Abstract
During activation of the renin-angiotensin system, hindbrain circumventricular organs such as the area postrema have been implicated in modulating the arterial baroreflex. This study was undertaken to test the hypothesis that the subfornical organ (SFO), a forebrain circumventricular structure, may also modulate the baroreflex. Studies were performed in rats with two-kidney, one-clip (2K,1C) hypertension as a model of endogenously activated renin-angiotensin system. Baroreflex function was ascertained during ramp infusions of phenylephrine and nitroprusside in conscious sham-clipped and 5-wk 2K,1C rats with either a sham or electrolytically lesioned SFO. Lesioning significantly decreased mean arterial pressure in 2K,1C rats from 158 ± 7 to 131 ± 4 mmHg but not in sham-clipped rats. SFO-lesioned, sham-clipped rats had a significantly higher upper plateau and range of the renal sympathetic nerve activity-mean arterial pressure relationship compared with sham-clipped rats with SFO ablation. In contrast, lesioning the SFO in 2K,1C rats significantly decreased both the upper plateau and range of the baroreflex control of renal sympathetic nerve activity, but only the range of the baroreflex response of heart rate decreased. Thus, during unloading of the baroreceptors, the SFO differentially modulates the baroreflex responses in sham-clipped vs. 2K,1C rats. Since lesioning the SFO did not influence plasma angiotensin II (ANG II), the effects of the SFO lesion are not caused by changes in circulating levels of ANG II. These findings support a pivotal role for the SFO in the sympathoexcitation observed in renovascular hypertension and in baroreflex regulation of sympathetic activity in both normal and hypertensive states.
Keywords: Goldblatt kidney, renal sympathetic nerve activity, angiotensin II, vasopressin
the role of the area postrema in angiotensin II (ANG II)-mediated modulation of arterial baroreflex control of blood pressure has been well established (10, 37, 49). Much less is known about whether other circumventricular organs also modulate baroreflex regulation of heart rate (HR) or renal sympathetic nerve activity (RSNA). ANG II given exogenously or induced from endogenous sources results in a decrease in the gain and resetting of baroreflex control of HR (4, 22, 32, 41, 48) and attenuation of the range of the baroreflex response of RSNA (2, 40, 41, 48). Considerable evidence suggests that the subfornical organ (SFO) located in the roof of the third ventricle is a target for circulating ANG II (19, 30, 33). Like the area postrema, the SFO lacks a blood-brain barrier. SFO neurons not only express AT1 receptor mRNA (27), but the SFO possesses one of the highest reported densities of ANG II receptors in the rat central nervous system (7). Moreover, the sensitivity of SFO neurons to ANG II exceeds that of neurons in the area postrema by an order of magnitude (11).
ANG II can influence blood pressure by several mechanisms, including direct vasoconstriction (5), retention of sodium and water by the kidney (9, 20), sympathoexcitation (49), and vascular remodeling (17). Numerous reports confirm an important role for ANG II in the regulation of thirst and sodium appetite via the SFO (13, 30, 47). Nevertheless, convincing data also exist showing that the chronic hypotensive effect of the AT1 receptor antagonist losartan is mediated, at least in part, via the SFO (8) and that this effect is independent of water and sodium intake and excretion (23). In addition, the peripheral vasoconstrictor activity of ANG II cannot entirely account for hypertension elicited by chronic low-dose ANG II (14). Acutely, intravenous ANG II evokes a pressor response that is attenuated by lesioning the SFO (30), and direct microinjection of ANG II into SFO evokes a pressor response that is blocked by pretreatment with saralasin, a peptide ANG II antagonist (29). Neurons within the SFO that are excited by ANG II send efferent projections to the parvocellular region of the paraventricular nucleus (PVN) (1, 19, 46). Outputs from the PVN, in turn, project to preganglionic sympathetic neurons in the spinal cord, either directly or via the rostral ventrolateral medulla (1, 43). Thus it is apparent that ANG II can act at the SFO, as well as the area postrema, to drive sympathetic nerve activity. Importantly, SFO neurons projecting to the PVN receive information via ascending projections from nucleus tractus solitarii (44), a site known to receive input from peripheral baroreceptors. Recently, a strong case has been made to examine the role arterial baroreflexes may play in the regulation of RSNA in chronic control of blood pressure, particularly ANG II-dependent hypertension (2). In turn, RSNA can modulate renin secretion by the kidney (25), thereby influencing circulating ANG II levels. To our knowledge, the role of the SFO in baroreflex control of sympathetic activity has gained scant attention.
