Abstract
This study examined critical periods in development to determine when offspring were most susceptible to dietary sodium manipulation leading to amphetamine sensitization. Wistar dams (n = 6–8/group) were fed chow containing low (0.12% NaCl; LN), normal (1% NaCl; NN), or high sodium (4% NaCl; HN) during the prenatal or early postnatal period (birth to 5 wk). Offspring were fed normal chow thereafter until testing at 6 mo. Body weight (BW), blood pressure (BP), fluid intake, salt preference, response to amphetamine, open field behavior, plasma adrenocorticotropin hormone (ACTH), plasma corticosterone (Cort), and adrenal gland weight were measured. BW was similar for all offspring. Offspring from the prenatal and postnatal HN group had increased BP, NaCl intake, and salt preference and decreased water intake relative to NN offspring. Prenatal HN offspring had greater BP than postnatal HN offspring. In response to amphetamine, both prenatal and postnatal LN and HN offspring had increased locomotor behavior compared with NN offspring. In a novel open field environment, locomotion was also increased in prenatal and postnatal LN and HN offspring compared with NN offspring. ACTH and Cort levels 30 min after restraint stress and adrenal gland weight measurement were greater in LN and HN offspring compared with NN offspring. These results indicate that early life experience with low- and high-sodium diets, during the prenatal or early postnatal period, is a stress that produces long-term changes in responsiveness to amphetamines and to subsequent stressors.
Keywords: behavioral sensitization, psychomotor stimulant, salt appetite
drugs of abuse and natural motivators or stimuli, such as sodium deprivation or stress, activate similar neural circuits involved in mediating reward and drive-related responses (12, 18, 28). One of the main systems believed to be involved in triggering these behaviors is the mesocorticolimbic dopamine system, with dopaminergic projections traveling from the midbrain ventral tegmental area to the nucleus accumbens and other limbic and cortical areas (38, 44). With repeated exposure to both amphetamines and sodium deprivation, behavioral sensitization develops (38, 48). Sensitization can be described as an increased and enduring response to the same stimuli with repeated exposures (38). With amphetamines, sensitization manifests itself as increased locomotor behavior (21, 53). Repeated amphetamine treatment leading to sensitization is associated with morphological changes in nucleus accumbens neurons, including increased spine density, dendritic length, and dendritic branching (46). Repeated sodium deprivation can lead to an enduring daily increase in the ingestion of hypertonic NaCl in otherwise sodium-replete rats (48), and a history of sodium depletion is also associated with morphological changes in neurons in the shell of the nucleus accumbens, an area that receives dopaminergic input (47). This suggests that the behavioral sensitization to repeated amphetamines and sodium-state manipulations share a common neural basis involving changes in the dopaminergic system.
Once behavioral sensitization has developed to one substance, an animal may also be more responsive to other substances that activate the dopaminergic system, termed cross-sensitization. Clark and Bernstein (12) showed that rats exposed to repeated sodium deficiencies were more responsive to amphetamines and vice versa. This process of cross-sensitization can also occur during early development. Previous work in our laboratory (33) revealed that offspring from dams maintained on sodium-deficient diets during the prenatal and early postnatal period were more responsive to amphetamines when tested as adults than their normal counterparts. This implies that early life experience with sodium deficiency has long-lasting effects on the organization of the developing brain, sensitizing offspring to amphetamines.
In our previous experiments (33), it also was observed that Wistar-Kyoto (WKY) offspring exposed to the low-sodium diet during the perinatal period had increased adrenal gland weights as adults in addition to altered amphetamine sensitivity. Adrenal hypertrophy can reflect hydromineral states and/or stress. Low-sodium diets can increase both aldosterone and corticosterone (Cort) levels in plasma (20), and high-sodium diets increase Cort levels in the blood, and this leads to hyperresponsiveness to stress in offspring (41). Therefore, adrenal hypertrophy due to periods of sodium deficiency or sodium excess could result in increased glucocorticoid release due to diet-induced stress.
Maternal malnutrition is a stressor for both mothers and offspring (3, 4, 7). Prenatal stress increases the responsiveness of offspring to amphetamines when tested as adults (18, 19, 29). Therefore, the present experiments examined whether the increased responsiveness to amphetamines observed in sodium-manipulated offspring was a direct effect of dietary sodium manipulation or diet-induced stress.
In addition, there appears to be critical periods in development for the effects of stress. Therefore, we chose to examine offspring exposed to dietary sodium manipulation during either the prenatal period or the early postnatal period in development. Prenatal maternal stress, indicated by increased glucocorticoid levels, can alter responsiveness of offspring to stressful experiences and to drugs of abuse (18, 19, 29, 52). Furthermore, prenatal stress occurring during specific time windows, late gestation in particular, is sufficient to cause this (18, 19, 29, 52). Likewise, maternal separation or poor mother-pup interactions during the early postnatal period have detrimental effects on pups, rendering them more sensitive to psychostimulant drugs and environmental stressors (9, 10). Therefore, if dietary sodium manipulation is a physiological stress affecting the mother and pup during either the prenatal or early postnatal period, then the offspring should show enhanced responsiveness to amphetamines and to stressful challenges as adults.
