Abstract
Leptin-resistant rats, when given a high-fat (HF) diet, have a delayed normalization of caloric intake and greater weight gain than those on a chow diet. Because aged, obese rats are leptin resistant, these data predict that they will also have a delayed normalization of caloric intake and exacerbated weight gain when provided a HF diet. To investigate this hypothesis, along with the consequences of a HF diet on voluntary wheel running, we compared various ages of rats on a HF or chow diet. HF-fed young rats spontaneously divided into diet-induced obese and diet-resistant rats. However, all aged rats were susceptible to the weight-gaining effects of HF feeding. Rate of initial weight gain was proportional to age, and peak caloric intake on the HF diet and the days required to normalize caloric intake to basal levels increased with age. Responsiveness to peripheral leptin before HF feeding revealed a dose-response decrease in food intake and body weight in the young but no responses in the aged to even the highest dose, 0.5 mg/day. In addition, both age and HF feeding decreased the tendency for wheel running, suggesting the propensity for inactivity with age and HF feeding may contribute to age-related obesity and accelerate the rate of diet-induced obesity. These results demonstrate that aged rats are more susceptible to the detrimental effects of a HF diet.
Keywords: leptin resistance, voluntary wheel running, diet-induced obese, peripheral leptin dose response
the typical western diet contains an excess of fat, and this is believed to be one contributor to the prevalence of obesity. Dietary obesity in adult humans and adult rats has been the subject of intense research, but the role of a high-fat (HF) diet with aging has largely been ignored. In some rodents, on initiation of a HF diet, there is a transient increase in caloric intake that returns to pretreatment levels, usually within weeks, despite continuation of the HF diet (6, 13, 17). One factor necessary for this normalization of caloric intake after HF feeding is leptin receptor activity (21). Our laboratory previously established that blockade of the leptin receptor with a specific leptin receptor antagonist prevents the normalization of caloric intake after HF feeding (21). Furthermore, rats made leptin resistant by central overexpression of leptin also display a prolonged hyperphagia with HF feeding and an exacerbated weight gain compared with leptin-responsive rats fed a HF diet (17). Collectively, these studies suggest that the normalization of caloric intake after exposure to a HF diet is mediated by leptin action.
The F344xBrown Norway (F344xBN) rat is a rodent model for late-onset obesity, whose weight gain with age parallels that observed in humans. This rat strain demonstrates a steady gain in adiposity into early senescence, followed by a decline beginning at 30 mo that continues into late life (15, 22). This increase in body fat with age cannot be accounted for by an increase in food intake (15). However, locomotor activity declines considerably with age (19) and potentially could contribute to the increase in obesity. Additionally, parallel to the increase in body fat with age, there is a rise in serum leptin levels (15, 22). This elevation in serum leptin with age should normally serve to lower body weight, but aged rats demonstrate reduced responsiveness to leptin, including an impaired anorexic response and little increase in energy expenditure, indicative of leptin resistance (6, 15).
We sought to address two hypotheses. First, because aged obese rats are leptin resistant (6, 15), these data predict that they will have a delayed normalization of caloric intake and exacerbated weight gain when provided a HF diet. Second, because both age and body weight impair locomotor activity, we hypothesized that both age and HF feeding would reduce voluntary wheel running. To evaluate these hypotheses, we chow or HF fed rats of various ages and examined caloric intake, body weight, and body composition over a 5-mo period, as well as voluntary wheel running.
MATERIALS AND METHODS
Animals.
Male F344xBN rats of various ages were obtained from the National Institute on Aging. Rats were cared for in accordance with the principles of the “Guide to the Care and Use of Experimental Animals”. Rats were housed individually on a 12:12-h light-dark cycle (lights on from 0700 to 1900). Protocols were approved by the University of Florida Institutional Animal Care and Use Committee.
Experimental design.
Six- (n = 5), 12- (n = 5), 18- (n = 5), 24- (n = 7), and 30-mo old rats (n = 5) were provided a HF diet (60% fat; 5.2 kcal/g D12492; Research Diets, New Brunswick, NJ) ad libitum for up to 5 mo, and additional 24-mo-old rats (n = 7) were fed a standard rat chow (15% fat; 3.3 kcal/g diet 2018; Harlan Teklad, Madison, WI) ad libitum. Body weight and food intake were recorded daily. A separate group of 3- (n = 32) and 30-mo-old (n = 23) rats was placed on either a standard chow or HF diet for 60 days and killed for assessment of serum leptin levels, hypothalamic protein tyrosine phosphatase 1B (PTP1B) levels, hypothalamic suppressor of cytokine signaling (SOCS-3) mRNA expression levels, brown adipose tissue (BAT) uncoupling protein 1 (UCP1) levels, and fat depot sizes.
Body composition (fat/lean mass) was determined before initiation of the experimental diets and periodically thereafter. Toward the end of the 150-day experimental period, physical performance tests and voluntary wheel running were assessed.
Additional groups of 3- (n = 36) and 30-mo-old rats (n = 24) on a chow diet were used to examine a 7-day dose response to peripheral leptin infusion.
Body composition.
Body composition was determined by time domain-nuclear magnetic resonance measurements on restrained but awake and alert animals (TD-NMR Minispec, Bruker Optics, The Woodlands, TX). The MiniSpec provides three components of body composition (fat, free body fluid, and lean tissue) by acquiring and analyzing TD-NMR signals from all protons in the sample area.
Serum leptin.
Serum was collected by cardiac puncture at death. Serum leptin was measured using rat radioimmunoassay kits (Linco Research).
Wheel running.