Together, these data provide a basis for testing the hypothesis that the SFO is involved in regulation of sympathetic activity and modulation of baroreflex function and, furthermore, that this regulation is influenced by endogenous circulating ANG II. To ascertain whether SFO involvement in baroreflex function depends on the concentration of endogenous ANG II, we chose to study two groups of rats: sham-clipped normotensive rats and two-kidney, one-clip (2K,1C) hypertensive rats that have an activated renin-angiotensin system. Specifically, we examined the effect of lesioning the SFO on arterial pressure and baroreflex control of RSNA and HR in conscious sham-clipped normotensive and 2K,1C hypertensive rats.
MATERIALS AND METHODS
Male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN), were used for all experiments. They were housed under controlled conditions (21–23°C, lights on 0700-1900) and had free access to water and standard rat chow. The rats were cared for in accordance with the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by our Institutional Animal Investigation Committee.
Surgical procedures.
In 5-wk-old rats under combined ketamine (80 mg/kg) and xylazine (5 mg/kg) intravenous anesthesia, a silver clip (0.2 mm) was surgically placed around the right renal artery via a flank incision (2K,1C group). Rats in the sham-clipped group underwent identical surgery but were not clipped. All rats were returned to their home cages.
Five weeks later, rats that were to undergo baroreflex testing were equipped with left carotid artery and jugular vein catheters while under similar ketamine and xylazine anesthesia. The catheters were filled with heparinized saline (100 U/ml), secured, tunneled subcutaneously, and exteriorized at the base of the neck. HR and blood pressure were monitored continuously during surgery to assure adequacy of anesthesia. In this and other procedures described below, supplemental doses were given if HR increased more than 20 beats/min or mean arterial pressure (MAP) more than 10 mmHg. After surgery, the rats were allowed to recover fully.
Two days later, rats from the 2K,1C and sham-clipped groups were randomly assigned to undergo electrolytic lesioning of the SFO (SFOx) or sham lesioning. For SFO lesions, rats were anesthetized with ketamine and xylazine as before and secured in a stereotaxic apparatus with the skull leveled between the bregma and lambda. A monopolar electrode insulated to within 0.5 mm of the tip was inserted into the SFO using stereotaxic coordinates: ±1.0 lateral to midline at an 11° angle, −0.9 caudal to bregma, and −5.8 ventral to skull surface. A direct current (3 mA for 50 s) was passed between the monopolar electrode in the SFO and a reference electrode. For sham-lesioned animals, the electrode was inserted only to a depth of −4.8 (dorsoventral) and no current was passed. The rats were permitted to recover for 2 days, and each rat was conditioned over the next 2 days to remain for 2 h within a custom-made Plexiglas chamber that would be used during the experiment. This chamber restricted the animal's movement but did not restrain it.
On the fourth day after SFO lesioning (or sham lesioning), RSNA electrodes were implanted under pentobarbital sodium anesthesia (40 mg/kg ip). HR and arterial pressure were monitored continuously via the previously implanted catheters, and supplemental doses of pentobarbital sodium were administered in accordance with the criteria mentioned above. A retroperitoneal approach was used to isolate the left renal nerve branch and carefully dissect it free. The nerve was placed on electrodes constructed of Teflon-coated silver wire (0.0055-in. diameter; A-M Systems) with the exposed ends wound into single loops. The nerve and electrodes were covered with silicone gel (Kwik-Sil; World Precision Instruments), which was allowed to harden before closure. A ground wire was sewn into the surrounding tissue. The electrodes and ground wire were tunneled subcutaneously and exteriorized at the base of the neck. A minimum of 24 h was permitted after the renal nerve surgery for full recovery from anesthesia before any assessment of baroreflex function (28). In all cases, the animals were grooming themselves normally, eating, drinking, and displaying normal cage activity.