In human populations and in rats, there are individual differences in susceptibility to psychostimulant drugs and development of behavioral sensitization (45, 51, 54). Previous studies from our laboratory and others have examined differences between WKY and spontaneously hypertensive rats (SHR) in response to amphetamine, because the SHR show altered dopamine function, which could contribute to differences in response to amphetamine (26, 34, 49, 54). Wistar rats, which were used in the current experiment, are commonly used in the study of psychomotor stimulants and display physiological distinctions from inbred WKY rats (37, 43). Examining substrains of Wistar rats allowed us to determine whether the effects of dietary sodium manipulation generalized across substrains of Wistar rats.
Three sets of experiments were performed to test our hypotheses 1) that dietary sodium manipulation during the prenatal or postnatal period affects behavioral responses to amphetamine in offspring and 2) that dietary sodium manipulation is a physiological stress that is an underlying factor mediating amphetamine sensitivity in the offspring. First, systolic blood pressure (BP), fluid intake, and salt preference were collected to determine whether there were permanent changes in behavior and physiology of adult offspring. Second, behavioral responses to amphetamine were tested in normal and sodium-manipulated offspring to test amphetamine sensitization. Third, measures of stress were evaluated, including locomotor behavior in a novel environment, plasma adrenocorticotropin hormone (ACTH) and Cort levels before and after a restraint stress, and adrenal gland weight.
MATERIALS AND METHODS
Procedure.
Adult male and female Wistar rats were obtained from Charles River Laboratories and housed in clear plastic cages in a temperature-controlled room with a 12:12-h light-dark cycle with lights on at 0700. Wistar dams (n = 6–8/group) were fed chow containing either low salt (0.12% NaCl; LN), normal salt (1% NaCl; NN), or high salt (4% NaCl; HN) ad libitum during either the prenatal period, from conception to birth, or the early postnatal period, from birth through weaning. Offspring in the postnatal groups were maintained on the test diet for an additional 2 wk postweaning. The dietary groups were prenatal LN, NN, and HN and postnatal LN, NN, and HN. During breeding, male rats were introduced into a cage with four to five females during the evening and returned to their home cage in the morning. Day 1 of pregnancy was determined by a vaginal smear and positive evidence of sperm. Pregnant dams were placed in a solitary plastic cage at this time, and weight of the dam was recorded weekly during gestation to ensure progression of pregnancy. Shortly after birth, litters were adjusted to eight pups per dam, with as many male pups retained as possible, because males were used in the experiment. Litters were culled to an equal number to reduce differences in the postnatal environment that might affect maternal care and growth rate. After weaning at postnatal day 21, male pups (n = 4–5) were housed with their littermates in suspended wire cages. Thereafter, all offspring were maintained ad libitum on normal Purina chow until testing at 6 mo of age. There were six to eight litters for each dietary group, and one male offspring from each litter was used for a specific test to prevent carryover effects; offspring used in one experiment were not used in subsequent tests.
All diets used in this study allow for normal reproduction, growth, and litter size. To produce diets with varied salt content, we added NaCl to sodium-deficient powdered rat chow (MP Biomedicals, Aurora, OH) by weight and mixed for 10 min to ensure homogeneity. Mixed diets were sealed in plastic containers and stored in a cold room. The 0.12% NaCl diet was chosen as the low-sodium diet because this amount is close to the minimum amount required for successful reproduction (0.08% NaCl), and amounts below this lead to high levels of mortality (8, 15). The 1% NaCl diet was chosen as the normal diet because it is similar to standard rat chow. The 4% NaCl diet was chosen as the high-sodium diet because this concentration is not normally preferred and is sufficiently high to elevate blood pressure (8, 15). In addition, the various NaCl levels used in this experiment mimic salt intake levels in humans. The 0.12% NaCl diet corresponds to reduced salt diets recommended for people with hypertension, the 1% NaCl diet corresponds to the recommended daily average for salt intake in the United States, and the 4% NaCl diet reflects a high-salt diet that corresponds with elevated daily salt intake (40, 48a). This study was approved by the University of Wyoming Animal Care and Use Committee in accordance with federal regulations.
Systolic blood pressure.
Systolic BP was measured indirectly using a semiautomated tail-cuff device (IITC, Woodland Hills, CA). Rats (n = 6–8/dietary group) were placed in cylindrical Plexiglas restraining tubes and were acclimated to the testing chamber and restraining tubes for 30 min to reduce restraint stress. Three BP measurements were then taken for each rat, and the average of the three readings was used for the BP. BP was measured in the morning to midafternoon (between 0900 and 1400).
Two-bottle fluid intake and salt preference test.
At 6 mo of age, adult male offspring were examined for differences in fluid intake and NaCl preference [NaCl intake/(NaCl + water intake) × 100]. For this test, offspring (n = 6–8/dietary group) were removed from their clear plastic home cages into individual suspended steel-mesh cages and acclimated to the test cages for several hours before the 24-h test period began. Offspring were kept in a temperature controlled room with a 12:12-h light-dark cycle with lights on at 0700 and returned to their home cages after testing. During testing, rats had ad libitum access to Purina rat chow. Two calibrated glass bottles, one containing distilled water and one containing 0.3 M NaCl, were attached to the front of each cage and placed on opposite sides to prevent mixing of solutions. After 24 h, intake was measured to the nearest milliliter.