Voluntary wheel running was assessed by housing rats in cages equipped with Nalgene Activity Wheels (1.081-m circumference, Fisher Scientific, Pittsburgh, PA) that allowed free access to the wheel. Each wheel was equipped with a magnetic switch and counter, and the number of revolutions was recorded daily. Data are presented as meters ran per day for each age/diet group.
Physical performance tests.
Forelimb grip strength was measured using an automated grip strength meter (Columbus Instruments, Columbus, OH), as described previously (2). Data are expressed as kilograms of force per kilograms of body weight. Muscle tone and endurance were determined by use of an inclined plane, as described previously (2). Data are presented as latency time per kilogram body weight.
Peripheral leptin infusion.
Rats were anesthetized with isoflurane, and an osmotic minipump (model 2001, Durect, Cupertino, CA) containing either murine recombinant leptin or saline was implanted in a subcutaneous pocket on the dorsal surface of the rat.
Tissue harvesting and preparation.
Rats were killed by thorocotomy under a ketamine (75 mg/kg)/xylazine (7 mg/kg) cocktail anesthesia, and the circulatory system was perfused with 30 ml of cold saline. The epididymal, perirenal, and retroperitoneal white adipose tissues, hypothalamus, and BAT were excised. Protein concentrations were determined using the DC protein assay kit (Bio-Rad, Hercules, CA).
Western analysis.
Protein homogenate was separated on an SDS-PAGE gel and electrotransferred to polyvinylidene difluoride membranes, as described previously (21). Immunoreactivity was assessed with antibodies specific to PTP1B (Calbiochem, Sand Diego, CA) in the hypothalamus or UCP1 (Linco Research, St. Charles, MO) in BAT.
RT-PCR.
Expression levels of SOCS-3 in the hypothalamus were identified by relative quantitative RT-PCR using QuantumRNA 18S Internal Standards kit (Ambion, Austin, TX), as described previously (20). The optimum ratio of 18S primer to competimer was 1:9, and the primers used were sense 5′-ACCAGCGCCACTTCTTCACA-3′ and antisense 5′-GTGGAGCATCATACTGGTCC-3′. PCR was performed at 94°C denaturation for 90 s, 59°C annealing temperature for 60 s, and 72°C elongation temperature for 120 s for 28 cycles.
Statistical analysis.
Data were analyzed by one-way and two-way ANOVA. When the mean effect was significant, a post hoc test (Newman-Keuls, Bonferroni, or Dunnett) was applied to determine individual differences between the means. A value of P < 0.05 was considered significant.
To assess the hypothesis of diet-induced obese (DIO) rats gaining more weight on a HF diet than diet-resistant (DR) rats, we fit two normal mixture models. The first assumed separate normal distributions for the chow fed and for the HF fed; the second assumed a normal distribution for the chow-fed, together with the HF-fed DR rats and a separate normal distribution for the HF-fed DIO-prone rats. We used the maximized value of the log likelihood as a measure of model fit (larger values indicate better fit).
RESULTS
Body weight change with HF feeding.
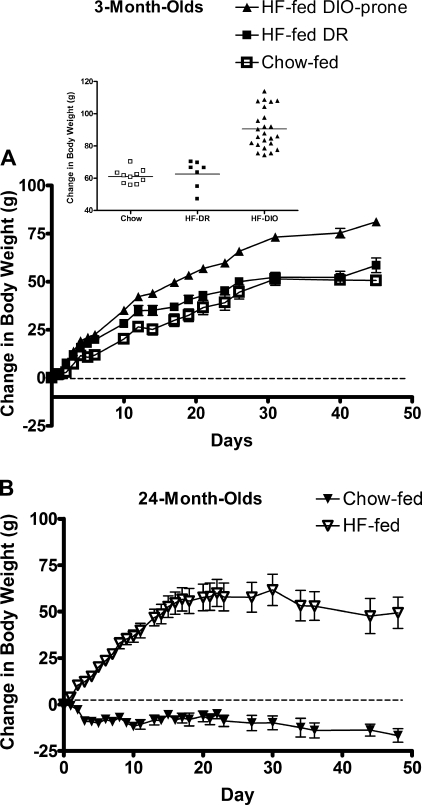
Normally, when young rats are provided with a HF diet, they spontaneously divide into two groups, DIO prone and DR, based on the amount of weight gained (11). This phenomenon has been well described in the Sprague-Dawley rat strain (7, 11, 12) and recently described in the F344xBN rat strain (20). In the present study, the 3-mo-old rats fed the HF diet spontaneously divided into two distinct groups, consisting of the top 75% of weight gainers (DIO prone), and the lowest 25% (DR) (Fig. 1A). The latter were more statistically similar to the chow fed as measured by the maximized value of the log likelihood. Surprisingly, this phenomenon was not seen in the aged rats. All of the aged rats provided the HF diet gained similar and considerable amounts of weight, and thus aged, as opposed to young rats, are all susceptible to the weight-gaining effects of HF feeding (Fig. 1B).
Fig. 1.
A: body weight gain in young rats on chow (open symbols) or high-fat (HF) (solid symbols) diet. Values are means ± SE of HF-fed diet-induced obese (DIO)-prone (n = 24, triangles), HF-fed diet-resistant (DR) (n = 8, solid squares), and chow-fed rats (n = 10, open squares). Inset: young rats were either prone or resistant to the effects of the HF diet. We used the maximized value of the log likelihood as a measure of model fit (larger values indicate better fit). When we assumed one normal distribution for the chow-fed together with the HF-fed DR rats, and a separate normal distribution for the HF-fed DIO-prone rats, the model fit yielded a log likelihood value of −530 compared with −542 when one normal distribution was assumed for the chow-fed rats and one normal distribution for the all of the HF-fed rats (DIO prone plus DR). This suggests that HF-fed DR rats are more similar to the chow-fed rats than the HF-fed DIO-prone rats, and thus the latter should be considered a separate group. By day 3 on the diet, the body weight gain in HF-fed DIO-prone animals was significantly greater than that of the chow-fed group (P < 0.0001 by t-test). The change in body weight was significant between the two HF-fed groups by day 15 (P < 0.05 by ANOVA). B: body weight change in aged rats on either chow (solid triangles) or HF diet (open triangles). Values are means ± SE of chow-fed (n = 5) and HF-fed rats (n = 7). All aged rats were susceptible to the detrimental effects of the HF diet. By day 1 on the diet, the HF-fed aged animals had experienced a weight gain significantly greater than that of their chow-fed counterparts (P < 0.05 by ANOVA).