A separate set of rats had implantation of radiotelemetry transducer (TA11PA-C40; Data Sciences International) at the time of renal artery clipping or sham clipping. After the femoral artery was exposed and the proximal end temporarily occluded, the gel-filled catheter attached to the transmitter device was inserted into the distal aorta via a 21-gauge needle. The catheter was advanced and secured with medical adhesive, and the transmitter was placed subcutaneously and sutured to the underlying muscle. The skin was closed with surgical staples. At the end of surgery, each rat received butorphanol tartrate (0.075 mg sc) for analgesia. Each rat was returned to its home cage with its individual receiver. Hemodynamic data was recorded continuously for the 5 wk preceding SFO lesioning and 5 days postlesioning (or sham lesioning) until the time of death for collection of ANG II plasma samples.
Hemodynamic and nerve activity recordings.
Arterial pressure was measured by connecting the arterial catheter with a pressure transducer (Gould P23 XL), which was coupled to an amplifier (Digi-Med BPA-200). HR and MAP were derived by data acquisition software (DasyLab; Biotech Products) using the arterial pressure pulse and averaged over 1-s intervals.
Renal nerve activity was amplified (5,000–20,000 times) and filtered (100–1,000 Hz) with a Grass P511 differential preamplifier and a high-impedance probe (HIP511GB). The probe and animals were located inside a shielded Faraday cage. The amplified and filtered neurogram signal was channeled to an oscilloscope and Grass AM8 audio monitor for visual and auditory evaluation, respectively. The amplified nerve activity was digitized, rectified, integrated, and averaged over 1-s intervals by the computer data acquisition software (DasyLab). Background noise was determined at the end of experiment after administration of a bolus dose of the ganglionic blocker trimethaphan camsylate (20 mg/kg iv; Hoffman-La Roche). RSNA was defined as the amount of recorded nerve activity after subtraction of background noise.
Baroreflex measurements.
All baroreflex testing was performed in conscious animals. Resting MAP and HR were measured in each of the sham-clipped and 2K,1C rats just before the SFO lesion and then again 4 days after the lesion. Baroreflex control of RSNA and HR was tested in four groups of rats: sham-clipped, sham-lesioned (n = 7); sham-clipped SFOx (n = 6); 2K,1C sham-lesioned (n = 8); and 2K,1C SFOx (n = 5). Baroreceptor reflex curves were generated by producing ramp increases and decreases in arterial pressure using intravenous infusions of phenylephrine hydrochloride (200 μg/ml; Sigma RBI) and sodium nitroprusside (200 μg/ml; Ohmeda), respectively. Phenylephrine was infused in increasing rates of 5–50 μg·kg−1·min−1 and nitroprusside at rates of 7.5–100 μg·kg−1·min−1. The ramp increase or decrease of MAP was completed in ∼2 min. Fifteen to thirty minutes were allowed between the infusions to permit all parameters to return to baseline values. Infusions of phenylephrine or nitroprusside were performed randomly.
When all experiments were completed, each rat was euthanized with an overdose of pentobarbital sodium (100 mg/kg iv) and perfused transcardially with 0.9% saline followed by 10% neutral buffered formalin. The brain was then removed, dehydrated using an alcohol series, and embedded in paraffin. Coronal sections (4 μm) were stained with hematoxylin and eosin, and the site of lesion was verified histologically.
Evaluation of SFO lesioning on plasma ANG II.
To ascertain whether lesioning the SFO would alter plasma ANG II levels, we performed experiments in a second set of sham-clipped, sham-lesioned; sham-clipped SFOx; 2K,1C sham-lesioned; and 2K,1C SFOx rats. At the time of renal artery clipping (or sham clipping), these animals were equipped with radiotelemetry transmitters for HR and arterial pressure. Baroreflex tested animals were not used in these experiments to avoid any influence of the changes in blood pressure during baroreflex testing protocols on plasma ANG II levels. Five days after recovery from SFO lesioning or sham lesioning, the rats were anesthetized with isoflurane and aortic blood for measurement of ANG II was collected into prechilled tubes containing a solution with inhibitors of proteolytic enzymes and angiotensin-converting enzyme (5 mM EDTA, 10 μM pepstatin, 25 mM phenanthroline, and 20 μM enalaprilat) to block degradation. The blood samples were immediately centrifuged at 4°C, and the plasma was removed and stored at −70°C until assay (3).
Evaluation of SFO lesioning on water balance.