Behavioral activity testing and amphetamine challenge.
Locomotor activity was recorded using an activity monitoring system (Omniscan Instruments, Columbus, OH) similar to that commonly used to characterize the effects of psychomotor stimulants (12, 21, 30, 53). Previous studies examining the effects of nicotine on locomotor activity have employed these monitors and validated their data collection (30). The Digiscan monitor uses arrays of infrared photobeams mounted on the sides (2.54–7.62 and 16.51–21.59 cm) of the clear acrylic test chambers (41.91 × 41.91 × 30.48 cm). Locomotion or horizontal activity was detected by beam breaks of lower photocells (30). Locomotor behavior is associated with the psychomotor stimulant effects of amphetamines and is a commonly used indicator of amphetamine effects (21, 53).
Activity tests were conducted during the daytime, between 0900 and 1400. Before testing, adult offspring (n = 6–8/dietary group, 6 mo of age) were placed in the activity chamber and allowed to acclimate to the chamber for 30 min. After acclimation, rats were removed from the chamber, given an intraperitoneal injection of isotonic saline, and returned to the chamber for 30 min, when behavior was measured. After 30 min, rats were again removed and given an intraperitoneal injection of 3 mg/kg amphetamine (d-amphetamine sulfate; Sigma, St. Louis, MO). Rats were returned to the activity chamber for 30 min, and behavior was measured. In our previous study (33), two different doses of amphetamine were used to determine whether there were dose-dependent interactions with perinatal dietary sodium history. In that study, the 3 mg/kg dose was found most effective, so for this experiment, we used only that dose. In addition, this dose is consistent with those typically used to show psychomotor effects of amphetamine and cross-sensitization to NaCl in adult rats (12, 53).
Locomotion in a novel open field.
Locomotor behavior was measured using the same method described above for behavioral activity testing, with the exception that bright lights were placed above the activity chambers. To measure behavior in a novel open field, offspring (n = 6–8/dietary group) were placed in the center of the testing chambers and measurements were started immediately afterward. Behavior in a novel environment varies the most within the first 20 min; therefore, measurements from 0 to 20 min were analyzed (18, 42). After this time period, rats are mainly sedentary (42).
Plasma ACTH and Cort levels before and after restraint stress.
Experiments were performed between 0900 and 1400 to minimize time-of-day differences in ACTH and Cort levels. Offspring used to establish baseline measurements were not exposed to restraint stress. Offspring exposed to restraint stress were placed in a cylindrical Plexiglas restraining tube for 30 min. At the end of this period, offspring were removed from the restraining tube and blood was collected. Blood was collected again 120 min after removal from the restraining tube (150 min from the start of the experiment), similar to the protocol described by Koenig et al. (29). Offspring (n = 4/dietary group) from the same litter were used for the repeated baseline, 30 min, and 150 min poststress measurements because these offspring should be genetically similar and were exposed to the same early life experiences.
For blood sampling, adult male offspring were anesthetized with a mixture of ketamine HCl (90.1 mg/kg) and acepromazine maleate (9.1 mg/kg ip) before cardiac stick. Samples were collected into plastic tubes containing heparinized saline (50 U/ml) and 10 μM phosphoramidon, a protease inhibitor. Samples were centrifuged, and plasma was stored at −80°C until enzyme immunoassay (EIA) analysis. Plasma ACTH was extracted using a 1% trifluoroacetic acid buffer and C-18 column technique, and plasma Cort was extracted using a methylene chloride liquid extraction method. Extracted samples were dehydrated using an Eppendorf vacufuge, and pelleted samples were stored at −80°C. Samples were reconstituted 24 h before the EIA assay in assay buffer. Cort samples were diluted 1:100 for the assay. Each sample was analyzed in triplicate using an ACTH EIA (Phoenix Pharmaceuticals, Belmont, CA) or Cort EIA (Assay Designs, Ann Arbor, MI). Intra-assay variability on triplicate samples and interassay variability were determined, and in both assays the values were <5%.
Adrenal gland weight.
After the completion of blood sampling, rats (n = 4/dietary group) were killed and the left adrenal gland was removed from each rat. Wet weight (nearest 0.01 g) of the adrenal gland was obtained to determine the effects of prenatal and early postnatal dietary sodium manipulation on adrenal gland weight.
Data analysis.
Data are means ± SE and were analyzed using SPSS software with a P value <0.05 considered significant. For comparisons of prenatal groups (LN, NN, and HN), a one-way ANOVA with subsequent post hoc least significant difference analysis was used to break down significant differences and interactions between groups. The same statistical analysis was applied to examine differences among postnatal groups (LN, NN, and HN). For comparisons between the prenatal and the postnatal groups (i.e., prenatal LN and postnatal LN, and so on), an independent-samples t-test was used.
RESULTS
Body weight of dams and offspring.
Table 1 shows the body weight and overall weight gain of the dams from the beginning of pregnancy (week 1) through the end of pregnancy (week 3). No differences were found among the different groups. In addition, body weights were similar for all offspring at the time of testing (Table 2).