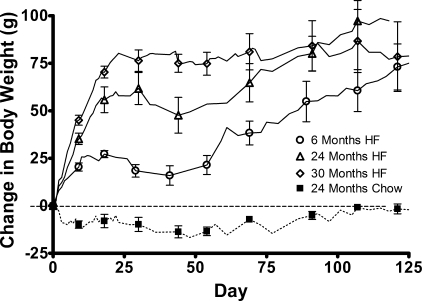
This age-related susceptibility to HF feeding was also evident in the body weight gain across different rat ages. Particularly, age exacerbated the initial body weight gain seen with HF feeding (Fig. 2). Both the 6-mo-old young rats and the 24-mo-old mature rats steadily gained weight on the HF diet and continued to gain weight throughout the experimental period, but the weight gain in the 24-mo-old rats was significantly greater throughout the study period, except during the last 2 wk. This diet- and age-related weight gain was most pronounced in the oldest age group, especially during the initial phase of HF feeding. In the first 30 days of HF feeding, the 30-mo-old rats gained the most weight, but this increase in body weight reached a plateau by day 30 and at ∼31 mo of age.
Fig. 2.
Body weight change in rats of differing ages on chow (dotted line) or HF (solid lines) diet. Values are means ± SE of 6-mo-old HF-fed (n = 5, circles), 24-mo-old HF-fed (n = 7, triangles), 30-mo-old HF-fed (n = 5, diamonds), and 24-mo-old chow-fed (n = 7, squares) rats. The 30-mo-old rats fed a HF diet experienced a body weight gain greater than that of the 6- and 24-mo-old rats fed a HF diet by day 3 (P < 0.01, one-way ANOVA) and day 5 (P < 0.01), respectively. The change in body weight was no longer significantly different between the 24- and 30-mo-old rats fed a HF diet by day 69 (P > 0.05). The change in body weight in the 3-, 24-, and 30-mo-old rats fed a HF diet was no longer significantly different by day 89 (P > 0.05) and for the duration of the experiment.
It should be noted that initial body weights, before initiation of HF feeding, were greater with increasing age (3 mo olds: 324.35 ± 3.68 g; 6 mo olds: 385.26 ± 11.28 g; 12 mo olds: 435.58 ± 14.24 g; 18 mo olds: 500.86 ± 13.47 g; 24 mo olds: 551.23 ± 9.47 g; 30 mo olds: 568.33 ± 9.17 g).
Caloric intake with HF feeding.
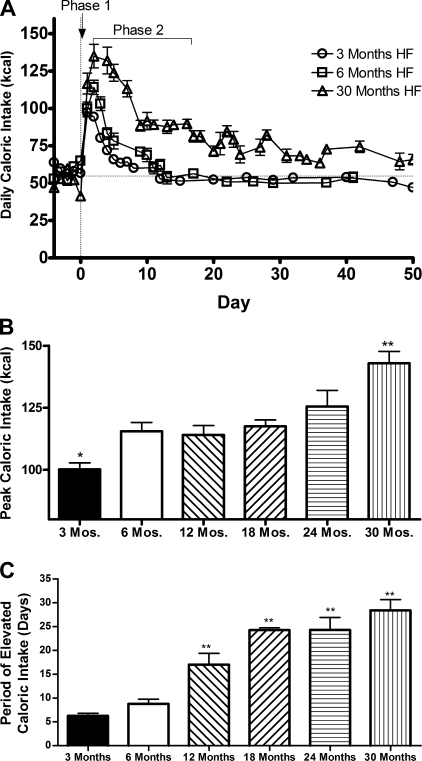
The age-related disproportionate increase in initial weight gain suggests that this may be related to the hyperphagia normally observed after introduction of a HF diet. When 3-, 6-, and 30-mo-old rats were provided the HF diet, all ages experienced an immediate increase in daily caloric intake that gradually normalized to basal levels, or near basal level in the case of the oldest age group, over time (Fig. 3A). For ease in analyzing the data, the daily caloric intake was divided into two phases, with phase 1 representing the peak caloric intake on initiation of the HF diet, and phase 2 being the days necessary to normalize the elevated caloric intake back to levels before HF feeding. It is important to note that the animals of all ages consumed a similar amount of calories on the regular chow diet before the introduction of the HF diet.
Fig. 3.
A: daily caloric intake of 3- (circles), 6- (squares), and 30-mo-old (triangles) rats on a HF diet. Values are means ± SE of 3- (n = 9), 6- (n = 5), and 30-mo-HF-fed rats (n = 5). B: the peak caloric intake after the initiation of HF feeding. Values are means ± SE of 3- (n = 24), 6- (n = 5), 12- (n = 5), 18- (n = 5), 24- (n = 7), and 30-mo-old HF-fed (n = 5) rats. P < 0.0001 for difference with age by one-way ANOVA. *P < 0.05 for the difference between 3-mo-old rats and all other ages by post hoc analysis. **P value < 0.001 for the difference between the 30-mo-old rats and all other ages by post hoc analysis. C: the days required to normalize the elevated caloric intake following HF feeding. Values are means ± SE of 3- (n = 11), 6- (n = 5), 12- (n = 5), 18- (n = 4), 24- (n = 7), and 30-mo-old HF-fed (n = 5) rats. P < 0.0001 for difference with HF feeding by one-way ANOVA. **P value < 0.001 for the difference compared with 3-mo-old rats by one-way ANOVA and post hoc.
