Abstract
Previous studies suggest that the chorda tympani nerve (CT) is important in transmitting fat taste information to the central nervous system. However, the contribution of the CT in this process may depend upon the presence of other taste stimuli and/or differ in males and females. Accordingly, the present study investigated the role of the CT in free fatty acid taste processing by examining electrophysiological activity of the CT in response to the free fatty acid linoleic acid (LA), as well as by measuring behavioral responses to LA-taste mixtures. We recorded whole nerve responses from the CT in response to lingual application of LA with or without monosodium glutamate (MSG) in anesthetized male and female rats. In addition, we examined preferences for MSG + LA taste mixtures in behavioral tests. Although lingual application of LA alone did not produce CT whole nerve responses, coapplication of LA and MSG elicited greater CT responses than did MSG alone. These findings were paralleled by greater preferences for MSG + LA taste mixtures than for MSG alone. In both cases, the effect was particularly pronounced in male rats. Thus LA enhances CT activity and behavioral responses to LA + MSG taste mixtures, although there are sex differences in the effects. These results suggest that CT input is important in mediating behavioral responses to fat taste, but the effects depend upon other taste stimuli and differ in males and females.
Keywords: fat taste, sex differences, free fatty acids, electrophysiology
as obesity reaches epidemic proportion in the United States and in other developing countries, efforts are made to reduce, if not eliminate, fats from the diet. However, fats have critical biological functions, from nerve conduction to reproduction. Importantly, fats, and in particular, essential free fatty acids (FFAs), must be obtained from the diet, since they cannot be synthesized by the body. Thus, the detection of FFAs in food is necessary for survival. Conventional wisdom holds that dietary fats are detected by textural or olfactory attributes, a proposal supported by both personal experience and substantial research (e.g., 16, 32, 43, 56); however, rats can discriminate between different kinds of oils in behavioral tests (36) and prefer fat solutions even when texture and olfaction are minimized (19). In fact, increasing evidence suggests that FFAs may be the prominent feature in the detection of ingested fats. Rats' preferences for fat solutions are greatly reduced by the addition of a lipase inhibitor (31), which prevents the breakdown of ingested fats, and rats demonstrate a robust preference for FFAs (31). More importantly, ingested fats are rapidly (within 1–5 s) broken down into FFAs in the oral cavity by lingual lipase (31), suggesting that FFAs, themselves, have taste qualities. Neither texture nor smell appears to be necessary for FFA discrimination by rats, since FFAs have minimal viscosity (42) and olfactory bulbectomy does not affect the ability to discriminate FFAs at very low concentrations (53).
These results suggest that FFAs activate taste receptor cells (TRCs) located on the tongue, although the mechanism by which this activation occurs remains the subject of ongoing investigations. It has been postulated that TRC activation by FFAs involves an intracellular transduction cascade that is attributable to an increase in intracellular FFAs, rather than FFA binding to membrane receptor proteins. This possibility is supported by the ready entry of FFAs into cells (25); however, an alternative possibility involves the fatty acid transporter/translocase CD36, which is localized to taste buds of the gustatory epithelium (18) and recently was implicated in TRC activation based on genetic and behavioral studies in mice (38).
In contrast to the developing evidence for the detection of FFAs in the oral cavity and the cellular effects of FFAs in TRC responses, little is known about the peripheral neural pathways that transmit fat taste information to the central nervous system (CNS), and thereby mediate behavioral responses. Work from our laboratory (55) and others (42) show that male rats can discriminate low concentrations of the essential FFA linoleic acid (LA) from water; however, when the chorda tympani (CT) is transected bilaterally (CTX), rats are unable to discriminate LA from water until the LA concentration is substantially greater. Interestingly, female rats have a lower discrimination threshold for LA taste than do male rats (55; see also Ref. 46), and their ability to detect LA also is affected by CTX. In fact, CTX shifts the LA discrimination threshold to the same LA concentration in female and male rats, so the magnitude of the shift in LA detection after CTX is greater in females. Taken together, these results suggest that the CT is important in transmitting fat taste information to the CNS in both males and females and that the CT may be more important for fat taste discrimination in females.
Surprisingly, then, our recent study (2) found that neurons in the geniculate ganglion, the location of the cell bodies of CT gustatory sensory neurons, are unresponsive to lingual LA stimulation. It should be noted that those extracellular recordings were exclusively from male rats. Thus, the apparent discrepancy between the effect of CT on LA detection thresholds and the lack of responses by CT neurons to LA stimulation may be explained, in part, by sex differences in gustatory responses. There is an important methodological difference between behavioral and physiological studies of taste function that raises an intriguing alternative explanation. It is standard practice during electrophysiological recording to apply taste stimuli only after prolonged water rinses, thereby placing taste receptors in an environment completely devoid of the complex chemical background of saliva naturally present in behavioral studies. With this in mind, FFAs may be an effective taste stimulus only when combined with other chemical molecules, perhaps saliva or even in other taste stimuli. Consistent with this idea, our recent study (55) showed that LA-sucrose taste mixtures elicited greater behavioral responses than did sucrose alone. Electrophysiological studies of isolated TRC (23) suggest that LA stimulation results in prolonged neurotransmitter release, which provides further support for this idea.
Accordingly, the present study examined the role of the CT in interactions between LA and other taste stimuli in male and female rats using both behavioral tests and electrophysiological recordings from the CT. Based on our previous work showing that LA enhances behavioral taste responses to sucrose (55), and the idea that salivary sodium influences responses to LA, we hypothesized that interactions between LA and other taste stimuli are not limited to one taste quality. Therefore, we combined LA with a more complex taste stimulus to maximize electrophysiological responses and to better evaluate behavioral responses. More specifically, we mixed LA with monosodium glutamate (MSG). MSG is a classic stimulus for umami taste (34, 45, 49, 61) and is known to activate the T1R1+ T13 receptor (44, 63). In addition to umami, behavioral studies indicate that MSG also has sweet and salty components. Rats cannot distinguish between sucrose and MSG when the sodium taste of MSG is reduced using the sodium channel blocker amiloride (29). Moreover, an aversion conditioned to the taste of MSG generalizes to sweet stimuli when amiloride is added (60), but to NaCl when amiloride is not added (61). Thus, use of LA + MSG taste mixtures allows us to extend our previous studies of sex differences in behavioral responses to the taste of LA, including combinations of fat and umami, salt and sweet. At the same time, evaluation of the role of the CT in LA taste responses to combinations of LA and MSG will further understanding of the peripheral gustatory pathways that transmit LA taste information to the CNS.