Table 1.
Maternal weight during pregnancy
| Treatment |
Maternal Weight Gain, g |
|||
|---|---|---|---|---|
| Gestation week 1 | Gestation week 2 | Gestation week 3 | Total | |
| Prenatal | ||||
| LN | 242±9 | 266±8 | 298±11 | 56±4 |
| NN | 235±5 | 259±7 | 288±7 | 53±2 |
| HN | 233±9 | 256±9 | 278±8 | 45±4 |
| Postnatal | ||||
| LN | 235±5 | 251±6 | 269±6 | 34±3 |
| NN | 245±11 | 263±8 | 295±8 | 50±6 |
| HN | 237±4 | 265±9 | 285±11 | 48±7 |
Data are means ± SE. LN, low-sodium diet; NN, normal sodium diet; HN, high-sodium diet.
Table 2.
Effects of dietary sodium manipulation during early development on physiology and behavior of offspring
| Treatment | BP, mmHg | Body Weight, g |
Fluid Intake Over 24 h, ml |
NaCl Preference, % | ||
|---|---|---|---|---|---|---|
| dH2O | 0.3M NaCl | Total | ||||
| Prenatal | ||||||
| LN | 123±3 | 364±12 | 34±2 | 6±2 | 40±4 | 15±3 |
| NN | 121±3 | 344±11 | 33±4 | 4±1 | 37±3 | 11±2 |
| HN | 150±3‡§ | 380±22 | 24±2* | 17±4† | 41±2 | 42±7‡ |
| Postnatal | ||||||
| LN | 121±2 | 383±18 | 40±2 | 5±1 | 45±2 | 11±3 |
| NN | 125±4 | 372±16 | 37±1 | 5±1 | 42±1 | 12±3 |
| HN | 138±2† | 367±11 | 24±6* | 11±3* | 35±7 | 31±12† |
Data are means ± SE. BP, blood pressure; dH2O, distilled water.
P < 0.05;
P < 0.01;
P < 0.001 compared with NN offspring.
P < 0.05, prenatal vs. postnatal HN offspring.
Systolic BP.
As shown in Table 2, offspring exposed to HN during either the prenatal or the early postnatal period had significantly increased BP compared with the NN offspring (P < 0.001 or P < 0.003, respectively). The offspring prenatally exposed to the HN diet were affected to a greater extent than the offspring postnatally exposed to HN and consequently had a significantly greater BP than those offspring (P < 0.005). Both the prenatal and postnatal LN and NN offspring had similar BP, indicating that only the HN diet permanently affected BP.
Two-bottle fluid intake and salt preference test.
Overall, the responses of the prenatally and postnatally exposed offspring to altered dietary sodium were very similar, and no statistical differences were observed between the prenatal and postnatal groups. As observed in Table 2, early dietary sodium manipulation affected water intake, 0.3 M NaCl intake, and salt preference in both prenatally and postnatally exposed offspring. Offspring that were prenatally or postnatally exposed to HN had significantly decreased water intake compared with the corresponding NN offspring (both P < 0.03). Offspring exposed to HN during either the prenatal or the postnatal period had increased intake of 0.3 M NaCl compared with the corresponding NN offspring (P < 0.006 or P < 0.04, respectively). In accordance with this, both prenatal and postnatal HN offspring had a greater salt preference compared with the NN offspring (P < 0.001 and P < 0.01, respectively). LN and NN offspring had similar water intake, 0.3 M NaCl intake, and salt preference whether the manipulation occurred during the prenatal or the postnatal period. Total fluid intake (water + 0.3 M NaCl) was similar for all groups.
Behavioral activity testing and amphetamine challenge.
There were no differences among the groups in their locomotor behavior following saline injection. However, there were group differences in locomotion following injection of amphetamine in both prenatal and postnatal offspring [F(2, 21) = 4.9, P < 0.02 and F(2, 19) = 4.5, P < 0.03, respectively]. Amphetamine injection elicited a greater locomotor response in offspring prenatally exposed to LN and HN diets compared with the offspring prenatally exposed to the NN diet (both P < 0.01). Similarly, the postnatally exposed LN and HN offspring exhibited increased locomotor behavior in response to amphetamines compared with the NN offspring (P < 0.01 and P < 0.04, respectively). These results reveal that both LN and HN dietary manipulations during early life, whether during the prenatal or the early postnatal period, sensitized offspring to the psychomotor stimulant effects of amphetamine (Fig. 1).
Fig. 1.
Mean (±SE) number of locomotor counts (cts) over 30 min in rat offspring administered either isotonic saline or 3 mg/kg amphetamine. Dams were on the low-sodium (LN), normal sodium (NN), or high-sodium diet (HN) during either the prenatal or the early postnatal period. *P < 0.05; **P < 0.01 compared with NN offspring.
Locomotion in a novel open field.