To evaluate the effect of a discrete SFO lesion on water balance that could potentially impact the baroreflex, we first ascertained water intake and vasopressin (VP) secretion in a separate group of SFOx and sham-lesioned rats equipped with vascular catheters. Only sham-clipped rats were used in these experiments, since the high arterial blood pressures in the 2K,1C rats could independently inhibit VP secretion via baroreflex mechanisms and modify the response to osmotic stimulation. After 1 day was allowed for recovery from surgery, spontaneous water intake was assessed daily for 5 days after SFOx or sham lesioning. On day 5, each rat was weighed. After a stabilization period of ∼20 min as judged by MAP and HR, 100 μl of blood were removed for measurement of serum osmolality and sodium. An additional 800–900 μl of blood were removed slowly into prechilled, heparinized tubes via the arterial catheter for assessment of plasma VP. The blood was centrifuged at 4°C, the plasma was frozen at −70°C, and the red blood cells were reconstituted in an equal volume of 0.9% NaCl and immediately reinfused. A volume of 5% NaCl calculated to increase plasma osmolality ∼15 mosmol/kgH2O (range 1.8–2.2 ml) was then infused intravenously over 60 s. Ten minutes later, a second set of blood samples was obtained for osmolality, sodium, and VP.
Radioimmunoassays for ANG II and vasopressin.
Plasma samples for ANG II were processed as described by Navar and colleagues (3, 37). Briefly, 1 ml of plasma was extracted by adsorbing to a phenyl-bonded SPE column (Bond-Elut; Varian, Walnut Creek, CA) that had been prewashed with 90% methanol in water. The column was then washed with 2 ml of water, 1 ml of hexane, and 1 ml of chloroform. Angiotensin peptides were eluted with 2 volumes of 1 ml 90% methanol in water. The eluates were combined, taken to dryness under N2, and stored overnight at −20°C. The extracts were reconstituted in 0.5 ml of assay buffer consisting of 50 mM sodium phosphate, 1 mM EDTA, 0.25 mM thimerosal, and 0.25% peptidase-free human serum albumin. All samples were assayed in duplicate using 125I-labeled ANG II (Perkin-Elmer, Billerica, MA) as the tracer and anti-ANG II antibody (Peninsula Laboratories, San Carlos, CA) at a final dilution of 1:660,000. Nonspecific binding was 2.1%, the lower limit of detection was 0.5 fmol/tube, and 50% binding was 15.2 fmol/tube.
Plasma VP concentration was assessed using methods reported previously from our laboratory (39). Briefly, the plasma samples were extracted before assay. The samples and all standards were assayed in duplicate using purified VP (Ferring, Malmo, Sweden) as the standard, 125I-iodotyrosyl VP as the tracer (Amersham, Arlington Heights, IL), and anti-VP antibody (no. 2849; the gift of Drs. Durr and Lindheimer) at a dilution of 1:360,000.
Analyses and statistics.
Serum osmolality was assessed by freezing point depression (Precision Systems 5004; Sudbury, MA), and serum sodium concentration was determined using flame photometry with internal lithium standardization.
RSNA was normalized using resting nerve activity as the 100% value. MAP-RSNA and MAP-HR curves were constructed for each rat by fitting all individual data points (averaged over 1-s intervals) to a four-parameter logistic function using a standard software package (SigmaPlot; Jandel Scientific), according to the equation RSNA or HR = (P1 − P2)/{1 + exp[P3(MAP − P4)]} + P4, where P1 is the upper plateau of the curve, P2 is the lower plateau, P3 is the slope coefficient, and P4 is MAP at the midpoint of the curve (BP50). The range of the baroreflex curve was defined as P1 − P2, and the maximum gain was calculated as −(P1 − P2)P3/4. The gain of the function at any given MAP was calculated from the first derivative of the equation: gain = −(P1 − P2)P3·exp[P3(MAP − P4)]/{1 + exp[P3(MAP − P4)]}2. For each individual animal's curve, the four parameters (P1 to P4) were derived and then averaged across animals. The mean parameters were used to generate averaged baroreflex curves for each group of animals. Resting values recorded before phenylephrine or nitroprusside were averaged for each curve.
All data are means ± SE. A two-way ANOVA was used to determine the effect of the SFO lesion on baseline and baroreflex curve parameters in normotensive and 2K,1C rats. Comparison of water balance between groups was made using Student's t-test; comparisons within the same animal before and after lesioning or hypertonic saline were made using the paired t-test. A P value <0.05 was accepted as significant.
