Abstract
Kv1.3 channels are known to modulate many aspects of neuronal function. We tested the hypothesis that Kv1.3 modulates the function of postganglionic sympathetic neurons. RT-PCR, immunoblot, and immunohistochemical analyses indicated that Kv1.3 channels were expressed in these neurons. Immunohistochemical analyses indicated that Kv1.3 protein was localized to neuronal cell bodies, processes, and nerve fibers at sympathetic neurovascular junctions. Margatoxin (MgTX), a specific inhibitor of Kv1.3, was used to assess the function of the channel. Electrophysiological analyses indicated that MgTX significantly reduced outward currents [P < 0.05; n = 18 (control) and 15 (MgTX)], depolarized resting membrane potential, and decreased the latency to action potential firing [P < 0.05; n = 11 (control) and 13 (MgTX)]. The primary physiological input to postganglionic sympathetic neurons is ACh, which activates nicotinic and muscarinic ACh receptors. MgTX modulated nicotinic ACh receptor agonist-induced norepinephrine release (P < 0.05; n ≥ 6), and MgTX-sensitive current was suppressed upon activation of muscarinic ACh receptors with bethanechol (P < 0.05; n = 12). These data indicate that Kv1.3 affects the function of postganglionic sympathetic neurons, which suggests that Kv1.3 influences sympathetic control of cardiovascular function. Our data also indicate that modulation of Kv1.3 is likely to affect sympathetic control of cardiovascular function.
Keywords: adrenergic, blood pressure, catecholamine, potassium channel
the sympathetic nervous system is a major determinant of cardiovascular function and is implicated in cardiovascular disease (2–4, 7, 9, 15, 23, 24, 29, 32, 40, 41). Many effects of the sympathetic nervous system on cardiovascular function are mediated via neurotransmitters released from postganglionic sympathetic neurons innervating blood vessels. The mechanisms governing neurotransmitter release at sympathetic neurovascular junctions are not completely understood.
Kv1 family potassium channels consist of eight genes encoding distinct alpha subunit proteins, Kv1.1 through Kv1.8, and are expressed throughout the nervous system (5, 13, 14, 18, 20, 33, 37, 39, 43, 44, 46, 48). Functional Kv1 channels are formed when four Kv1 alpha subunits assemble as homotetramers or as heterotetramers with other members of the Kv1 family. These channels affect a range of neuronal functions (6, 8, 11, 16, 26, 34–36), including spike frequency adaptation (35, 36) and the regulation of cellular excitability in response to synaptic input (11). Postganglionic sympathetic neurons express Kv1 channels (5, 33, 43), and inhibition of these channels has been reported to modulate neurotransmitter release from these neurons (21, 22, 45). However, the mechanisms involved in Kv1 channel modulation of neurotransmitter release in sympathetic neurons are largely unexplored.
Kv1.3 channels play a key role in a wide range of physiological phenomena. Kv1.3 is required for the activation of T-lymphocytes and is thus a determinant of immune function (1). Inhibition of Kv1.3 facilitates translocation of the insulin-sensitive glucose transporter, GLUT4, to the plasma membrane of adipocytes (31) and skeletal muscle (50), and is thus a determinant of glucose homeostasis. Kv1.3 has also been shown to contribute to body weight regulation and energy homeostasis, processes that are regulated by the sympathetic nervous system (49). In neurons, Kv1.3 has been reported to modulate action potential firing (8, 26).
Here, we evaluate the role of Kv1.3 in postganglionic sympathetic neuron function. We demonstrate that this channel is present and functional in postganglionic sympathetic neurons. Kv1.3 was detected throughout the neurons, in both soma and processes. It contributed to outward currents recorded from the soma and was a determinant of resting membrane potential and neurotransmitter release. These studies show that Kv1.3 channels are important determinants of postganglionic sympathetic neuronal function and have important implications for understanding the effects of the sympathetic nervous system on cardiovascular function.
METHODS
Animals.
The use of animals in the present studies was in accordance with the National Institutes of Health guidelines for the humane care and use of animals in research and was approved by the Institutional Animal Care and Use Committee of the University of Vermont. Neonatal Sprague-Dawley rats were used to obtain superior cervical sympathetic ganglia (SCG). Adult postpartum female Sprague-Dawley rats were used to obtain SCG and tail arteries. The postpartum females used in the present studies were the mothers of the neonatal rats and were used to minimize the number of animals.
Neuronal culture.
Postganglionic sympathetic neurons were isolated from the SCG of neonatal (3 or 4 days) Sprague-Dawley rats (males and females). Ganglia were dissociated for 10 min at 37°C in a collagenase/hyaluronidase solution (10 mg/ml BSA, 4 mg/ml collagenase, 1 mg/ml hyaluronidase in Dulbecco's PBS) and then for 10 min in trypsin (3 mg/ml added to trypsin-EDTA). Dissociated cells were resuspended in neuronal growth medium [DMEM/F12 supplemented with 10% NuSerum (BD Biosciences), 5% fetal bovine serum (Invitrogen) and penicillin/streptomycin], supplemented with NGF (BD Biosciences; 50 ng/ml), and applied to collagen-coated tissue culture dishes. The cells were allowed to attach overnight in a humidified 5% CO2 environment maintained at 37°C. Nonneuronal cells were then growth arrested with mitomycin C (Sigma; 10 μg/ml for 1 h). These cultures of neurons will be subsequently referred to as dissociated neurons.
RT-PCR.