Figure 2 shows differences in locomotor behavior in prenatal and postnatal offspring when placed in a novel open field [F(2, 20) = 8.3, P < 0.003 and F(2, 19) = 4.9, P < 0.02, respectively]. Prenatally and postnatally exposed offspring responded similarly to the novel environment. Offspring prenatally exposed to both LN and HN exhibited greater locomotor activity compared with NN offspring in the novel environment (P < 0.04 and P < 0.001, respectively). Similarly, postnatal LN and HN offspring had increased locomotor behavior in a novel environment compared with NN offspring (P < 0.01 and P < 0.02, respectively).
Fig. 2.
Mean (±SE) number of locomotor counts over 20 min in rat offspring exposed to a novel open field environment. Dams were on the LN, NN, or HN diet during either the prenatal or the early postnatal period. *P < 0.05; **P < 0.01; ***P < 0.001 compared with NN offspring.
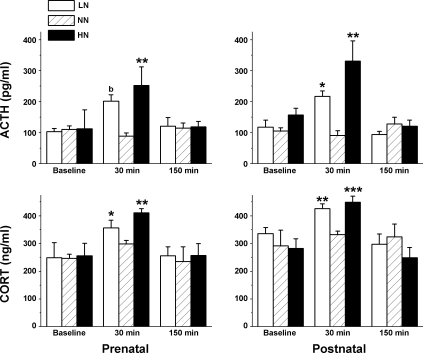
Plasma ACTH and Cort levels before and after restraint stress.
Plasma ACTH and Cort levels were measured before stress (baseline) and at 30 and 150 min following restraint stress. LN and HN offspring were compared with NN offspring at each time point (baseline, 30 min, and 150 min). Figure 3 summarizes the effects of restraint on ACTH and Cort levels in the offspring. Baseline levels of ACTH and Cort were similar for all groups but were higher than expected. To determine whether the blood sampling procedure stimulated the release of the hormones, we fitted normal Wistar rats with indwelling femoral catheters and collected blood samples from awake, behaving rats (24, 25). Under these conditions, plasma levels of ACTH (62 ± 10 pg/ml, n = 4) and Cort (21 ± 7 ng/ml, n = 4) were lower than those collected from anesthetized rats.
Fig. 3.
Plasma adrenocorticotropin hormone (ACTH) and corticosterone (Cort) levels (means ± SE) under basal conditions and following restraint stress for LN, NN, and HN offspring exposed to the diet during either the prenatal or the early postnatal period. *P < 0.05; **P < 0.01; ***P < 0.001 compared with NN offspring. bP < 0.06 indicates a marginally significant increase in ACTH levels in LN offspring compared with NN offspring.
At the 30-min poststress time period, ACTH levels in offspring prenatally exposed to LN and HN were higher than in NN offspring (P < 0.06 and P < 0.01, respectively). Similarly, Cort levels were also significantly higher in prenatal LN and HN offspring at 30 min poststress than in NN offspring (P < 0.05 and P < 0.002, respectively). Plasma ACTH and Cort levels in prenatal LN and HN offspring at 30 min were significantly higher than baseline (both P < 0.03). Plasma ACTH and Cort levels from prenatal NN offspring 30 min following restraint were not different from baseline (both P = 0.3).
Similarly, 30 min after restraint stress, the postnatal LN and HN offspring had significantly elevated ACTH (P < 0.05 and P < 0.002, respectively) and Cort levels (P < 0.006 and P < 0.001, respectively) compared with NN offspring. ACTH (P < 0.01 and P < 0.04, respectively) and Cort levels (P < 0.02 and P < 0.00l, respectively) levels in postnatal LN and HN offspring at 30 min were significantly higher than baseline. Plasma ACTH and Cort levels in postnatal NN offspring were not significantly different from baseline (both P = 0.5).
At 150 min following the restraint stress, no differences were detected in ACTH or Cort levels when comparing prenatal and postnatal LN and HN offspring with their NN counterparts or with baseline.
Adrenal gland weight.
Adrenal gland weight was expressed as a function of body weight. Figure 4 shows that the adrenal gland weight was increased in both the prenatal and postnatal LN and HN groups compared with the NN offspring (both P < 0.05). No differences were observed between the prenatal and postnatal groups in adrenal gland size, suggesting that both prenatal and postnatal dietary sodium manipulation were sufficient to affect adrenal gland weight.
Fig. 4.
Mean (±SE) adrenal gland weight as a function of body weight for LN, NN, and HN offspring exposed to the diet during the prenatal or the early postnatal period. *P < 0.05; ***P < 0.001 compared with NN offspring.
DISCUSSION
There is an increasing interest in the developmental factors that may alter an individual's response to amphetamines. Prenatal administration of amphetamines, maternal stress, and poor postnatal maternal care all enhance an offspring's behavioral response to amphetamines (1, 19, 23). In addition, maintaining SHR and WKY dams on a low-sodium diet during pregnancy and through the early postnatal period sensitizes the offspring to amphetamines when tested as adults. However, a high-sodium diet has no effect on behavioral sensitization to amphetamine in SHR and WKY offspring (33). The present study identified that the effects of dietary sodium manipulation in SHR and WKY substrains generalized to the Wistar rat.