RESULTS
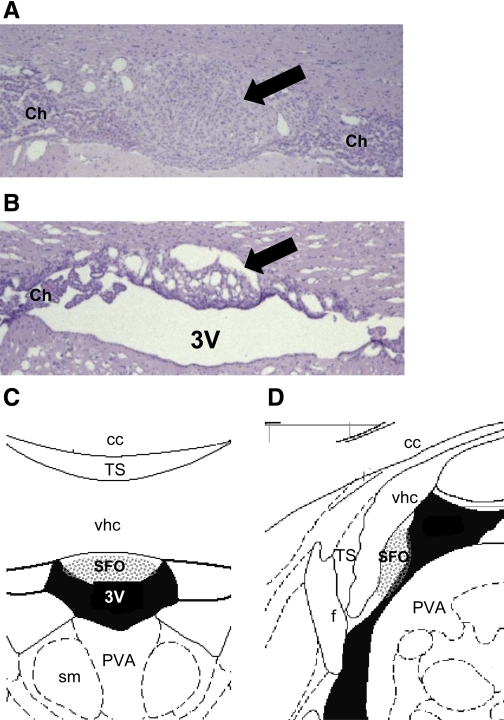
Sham-lesioned animals displayed an intact SFO (Fig. 1A). Only rats with a lesion (Fig. 1B) encompassing the areas depicted in Fig. 1, C and D, were included for analysis in the SFOx group.
Fig. 1.
Histological sections and schematic diagrams at the level of the subfornical organ (SFO). Representative coronal sections at the level of the SFO (arrows) from a sham-lesioned (A) and an SFO-lesioned rat (B) and corresponding coronal (C) and sagittal diagrams (D) are shown. All rats included in the final data analysis had lesions encompassing the stippled areas shown on the schematic. Staining in A and B is hematoxylin and eosin (×40 magnification). 3V, third ventricle; cc, corpus callosum; f, fornix; PVA, anterior part of the paraventricular thalamic nucleus; sm, stria medullaris; TS, triangular septal nucleus; vhc, ventral hippocampal commissure; Ch, choroid plexus. Diagrams in C and D are modified from the rat brain atlas of Paxinos and Watson (38a).
Effect of SFO lesion on resting heart rate and arterial pressure.
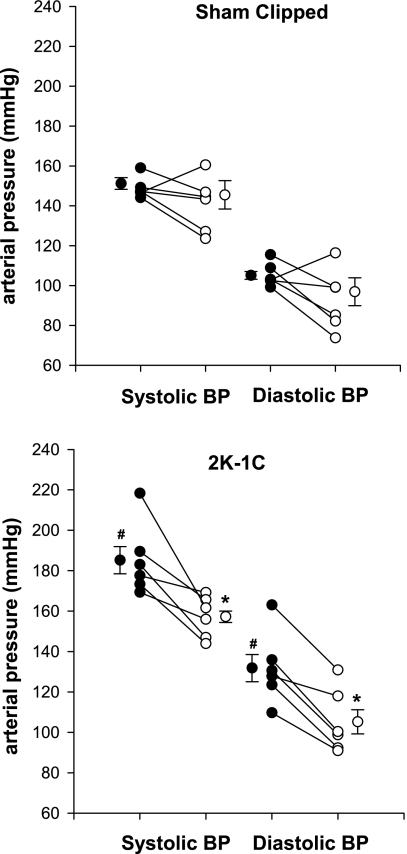
The effect of SFO lesioning on resting MAP and HR was evaluated using two approaches. First, in the longitudinal approach, resting arterial pressure (mean, systolic, and diastolic) and HR were evaluated in the same animal (sham-clipped or 2K,1C) before and 4 days after SFO lesioning in the awake state and before placement of the nerve electrodes (Fig. 2). (One rat with a subsequent unsuccessful nerve recording in the 2K,1C SFOx group was also included in this analysis). Before the SFO was lesioned, MAP was significantly higher in the 2K,1C rats (158 ± 7 mmHg) compared with the sham-clipped rats (128 ± 2 mmHg, P < 0.01). HR did not differ between the groups (P > 0.05). Ablation of the SFO did not change MAP in sham-clipped rats (120 ± 7 mmHg) but resulted in a significant decrease in MAP in 2K,1C rats (133 ± 5 mmHg, P < 0.01 vs. prelesion 2K,1C). There was a significant interaction between group and lesion effects (P = 0.0400). Thus, in the 2K,1C rats, MAP tended to normalize after the SFO lesion and did not differ from MAP in sham-clipped rats either pre- or postlesion (P > 0.05). HR was not affected by the SFO lesion in either the sham-clipped or the 2K,1C group. Rats whose lesions were outside the SFO, mostly in the region of the ventral hippocampal commissure, did not have a decrease in MAP or a change in HR.