RNA was isolated with RNeasy mini kits from Qiagen. Equal amounts of RNA were reverse transcribed (RetroScript, Ambion) and equal amounts of cDNA amplified (Amplitaq Gold, Applied Biosystems). PCR primers and annealing temperatures are indicated in Table 1. PCR products were electrophoresed on 1.2% agarose gels containing ethidium bromide and visualized with UV light. All PCR reactions included (−) RT and (−) template controls. Amplified PCR products were sequenced by the University of Vermont DNA facility to confirm the identity of the DNA.
Table 1.
Forward and reverse PCR primer sequences
| mRNA | Primer Sequences | Annealing Temperature, °C |
|---|---|---|
| Kv1.3 | 5′-GTA CTT CGA CCC GCT CCG CAA TGA-3′ | 59 |
| 5′-GGG CAA GCA AAG AAT CGC ACC AG-3′ |
Western blot analyses.
Tissues and cells were lysed and homogenized in enhanced RIPA buffer [50 mM Tris, 150 mM NaCl, 10 mM EDTA 0.25% deoxycholate 1% NP40, 10% glycerol, 1% protease inhibitor cocktail (Sigma), 1 mM dithiothreitol, 0.1% sodium dodecyl sulfate; pH 8.0]. Samples were diluted with equal volumes of electrophoresis running buffer, boiled for 5 min, and electrophoresed on 4–20% gradient acrylamide gels. Samples were then transferred to nitrocellulose membranes. The membranes were blocked with PBS containing 0.05% Tween and 3% nonfat dry milk (30 min at room temperature) and then incubated overnight at 4°C in PBS-Tween containing 3% nonfat dry milk, and primary antibody [0.2 μg/ml anti-Kv1.3 (NeuroMab) and 1 μg/ml anti-tyrosine hydroxylase (TH; Sigma)]. The Kv1.3 mouse monoclonal antibody, IgG2a isotype, was raised against synthetic peptide amino acids 485-506 of rat Kv1.3 (clone L23/27; Lot # 413-5RR-07). The TH mouse monoclonal antibody recognizes the N-terminal epitope between amino acids 40 and 152 of rodent and human TH (lot # 016K4857). Unbound primary antibody was removed with three 5-min washes (PBS-Tween). The membranes were then incubated in PBS-Tween containing 3% nonfat dry milk and horseradish peroxidase-conjugated secondary antibodies (1:3,000; Bio-Rad) for 1 h at room temperature. The horseradish peroxidase was detected with enhanced chemiluminescence (Pierce) and documented on autoradiographic film.
Immunofluorescence.
Cultures of postganglionic sympathetic neurons were rinsed in PBS and fixed in 4% formaldehyde in PBS for 12 min. SCG and tail arteries were dissected from adult postpartum female rats and fixed in 4% formaldehyde in PBS for 1 or 2 h, respectively. Ganglia were immersed in 30% sucrose in PBS overnight and frozen in Tissue-Tek O.C.T. compound (Electron Microscopy Sciences) and sliced to a thickness of 30 μm at −20°C. SCG sections and dissociated neurons were permeabilized with 0.2% Triton X-100 in PBS for 5 min and subsequently rinsed in PBS. Tail arteries were permeabilized with acetone for 5 min and rinsed in PBS. Cultures, SCG sections, and whole mount tail arteries were blocked for 1 h in 3% goat serum and 0.1% fish skin gelatin in PBS. A rabbit polyclonal TH antibody (0.16 μg/ml; Chemicon), raised against denatured rat TH (Lot # 0512016843), was used to identify neurons. A rabbit polyclonal GM130 antibody (0.7 μg/ml; Calbiochem), raised against recombinant protein containing amino acids 371-990 of human GM130 (lot # D00004465), was used to identify the Golgi apparatus. The same monoclonal Kv1.3 antibody (0.84 μg/ml; NeuroMab) used for Western blot analyses was also used to identify Kv1.3. Cells were incubated in primary antibodies overnight at 4°C, followed by three 5-min washes in PBS. Alexa Fluor Goat anti-mouse (cultures 568 nm; SCG and tail arteries 647 nm) and goat anti-rabbit (488 nm) secondary antibodies (4 μg/ml; Invitrogen) were applied for 1 h at room temperature, and all samples were mounted using ProLong Gold antifade reagent (Invitrogen). All images were taken using the Olympus IX70 microscope and DeltaVision Restoration Imaging System (Applied Precision, LLC) and background subtracted with an IgG2a isotype control (R&D Systems).
Neuronal transfection.
Neurons were transfected using the Helios Gene Gun (Bio-Rad) with pEGFP or pEGFP-Kv1.3. pEGFP-coated bullets were a generous gift from Dr. Victor May (University of Vermont, Department of Anatomy and Neurobiology). pEGFP-C1-Kv1.3 was a generous gift from Dr. Jürgen Kupper [(25); Max Planck Institute of Biochemistry, Martinsried, Germany]. Cells were studied 48 h after transfection.
Electrophysiology.