Similar to our previous experiments using WKY offspring, we found that early life exposure to a high-sodium diet permanently increased BP in the Wistar offspring (33). In addition, both WKY and Wistar offspring exposed to the high-sodium diet had an increased sodium appetite as adults compared with control offspring. SHR, with their overall high salt intake, did not show a dietary sodium effect on salt intake or preference (33).
The present results demonstrate that maintaining Wistar dams on high dietary sodium has permanent effects on the offspring's salt preference and BP. Similar findings show that maternal dietary sodium intake affects an offspring's salt preferences and BP when dams and offspring are maintained on a high-sodium diet during pregnancy, weaning, and through the early postnatal period (8, 14, 16, 17, 33). The present results show that a limited exposure to high sodium, during either the prenatal or postnatal period, is sufficient to significantly increase the salt preference and BP of offspring when tested as adults.
Although the high-sodium diet affected BP and salt intake in the WKY substrain, a history with the low-sodium diet caused a greater behavioral response to amphetamine when the WKY and SHR offspring were tested as adults (33). This outcome was also found in the Wistar strain, in which the low-sodium diet caused an exaggerated response to amphetamine. A strain difference arises when considering the effects of the high dietary sodium on behavioral responses to amphetamine. The high dietary sodium history had no effect on the SHR and WKY offsprings' responses to amphetamine (33). In contrast, both the low and high dietary sodium exposure in the Wistar strain potentiated the adult offsprings' behavioral responses to amphetamine. The susceptibility of different substrains to dietary sodium manipulation may be tied to differences in the expression of receptors, such as the tachykinins, in brain regions that mediate the psychomotor stimulant and reinforcing properties of amphetamine and sodium solutions (27, 32). These results add to the literature identifying strain differences in salt intake, responses to stress, and psychomotor stimulant sensitivity (2, 5, 35, 43, 54).
Our previous experiments identified that combined prenatal and postnatal exposure to dietary sodium manipulations sensitized adult offspring to amphetamine. This time period was divided into either the prenatal or the early postnatal period in the current experiment to determine whether dietary sodium manipulation during specific time windows in early development would affect the offsprings' responses to amphetamine, novelty, and restraint stress. During development, different neural systems develop at different times, and it is during these critical periods that they are most susceptible or vulnerable to change. The mesolimbic dopamine system, which has been implicated in drug abuse (38), develops during late gestation and the early postnatal period (22, 29). Early life manipulations that increase behavioral responses in adults to drugs alter dopamine function, and behavioral responsiveness to amphetamine is an indication of the responsiveness of dopamine neurons (9, 19). Sodium restriction alters dopamine function in adults (13), and dietary sodium manipulation during the period that the dopamine system is being established may produce enduring changes in dopamine activity, particularly in mediating responses to amphetamine. Our results show that dietary sodium manipulations during either the prenatal or the early postnatal period had similar effects, sensitizing the offspring to amphetamines. The psychomotor sensitization indicates that dietary sodium restriction or excess during the time that the dopamine system is developing causes long-lasting alterations in dopamine function that persist into adulthood.
The hypothalamic-pituitary-adrenal (HPA) axis is developing throughout the prenatal and early postnatal period (50). Early life exposure to a variety of stressors during this time, including prenatal nutritional stress, prenatal Cort administration, and prenatal environmental stressors (cold, noise, crowding, restraint), results in an exaggerated stress response, both behavioral and endocrine, in the offspring (4, 9, 11, 19, 29). Several of our results indicate that maternal dietary sodium restriction or excess is also a stress to the dams. First, like other stressors during the prenatal period (20, 41), dietary sodium manipulation during early life resulted in enlarged adrenal glands in LN and HN offspring (4, 15, 33). Second, when exposed to a novel environment, LN and HN offspring exhibited more activity than NN offspring. High locomotor responsiveness in a novel environment results from a prior history of stress (31) and is associated with increased susceptibility for drug self-administration (39). Third, LN and HN offspring had exaggerated ACTH and Cort responses to restraint stress compared with NN offspring. In comparing hormone levels at baseline and at 30 min, it appears that stress did not affect hormone release in the NN offspring. As pointed out in results, this may reflect the stressful effect of anesthesia on baseline levels, and the high baseline may have masked the effects of restraint stress in NN offspring.
Regardless of baseline values, at 30 min after restraint stress, prenatal and postnatal LN and HN offspring showed an increased activation of the HPA axis compared with NN offspring. Interestingly, in contrast to NN offspring, ACTH and Cort levels in LN and HN offspring did increase significantly over baseline. The exaggerated response to environmental stress observed in the LN and HN offspring may reflect the additive effects of stressors (36) and support the stressful nature of the altered dietary sodium in the experimental animals. Together, the results indicate that dietary sodium restriction or excess during the prenatal or postnatal period is similar to other early life stressors and causes long-term changes in the LN and HN offsprings' behavioral and hormonal responses to stressors.
Our results suggest that low or high dietary sodium intake during the prenatal or postnatal period is a stress. There is an association between early life stress and an offspring's subsequent psychomotor responses to amphetamine (23). Stressors, including maternal separation and poor maternal care, as well as prenatal administration of Cort, all increase an offspring's behavioral response to amphetamine (9, 10, 19). Offspring of dams on the low- and high-sodium diets show physiological changes associated with early life stress. This early life stress may contribute to increased responsiveness to amphetamines in the LN and HN offspring.