METHODS
Subjects
Age-matched adult male and female Sprague-Dawley rats (Charles River Laboratory) weighing 200–375 g at the beginning of testing were individually housed in a temperature-controlled (72°F) room and maintained on a 12:12-h light-dark cycle with lights on at 0700. Rats were given ad libitum access to Purina rodent chow (no. 5001) and water, except where noted. The Institutional Animal Care and Use Committee at Florida State University approved all procedures.
Chemicals
Reagent-grade chemicals were refrigerated and protected from light. Due to its lipophilic nature, LA (99% pure; Sigma) was dissolved in 5 mM ethanol (EtOH). All other chemicals were mixed in deionized water (dH2O) unless otherwise noted.
Experiment 1: CT Whole Nerve Electrophysiological Recordings
Whole nerve electrophysiological recordings were obtained from the CT in urethane-anesthetized (1.5 g/kg body wt) rats using methods from our laboratory, as previously described (2, 3, 8, 39, 40). The trachea was cannulated, and rats were placed in a nontraumatic head holder. Using a mandibular approach, the right CT branch of the facial nerve then was exposed and transected where it enters the tympanic bulla. The perineurium was removed to the point where the lingual nerve joins the CT, and the distal portion of the cut nerve was placed on a tungsten wire electrode. A silver indifferent electrode placed in the muscle near the nerve allowed differential amplification (×10,000) of nerve activity.
The tongue was slightly extended and held in place with a small suture attached to the ventral surface. Taste stimuli (see below) were applied across the tongue at a constant flow rate of 50 μl/s for 10 s. A custom computer program controlled input to a mixing platform, allowing rapid switching and/or mixing while maintaining continuous solution flow. Between stimuli, the tongue was continuously rinsed to minimize transient thermal or tactile responses. Each taste stimulus was followed by a 90-s rinse to ensure that nerve activity returned to stable baseline levels. NaCl (600 mM) was applied for 10 s at the beginning and at the end of the recording protocol, which typically was ∼40 min, to evaluate the viability of the nerve. If the response to NaCl at the end of the protocol varied by >15% from the initial NaCl response, the data from the recording were not included in the analysis.
Sensory nerve activity was recorded and stored on video tape for off-line analysis using a GW Instrument 15-s data acquisition board and custom software. Amplified nerve activity was integrated using a root mean square calculation and a 150-ms time constant. Baseline neural activity was recorded during rinses for ≥30 s preceding each stimulus. Average baseline activity (in μV) for the 15-s period immediately before each taste stimulus was used to calculate area under the curve (AUC), expressed as response above baseline, for the integrated response during each stimulus. Each response was then normalized to the average response to a standard stimulus, which was applied at the beginning and end of the recording protocol.
Experiment 1a: Electrophysiological response of the CT to lingual application of LA.
Whole nerve recordings were obtained from the CT of male (n = 7) and female (n = 6) rats. LA (11, 22, 44, and 88 μM) was applied in ascending order of concentration across the tongue for 10 s; responses were normalized to a 30 mM quinine hydrochloride (QHCl) standard. Between stimuli, the tongue was rinsed with dH2O (for NaCl and QHCl) or 5 mM EtOH (for LA).
Experiment 1b: Electrophysiological response of the CT to lingual coapplication of MSG and LA.
Male (n = 10) and female (n = 7) rats were used to obtain CT whole nerve responses to ascending concentrations of MSG (40, 100, and 300 mM) with and without coapplication of 88 μM LA (MSG + LA). To control for the possibility that EtOH affected CT responses, we also applied MSG mixed in 5 mM EtOH (MSG + EtOH). In all cases, responses were normalized to a 300 mM NH4Cl standard. In experiment 1a, we used QHCl as a standard, and the modest responses evoked by QHCl were sufficient to assess CT activity, particularly given the lack of responses to LA. In this experiment, we opted to use NH4Cl, which produces greater CT responses, to provide better resolution for analyses of the mixed stimulus responses. Between stimuli, the tongue was rinsed with dH2O (for NaCl, NH4Cl, and MSG), EtOH (for MSG + EtOH), or 88 μM LA (for MSG + LA).
To provide more detailed information about the time course of CT responses, we also measured rise time (the time between stimulus onset and peak response), peak response, and response duration (the time from stimulus onset to return to baseline, including the initial 10-s stimulation), each of which was normalized to the corresponding averaged NH4Cl response.
Experiment 2: MSG and LA Taste Preferences
Male (n = 9) and female (n = 7) rats were placed on a water restriction schedule during which they had daily access to dH2O for 10 min in the morning and 30 min in the afternoon until they reliably drank >7 ml of dH2O during the 10-min morning access period. Animals then were given a series of 10-min, three-bottle preference tests, conducted in the morning, in which taste solution location was random.
To control for order effects, rats were randomly assigned to one of two testing sequences. In one sequence, rats were first tested for their preference for the combination of MSG and LA by providing one bottle of dH2O, one bottle of 40 mM MSG + EtOH, and one bottle of 40 mM MSG + EtOH + 88 μM LA on two consecutive days (D1 and D2). On the following days (D3 and D4), the preference for MSG alone was evaluated by providing one bottle of dH2O and two bottles of 40 mM MSG + EtOH. It was necessary to use two bottles of MSG (MSG1 and MSG2) to be able to draw direct comparisons to our three-bottle water, MSG and MSG + LA tests. We were unable to directly compare preferences for LA and MSG because our preference tests were limited to three bottles; however, in a previous study (55), we found that the preference for LA in both males and females is slightly greater than that for water (see also Ref. 46). The 4-day protocol was repeated with 100 and 300 MSG, for a total of 12 test days. In the second testing sequence, MSG preference tests were conducted on D1 and D2, and MSG + LA preference tests were conducted on D3 and D4. To further ensure that there was no bottle preference, rats had to sample each bottle before the subsequent bottle was put on the cage during all of the three-bottle preferences tests.