Electrophysiological recordings were performed at room temperature, using the whole cell patch-clamp technique. Data acquisition and analysis were obtained using the Axopatch 200B (Axon Instruments) patch-clamp amplifier and pCLAMP 9.2 (Axon Instruments) software. Electrodes were pulled in two stages from thin-wall filament glass capillary tubing (Warner Instruments) and fire polished to a resistance ranging from 1 to 2 μM. Voltage-clamp recording solutions were as follows (in mM): external (bath) solution 100 NaCl, 5.4 KCl, 1.8 CaCl2. 0.8 MgCl2, 23 glucose, 5 Na HEPES, 0.001 TTX, 10 tetraethylammonium (TEA), pH 7.4; internal (pipette) solution: 120 KCl, 3.69 CaCl2, 0.094 MgCl2, 5 BAPTA, 5 EDTA, 5 Na HEPES, 5 glucose, pH 7.2. Pharmacological agents were applied at the following concentrations: 1 nM margatoxin (MgTX; Alomone Labs), 100 nM α-dendrotoxin (DTX; Research Biochemicals International), and 100 μM bethanechol (BeCh; Sigma). Cells were held at −60 mV, followed by a 20-ms hyperpolarization to −90 mV, and stepped from −70 mV to +50 mV in 10-mV increments. Leak currents (P/8) were subtracted from all traces. Averaged MgTX traces were subtracted from averaged control traces to obtain the resolved Kv1.3 current. Current clamp recording solutions were the same as listed above, except that TTX and TEA were omitted. Resting membrane potential was monitored for 100 ms, followed by 400-ms current injections of the following magnitudes (in pA): −500, 500, 1,000, 1,500, 2,000, and 2,500. Latency to action potential firing was defined as the time between the start of the current injection and the peak of the action potential. Action potential width was measured at half the action potential amplitude as previously described (12).
Norepinephrine release.
Norepinephrine (NE) release was assessed using tritiated norepinephrine purchased from Amersham. These assays were performed using HEPES-buffered Krebs solution [122 mM NaCl, 3 mM KCl, 0.4 mM MgSO4·H2O, 1.2 mM KH2PO4, 10 mM glucose, 20 mM HEPES, 1.3 mM CaCl2·2H2O, 1 mM ascorbic acid, 10 μM pargyline, pH 7.4]. Cells were preincubated at 37°C with 100-nM tritiated norepinephrine for 30 min. The cells were then washed (6 × 5 min) and stimulated with a nicotinic agonist, dimethylphenylpiperazinium (DMPP; 30 μM; Sigma). The remaining cell-associated NE was then extracted with acidified ethanol. NE in all samples was collected and analyzed using a Beckman LS6000IC Liquid Scintillation Counter (Beckman Instruments). Stimulated release was calculated using the following equation: (stimulated cpm − background cpm)/(total cpm available for release).
Statistics.
Data are presented as means ± SE. Unpaired Student's t-tests assuming unequal variances were used to determine statistical differences. Differences were considered significant if P ≤ 0.05.
RESULTS
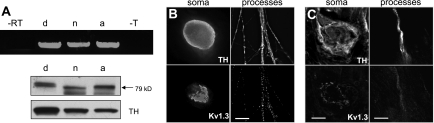
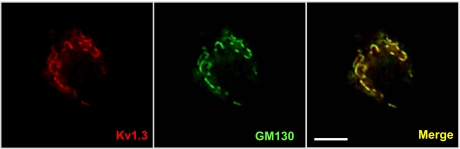
To begin to assess the function of Kv1.3 channels in postganglionic sympathetic neurons, we first characterized the expression and subcellular localization of these channels. Kv1.3 mRNA and protein were detected in dissociated neurons (d) and in intact neonatal (n) and adult (a) ganglia (Fig. 1A). Potassium channel effects on neuronal physiology are strongly influenced by their location within the cell; therefore, we used immunofluoresence microscopy to examine the subcellular localization of Kv1.3 protein (Fig. 1, B and C). Neurons were identified with antibodies directed against TH. In dissociated neurons and freshly isolated tissues, Kv1.3 was detected in both the soma and processes. In the soma, Kv1.3 exhibited a striking pattern of localization to a discrete intracellular compartment that overlaps with the GM130 Golgi apparatus marker in dissociated neurons (Fig. 2). All dissociated neurons observed expressed Kv1.3 and exhibited this intracellular localization pattern.
Fig. 1.
Kv1.3 channels are present in postganglionic sympathetic neurons. A: RT-PCR (top; n = 2) shows expression of mRNA for Kv1.3 in neonatal dissociated neurons (d), neonatal sympathetic superior cervical ganglia (SCG) (n) and adult sympathetic SCG (a). PCR reactions included minus reverse transcriptase (-RT) and minus template (-T) controls. Kv1.3 and tyrosine hydroxylase (TH) immunoblots (bottom; n = 2) show corresponding protein expression. TH was used as a marker of postganglionic sympathetic neurons. Approximate molecular weight is noted with arrows. B: immunolocalization of TH (top) and Kv1.3 (bottom) in the soma (left) and processes (right) of dissociated postganglionic sympathetic neurons in vitro (n = 2). Scale bar = 10 μm. C: immunolocalization of TH (top) and Kv1.3 (bottom) in neuronal soma of intact adult sympathetic SCG (left) and of processes innervating adult rat tail arteries (right) (n = 2). SCG scale bar = 10 μm; tail artery scale bar = 30 μm.
Fig. 2.
Kv1.3 overlaps with a marker for the Golgi apparatus in postganglionic sympathetic neurons. Immunoreactivity of endogenous Kv1.3 (red) overlaps with the Golgi marker, GM130 (green), in the soma of dissociated postganglionic sympathetic neurons. Right: overlap between these two antibodies (merge; n = 3). Scale bar = 10 μm.