Fig. 2.
Changes in arterial pressure after lesioning of the SFO in individual rats. Systolic and diastolic arterial pressures in conscious sham-clipped (n = 6, top) and 2 kidney, 1 clip (2K,1C; n = 6, bottom) rats before (•) and 4 days after (○) lesioning of the SFO. Values for individual rats as well as means ± SE are shown. *P < 0.01 vs. prelesion in the same group. #P < 0.01 vs. prelesion in the sham-clipped group.
Second, similar results were observed when resting MAP and HR were compared among all four groups on the day of baroreflex testing (Table 1). The interaction between group and lesion effects was also significant (P = 0.0171). In this set of comparisons, MAP was still significantly lower in the 2K,1C SFOx group than in the 2K,1C sham-lesioned group. Although MAP in the 2K,1C SFOx group did not differ significantly from that in the sham-clipped SFOx group (P > 0.05), it was still significantly higher than in the sham-clipped, sham-lesioned group (Table 1).
Table 1.
Resting MAP and HR in all 4 groups of rats on the day baroreflex curves were assessed
| Group | n | MAP, mmHg | HR, beats/min |
|---|---|---|---|
| Sham clipped, sham lesioned | 7 | 123±2 | 400±8 |
| Sham clipped, SFOx | 6 | 127±6 | 405±19 |
| 2K,1C, sham lesioned | 8 | 162±6c | 404±8 |
| 2K,1C, SFOx | 5 | 138±3a,b | 415±7 |
Values are means ± SE; n = no. of animals. Mean arterial pressure (MAP) and heart rate (HR) were measured in sham-clipped, sham-lesioned; sham-clipped subfornical organ-lesioned (SFOx), two-kidney, one-clip (2K,1C) sham-lesioned, and 2K,1C SFOx rats.
P < 0.025 vs. 2K,1C sham-lesioned group.
P < 0.05;
P < 0.001 vs. sham-clipped, sham-lesioned group.
Effect of SFO lesion on baroreflex control of RSNA and HR.
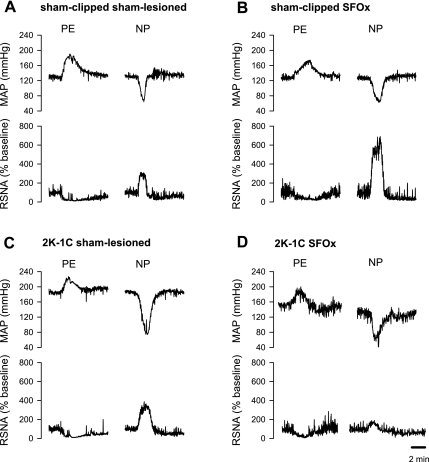
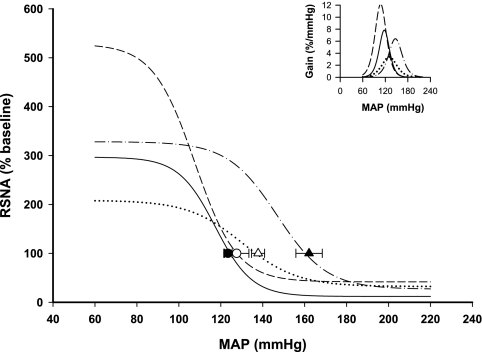
Figure 3 shows the response of integrated RSNA to ramp increases and decreases in arterial pressure in individual rats representative of each group. In sham-lesioned rats, baroreflex regulation of both RSNA and HR was reset to higher levels of arterial pressure in the 2K,1C as attested by the higher BP50 in this group compared with sham-clipped rats (Table 2). These effects on midpoint were consistent with the higher resting MAP in 2K,1C rats. A significant reduction in the range of baroreflex control of HR was observed in 2K,1C sham-lesioned rats due to the higher value of the lower plateau of the MAP-HR curve (Fig. 4 and Table 2). All other parameters of the RSNA and HR baroreflex curves were similar in both groups of sham-lesioned rats.