In both sequences, intakes were recorded after 10 min, and preferences for each solution were calculated as follows: [solution intake (ml)/total intake (ml)]. For each rat, preference scores for each solution at each MSG concentration were averaged over the two test days. Unlike the traditional two-bottle preference test in which a preference score of ∼0.5 indicates an indifference to a test solutions, a preference score of ∼0.33 indicates an indifference between test solutions, and a preference score >0.33 indicates a preference for a test solution; a preference score <0.33 indicates an aversion to a test solution.
Statistical Analyses
Data are presented as group means ± SE. Data were analyzed using appropriate ANOVA (Statistica; StatSoft, Tulsa, OK), as described below. Pairwise comparisons of statistically significant (P < 0.05) main effects or interactions were evaluated using Student Newman-Keul's tests.
Experiment 1: Electrophysiological activity of the CT.
Whole nerve responses to the standards (NaCl and QHCl or NaCl and NH4Cl) at the beginning and end of testing were compared using a three-factor (sex × solution × time) ANOVA, repeated for solution and time. Normalized CT responses to LA by male and female rats were compared using a two-factor (sex × concentration) ANOVA, repeated for concentration.
In initial comparisons of the CT response to MSG with that to MSG + EtOH using three-factor (sex × solution × concentration) ANOVA, it was apparent that EtOH did not alter CT responses to MSG for AUC, rise time, peak response, or response duration (all P > 0.70; see Fig. 3). Therefore, for each rat at each MSG concentration, a single value was calculated for each of these measures by averaging the responses to MSG and to MSG + EtOH. These values were used in comparisons with CT responses to MSG + LA using three-factor (sex × solution × concentration) ANOVAs, repeated for solution and concentration.
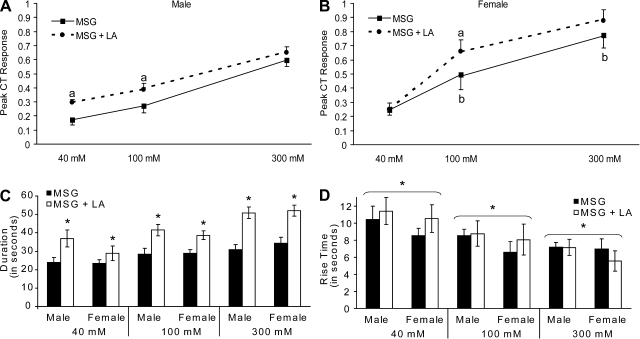
Fig. 3.
Mean ± SE peak response (A and B), duration (C), and rise time (D) of CT response to MSG and MSG + 88 μM LA by male and female rats. Significantly different from MSG (a). Female significantly different from male (b).
Experiment 2: MSG and LA taste preferences.
Initial four-factor (test sequence × sex × solution × concentration) ANOVA revealed no effect of test sequence; thus, these data were combined for subsequent analyses. Fluid intake for MSG and for MSG + LA by male and female rats was analyzed using three-factor (sex × solution × concentration) ANOVA, repeated for solution and concentration. Preferences for MSG and for MSG + LA by male and female rats were analyzed using three-factor (sex × solution × concentration) ANOVA, repeated for solution and concentration.
RESULTS
Experiment 1a: Electrophysiological Response of the CT to Lingual Application of LA
As illustrated by the representative raw trace of CT electrophysiological activity (Fig. 1A), the CT was highly responsive to NaCl and, to a lesser degree, to QHCl. The response to NaCl was significantly greater than that to QHCl [F(1, 11) = 73.06, P < 0.001], but responses to NaCl and QHCl did not differ between male and female rats [F(1, 11) = 0.16, P = 0.70]. This is particularly important because behavioral studies have reported sex differences in taste responses to quinine (5, 14). Moreover, these responses were comparable at the beginning and end of testing [F(1, 11) = 4.52, P = 0.07], confirming continued viability of the nerve. Consistent with our recent geniculate ganglion study (2), LA did not elicit a detectable CT response at any concentration [F(3, 33) = 0.01, P = 0.99] in either sex [F(1, 11) = 0.00, P = 0.42] (Fig. 1B).
Fig. 1.
Chorda tympani (CT) whole nerve activity in response to lingual application of NaCl, quinine hydrochloride (QHCl), and linoleic acid (LA) (11, 22, 44, and 88 μM). A: representative trace of CT whole nerve activity (μV) from a male rat. Gray, raw nerve activity; black, integrated, rectified activity. B: mean ± SE normalized CT responses to lingual application of NaCl and LA in male and female rats.
Experiment 1b: Electrophysiological Response of the CT to Lingual Coapplication of MSG and LA
AUC.
The CT was responsive to MSG in a concentration-dependent manner [F(2, 30) = 82.99, P < 0. 001], and LA enhanced the response to MSG [F(1, 15) = 7.64, P < 0.05] (Fig. 2, A and C). Moreover, this effect was different between male and female rats [F(1, 15) = 7.60, P < 0.05]. Post hoc analyses of the interaction between sex, solution, and concentration [F(2, 30) = 4.60, P < 0.05] revealed that responses to both MSG and to MSG + LA increased significantly with each increase in MSG concentration (all P < 0.01). However, female rats had significantly greater responses to all concentrations of MSG alone than did males (P < 0.01). Finally, there were sex differences in the increased response to MSG when LA also was applied. In male rats, 88 μM LA enhanced CT responses to MSG at 40 and 100 mM (both P < 0.05; Fig. 2B), whereas 88 μM LA enhanced responses in female rats only at 100 mM (P < 0.01; Fig. 2D).