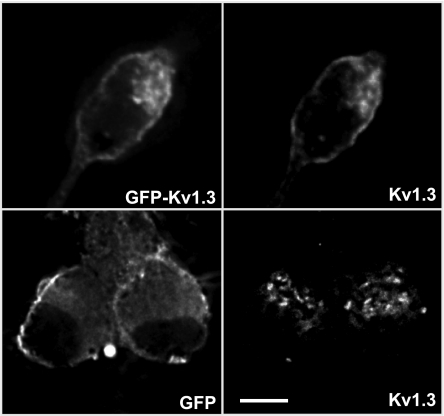
Because the striking intracellular localization detected with anti-Kv1.3 is not typical for a membrane-associated ion channel, we used GFP-Kv1.3 as an alternative approach to assess the subcellular localization of Kv1.3. The distribution of overexpressed GFP-Kv1.3 overlapped with that detected by Kv1.3 immunofluorescence (Fig. 3). The distribution of overexpressed GFP differed considerably from GFP-Kv1.3 and Kv1.3 immunofluorescence (Fig. 3). Intracellular compartmentalization of Kv1.3 was also observed in sympathetic neurons in adult ganglia (Fig. 1C).
Fig. 3.
Localization of transfected GFP-Kv1.3 vs. endogenous Kv1.3 in postganglionic sympathetic neurons. GFP-Kv1.3 (top left) and immunoreactive Kv1.3 (top right) overlap in postganglionic sympathetic neurons (n = 3). Neurons transfected with GFP only (bottom left) do not overlap with immunoreactive Kv1.3 (bottom right) (n = 3). Scale bar = 10 μm.
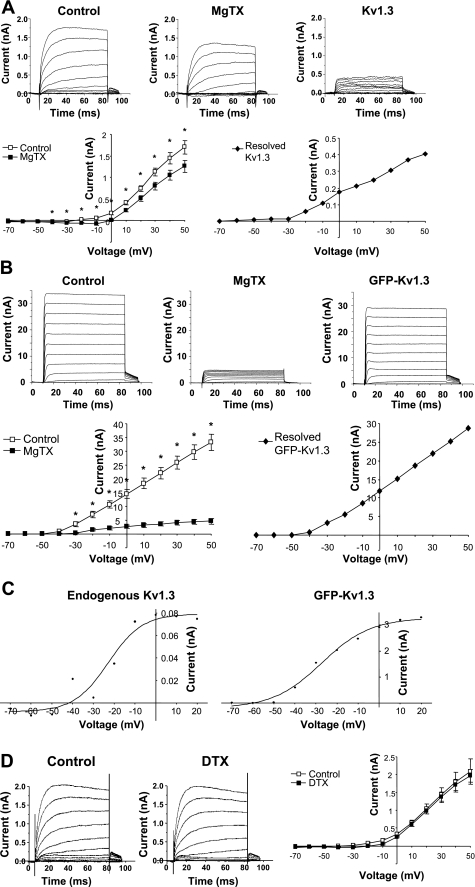
We next determined the function of Kv1.3 in postganglionic sympathetic neurons. We used whole cell patch-clamp electrophysiology in the absence and presence of MgTX, a specific inhibitor of Kv1.3 (10). To resolve Kv1.3 currents, all measurements were recorded in the presence of 1 μM TTX and 10 mM TEA to block currents generated by sodium and non-Kv1 family potassium channels. Steady-state currents measured in untransfected dissociated postganglionic sympathetic neurons were significantly suppressed by MgTX (1 nM; n = 15) relative to control (n = 18; P < 0.02) (Fig. 4A). This indicates that endogenous Kv1.3 channels contribute to outward current recorded from the soma of postganglionic sympathetic neurons. To confirm that MgTX inhibited Kv1.3, steady-state currents were also measured in dissociated postganglionic sympathetic neurons expressing GFP-Kv1.3. Outward currents in transfected cells were markedly increased, indicating that GFP-Kv1.3 was functional in these cells. MgTX (1 nM) elicited an 86% decrease in this current (Fig. 4B; n = 3; P < 0.002). Activation curves were generated from tail currents and fit to a Boltzmann function. These curves are shown in Fig. 4C for both endogenous and GFP-Kv1.3. Half-activation voltages (V1/2) determined from these curves were −22.8 mV for endogenous MgTX-sensitive current and −27.2 mV for GFP-Kv1.3, values consistent with previously published reports (47).
Fig. 4.
Voltage-clamp analyses of Kv1.3 in postganglionic sympathetic neurons. A: ionic current measured in untransfected dissociated sympathetic neurons in the absence (control; n = 18) and presence of 1 nM margatoxin (MgTX; n = 15). Current traces for each condition represent the average of unpaired measurements made in multiple cells. *Significant difference between MgTX and control (P ≤ 0.05; unpaired t-test). Resolved Kv1.3 current was obtained by subtracting MgTX from control. B: ionic current measured in dissociated sympathetic neurons transfected with GFP-Kv1.3 in the absence (control; n = 3) and presence of 1 nM MgTX (n = 3). Current traces for each condition represent the average of unpaired measurements made in multiple cells. *Significant difference between MgTX and control (P ≤ 0.02; unpaired t-test). Resolved GFP-Kv1.3 current was obtained by subtracting MgTX from control. C: activation curves were plotted from the tail current of endogenous Kv1.3 and GFP-Kv1.3 and fit to a Boltzmann function. D: ionic current measured in dissociated sympathetic neurons in the absence (control; n = 10) and presence of 100 nM DTX (n = 18; P > 0.05).