The peak daily caloric intake with HF feeding increased with age (R2 = 0.85, P = 0.009), as is evident in a comparison of phase 1 across ages (Fig. 3B). The 3-mo-old rats reached a peak caloric intake of 100 kcal. The 6-, 12-, and 18-mo-old rats all reached a peak of ∼115 kcal, and the 24-mo-old rats reached a peak caloric intake of 125 kcal. The 30-mo-old rats, the oldest age tested in this experiment, reached a maximal caloric intake of 142 kcal.
A similar trend was seen in phase 2 (Fig. 3C), where the days required to normalize caloric intake to basal levels increased with age (R2 = 0.93, P = 0.002). In this F344xBN rodent strain, the 3-mo-old rats experienced a complete normalization of caloric intake by day 6. The 6-mo-old rats and the 12-mo-old rats required 9 and 17 days, respectively, whereas the 18- and 24-mo-old rats did not experience normalization until day 25. In contrast to the younger ages, the 30-mo-old rats were unable to normalize to pre-HF caloric intake, but stabilized at a new slightly elevated plateau by day 29.
Body composition.
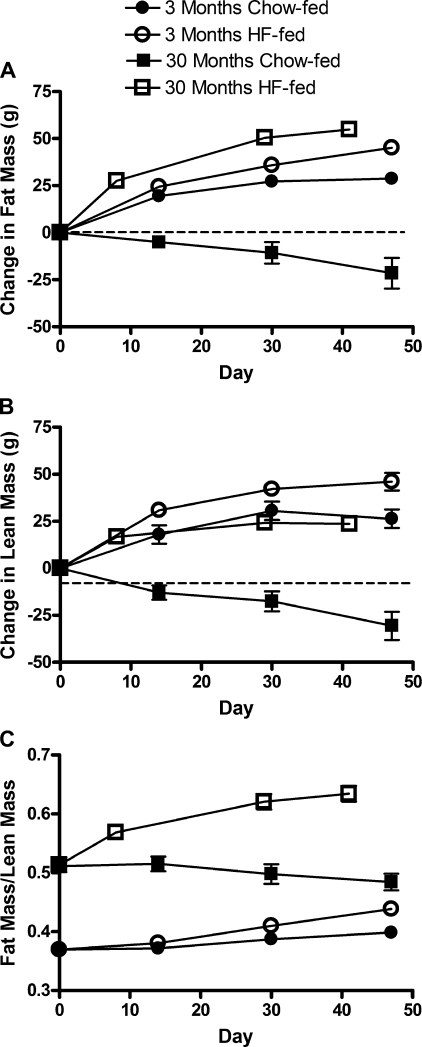
Absolute fat and lean body mass were greater in the 30-mo-old rats compared with the 3-mo-old rats at the beginning of the study (fat mass: 175.67 ± 4.38 vs. 73.56 ± 1.86 g, P < 0.0001; lean mass: 339.78 ± 5.33 vs. 199.05 ± 4.17 g, P < 0.0001). By examining the change in body composition, the increase in fat and lean mass was significantly greater in both young and old HF-fed groups compared with their age-matched, chow-fed controls (Fig. 4). In addition, the HF-fed aged rats gained significantly more fat mass than the HF-fed young rats. Conversely, the HF-fed young rats gained significantly more lean mass than the HF-fed aged rats. This resulted in a significantly larger fat-to-lean mass ratio in the aged, HF-fed rats compared with all other age/diet groups (Fig. 4C). In contrast, the ratio of fat mass to lean mass was relatively constant over time in the chow-fed aged rats (Fig. 4C).
Fig. 4.
Change in fat mass (A), change in lean mass (B), and the ratio of fat to lean mass over time (C) in 3- (circles) and 30-mo-old (squares) rats on either a HF (open symbols) or a standard chow (solid symbols) diet. Values are mean ± SE of 3-mo-old chow-fed (n = 10), 3-mo-old HF-fed (n = 12–32), 30-mo-old chow-fed (n = 4–6), and 30-mo-old HF-fed (n = 5) rats. A: P < 0.0001 for interaction, for difference with HF feeding, and for difference with age by two-way ANOVA. P < 0.001 for the difference between HF-fed aged rats and HF-fed young rats by day 30 by post hoc analysis. P < 0.01 for the difference between HF-fed young rats and chow-fed young rats by day 14 by post hoc analysis. Note: P < 0.05 for the difference between the day 0 fat mass values and the day 60 fat mass values in each age/diet group by paired T-test. B: P = 0.0003 for the interaction; P < 0.0001 for the difference with HF feeding and difference with age by two-way ANOVA. P < 0.05 for the difference between HF-fed aged rats and HF-fed young rats by day 30 by post hoc analysis. P < 0.05 for the difference between HF-fed young rats and chow-fed young rats by day 14 by post hoc analysis. Note: P < 0.01 for the difference between the day 0 lean mass values and the day 60 lean mass values in each age/diet group by paired T-test. C: P = 0.0003 for the interaction; P < 0.0001 for the difference with HF feeding and difference with age by two-way ANOVA. P < 0.001 for the difference between HF-fed aged rats and HF-fed young rats by day 14 by post hoc analysis. P < 0.05 for the difference between HF-fed young rats and chow-fed young rats by day 30 by post hoc analysis. Note: P < 0.001 for the difference between the day 0 fat/lean mass values and the day 60 fat/lean mass values in the 3-mo-old chow-fed, 3-mo-old HF-fed, and 30-mo-old HF-fed groups by paired T-test.