Fig. 2.
CT whole nerve activity in response to lingual application of NaCl, QHCl, monosodium glutamate (MSG) + water, MSG + ethanol (EtOH) and MSG + 88 μM LA. A and C: representative traces of CT whole nerve activity (μV) from a male (A) and a female (C) rat. Gray, raw nerve activity; black, integrated, rectified activity. B and D: mean ± SE CT response to MSG and MSG + 88 μM LA by male (B) and female (2D) rats. For each concentration of MSG: significantly different from MSG (a) and female significantly different from male (b).
The CT was highly responsive to both NaCl and NH4Cl (data not shown); however, the response to NaCl was significantly greater than the response to NH4Cl [F(1, 15) = 24.51, P < 0.001]. There were no differences in the responses to these salts (i.e., NaCl and NH4Cl) between male and female rats [F(1, 15) = 0.30, P = 0.59], and these responses did not change during the course of testing [F(1, 15) = 2.14, P = 0.16].
Peak response, rise time, and response duration.
To determine whether the observed LA enhancement of responses to MSG reflected an increase in rise time, peak response, or response duration, we analyzed each of these measures independently.
Comparisons of peak responses yielded results very similar to those of the AUC (Fig. 3, A and B) : responses were significantly greater in female rats than in males [F(1,15) = 6.43, P < 0.05], and depended on solution [F(1,15) = 11.86, P < 0.01], concentration [F(2, 30) = 87.61, P < 0.001], and the interaction between sex, solution, and concentration [F(2, 30) = 5.10, P < 0.05].
The duration of the CT response to MSG (Fig. 3C) was not different between males and females [F(1, 15) = 0.11, P = 0.74], but increased both with increasing MSG concentration [F(2,30) = 30.00, P < 0.001] and when LA was added [F(1, 15) = 47.63, P < 0.001]. Moreover, there was a significant interaction between solution and concentration [F(2, 30) = 5.48, P < 0.01]. Post hoc analyses of this interaction revealed that, at each concentration, the duration of the CT response to MSG was significantly greater when LA was added (all P < 0.01).
Rise time (Fig. 3D) was not different between males and females [F(1, 15) = 1.31, P = 0.27] and was not changed by the addition of LA [F(1, 15) = 0.300, P = 0.59], but did decrease significantly as MSG concentration increased [F(2, 30) = 12.22, P < 0.001].
Experiment 2: MSG and LA Taste Preferences
MSG and LA: Three-bottle tests.
In these three-bottle tests, LA increased the preference for MSG [F(2, 28) = 9.89, P < 0.001]. Post hoc analysis of the significant interaction between sex, solution, and concentration [F(4, 56) = 4.12, P < 0.001] revealed that LA enhanced the preference for 40 mM MSG in male (P < 0.05) but not female (P = 0.13) rats (Fig. 4, A vs. C). However, LA enhanced the preference for 100 mM MSG in both male and female rats (both P < 0.01). Finally, water was preferred over MSG and MSG + LA at 300 mM (all P < 0.01) by both male and female rats.
Fig. 4.
Mean ± SE preference scores of male and female rats for water, MSG, and MSG + LA (A and C) and for water and two bottles of MSG (MSG 1 = MSG bottle 1 and MSG 2 = MSG bottle 2; B and D). For each MSG concentration: significantly greater than MSG and water (a), significantly greater than MSG and MSG + LA (b), significantly greater than water (c), and significantly greater than MSG (d).
Intake (10 min) of test solutions by male and female rats (Table 1) closely resembled the values calculated for preference scores: overall, LA increased intake of MSG [F(2, 28) = 4.56, P < 0.001]. Post hoc analyses of the significant interaction between solution, concentration, and sex [F(4, 56) = 2.28, P < 0.05] showed that LA increased intake of 40 mM MSG by male (P < 0.01) but not female (P = 0.51) rats. However, LA increased intake of 100 mM MSG by both male and female rats (both P < 0.01). Finally, intake of water was greater than that of 300 mM MSG by male and female rats, regardless of whether LA was added (all P < 0.01; Table 1).
Table 1.
Mean fluid intake of test solutions
|
40 mM |
100 mM
|
300 mM
|
||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Water | 4.3±1.5 | 3.4±1.3 | 4.9±1.5 | 3.2±0.9 | 11.2±1.4† | 10.3±0.8† |
| MSG | 2.7±0.4 | 6.4±1.3 | 3.8±0.8 | 1.3±0.4 | 2.0±0.5 | 1.9±0.3 |
| MSG + LA | 11.2±2.0* | 4.4±0.7 | 11.9±1.7* | 9.3±1.2* | 4.1±1.2 | 2.0±1.6 |
| Water | 3.9±1.1 | 2.6±0.7 | 6.4±1.5 | 3.2±0.5 | 9.3±1.4§ | 8.4±1.2§ |
| MSG 1 | 7.4±1.7‡ | 5.3±0.8‡ | 4.8±1.0 | 5.9±1.6 | 2.6±0.5 | 2.1±0.4 |
| MSG 2 | 7.4±1.8‡ | 4.5±0.6‡ | 8.2±1.9 | 3.6±0.9 | 3.6±1.0 | 2.2±0.7 |
Values are means ± SE. MSG, monosodium glutamate; LA, linoleic acid; MSG 1, MSG bottle 1; MSG 2, MSG bottle 2.
Significantly greater than water and MSG.
Significantly greater than MSG and MSG + LA.
Significantly greater than water.
Significantly greater than MSG.
MSG: Three-bottle tests.
There were no sex differences in these three-bottle tests (Fig. 4, B and D); however, post hoc analyses of the significant interaction between solution and concentration [F(4, 56) = 0.04, P < 0.001] revealed that rats preferred 40 mM MSG over water (both MSG bottles, P < 0.01) but did not prefer 300 mM MSG over water (both MSG bottles, P < 0.01). There were no differences in preference scores at 100 mM MSG.