At concentrations higher than that used in the present studies, MgTX has been reported to inhibit Kv1.6 (10). To evaluate the contribution of Kv1.6 to the MgTX-sensitive current measured in Fig. 4A, we measured steady-state ionic currents in dissociated postganglionic sympathetic neurons in the presence of DTX, an inhibitor of Kv1.6, as well as Kv1.1 and Kv1.2 channels (17). DTX (100 nM) had no effect on steady-state currents in these cells (P > 0.05; n ≥ 10) (Fig. 4D). Steady-state outward currents recorded in HEK 293 cells stably transfected with Kv1.2 were significantly suppressed in the presence of DTX (100 nM; n = 4; P < 0.05; data not shown), confirming the effectiveness of this inhibitor. These findings support our conclusion that the MgTX-sensitive current shown in Fig. 4A is attributable to Kv1.3.
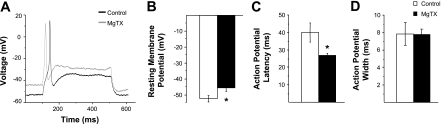
Current clamp electrophysiology was used to elucidate the physiological role of Kv1.3 current in dissociated postganglionic sympathetic neurons. Resting membrane potential measured in the presence of MgTX (−45.7 mV ± 2.1; n = 13) was significantly depolarized relative to control (−51.9 mV ± 2.3; n = 11; P < 0.05) (Fig. 5B). In addition, the latency to action potential firing in the presence of MgTX (26.9 ms ± 1.3; n = 13) was significantly less than control (39.9 ms ± 5.4; n = 11; P < 0.05) (Fig. 5C). MgTX did not alter the width of the action potential measured at half-peak amplitude (Fig. 5D).
Fig. 5.
Current-clamp analyses of Kv1.3 in postganglionic sympathetic neurons. A: representative traces of phasic action potentials recorded from the soma of dissociated postganglionic sympathetic neurons in the absence (control; black line) and presence (MgTX; gray line) of 1 nM MgTX. B: resting membrane potential. C: the latency to action potential firing. D: the action potential width as measured at half the action potential amplitude in the absence (open bars; n = 11) and presence (solid bars; n = 13) of 1 nM MgTX. *Significant difference between MgTX and control (P < 0.05; unpaired t-test).
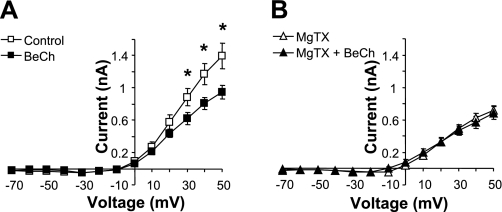
ACh is the preganglionic neurotransmitter for postganglionic sympathetic neurons. ACh activates both nicotinic (nAChR) and muscarinic (mAChR) ACh receptors in these neurons (28). Activation of mAChRs is known to suppress ionic current of Kv1 family channels (19). We found that BeCh (100 μM), a mAChR-selective agonist, significantly decreased outward current measured in dissociated neonatal sympathetic neurons (Fig. 6A; n = 12; P < 0.05). Inhibition of Kv1.3 with MgTX abrogated the effect of BeCh, suggesting that activation of mAChRs suppresses Kv1.3 current in these cells (Fig. 6B; n = 12; P > 0.05). In HEK cells lacking muscarinic receptors, but transfected with GFP-Kv1.3, BeCh did not decrease Kv1.3 current at any voltage (data not shown; n = 8; P > 0.05). These data indicate that BeCh did not directly affect Kv1.3.
Fig. 6.
Preganglionic modulation of Kv1.3 in postganglionic sympathetic neurons. A: steady-state current-voltage curves for postganglionic sympathetic neurons in the absence (open symbols) and presence (closed symbols) of muscarinic ACh receptor activation using 100 μM bethanechol (BeCh). B: steady- state current-voltage curves for postganglionic sympathetic neurons treated with 1 nM MgTX in the absence (open symbols) and presence (closed symbols) of 100 μM BeCh. *Significant difference between BeCh and control (n = 12; P < 0.05; unpaired t-test).
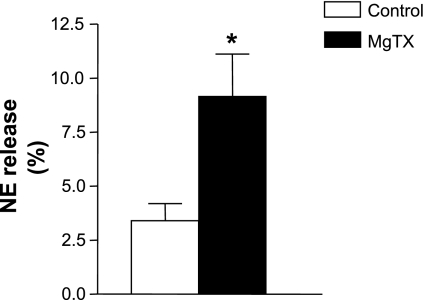
The ultimate function of postganglionic neurons is to release neurotransmitters, thereby affecting the function of sympathetic targets. Therefore, we assessed the effect of Kv1.3 on NE release from these neurons. ACh is the physiological stimulus for neurotransmitter release from these neurons. This effect is primarily mediated by activation of nAChRs. Therefore, we used DMPP, a nAChR-selective agonist to stimulate neurotransmitter release. Application of 30 μM of DMPP stimulated NE release from dissociated neonatal neurons. Inhibition of Kv1.3 with MgTX (1 nM) increased NE release from these cells (Fig. 7; n = 6; P < 0.05).
Fig. 7.
Kv1.3 affects norepinephrine release from postganglionic sympathetic neurons. Tritiated norepinephrine release from neuronal cultures in response to a 30-μM dimethylphenylpiperazinium (DMPP) treatment in the absence (open bar) and presence (closed bar) of MgTX (1 nM, 10 min). *Significant difference between MgTX and control (P < 0.05; unpaired t-test).