Serum leptin and adiposity.
With HF feeding in young rats, by day 60, serum leptin levels rose greater than twofold compared with that in the chow-fed counterparts. Interestingly, after 60 days of HF feeding in the young, serum leptin reached the same levels as that in the aged chow-fed rats (Fig. 5A). Similar to the young, the HF-fed aged rats had serum leptin levels more than twofold greater than the aged chow-fed rats by day 60. Serum leptin levels at day 60 paralleled that of the amount of white adipose tissue (sum of perirenal, retroperitoneal, and epididymal white adipose tissues) present in each group at death (Fig. 5B). The young HF-fed and aged chow-fed rats had the same amount of white adipose tissue, whereas the young chow-fed rats had significantly less and the aged HF-fed rats had significantly more. This is consistent with previous reports that leptin circulates in proportion to whole body fat depots (1).
Fig. 5.
A: serum leptin at day 60 in 3- and 30-mo-old rats on chow or HF diets. Ages represent the age of the animal when HF or chow feeding was begun. Assessments were determined 60 days later. Values are means ± SE of 3- (n = 9–10) and 30-mo-old (n = 9–13) rats. P < 0.0001 for the difference with HF feeding and difference with age by two-way ANOVA; P = 0.0041 for the interaction. *P < 0.05 for the difference between chow-fed young and aged rats by post hoc analysis. **P < 0.001 for the difference between HF-fed young and aged rats by post hoc analysis. †P < 0.05 for the difference between chow-fed and HF-fed young rats by post hoc analysis. ††P < 0.001 for the difference between chow-fed and HF-fed aged rats by post hoc analysis. B: white adipose tissue at death from 3- and 30-mo-old rats following chow or HF feeding. Ages represent the age of the animal when HF or chow feeding was begun. Assessments were determined 60 days later. Values are means ± SE of 3- (n = 10–12) and 30-mo-old (n = 8–14) rats. P < 0.0001 for the difference with HF feeding and difference with age by two-way ANOVA. *P < 0.001 for the difference between chow-fed young and aged rats by post hoc analysis. **P < 0.001 for the difference between HF fed young and aged rats by post hoc analysis. †P < 0.001 for the difference between chow-fed and HF-fed young rats by post hoc analysis. ††P < 0.001 for the difference between chow-fed and HF-fed aged rats by post hoc analysis.
Wheel running and physical performance tests.
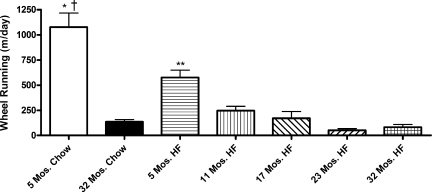
From 2–5 mo after HF or chow feeding, three performance tests were administered: wheel running and two measures of forelimb grip strength. Each age group was provided access to running wheels for 4 consecutive days, but the rats were not provided any training or encouragement to run. The youngest chow-fed rats ran >1,000 m/day (Fig. 6). In contrast, the oldest chow-fed rats ran eightfold less, only 136 m/day during the same period. HF feeding also impacted wheel running, but not to the same degree as age. The youngest HF-fed rats ran twofold less than the corresponding chow fed. With increasing age and HF feeding, wheel running declined with the oldest HF-fed rats running only 81.6 m/day (Fig. 6).
Fig. 6.
Voluntary wheel running over a 4-day period. Wheel running activity was determined 2 mo after HF or chow feeding for rats initially 3 or 30 mo of age and after 5 mo of HF or chow feeding in rats initially 6, 12, or 18 mo of age. Ages represent the age at the time of wheel running. Values are means ± SE. *P < 0.001 for the difference between 5- and 32-mo-old chow-fed rats. †P < 0.001 for the difference between 5-mo-old chow-fed and 5-mo-old HF fed rats. **P < 0.01 for the difference between 5-mo-old HF fed and all other HF-fed groups by post hoc analysis.
Because wheel running may be dependent on muscle strength, we subjected the 24-mo-old rats to a grip strength and inclined plane test at approximately day 130 on the standard chow or HF diet. There was no significant difference in either grip test or ability to perform in the inclined plane test between the diet groups in the 24-mo-old rats (data not shown).
Hypothalamic measures of leptin action.
We examined two factors that participate in leptin signaling, SOCS-3, a negative regulator of leptin signaling, and PTPIB, a phosphatase that dephosphorylates activated components in the leptin signaling cascade. Consistent with models of leptin resistance, both SOCS-3 mRNA levels and PTP1B protein levels were significantly increased in aged rats compared with young rats (Table 1). In addition, both hypothalamic SOCS-3 expression and PTP1B levels were significantly elevated with HF feeding (Table 1).
Table 1.
Hypothalamic PTP1B protein levels, hypothalamic SOCS-3 mRNA levels, and BAT UCP1 protein levels at death from 3- and 30-mo-old rats following chow or HF feeding
|
Young (3 mo old) |
Aged (30 mo old)
|
|||
|---|---|---|---|---|
| Chow Fed | HF Fed | Chow Fed | HF Fed | |
| PTP1B protein | 1.00±0.04 | 1.25±0.06 | 1.31±0.04* | 1.27±0.09 |
| SOCS-3 mRNA | 1.00±0.04 | 1.27±0.09† | 1.20±0.04 | 1.41±0.08 |
| BAT UCP1 | 1.00±0.14 | 2.53±0.40‡ | 1.30±0.25 | 3.19±0.48‡ |
Values are means ± SE in arbitrary units; n = 3–9 rats/group. Levels in young chow-fed rats are set to 1.0 with SE adjusted accordingly. HF, high fat; PTP1B, protein tyrosine phosphatase 1B; SOCS-3, suppressor of cytokine signaling-3; BAT, brown adipose tissue; UCP1, uncoupling protein 1. PTP1B: P = 0.017 for difference with HF feeding and P = 0.038 for difference with age by two-way ANOVA.