Intake of test solutions by male and female rats in these 10-min, three-bottle tests are shown in Table 1. Similar to preference scores, intake of the solutions was not different between males and females. However, post hoc analysis of the significant interaction between solution and concentration [F(4, 56) = 9.25, P < 0.01] revealed that rats consumed more 40 mM MSG than dH2O (P < 0.05) and that intake of 100 mM MSG was similar to that of dH2O. However, rats consumed more dH2O than 300 mM MSG (P < 0.01).
DISCUSSION
To continue our exploration of fat as a possible taste stimulus, we measured the integrated responses of the CT nerve to a broad range of LA concentrations in male and female rats. Although the CT nerve was unresponsive to lingual application of the FFA alone, CT responses to MSG were larger with LA coapplication. Furthermore, this LA enhancement of MSG neural responses was matched by a parallel increase in preference for a LA + MSG mixture over MSG alone. In addition, LA enhancement of behavioral and neural responses was greater in male than in female rats, especially for moderate MSG concentrations. Thus our data show that electrophysiological responses to LA + MSG mixtures have a direct and striking corresponding effect on behavior.
CT Responses
Consistent with our recent study showing that individual neurons from the geniculate ganglion of male rats were unresponsive to LA (2), we discovered that the whole CT nerve, which innervates the complete taste receptor field on the anterior two-thirds of the tongue, was also unresponsive to lingual application of LA (Fig. 1). These results are surprising to us given our previous findings that CTX impaired LA taste discrimination in male and female rats (55). This may be reconciled by the fact that CTX not only eliminates the afferent nerve supply to the anterior tongue but it also denervates the submaxillary and sublingual glands, thereby decreasing saliva production and secretion (7). This opens the possibility that LA may be unique compared with other stimuli and require a chemical background such as the constitutive components of saliva (27) or the presence of other taste stimuli to activate TRCs. In this regard, saliva is rinsed away with water before stimulus application in our study to ensure a uniform background for all animals during electrophysiological recordings, reducing the likelihood of a CT response to LA. Accordingly, CT nerve responses to MSG were greater in a mixture with LA than without in both male and in female rats (Fig. 2). In fact, this enhancement was apparent in both the integrated 10-s response (AUC) and in the amplitude of the peak response, reflecting an increase in stimulus intensity we well as in the duration of the tonic response (Fig. 2, B and D, and 3, A–C), reflecting a decrease in stimulus adaptation (1). In contrast, rise time, which correlates with the initial transduction of MSG taste (1), was not affected by LA (Fig. 3D). In summary, these data provide the first electrophysiological evidence that fats and, more specifically, FFAs enhance responses to gustatory stimuli and suggest that the CT plays a role in such enhancement.
The overall pattern of results was similar for male and females rats, although there were also notable differences. First, the amplitude of the CT nerve response to MSG was greater in females than in males across MSG concentrations both alone and in mixture with LA (Fig. 2, A–D), except to LA + 40 mM MSG. Although it remains unknown why the female CT is more responsive to MSG, the difference may be related to reported sex differences in response to salt and sweet (4, 5, 8–10, 35, 51), two components that contribute to umami taste in rodents. Second, there were concentration-specific sex differences in LA enhancement of MSG responses that differed across response measures (i.e., integrated AUC, peak, and duration). For example, in males, LA enhanced the responses to 40 and 100 mM MSG in all three response measures, whereas in females a similar enhancement was evident only for stimulus duration. Together, these findings suggest that LA increased the intensity of 40 and 100 mM MSG in males and 100 mM in females. Moreover, LA prolonged MSG taste before adaptation for all three concentrations in both sexes. It remains to be seen whether corresponding work from individual TRCs of the taste bud or single neurons from the geniculate ganglion also will reveal a similar interaction between LA and MSG and the mechanism by which it occurs.
Taste Preferences
Both male and female rats preferred 40 and 100 mM MSG to water, but neither preferred the more concentrated 300 mM MSG. In fact, both males and females found this MSG concentration to be mildly aversive (Fig. 4), which is consistent with some (24, 34), but not all (45, 57), behavioral studies. The explanation for discrepancies in taste preferences for 300 mM MSG is unclear but could be related to methodological differences, including test length, maintenance diet, or experience with other tastes. In any event, LA did not influence the aversion to 300 mM MSG in the current study. In contrast, LA increased the preference for lower MSG concentrations in male and female rats. These results parallel the findings observed in experiment 1b, particularly the concentration-specific sex differences in the effect: wherever LA increased the CT nerve response to MSG, it also increased MSG preference. As with CT responses, the addition of LA increased the preference for 40 and 100 mM MSG in male rats, but only for 100 mM MSG in female rats (Fig. 4, A and C). Thus CT nerve responses to LA + MSG mixtures predict the fluid preferences of LA + MSG solutions, especially for lower MSG concentrations.
If the main role of LA is to augment the intensity of other solutions in a complex mixture, then it is logical to expect that LA should increase preference for lower MSG concentrations until the intensity becomes too great, as seen with higher MSG concentrations, and causes an aversion. Extending this logic more broadly, with LA, preferred tastes become more preferred, whereas aversive tastes become more aversive. This is demonstrated compellingly by a recent study showing that LA increases licking responses to sucrose and glucose but decreases licking to sodium chloride, citric acid, and quinine by rats (48).
These findings appear to contradict work by Gilbertson and colleagues (23) showing an inverse relationship between fatty acid sensitivity in fungiform taste cells and taste preference. They found that the addition of LA decreased saccharin preference in obesity-prone rats. However, rats prefer saccharin at low concentrations and avoid it at high concentrations (54), which is attributable to a bitter taste component. Consequently, the LA-saccharin mixture will be even more intensely bitter, leading to decreased preference. In fact, obesity-prone rats appear to be highly sensitive to FFAs, since they develop stronger conditioned taste aversions to LA compared with obesity-resistant rats (47).