DISCUSSION
The present studies provide novel evidence that Kv1.3 determines the function of postganglionic sympathetic neurons. Expression analyses indicated that Kv1.3 is present in these neurons. Electrophysiological analyses indicated that this channel contributes to outward current and the maintenance of resting membrane potential. Pharmacological inhibition of Kv1.3 increased neurotransmitter release. In addition, we demonstrated that this channel was modulated by preganglionic mechanisms. Postganglionic sympathetic neurons are important determinants of cardiovascular function. Thus, our studies strongly suggest that Kv1.3 channels and their modulation are important determinants of sympathetic control of cardiovascular function.
The present studies are the first to demonstrate that Kv1.3 protein is expressed in postganglionic sympathetic neurons. RT-PCR, immunoblot, and immunohistochemical analyses indicate that Kv1.3 was in the soma. Kv1.3 was concentrated in an intracellular compartment (Figs. 1, 2, and 3). Similar localization of Kv1.3 has been reported in other cells and tissues (13, 38). Figure 2 indicates that this compartment is the Golgi apparatus. Kv1.3 was also detected in the processes of dissociated neurons, as well as in nerve fibers on the adventitial surface of freshly isolated tail arteries. These data suggest that Kv1.3 channels are likely to affect the function of postganglionic sympathetic neurons in general and, in particular, to affect sympathetic control of vascular function.
Functional analyses of Kv1.3 indicate that this channel is a determinant of the electrophysiological properties of postganglionic sympathetic neurons. MgTX, a specific inhibitor of Kv1.3 (10), decreased outward currents measured in these cells (Fig. 4A). In contrast, DTX, an inhibitor of Kv1.6, Kv1.1, and Kv1.2 (17), had no effect on outward currents in these neurons (Fig. 4D). This indicates that MgTX is specific for Kv1.3 and that Kv1.1, Kv1.2, and Kv1.6 do not significantly contribute to the outward current measured in these neurons. Action potential analyses presented in Fig. 5 indicate that Kv1.3 contributes to the maintenance of resting membrane potential. MgTX significantly decreased the latency to action potential firing, consistent with the depolarization of resting membrane potential. This is the first demonstration that Kv1.3 affects the function of postganglionic sympathetic neurons.
Our immunohistochemical and functional analyses indicate that Kv1.3 is localized to both the plasma membrane and Golgi apparatus in postganglionic sympathetic neurons. We detected Kv1.3 current in nontransfected cells, indicating surface localization of the channel (Fig. 4A). In nontransfected cells, surface expression of Kv1.3 was below the level of detection of our immunohistochemical analyses (Fig. 1B). In neurons that were transfected with GFP-Kv1.3, surface expression was easily detectable (Fig. 3). In both nontransfected and transfected neurons, our immunohistochemical analyses detected a fraction of Kv1.3 localized to the Golgi apparatus (Figs. 2 and 3). Cellular localization is an important determinant of ion channel function (27). Our data thus suggest that Golgi localization or retention may be a determinant of surface expression and function of Kv1.3.
The primary preganglionic input to these neurons is ACh. It is well known that activation of mAChRs modulates KCNQ potassium channels in postganglionic sympathetic neurons and that this modulation affects neurotransmitter release (28). In addition, previous studies indicate that activation of mAChRs modulates the function of Kv1 channels (19). We show that activation of mAChRs suppresses Kv1.3 current in postganglionic sympathetic neurons (Fig. 6). These data demonstrate a novel effect of mAChR activation, a novel mechanism of Kv1.3 modulation, and suggest a new mechanism by which mAChR activation modulates membrane excitability.
In addition to activating mAChRs, release of ACh from preganglionic neurons activates nAChRs. Nicotinic AChR activation is the primary mechanism for generating action potentials and eliciting neurotransmitter release from postganglionic sympathetic neurons. We assessed the effects of Kv1.3 on nicotinic receptor-induced NE release. Our data indicate that inhibition of Kv1.3 enhanced NE release (Fig. 7). This suggests that Kv1.3 is acting to suppress membrane excitability and thereby inhibit neurotransmitter release. These findings are consistent with previous reports demonstrating that inhibition of Kv1 family channels enhances neurotransmitter release. The work of Jackson et al. (22) and Uhrenholt and Nedergaard (45) indicates that inhibition of Kv1 channels enhances NE release at sympathetic neurovascular junctions. Our findings that Kv1.3 channels are present in processes innervating arteries and that MgTX enhances NE release, suggest that these channels play a role in modulating sympathetic neurovascular transmission.
Our data clearly indicate that Kv1.3 channels influence the function of postganglionic sympathetic neurons derived from the SCG of the rat, a representative paravertebral sympathetic ganglion. The postganglionic sympathetic neurons in this ganglion innervate many targets, including blood vessels (30). Kv1.3 was expressed in all of the neurons that were studied, suggesting that Kv1.3 affects sympathetic regulation of all SCG targets. The studies of Dixon and McKinnon (5) indicate that Kv1.3 is also expressed in prevertebral ganglia, suggesting a general role for Kv1.3 in postganglionic sympathetic neuronal function. Our finding that Kv1.3 is expressed in the fibers innervating the tail artery (Fig. 1C) strongly suggests that Kv1.3 affects neurotransmitter release at sympathetic neurovascular junctions. This would suggest that Kv1.3 is a determinant of sympathetic control of blood flow and blood pressure.