P < 0.05 for the difference between chow-fed 3- and 30-mo-old rats by post hoc analysis. SOCS-3: P = 0.006 for difference with HF feeding and P = 0.022 for difference with age by two-way ANOVA.
P < 0.05 for the difference between 3-mo-old HF-fed and 3-mo-old chow-fed rats. BAT UCP-1: P < 0.0001 for the difference with HF feeding by two-way ANOVA.
P < 0.01 for the difference with HF feeding in both 3- and 30-mo-old rats by post hoc analysis.
BAT UCP1 levels.
The induction of UCP1 in BAT is a marker for enhanced thermogenesis in rodents and is often used as an indicator of energy expenditure. Consistent with our laboratory's previous findings (20, 21), HF feeding increased UCP1 protein levels in BAT in young rats (Fig. 7). Surprisingly, the HF diet also induced increased UCP1 protein levels in BAT in the aged leptin-resistant rats.
Fig. 7.
A: change in body weight during a 7-day peripheral leptin infusion in 3-mo-old chow-fed rats. Values are means ± SE of 7 rats per group. P < 0.0001 for the difference with leptin treatment by one-way ANOVA. Each individual dose is significantly different from control (P < 0.0002 for the difference in slope). B: change in food intake in young rats during a 7-day peripheral leptin infusion. Values are means ± SE of 3-mo-old chow-fed (n = 7 per group) rats. P < 0.0001 for the difference with leptin treatment by one-way ANOVA. P < 0.01 for the difference between control and each dose at day 7 and between control and each dose for cumulative food intake.
Dose response to peripheral leptin infusion.
Because previous data suggest that leptin resistance prolongs or prevents the renormalization of caloric intake following HF feeding, we evaluated the responsiveness to leptin before initiation of the HF feeding in both young and aged rats. The doses used were 0.03, 0.05, 0.07, 0.1, and 0.5 mg/day (0.1, 0.167, 0.233, 0.33, and 1.67 mg·kg−1·day−1, respectively) in the young rats and 0.05, 0.1, and 0.5 mg/day (0.091, 0.182, and 0.909 mg·kg−1·day−1, respectively) in the aged rats. In the young rats (initially 3 mo old and weighing 308.79 ± 3.19 g), all doses >0.03 mg/day of peripheral leptin significantly increased serum leptin levels compared with saline-infused rats (Table 2). The leptin-infused rats displayed a dose-dependent, anorectic, and body weight reduction in response to a peripheral leptin up to a dose of 0.07 mg/day, above which there was no additional effect (Fig. 7, A and B, respectively). The decrease in body weight for each of the leptin-infused groups was significantly greater than that of the saline-infused group. In addition, the reduction in cumulative food intake due to leptin infusion was greater with each leptin dose, except for the lowest dose compared with the saline-infused group. The anorectic and weight reduction responses to doses of 0.07, 0.1, and 0.5 mg/day were not statistically different from each other and appear to represent the maximum response to leptin.
Table 2.
Serum leptin levels in young and aged rats on a chow diet after 7-day saline or leptin infusion
| Dose (mg/day) | Young Rats | Aged Rats |
|---|---|---|
| Control | 6.91±1.12 | 13.25±2.00 |
| 0.03 | 10.10±0.47 | |
| 0.05 | 14.05±0.86* | 19.56±1.34 |
| 0.07 | 19.75±1.38* | |
| 0.1 | 20.16±1.08* | 25.38±2.86† |
| 0.5 | 22.23±2.15* | 24.62±2.26† |
Values are means ± SE in ng/ml; n = 7–9 rats/group. Young rats: P < 0.0001 for the difference with leptin infusion.
P < 0.01 for the increase in serum leptin levels compared with controls. Aged rats: P = 0.0008 for the difference with leptin infusion.
P < 0.01 for the increase in serum leptin levels compared with control values by post hoc analysis.
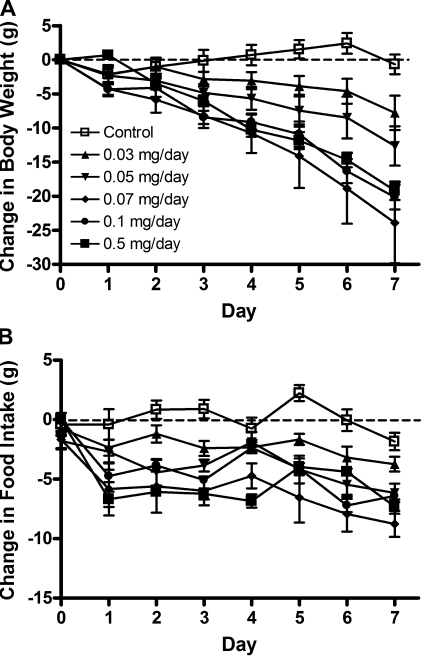
In contrast, the aged rats (initially 30 mo old and weighing 553.39 ± 5.48 g) did not respond to even the largest dose of leptin. In particular, cumulative food consumption was unchanged across leptin doses (Fig. 8B). All of the aged rats lost weight, likely in response to the detrimental effects of surgery (Fig. 8A). However, the leptin-infused rats did not differ from the saline-infused rats with respect to either body weight or food intake reduction, even though the doses of both 0.1 and 0.5 mg/day of leptin significant increased serum leptin compared with saline-infused rats (Table 2).