Other receptive fields and taste nerves must play a significant role in LA taste transmission because CTX does not completely abolish LA discrimination in behavioral tests. In particular, CD36 is highly expressed in the circumvallate papillae on the posterior tongue innervated by the glossopharyngeal nerve (GL; see Ref. 15) and consequently may be especially sensitive to FFA stimulation. In addition, lingual lipase is secreted from von Ebner's glands (15, 38), which also are located in the posterior oral cavity. In this regard, the FFA oleic acid is reported to activate the GL (33), although in that study, recordings were from the pharyngeal branch of the GL, which innervates receptors in the pharynx that are important in the control of reflexes (33). Nonetheless, bilateral transection of the GL impairs the ability of mice to discriminate LA from a control solution (20), which provides additional support for GL involvement in FFA taste processing.
Interestingly, fungiform papillae, which are innervated by the CT, show little CD36 expression, an observation that suggests FFAs play multiple roles in gustatory processing, depending on the location of the “fat taste receptors.” As first proposed by Laugerette et al. (37), FFA actions on taste receptors in the anterior part of the tongue may enhance the intensity of other taste stimuli, as seen in the present study; however, FFAs may directly activate taste receptors in the posterior oral cavity, thereby producing a unique “fat taste.” In support of the idea of multiple roles for FFA in taste, both CD36 knockout mice and Trpm5 knockout mice demonstrate significantly reduced preferences for fat solutions (50, 52), suggesting that both CD36 and Trpm5, which is expressed in circumvallate papillae and is also found in other types of TRCs (12), are important in fat taste processing. Moreover, FFA stimulation of circumvallate papillae results in a dramatic increase of intracellular Ca2+, resulting in release of neurotransmitters, including 5-hydroxytryptamine and norepinephrine (17). On the other hand, stimulation of fungiform papillae with LA alone does not elicit a response in the geniculate ganglion (2) or the CT (current study) but does increase CT responses to MSG, as seen in our current study. Also, the addition of LA significantly increases the preference for saccharin concentrations that apparently are undetectable without LA (22). Finally, FFAs inhibit delayed-rectifying potassium channels (DRKs) in isolated TRC from fungiform papillae (21), which presumably prolong evoked neurotransmitter release. This latter process may involve interactions between DRKs and inhibitory G proteins, since mRNA for several orphan G proteins increases in response to FFA stimulation of fungiform TRCs (26).
Integration of these complex results from a diverse body of work leads us to believe that LA enhancement of CT and behavioral responses to MSG are not attributable to direct actions of LA at MSG receptors. Rather, we propose that MSG-stimulated neurotransmitter release on gustatory afferents is increased by LA action on DRKs. More specifically, when LA and MSG are applied together, MSG acts at T1R1+ T1R3 receptors while LA acts separately to inhibit DRKs. The combined actions of LA and MSG on fungiform TRCs result in an even greater increase in intracellular Ca2+ and, thus, prolonged release of even greater amounts of neurotransmitter. However, CD36 is not present in fungiform papillae and is rarely coexpressed with α-gustducin (38), a G protein that is expressed at high levels in fungiform papillae and is activated by MSG stimulation (28). Thus the effect of LA on fungiform TRCs does not involve CD36. Although this model focuses on LA enhancement of MSG responses as observed in the present study, it also predicts that LA will enhance responses to other taste stimuli by augmenting neurotransmitter release evoked by the actions of those taste stimuli at their receptors. Clearly, however, this effect may be more pronounced for taste information primarily transmitted via the CT (e.g., NaCl; see Ref. 6), as opposed to the GL or the greater superficial petrosal nerve, and ongoing studies are examining this issue by adding LA to other taste stimuli.
In summary, the results of the current study demonstrate that, although lingual administration of LA alone did not affect CT whole nerve responses, CT responses to MSG were enhanced by the presence of LA. More specifically, LA enhanced the intensity of MSG taste, particularly at low MSG concentrations. The enhancement of gustatory sensory input appears to be behaviorally relevant, since preferences for the same concentrations of MSG also were enhanced when LA was present. Moreover, in both CT whole nerve recordings and in behavioral preferences, male rats had enhanced responses to the combination of LA and MSG at a lower concentration than did female rats. Thus, there are sex differences in the enhancement of behavioral responses to MSG by the FFA and LA, and the CT is important in this difference.
MSG is a complex taste stimulus; therefore, increased CT and behavioral responses to LA + MSG combinations may reflect an enhancement of responses to the sodium component, to the glutamate component, or to both. Moreover, LA may enhance responses to one component while decreasing responses to the other. Some insights into the relative weights of the MSG components in the enhancement of responses observed in the present study may be obtained from the results of our previous study in which we found that female rats increase licking to LA-sucrose taste mixtures at lower concentrations than do males (55). Comparison of these two studies leads to the obvious conclusion that sex differences in behavioral responses to LA-taste mixtures may depend on the solution in which LA is mixed. However, a “subtractive analysis” also allows the deduction that increased preferences for LA + MSG combinations is explained by LA enhancement of the sodium component of MSG taste. Unfortunately, little work has examined sex differences in preferences to MSG, and the few studies that did (30, 45) suggest that males prefer lower MSG concentrations than do females. We did not find sex differences in preferences for MSG alone. Rather, differences were apparent only with the addition of LA and only at lower MSG concentrations. Sex differences in preferences for LA + MSG mixtures clearly reflected sex differences in CT responses; nonetheless, additional behavioral and electrophysiological experiments will be necessary to determine more precisely which taste component of MSG is enhanced by LA and how gender influences electrophysiological and behavior responses.