Perspectives and Significance
There are individual differences in human susceptibility to drug abuse (44, 51), and the differential susceptibility to drugs as adults may be tied to differences in early life experiences. Uncovering the factors that contribute to vulnerability to drug taking may lead to preemptive treatments to curtail drug abuse. One early life experience that is clearly linked to enhanced amphetamine sensitivity is stress (9, 19, 23). We demonstrated in both the previous and current work that manipulation of dietary sodium, either restriction or excess, during either the prenatal or early postnatal period is a stress that causes long-term changes in how the adult animal responds to environmental stressors and amphetamine. Differences in the animal's dietary sodium environment during early development may contribute to individual differences in drug taking as adults. The results should direct studies that identify the effects of the altered sodium environment during early development on the responsiveness of specific neural pathways, such as the mesocorticolimbic dopamine system, to psychostimulants.
GRANTS
This study was supported by National Institutes of Health Grants R01 DK50586, NS57823, and P20 RR15640 (to F. W. Flynn).
Acknowledgments
We thank Xiaochun Zhang, David Bruch, Gwendolen Haley, Don Pratt, and Bryan Stevens for help and technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams J, Buelke-Sam J, Kimmel CA, LaBorde JB. Behavioral alterations in rats prenatally exposed to low doses of d-amphetamine. Neurobehav Toxicol Teratol 4: 66–70, 1982. [PubMed] [Google Scholar]
- 2.Alemayehu A, Breen L, Printz MP. A new inbred Wistar-Kyoto rat substrain exhibiting apparent salt sensitivity and borderline hypertension. Am J Physiol Heart Circ Physiol 283: H1181–H1190, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Almeida SS, Tonkiss J, Galler JR. Malnutrition and reactivity to drugs acting in the central nervous system. Neurosci Biobehav Rev 20: 389–402, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Almeida SS, Tonkiss J, Galler JR. Prenatal protein malnutrition affects exploratory behavior of female rats in the elevated plus-maze test. Physiol Behav 60: 675–680, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Amini B, Yang PB, Swann AC, Dafny N. Differential locomotor responses in male rats from three strains to acute methylphenidate. Int J Neurosci 114: 1063–1084, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJP Maternal nutrition, fetal nutrition, and disease in later life. Nutrition 13: 807–813, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Bird E, Contreras RJ. Maternal dietary NaCl intake influences weanling rats salt preferences without affecting taste nerve responsiveness. Dev Psychobiol 20: 111–130, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci 19: 1863–1874, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci 24: 4113–4123, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry 59: 1227–1235, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Clark JJ, Bernstein IL. Reciprocal cross-sensitization between amphetamine and salt appetite. Pharmacol Biochem Behav 78: 691–698, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Clark JJ, Bernstein IL. A role for D2 but not D1 dopamine receptors in the cross-sensitization between amphetamine and salt appetite. Pharmacol Biochem Behav 83: 277–284, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Contreras RJ Differences in perinatal NaCl exposure alters blood-pressure levels of adult rats. Am J Physiol Regul Integr Comp Physiol 256: R70–R77, 1989. [DOI] [PubMed] [Google Scholar]
- 15.Contreras RJ, Kosten T. Prenatal and early postnatal sodium chloride intake modifies the solution preferences of adult rats. J Nutr 113: 1051–1062, 1983. [DOI] [PubMed] [Google Scholar]
- 16.Contreras RJ, Ryan KW. Perinatal exposure to a high NaCl diet increases the NaCl intake of adult-rats. Physiol Behav 47: 507–512, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Curtis KS, Krause EG, Wong DL, Contreras RJ. Gestational and early postnatal dietary NaCl levels affect NaCl intake, but not stimulated water intake, by adult rats. Am J Physiol Regul Integr Comp Physiol 286: R1043–R1050, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Deminiere JM, Piazza PV, Guegan G, Abrous N, Maccari S, Lemoal M, Simon H. Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res 586: 135–139, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Diaz R, Ogren SO, Blum M, Fuxe K. Prenatal corticosterone increases spontaneous and d-amphetamine induced locomotor-activity and brain dopamine metabolism in prepubertal male and female rats. Neuroscience 66: 467–473, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Francesconi RP, Hubbard RW, Mager M. Chronic low-sodium diet in rats: hormonal and physiological effects during exercise in the heat. J Appl Physiol 55: 870–874, 1983. [DOI] [PubMed] [Google Scholar]
- 21.Fray PJ, Sahakian BJ, Robbins TW, Koob GF, Iversen SD. An observational method for quantifying the behavioural effects of dopamine agonists: contrasting effects of d-amphetamine and apomorphine. Psychopharmacology (Berl) 69: 253–259, 1980. [DOI] [PubMed] [Google Scholar]