Fig. 8.
A: change in body weight during a 7-day peripheral leptin infusion in 30-mo-old chow-fed rats. Values are means ± SE of 7–8 per group. P = 0.9975 for the difference with leptin treatment by one-way ANOVA. B: change in food intake in aged rats during a 7-day peripheral leptin infusion. Values are means ± SE of 30-mo-old chow-fed rats (n = 7–8 per group). There was no difference (P = 0.087) in cumulative food intake with leptin treatment.
DISCUSSION
Rodents provided with a highly palatable caloric-dense diet initially consume an elevated level of calories, but within several weeks adjust their total food consumption, such that their diet becomes isocaloric to that of chow-fed companions (6, 13, 17). Leptin-resistant animals, however, display impaired normalization of caloric intake with HF feeding (21). Because aged obese rats are also leptin resistant (17), we predicted that aged rats would also fail to properly normalize caloric intake after exposure to HF feeding. The present investigation confirms this hypothesis by examining the physiological effects of a HF diet on rats of various ages between 3 and 33 mo of age and provides several salient findings.
First, our data are consistent with several previous studies indicating that aging increases the susceptibility to obesity and fat storage (9, 10). Iossa et al. (9) showed that young male Wistar rats that are naturally growing to maturity have the ability to store both proteins and lipids. However, as the rats age, from 1 to 6 mo old, the protein deposition eventually becomes almost nonexistent, and all excess energy consumed is stored as fat (9). They propose that this is one mechanism underlying age-associated obesity. Moreover, these adult rats were more prone to obesity when fed a HF diet than were younger counterparts (9, 10). It is reasonable that these trends toward obesity continue as rats age even further. Supporting this hypothesis is our demonstration that aged rats do not divide into DIO and DR, as previously and currently seen in young F344xBN rats (20). Whereas young rats are either susceptible or resistant to weight gain, all aged rats are susceptible to this negative effect of a HF diet. Moreover, body composition analysis indicates that the older rats gain a disproportionate amount of body fat compared with younger counterparts when provided a HF diet. Hence, these data support the concept that energy storage shifts toward fat deposition with aging, implicating one mechanism underlying age-related obesity. Moreover, aged F344xBN rats, in our case both 24- and 30-mo old rats, provided a HF diet, gain more weight than correspondingly fed young rats. Together, these data indicate that all aged rats, as opposed to only some young rats, are prone to develop obesity on a HF diet, and furthermore the degree of weight gain is greater in the older rats.
Second, our data indicate that the nature of the transient increase in caloric intake on initiation of HF feeding is dependent on age. Both the peak increase in caloric intake on initiation of HF feeding and the time to normalization increase with age. Moreover, this hyperphagia was a specific result of the HF diet: before initiation of the HF diet, all rats, regardless of age, consumed the same amount of chow diet, confirming earlier studies (8, 15, 22).
Thus the greater initial body weight gain in the 24- and 30-mo-old rats appears to be a consequence of this failure to normalize caloric intake after initiation of HF feeding, and we suggest the latter is a direct result of leptin resistance in these aged animals. Previous experiments demonstrated that the simultaneous administration of a leptin receptor antagonist along with HF feeding prevents the normalization of caloric intake after HF feeding, indicating that leptin receptor activity is necessary for this normalization (21). Older rats have reduced numbers of leptin receptors and diminished leptin signaling (16). In addition, in the present study, we found increases with age in both SOCS-3, a negative regulator of leptin signaling, and PTPIB, a phosphatase that dephosphorylates activated components in the leptin signaling cascade. These changes with age likely impair the native responses to the endogenous elevation in leptin triggered by HF feeding. We examined the status of leptin resistance at the point before initiation of the HF feeding by assessing the leptin dose-response decrease in food consumption and body weight over the course of 7 days in young and aged rats. As expected, the young rats responded in a dose-response fashion, whereas there were no responses in the aged rats, thus confirming that, before initiating HF feeding, the aged rats were unresponsive to leptin. As such, these leptin-resistant animals display a delayed normalization of caloric intake on a HF diet, strongly suggesting that preexisting leptin resistance is causal to the exacerbated weight gain with age. It should be noted, however, that these measures of leptin responsiveness were examined only in the 3- and 30-mo-old rats. While they demonstrate impaired leptin responsiveness by 30 mo of age, we cannot dismiss the possibility that the leptin resistance may be fully manifested before this age. If this is the case, the leptin resistance may be only one factor in the progressive exacerbated weight gain with age to HF feeding.
Our data indicating that leptin receptor activity is necessary for the normalization of caloric intake predicts that any impaired normalization should be proportional to the degree of leptin resistance and possibly even never occur in aged rats that are fully leptin resistance. Our data support this prediction. The delay in normalization is proportional to advancing age, with the oldest group achieving only a partial normalization. Similarly, leptin resistance is greater in 30-mo-old rats compared with 18-mo-old ones (18). We suspect the partial normalization in the oldest age group represents some residual leptin receptor activity or compensation by another anorexic pathway.