Perspectives
Although investigations of sex differences in taste responses span four decades and have examined taste stimuli from sweet to salt to bitter (4, 5, 8, 9, 11, 13, 14, 35, 51, 58, 59), little work has focused on the mechanisms that underlie such differences. Certainly, this is true of sex differences in the taste of MSG and of FFAs such as LA, taste stimuli that have received much less attention. Not surprisingly, then, although there are sex differences in the LA enhancement of CT and behavioral responses to MSG, the mechanism remains unknown. Sex differences in CT responses to LA + MSG taste combinations suggest that the behavioral differences are attributable, at least in part, to differences in peripheral taste processing. Thus, sex differences in receptor affinity and/or in the number of MSG receptors (or in putative LA receptors) might account for the sex differences we observed. Circulating reproductive hormones also could be involved, since estrogen decreases CT responses to NaCl (9). This possibility seems unlikely to explain sex differences in the LA enhancement of responses to LA, however, since estrogen treatment does not affect licking responses to LA-sucrose mixtures (55) in ovariectomized female rats. Finally, sex differences in peripheral input do not preclude the possibility of sex differences in the central processing of LA + MSG taste mixtures.
Regardless of the mechanism, these findings of sex differences in LA effects on taste processing and on related taste preferences are especially important because taste is a major determinant of food choice and food consumption. Although there may be species differences in the specific tastes that are influenced by FFAs (see, e.g., Refs. 41 and 48), if FFAs change taste intensity, the result may be increased consumption of preferred foods and/or greater willingness to consume foods normally not preferred. In this regard, men prefer “protein fats” such as steak (62), whereas women prefer “sweet fats” such as chocolate. Given that MSG is considered by many to be the prototypical protein taste, our findings of sex differences in preferences for LA + MSG taste mixtures, essentially a protein and fat taste, cast new light on sex differences in food choices by humans.
GRANTS
This research was supported by National Institute on Deafness and Communication Disorders Grants DC-04785 (R. J. Contreras), DC-06360 (K. S. Curtis), T-32 DC-00044 and DC-008934-02 (J. M. Stratford).
Acknowledgments
Present address for K. Curtis: Oklahoma State University Center for Health Sciences, Tulsa, Oklahoma.
Portions of these data were presented in preliminary form at the 35th annual meeting of the Society for Neuroscience, Nov. 12–16, 2005, and the 28th annual meeting of the Association for Chemoreception Sciences, April 26–30, 2006, in Sarasota, FL.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bealer SL Intensity coding in the transient portion of the rat chorda tympani response. J Comp Physiol Psychol 92: 185–195, 1978. [DOI] [PubMed] [Google Scholar]
- 2.Breza JM, Curtis KS, Contreras RJ. Monosodium glutamate but not linoleic acid differentially activates gustatory neurons in the rat geniculate ganglion. Chem Senses 32: 833–846, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol 95: 674–685, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Chow SY, Sakai RR, Witcher JA, Adler NT, Epstein AN. Sex and sodium intake in the rat. Behav Neurosci 106: 172–180, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Clarke SN, Ossenkopp KP. Taste reactivity responses in rats: influence of sex and the estrous cycle. Am J Physiol Regul Integr Comp Physiol 274: R718–R724, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Contreras RJ, Frank M. Sodium deprivation alters neural responses to gustatory stimuli. J Gen Physiol 73: 569–594, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contreras RJ, Gomez MM, Norgren R. Central origins of cranial nerve parasympathetic neurons in the rat. J Comp Neurol 190: 373–394, 1980. [DOI] [PubMed] [Google Scholar]
- 8.Curtis KS, Contreras RJ. Sex differences in electrophysiological and behavioral responses to NaCl taste. Behav Neurosci 120: 917–924, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Curtis KS, Davis LM, Johnson AL, Therrien KL, Contreras RJ. Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats. Physiol Behav 80: 657–664, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Curtis KS, Krause EG, Contreras RJ. Altered NaCl taste responses precede increased NaCl ingestion during Na(+) deprivation. Physiol Behav 72: 743–749, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Curtis KS, Stratford JM, Contreras RJ. Estrogen increases the taste threshold for sucrose in rats. Physiol Behav 86: 281–286, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses 31: 253–264, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Di Lorenzo PM, Monroe S. Taste responses in the parabrachial pons of male, female and pregnant rats. Brain Res Bull 23: 219–227, 1989. [DOI] [PubMed] [Google Scholar]
- 14.Di Lorenzo PM, Monroe S. Taste responses in the parabrachial pons of ovariectomized rats. Brain Res Bull 25: 741–748, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Doty RL Handbook of Olfaction and Gustation. New York, NY: Dekker, 2003.
- 16.Drewnowski A Sensory properties of fats and fat replacements. Nutr Rev 50: 17–20, 1992. [DOI] [PubMed] [Google Scholar]
- 17.El-Yassimi A, Hichami A, Besnard P, Akhtar Khan N. Linoleic acid induces calcium signaling, SRC-kinase phosphorylation and neurotransmitters release in mouse CD36-positive gustatory cells. J Biol Chem 283: 12949–12959, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Fukuwatari T, Kawada T, Tsuruta M, Hiraoka T, Iwanaga T, Sugimoto E, Fushiki T. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett 414: 461–464, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Fukuwatari T, Shibata K, Iguchi K, Saeki T, Iwata A, Tani K, Sugimoto E, Fushiki T. Role of gustation in the recognition of oleate and triolein in anosmic rats. Physiol Behav 78: 579–583, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Gaillard D, Laugerette F, Darcel N, El-Yassimi A, Passilly-Degrace P, Hichami A, Khan NA, Montmayeur JP, Besnard P. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J 22: 1458–1468, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Gilbertson TA Gustatory mechanisms for the detection of fat. Curr Opin Neurobiol 8: 447–452, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Gilbertson TA, Liu L, Kim I, Burks CA, Hansen DR. Fatty acid responses in taste cells from obesity-prone and -resistant rats. Physiol Behav 86: 681–690, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Gilbertson TA, Liu L, York DA, Bray GA. Dietary fat preferences are inversely correlated with peripheral gustatory fatty acid sensitivity. Ann NY Acad Sci 855: 165–168, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses 30: 299–316, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton JA, Kamp F. How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes 48: 2255–2269, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Hansen DR, McKenna L, Shaw BP, Gilbertson TA. Expression of fatty acid-activates g protein coupled receptors in chemosensory cells (Abstract). Chem Senses 31: A105, 2006. [Google Scholar]