Perspectives and Significance
Compelling evidence suggests that the sympathetic nervous system contributes to the development and/or maintenance of cardiovascular disease (2–4, 7, 9, 15, 23, 24, 29, 32, 40–42). Sympathetic activity is increased in hypertensive animals and humans, and sympathoinhibition decreases blood pressure (4, 15, 23, 24, 29, 40). Hypertension is also a complication of diabetes and obesity. Elevated sympathetic activity is thought to contribute to these forms of hypertension (2, 7). Sympathetic activity is increased in many patients with heart failure, and this increased activity contributes to the pathology of this disease (41, 42). The present studies suggest that Kv1.3 channels in postganglionic sympathetic neurons are determinants of sympathetic activity and therefore are potential therapeutic targets for the prevention and treatment of cardiovascular disease.
GRANTS
This work was supported by National Institutes of Health Grants HL-076774 (D. H. Damon) and NS-050623 (A. D. Morielli.).
Acknowledgments
We would like to acknowledge the expert technical assistance of Stephen Marko and Jen Wlodarski. We would also like to thank Emilee Connors, Brandon Field, James Kilcoyne, Lee Stirling, and Michael Williams for helpful editorial comments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR, Wang PH, LeeHealey CJ BSA, Sankaranarayanan A, Homerick D, Roeck WW, Tehranzadeh J, Stanhope KL, Zimin P, Havel PJ, Griffey S, Knaus HG, Nepom GT, Gutman GA, Calabresi PA, Chandy KG. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci USA 103: 17414–17419, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson SH, Shelton J, White CR, Wyss JM. Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension 35: 403–408, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO, 3rd. Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol 76: 125–130, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Dhital KK, Gerli R, Lincoln J, Milner P, Tanganelli P, Weber G, Fruschelli C, Burnstock G. Increased density of perivascular nerves to the major cerebral vessels of the spontaneously hypertensive rat: differential changes in noradrenaline and neuropeptide Y during development. Brain Res 444: 33–45, 1988. [DOI] [PubMed] [Google Scholar]
- 5.Dixon JE, McKinnon D. Potassium channel mRNA expression in prevertebral and paravertebral sympathetic neurons. Eur J Neurosci 8: 183–191, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Dodson PD, Barker MC, Forsythe ID. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J Neurosci 22: 6953–6961, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 48: 787–796, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Fadool DA, Tucker K, Perkins R, Fasciani G, Thompson RN, Parsons AD, Overton JM, Koni PA, Flavell RA, Kaczmarek LK. Kv1.3 channel gene-targeted deletion produces “Super-Smeller Mice” with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron 41: 389–404, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fingerle J, Sanders KH, Fotev Z. Alpha 1-receptor antagonists urapidil and prazosin inhibit neointima formation in rat carotid artery induced by balloon catheter injury. Basic Res Cardiol 86 Suppl1: 75–81, 1991. [PubMed] [Google Scholar]
- 10.Garcia-Calvo M, Leonard RJ, Novick J, Stevens SP, Schmalhofer W, Kaczorowski GJ, Garcia ML. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J Biol Chem 268: 18866–18874, 1993. [PubMed] [Google Scholar]
- 11.Glazebrook PA, Ramirez AN, Schild JH, Shieh CC, Doan T, Wible BA, Kunze. Potassium channels Kv1 DL.1, Kv1.2 Kv1.6 influence excitability of rat visceral sensory neurons. J Physiol 541: 467–482, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan D, Lee JC, Higgs MH, Spain WJ, Foehring RC. Functional roles of Kv1 channels in neocortical pyramidal neurons. J Neurophysiol 97: 1931–1940, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Guan D, Lee JC, Tkatch T, Surmeier DJ, Armstrong WE, Foehring RC. Expression and biophysical properties of Kv1 channels in supragranular neocortical pyramidal neurones. J Physiol 571: 371–389, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, Covarriubias M, Desir GV, Furuichi K, Ganetzky B, Garcia ML, Grissmer S, Jan LY, Karschin A, Kim D, Kuperschmidt S, Kurachi Y, Lazdunski M, Lesage F, Lester HA, McKinnon D, Nichols CG, O'Kelly I, Robbins J, Robertson GA, Rudy B, Sanguinetti M, Seino S, Stuehmer W, Tamkun MM, Vandenberg CA, Wei A, Wulff H, Wymore RS. International Union of Pharmacology XLI Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev 55: 583–586, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Guyenet PG The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Haghdoust H, Janahmadi M, Behzadi G. Physiological role of dendrotoxin-sensitive K channels in the rat cerebellar Purkinje neurons. Physiol Res 2006. [DOI] [PubMed]
- 17.Harvey AL Twenty years of dendrotoxins. Toxicon 39: 15–26, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Hatton WJ, Mason HS, Carl A, Doherty P, Latten MJ, Kenyon JL, Sanders KM, Horowitz B. Functional and molecular expression of a voltage-dependent K(+) channel (Kv1.1) in interstitial cells of Cajal. J Physiol 533: 315–327, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang XY, Morielli AD, Peralta EG. Tyrosine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell 75: 1145–1156, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa H, Sugimoto T. Kv1. 2-immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res 974: 222–227, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Ikushima S, Muramatsu I, Fujiwara M. Effects of 4-aminopyridine on the adrenergic nerve terminals of rabbit arteries. J Pharmacol Exp Ther 219: 792–797, 1981. [PubMed] [Google Scholar]
