduced NOx metabolite relative to ET-1 levels likely contribute to the smaller UA diameter and blood flow apparent at high altitudes which, in turn, plays a role in reduced fetal growth at high altitude.
We also hypothesized that the smaller UA diameters seen at high altitude were influenced by a reduction in UA growth. Supporting such an hypothesis were prior studies by Rockwell et al. (24), demonstrating only half as much pregnancy-associated rise in UA DNA synthesis in guinea pigs gestated at high compared with low altitude. In addition, production of the antiangiogenic factor sFlt-1 is stimulated by hypoxia, and elevated sFlt-1 levels have been reported in pregnancies complicated by preeclampsia and/or SGA (17, 19). Contrary to our expectations, there were no differences between altitudes in sFlt-1 levels, PlGF levels, or their ratios in the nonpregnant state or during pregnancy. While both substances increased from 20 to 36 wk of pregnancy, they did so similarly at the two altitudes and neither was related to variation in UA blood flow or fetal growth at any time. Perhaps altitudinal differences in uteroplacental levels of these substances were obscured by production from other vascular beds or by increases in other growth factors sufficient to overcome any inhibitory effects of sFlt-1 (38). Nonetheless, the present study results supported the likelihood that smaller UA diameters were more likely due to an imbalance of vasoconstrictor relative to vasodilator substances than to excess sFlt-1 relative to PlGF levels.
Perspectives and Significance
In summary, our principal findings indicated that chronic hypoxia reduced the pregnancy-associated rise in UA diameter by midpregnancy, well before the slowing of fetal growth known to be responsible for reducing birth weight at high altitude. Further, the smaller pregnancy-associated rise in UA diameter and blood flow at high altitude and the positive relationship between UA blood flow and birth weight support the idea that the lower UA flows were the cause, not the consequence, of the lesser fetal growth observed at altitude. Alterations in circulating vasoactive factors were implicated in these altitude differences in UA diameter; NOx metabolite levels were lower and near-term ET-1 levels higher in the high- than low-altitude residents, but no differences were apparent in the levels of the antiangiogenic sFlt-1 or proangiogenic PlGF between altitude groups. On balance, these data suggest that a deficiency of vasodilator relative to vasoconstrictor levels may contribute to hypoxia-associated reduction in birth weight. Since multigenerational high-altitude populations appear protected from hypoxia-associated reductions in UA blood flow and fetal growth (32), an interesting question for future investigation is whether such protection is due to variation in circulating vasoactive and/or angiogenic factors. Also of interest is whether variation in such factors can dissociate the effects of hypoxia on SGA vs. preeclampsia, both conditions in which hypoxia is suspect but fetal growth is variably affected.
GRANTS
Financial support was received through National Institutes of Health Grant HL-060131 and a predoctoral fellowship from the American Heart Association (0610129Z).
Acknowledgments
We would like to thank the many women who graciously participated in this study, as well as the hospital staff and technicians whose dedication to the project made it possible. In particular, we thank Dr. Lisa Zwerdlinger and Dr. Christopher Wenner, both local physicians in Leadville, for their enduring support of our research, and Rhonda Mouser and Jennifer Hageman for their invaluable laboratory and administrative contributions to this project.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arslan M, Yazici G, Erdem A, Erdem M, Arslan EO, Himmetoglu O. Endothelin 1 and leptin in the pathophysiology of intrauterine growth restriction. Int J Gynaecol Obstet 84: 120–126, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ Fetal origins of cardiovascular disease. Ann Med 31: 3–6, 1999. [PubMed] [Google Scholar]
- 3.Carter A Factors affecting gas transfer across the placenta and the oxygen supply to the fetus. J Dev Physiol 12: 305–322, 1989. [PubMed] [Google Scholar]
- 4.Clapp JF Influence of endurance exercise and diet on human placental development and fetal growth. Placenta 27: 527–534, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Conrad K, Kerchner L, Mosher M. Plasma and 24-h NO(x) and cGMP during normal pregnancy and preeclampsia in women on a reduced NO(x) diet. Am J Physiol Renal Physiol 277: F48–F57, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Dupuis J, Stewart D, Cernacek P, Gosselin G. Human pulmonary circulation is an important site for both clearance and production of endothelin-1. Circulation 94: 1578–1584, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Erdem A, Erdem M, Himmetoglu O, Yildirim G, Arslan M. Maternal and fetal plasma endothelin levels in intrauterine growth restriction: relation to umbilical artery Doppler flow velocimetry. J Perinat Med 31: 52–59, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Furuhashi N, Kimura H, Nagae H, Yajima A. Maternal plasma endothelin levels and fetal status in normal and preeclamptic pregnancies. Gynecol Obstet Invest 39: 88–92, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Galan HL, Rigano S, Chyu J, Beaty B, Bozzo M, Hobbins JC, Ferrazzi E. Comparison of low- and high-altitude Doppler velocimetry in the peripheral and central circulations of normal fetuses. Am J Obstet Gynecol 183: 1158–1161, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Galan HL, Rigano S, Radaelli T, Cetin I, Bozzo M, Chyu J, Hobbins JC, Ferrazzi E. Reduction of subcutaneous mass, but not lean mass, in normal fetuses in Denver, Colorado. Am J Obstet Gynecol 185: 839–844, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert WM Pregnancy outcomes associated with intrauterine growth restriction. Am J Obstet Gynecol 188: 1596–1599, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Giussani DA, Seamus P, Anstee S, Barker D. Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res 49: 490–494, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public Health 87: 1003–1007, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Villena M, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed 92: F372–F377, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young D, Villena M, Moore LG. Intrauterine growth restriction, preeclampsia and intrauterine mortality at high altitude in Bolivia. Pediatr Res 54: 20–25, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Krampl E, Lees C, Bland JM, Dorado JE, Gonzalo M, Campbell S. Fetal biometry at 4300 m compared to sea level in Peru. Ultrasound Obstet Gynecol 16: 9–18, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Maynard S, Min J, Merchan J, Lim K, Li J, Mondal S, Liberman T, Morgan J, Sellke F, Stillman I, Epstein F, Sukhatme V, Karumanchi S. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore LG, Rounds SS, Jahnigen D, Grover RF, Reeves JT. Infant birth weight is related to maternal arterial oxygenation at high altitude. J Appl Physiol 52: 695–699, 1982. [DOI] [PubMed] [Google Scholar]
- 21.Morawietz HTR, Szibor M, Rueckschloss U, Schubert A, Bartling B, Darmer D, Holtz J. Regulation of endothelin system by shear stress in human endothelial cells. J Physiol: 761–770, 2000. [DOI] [PMC free article] [PubMed]
- 22.Papageorghiou A, Yu CK, Nicolaides KH. The role of uterine artery Doppler in predicting adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol 18: 383–396, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Prefumo F, Bhide A, Sairam S, Penna L, Hollis B, Thilaganathan B. Effect of parity on second-trimester uterine artery Doppler flow velocity and waveforms. Ultasound Obstet Gynecol 23: 46–49, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Rockwell LC, Keyes LE, Moore LG. Chronic hypoxia diminishes pregnancy-associated DNA synthesis in guinea pig uteroplacental arteries. Placenta 21: 313–319, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Savvidou M, Yu C, Harland L, Hingorani A, Nicolaides K. Maternal serum concentration of soluble fms-like tyrosine kinase 1 and vascular endothelial growth factor in women with abnormal uterine artery Doppler and in those with fetal growth restriction. Am J Obstet Gynecol 195: 1668–1673, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Steiner DRS, Gonzalez NC, Wood JG. Interaction between reactive oxygen species and nitric oxide in the microvasculature response to systemic hypoxia. J Appl Physiol 93: 1411–1418, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Thaete LG, Dewey ER, Neerhof MG. Endothelin and the regulation of uterine and placental perfusion in hypoxia-induced fetal growth restriction. J Soc Gynecol Investig 11: 16–21, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Vargas M, Vargas E, Julian C, Armaza JF, Rodriguez A, Tellez W, Niermeyer S, Wilson M, Parra E, Shriver M, Moore L. Determinants of blood oxygenation during pregnancy in Andean and European residents of high altitude. Am J Physiol Regul Integr Comp Physiol 293: R1303–R1312, 2007. [DOI] [PubMed] [Google Scholar]
- 29.White MM, McCullough RE, Dyckes R, Robertson AD, Moore LG. Chronic hypoxia, pregnancy, and endothelium-mediated relaxation in guinea pig uterine and thoracic arteries. Am J Physiol Heart Circ Physiol 278: H2069–H2075, 2000. [DOI] [PubMed] [Google Scholar]
- 30.White MM, Zhang L. Effects of chronic hypoxia on maternal vascular changes in guinea pig and ovine pregnancy. High Alt Med Biol 4: 157–169, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol 59: 624–632, 1982. [PubMed] [Google Scholar]
- 32.Wilson MJ, Lopez M, Vargas M, Julian C, Tellez W, Rodriguez A, Bingham A, Armaza JF, Niermeyer S, Shriver M, Vargas E, Moore LG. Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. Am J Physiol Regul Integr Comp Physiol 293: R1313–R1324, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Yallampalli C, Garfield RE. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol 169: 1316–1320, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Yip R Altitude and birth weight. J Pediatr 111: 869–876, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA, Moore LG. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol 79: 15–22, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol 79: 7–14, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Zamudio S, Postigo L, Illsley N, Rodriguez C, Heredia G, Brimacombe M, Echalar L, Torricis T, Tellez W, Maldonado I, Balanza E, Alvarez T, Ameller J, Vargas E. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol 582: 883–895, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao B, Cai J, Boulton M. Expression of placenta growth factor is regulated by both VEGF and hyperglycaemia via VEGFR-2. Molec Res 68: 239–264, 2004. [DOI] [PubMed] [Google Scholar]