- 27.Hart PS Salivary abnormalities in Prader-Willi syndrome. Ann NY Acad Sci 842: 125–131, 1998. [DOI] [PubMed] [Google Scholar]
- 28.He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci 24: 7674–7680, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heyer BR, Taylor-Burds CC, Tran LH, Delay ER. Monosodium glutamate and sweet taste: generalization of conditioned taste aversion between glutamate and sweet stimuli in rats. Chem Senses 28: 631–641, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Hiji Y, Masayasu S. Preference-aversion function for sodium monoaminodicarboxylates in rats. Nippon Seirigaku Zasshi 29: 168–169, 1967. [PubMed] [Google Scholar]
- 31.Kawai T, Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am J Physiol Regul Integr Comp Physiol 285: R447–R454, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Kinney NE, Antill RW. Role of olfaction in the formation of preference for high-fat foods in mice. Physiol Behav 59: 475–478, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Kitagawa J, Shingai T, Kajii Y, Takahashi Y, Taguchi Y, Matsumoto S. Leptin modulates the response to oleic acid in the pharynx. Neurosci Lett 423: 109–112, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Kondoh T, Mori M, Ono T, Torii K. Mechanisms of umami taste preference and aversion in rats. J Nutr 130: 966S–970S, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Krecek J, Novakova V, Stibral K. Sex differences in the taste preference for a salt solution in the rat. Physiol Behav 8: 183–188, 1972. [DOI] [PubMed] [Google Scholar]
- 36.Larue C Oral cues involved in the rat's selective intake of fats. Chem Senses Flavour 3: 1–6, 1978. [Google Scholar]
- 37.Laugerette F, Gaillard D, Passilly-Degrace P, Niot I, Besnard P. Do we taste fat? Biochimie 89: 265–269, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest 115: 3177–3184, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundy RF Jr, Contreras RJ. Gustatory neuron types in rat geniculate ganglion. J Neurophysiol 82: 2970–2988, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Lundy RF Jr, Contreras RJ. Tongue adaptation temperature influences lingual nerve responses to thermal and menthol stimulation. Brain Res 676: 169–177, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Mattes RD Effects of linoleic acid on sweet, sour, salty, and bitter taste thresholds and intensity ratings of adults. Am J Physiol Gastrointest Liver Physiol 292: G1243–G1248, 2007. [DOI] [PubMed] [Google Scholar]
- 42.McCormack DN, Clyburn VL, Pittman DW. Detection of free fatty acids following a conditioned taste aversion in rats. Physiol Behav 87: 582–594, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Mela DJ Sensory assessment of fat content in fluid dairy products. Appetite 10: 37–44, 1988. [DOI] [PubMed] [Google Scholar]
- 44.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature 416: 199–202, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Ohara I, Naim M. Effects of monosodium glutamate on eating and drinking behavior in rats. Physiol Behav 19: 627–634, 1977. [DOI] [PubMed] [Google Scholar]
- 46.Pittman D, Crawley ME, Corbin CH, Smith KR. Chorda tympani nerve transection impairs the gustatory detection of free fatty acids in male and female rats. Brain Res 1151: 74–83, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Pittman D, Smith K, Crawley M, Corbin C, Hansen D, Fraiser Gilbertson K, T. Orosensory detection of fatty acids by obesity-prone and obesity-resistant rats. Chem Senses 33: 448–460, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Pittman DW, Labban CE, Anderson AA, O'Connor HE. Linoleic and oleic acids alter the licking responses to sweet, salt, sour, and bitter tastants in rats. Chem Senses 31: 835–843, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Sako N, Tokita K, Sugimura T, Yamamoto T. Synergistic responses of the chorda tympani to mixtures of umami and sweet substances in rats. Chem Senses 28: 261–266, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol 293: R1823–R1832, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Sclafani A, Hertwig H, Vigorito M, Feigin MB. Sex differences in polysaccharide and sugar preferences in rats. Neurosci Biobehav Rev 11: 241–251, 1987. [DOI] [PubMed] [Google Scholar]
- 52.Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of alpha-gustducin and Trpm5 taste-signaling proteins. Am J Physiol Regul Integr Comp Physiol 293: R1504–R1513, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith JC Gustation as a factor in the ingestion of sweet and fat emulsions by the rat. Physiol Behav 82: 181–185, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Smith JC, Sclafani A. Saccharin as a sugar surrogate revisited. Appetite 38: 155–160, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Stratford JM, Curtis KS, Contreras RJ. Chorda tympani nerve transection alters linoleic acid taste discrimination by male and female rats. Physiol Behav 89: 311–319, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Takeda M, Sawano S, Imaizumi M, Fushiki T. Preference for corn oil in olfactory-blocked mice in the conditioned place preference test and the two-bottle choice test. Life Sci 69: 847–854, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Tordoff MG, Rabusa SH. Calcium-deprived rats avoid sweet compounds. J Nutr 128: 1232–1238, 1998. [DOI] [PubMed] [Google Scholar]
- 58.Valenstein ES, Kakolewski JW, Cox VC. Sex differences in taste preference for glucose and saccharin solutions. Science 156: 942–943, 1967. [DOI] [PubMed] [Google Scholar]
- 59.Wade GN, Zucker I. Hormonal and developmental influences on rat saccharin preferences. J Comp Physiol Psychol 69: 291–300, 1969. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto T, Matsuo R, Fujimoto Y, Fukunaga I, Miyasaka A, Imoto T. Electrophysiological and behavioral studies on the taste of umami substances in the rat. Physiol Behav 49: 919–925, 1991. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste effects of “umami” substances in hamsters as studied by electrophysiological and conditioned taste aversion techniques. Brain Res 451: 147–162, 1988. [DOI] [PubMed] [Google Scholar]
- 62.Zellner DA, Garriga-Trillo A, Rohm E, Centeno S, Parker S. Food liking and craving: A cross-cultural approach. Appetite 33: 61–70, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003. [DOI] [PubMed] [Google Scholar]