Subsequent to the caloric normalization, rats of all ages continued to gain weight, suggesting that energy expenditure must be diminished. However, any such decrease in energy expenditure does not appear to be related to the thermic effect of food, because both the young and aged HF-fed rats responded equally with an increase in UCP1 protein level in BAT. In addition to thermogenesis, an important component of energy expenditure is physical activity levels. There is an inverse relationship between body weight and physical activity (19), and a decrease in locomotor activity, including volitional activity, may be an important contributor to age-related obesity. Voluntary wheel running is one form of volitional activity involving motivational, exploratory, muscular, age, and body size components (19). Data indicate that locomotor activity declines both with age and obesity (19). We hypothesized that both age and HF feeding would impact voluntary wheel running, and this hypothesis proved correct: wheel running activity declined with both age and HF feeding, the latter especially in young rats. It has previously been reported that Sprague-Dawley and S5B/P1Ras rats on a high-carbohydrate diet voluntarily run more than those fed a HF diet, but there was no comparison to rats on a standard chow diet (3). Another group showed that the introduction of sweet milk plus standard chow decreased voluntary wheel running in female rats, but not male rats (5). Research with hamsters indicated that aged hamsters run significantly less than young ones (4). These data are consistent with our findings that aged F344xBN rats run significantly less than young F344xBN rats on a standard chow diet. In addition, HF feeding can further reduce voluntary wheel running activity in young rats. Interestingly, the aging and HF feeding suppressive effect on voluntary wheel running do not appear to be independent; for instance, in the oldest age group that ran the least, HF feeding had little additional suppressive effect. Collectively, these data suggest that the propensity for inactivity with age may be one contributory factor in age-related obesity, and the inactivity with HF feeding may accelerate the rate of diet-induced obesity.
Perspectives and significance.
Obesity is a worldwide epidemic in developed countries, and the lure of palatable HF food presents as much of a threat to the aging population as it does to the young in our modern society. Our data indicate that, with HF feeding, aged obese rats experience a greater hyperphagia and a delayed normalization in caloric intake, therefore resulting in an exaggerated weight gain. The mechanism appears to be a consequence of the preexisting leptin resistance present with age and obesity. Moreover, both age and HF feeding diminish volitional activity. Obesity in males and females over 70 yr of age dramatically increases by nearly two-thirds the number of remaining years spent disabled (14). Our data provide a negative link between a HF diet and physical activity. Collectively, aged rats experience greater negative effects of a HF diet, and that leads to both obesity and diminished physical activity, which, in turn, contribute to an ever escalating cycle of weight gain. If these results are applicable to humans, there may be a greater need for intervention in our aging community to combat this heightened susceptibility to HF-induced obesity and subsequent decrease in volitional activity.
GRANTS
This study was supported by National Institute on Aging Grants AG26159 and AG20985; University of Florida Institute on Aging and the Claude D. Pepper Older Americans Independence Center Grant NIH P30 AG028740, and the Medical Research Service of the Department of Veterans Affairs.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahima RS, Flier JS. Leptin. Annu Rev Physiol 62: 413–437, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Carter CS, Cesari M, Ambrosius WT, Hu N, Diz D, Oden S, Sonntag WE, Pahor M. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci 59: 416–423, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Chang LT, Kras K, Suzuki K, Strasburg G, Rodgers CD, Schemmel RA. Voluntary running in male S5B/P1Ras rats fed HF or high carbohydrate diets. Physiol Behav 57: 501–508, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Coutinho AE, Fediuc S, Campbell JE, Riddell MC. Metabolic effects of voluntary wheel running in young and old Syrian golden hamsters. Physiol Behav 87: 360–367, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Eckel LA, Moore SR. Diet-induced hyperphagia in rats is influenced by sex and exercise. Am J Physiol Regul Integr Comp Physiol 287: R1080–R1085, 2004. [DOI] [PubMed] [Google Scholar]
- 6.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105: 1827–1832, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet induced obesity in susceptible and resistant rats. Obes Res 11: 845–51, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Gruenewald DA, Marck BT, Matsumoto AM. Fasting-induced increases in food intake and neuropeptide Y gene expression are attenuated in aging male brown Norway rats. Endocrinology 137: 4460–4467, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Iossa S, Lionetti L, Mollica MP, Barletta A, Liverini G. Energy intake and utilization vary during development in rats. J Nutr 99: 1593–1596, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Iossa S, Lionetti L, Mollica MP, Crescenzo R, Botta M, Barletta A, Liverini G. Effect of HF feeding on metabolic efficiency and mitochondrial oxidative capacity in adult rats. Br J Nutr 90: 953–960, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Levin BE, Hogan S, Sullivan AC. Initiation and perpetuation of obesity and obesity resistance in rats. Am J Physiol Regul Integr Comp Physiol 256: R766–R771, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol Regul Integr Comp Physiol 274: R412–R419, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds SL, Saito Y, Crimmins EM. The impact of obesity on average life expectancy in older American men and women. Gerontologist 45: 438–444, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Scarpace PJ, Matheny M, Moore RL, Tümer N. Impaired leptin responsiveness in aged rats. Diabetes 49: 431–434, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Scarpace PJ, Matheny M, Tümer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience 104: 1111–1117, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Scarpace PJ, Matheny M, Tümer N, Cheng KY, Zhang Y. Leptin resistance exacerbates diet-induced obesity and is associated with diminished maximal leptin signaling capacity in rats. Diabetologia 48: 1075–1083, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Scarpace PJ, Matheny M, Zhang Y, Shek EW, Prima V, Zolotukhin S, Tümer N. Leptin-induced leptin resistance reveals separate roles for the anorexic and thermogenic responses in weight maintenance. Endocrinology 143: 3026–3035, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Tou JC, Wade CE. Determinants affecting physical activity levels in animal models. Exp Biol Med (Maywood) 227: 587–600, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Wilsey J, Zolotukhin S, Prima V, Scarpace PJ. Central leptin gene therapy fails to overcome leptin resistance associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 285: R1011–R1020, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Matheny MK, Tümer N, Mitchell MK, Scarpace PJ. Leptin antagonist reveals that the normalization of caloric intake and the thermic effect of food after HF-feeding are leptin dependent. Am J Physiol Regul Integr Comp Physiol 292: R868–R874, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav 88: 249–256, 2006. [DOI] [PubMed] [Google Scholar]