- 22.Jackson VM, Trout SJ, Brain KL, Cunnane TC. Characterization of action potential-evoked calcium transients in mouse postganglionic sympathetic axon bundles. J Physiol 537: 3–16, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karoon P, Rubino A, Burnstock G. Enhanced sympathetic neurotransmission in the tail artery of 1,3-dipropyl-8-sulphophenylxanthine (DPSPX)-treated rats. Br J Pharmacol 116: 1918–1922, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolo LL, Westfall TC, Macarthur H. Modulation of neurotransmitter release by NO is altered in mesenteric arterial bed of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 287: H1842–H1847, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Kupper J Functional expression of GFP-tagged Kv1.3 and Kv14 channels in HEK 293 cells. Eur J Neurosci 10: 3908–3912, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Kupper J, Prinz AA, Fromherz P. Recombinant Kv13. potassium channels stabilize tonic firing of cultured rat hippocampal neurons. Pflügers Arch 443: 541–547, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci 7: 548–562, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Lechner SG, Mayer M, Boehm S. Activation of M1 muscarinic receptors triggers transmitter release from rat sympathetic neurons through an inhibition of M-type K+ channels. J Physiol 553: 789–802, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee RM, Triggle CR, Cheung DW, Coughlin MD. Structural and functional consequence of neonatal sympathectomy on the blood vessels of spontaneously hypertensive rats. Hypertension 10: 328–338, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Horn JP. Physiological classification of sympathetic neurons in the rat superior cervical ganglion. J Neurophysiol 95: 187–195, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Wang P, Xu J, Desir GV. Voltage-gated potassium channel Kv1.3 regulates GLUT4 trafficking to the plasma membrane via a Ca2+-dependent mechanism. Am J Physiol Cell Physiol 290: C345–C351, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Luo M, Hess MC, Fink GD, Olson LK, Rogers J, Kreulen DL, Dai X, Galligan JJ. Differential alterations in sympathetic neurotransmission in mesenteric arteries and veins in DOCA-salt hypertensive rats. Auton Neurosci 104: 47–57, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Malin SA, Nerbonne JM. Molecular heterogeneity of the voltage-gated fast transient outward K+ current, I(Af), in mammalian neurons. J Neurosci 21: 8004–8014, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McFarlane S, Pollock NS. A role for voltage-gated potassium channels in the outgrowth of retinal axons in the developing visual system. J Neurosci 20: 1020–1029, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKay BE, Molineux ML, Mehaffey WH, Turner RW. Kv1 K+ channels control Purkinje cell output to facilitate postsynaptic rebound discharge in deep cerebellar neurons. J Neurosci 25: 1481–1492, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mo ZL, Adamson CL, Davis RL. Dendrotoxin-sensitive K(+) currents contribute to accommodation in murine spiral ganglion neurons. J Physiol 542: 763–778, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nashmi R, Jones OT, Fehlings MG. Abnormal axonal physiology is associated with altered expression and distribution of Kv1.1 and Kv12 K+ channels after chronic spinal cord injury. Eur J Neurosci 12: 491–506, 2000. [DOI] [PubMed] [Google Scholar]
- 38.O'Connell KM, Tamkun MM. Targeting of voltage-gated potassium channel isoforms to distinct cell surface microdomains. J Cell Sci 118: 2155–2166, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Popratiloff A, Giaume C, Peusner KD. Developmental change in expression and subcellular localization of two shaker-related potassium channel proteins (Kv1.1 and Kv12) in the chick tangential vestibular nucleus. J Comp Neurol 461: 466–482, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Sauzeau V, Sevilla MA, Rivas-Elena JV, de Alava E, Montero MJ, Lopez-Novoa JM, Bustelo XR. Vav3 proto-oncogene deficiency leads to sympathetic hyperactivity and cardiovascular dysfunction. Nat Med 12: 841–845, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 341: 577–585, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med 347: 1135–1142, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Szulczyk B, Rola R, Witkowski G, Szulczyk P. Effects of ATP and GTP on voltage-gated K+ currents in glandular and muscular sympathetic neurons. Brain Res 1068: 82–93, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Tsaur ML, Sheng M, Lowenstein DH, Jan YN, Jan LY. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron 8: 1055–1067, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Uhrenholt TR, Nedergaard OA. Calcium channels involved in noradrenaline release from sympathetic neurones in rabbit carotid artery. Pharmacol Toxicol 92: 226–233, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur J Neurosci 7: 2189–2205, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Vicente R, Villalonga N, Calvo M, Escalada A, Solsona C, Soler C, Tamkun MM, Felipe A. Kv1.5 association modifies kv1.3 traffic and membrane localization. J Biol Chem 283: 8756–8764, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL. Localization of Kv1.1 Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J Neurosci 14: 4588–4599, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu J, Koni PA, Wang P, Li G, Kaczmarek L, Wu Y, Li Y, Flavell RA, Desir GV. The voltage-gated potassium channel Kv1.3 regulates energy homeostasis and body weight. Hum Mol Genet 12: 551–559, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Xu J, Wang P, Li Y, Li G, Kaczmarek LK, Wu Y, Koni PA, Flavell RA, Desir GV. The voltage-gated potassium channel Kv1.3 regulates peripheral insulin sensitivity. Proc Natl Acad Sci USA 101: 3112–3117, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]