- 22.Galineau L, Kodas E, Guilloteau D, Vilar MP, Chalon S. Ontogeny of the dopamine and serotonin transporters in the rat brain: an autoradiographic study. Neurosci Lett 363: 266–271, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Gordon HW Early environmental stress and biological vulnerability to drug abuse. Psychoneuroendocrinology 27: 115–126, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Haley GE, Flynn FW. Agonist and hypertonic saline-induced trafficking of the NK3-receptors on vasopressin neurons within the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol 290: R1242–R1250, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Haley GE, Flynn FW. Tachykinin NK3 receptor contribution to systemic release of vasopressin and oxytocin in response to osmotic and hypotensive challenge. Am J Physiol Regul Integr Comp Physiol 293: R931–R937, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Hynes MD, Langer DH, Hymson DL, Pearson DV, Fuller RW. Differential effects of selected dopaminergic agents on locomotor activity in normotensive and spontaneously hypertensive rats. Pharmacol Biochem Behav 23: 445–448, 1985. [DOI] [PubMed] [Google Scholar]
- 27.Jocham G, Lauber AC, Muller CP, Huston JP, Silva MAD. Neurokinin(3) receptor activation potentiates the psychomotor and nucleus accumbens dopamine response to cocaine, but not its place conditioning effects. Eur J Neurosci 25: 2457–2472, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug-induced and stress-induced sensitization of motor-activity. Brain Res Brain Res Rev 16: 223–244, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res 156: 251–261, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Ksir C Acute and chronic nicotine effects on measures of activity in rats- a multivariate-analysis. Psychopharmacology (Berl) 115: 105–109, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Lepsch LB, Gonzalo LA, Magro FJB, Delucia R, Scavone C, Planeta CS. Exposure to chronic stress increases the locomotor response to cocaine and the basal levels of corticosterone in adolescent rats. Addict Biol 10: 251–256, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Massi M, Perfumi M, Polidori C, Pompei P, Decaro G. Inhibition of ethanol intake in the rat by central injection of the tachykinin NH2-senktide. Eur J Pharmacol 183: 488–489, 1990. [Google Scholar]
- 33.McBride SM, Culver B, Flynn FW. Prenatal and early postnatal dietary sodium restriction sensitizes the adult rat to amphetamines. Am J Physiol Regul Integr Comp Physiol 291: R1192–R1199, 2006. [DOI] [PubMed] [Google Scholar]
- 34.McCarty R, Chiueh CC, Kopin IJ. Differential behavioral responses of spontaneously hypertensive (SHR) and normotensive (WKY) rats to d-amphetamine. Pharmacol Biochem Behav 12: 53–59, 1980. [DOI] [PubMed] [Google Scholar]
- 35.McDougall SJ, Widdop RE, Lawrence AJ. Differential gene expression in WKY and SHR brain following acute and chronic air-puff stress. Mol Brain Res 133: 329–336, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Nock B, Cicero TJ, Wich M. Chronic morphine increases the pituitary-adrenocortical response of juvenile rats to mild stress. Pharmacol Biochem Behav 80: 77–85, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Novick A, Yaroslavsky I, Tejani-Butt S. Strain differences in the expression of dopamine D1 receptors in Wistar-Kyoto (WKY) and Wistar rats. Life Sci 83: 74–78, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25: 192–216, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berl) 129: 277–284, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Pittman DW, Contreras RJ. Dietary NaCl influences the organization of chorda tympani neurons projecting to the nucleus of the solitary tract in rats. Chem Senses 27: 333–341, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Porter JP, King SH, Honeycutt AD. Prenatal high-salt diet in the Sprague-Dawley rat programs blood pressure and heart rate hyperresponsiveness to stress in adult female offspring. Am J Physiol Regul Integr Comp Physiol 293: R334–R342, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463: 3–33, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Redei E, Pare WP, Aird F, Kluczynski J. Strain differences in hypothalamic-pituitary-adrenal activity and stress-ulcer. Am J Physiol Regul Integr Comp Physiol 266: R353–R360, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol 54: 25–53, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 11: 1598–1604, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Roitman MF, Na E, Anderson G, Jones TA, Bernstein IL. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. J Neurosci 22: RC225–RC229, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakai RR, Frankmann SP, Fine WB, Epstein AN. Prior episodes of sodium depletion increase the need-free sodium-intake of the rat. Behav Neurosci 103: 186–192, 1989. [DOI] [PubMed] [Google Scholar]
- 48a.U.S. Department of Agriculture. Nutrition and Your Health: Dietary Guidelines for Americans (Home and Garden Bulletin N0. 32). Washington, DC: U.S. Department of Agriculture and U.S. Department of Health and Human Services, 2000, p. 1–44.
- 49.Van den Buuse M, Jones CR, Wagner J. Brain dopamine D-2 receptor mechanisms in spontaneously hypertensive rats. Brain Res Bull 28: 289–297, 1992. [DOI] [PubMed] [Google Scholar]
- 50.Vazquez DM Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology 23: 663–700, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Wagner FA, Anthony JC. From first drug use to drug dependence: Developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology 26: 479–488, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Welberg LAM, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: Possible implications for behaviour. Neuroscience 104: 71–79, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Welge JA, Richtand NM. Regression modeling of rodent locomotion data. Behav Brain Res 128: 61–69, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Yang PB, Amini B, Swann AC, Dafny N. Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Res 971: 139, 2003. [DOI] [PubMed] [Google Scholar]