Fig. 3.
Representative reflex responses of renal sympathetic nerve activity (RSNA) in each of the 4 groups of rats. Reflex response of RSNA to phenylephrine (PE)-induced increases and nitroprusside (NP)-induced decreases in mean arterial pressure (MAP) are shown for sham-clipped, sham-lesioned (A), sham-clipped SFO-lesioned (SFOx; B), 2K,1C sham-lesioned (C), and 2K,1C SFOx rats (D).
Table 2.
HR and RSNA baroreflex curve parameters in sham-lesioned and SFOx sham-clipped and 2K,1C rats
|
Sham Clipped |
2K,1C
|
|||
|---|---|---|---|---|
| Sham Lesioned | SFOx | Sham Lesioned | SFOx | |
| n | 7 | 6 | 8 | 5 |
| HR | ||||
| Upper plateau, beats/min | 505±19 | 532±8 | 523±13 | 484±14e |
| Lower plateau, beats/min | 267±17 | 308±25 | 331±14c | 348±19 |
| Range, beats/min | 238±11 | 224±33 | 190±8c | 135±15b |
| BP50, mmHg | 131±5 | 121±4 | 149±4c | 135±9 |
| Slope coefficient | 0.06±0.01 | 0.06±0.01 | 0.05±0.01 | 0.07±0.01 |
| Gmax, −beats·min−1·mmHg−1 | 3.39±0.48 | 2.91±0.40 | 2.50±0.33 | 2.27±0.40 |
| RSNA | ||||
| Upper plateau, % | 297±31 | 528±56b | 328±28 | 209±21a,e |
| Lower plateau, % | 12±3 | 42±6b | 27±8 | 32±11 |
| Range, % | 285±31 | 487±53b | 302±27 | 176±23b,e |
| BP50, mmHg | 117±3 | 108±5 | 147±5d | 131±3a,e |
| Slope coefficient | 0.11±0.02 | 0.10±0.01 | 0.08±0.01 | 0.08±0.01 |
| Gmax, −%/mmHg | 8.25±1.52 | 12.68±2.40 | 6.18±0.48 | 3.52±0.74b,e |
Values are means ± SE; n = no. of animals. BP50, MAP at midpoint of curve; Gmax, maximum gain; RSNA, renal sympathetic nerve activity;
P < 0.05;
P < 0.01 vs. corresponding sham-lesioned group.
P < 0.05;
P < 0.005 vs. sham-clipped, sham-lesioned group.
P < 0.02 vs. sham-clipped SFOx group.
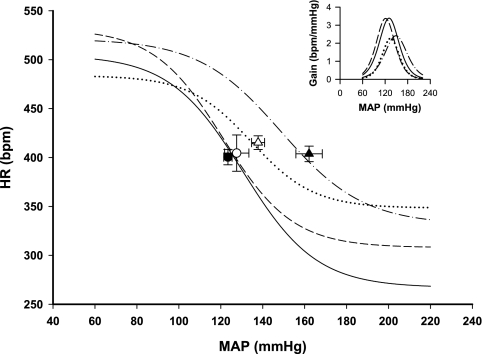
Fig. 4.
Baroreflex curves of the heart rate (HR) response in the 4 groups of rats. Mean baroreflex curves of the HR response are shown for sham-clipped, sham-lesioned (•), sham-clipped SFOx (○), 2K,1C sham-lesioned (▾), and 2K,1C SFOx rats (▿). Inset depicts the baroreflex gain in the same groups. Symbols on the curves represent resting values (means ± SE). See Tables 2 and 3 for complete statistics and numbers in each group.
The most pronounced effect of the SFO lesion was on the baroreflex control of RSNA, notably on the upper plateau and the range. In sham-clipped rats, the maximum RSNA response to a decrease in arterial pressure was almost two times greater in SFOx animals than in sham-lesioned rats and was accompanied by a significant increase in the range of the baroreflex of RSNA (Fig. 4 and Table 2). Interestingly, in 2K,1C rats, the effect of SFO ablation on the baroreflex control of RSNA was qualitatively different. The sympathoexcitatory response to baroreceptor unloading and the range of RSNA baroreflex were significantly smaller in 2K,1C SFOx rats than in 2K,1C sham-lesioned rats (Fig. 5 and Table 2). Furthermore, the gain of baroreceptor control of RSNA was also diminished in 2K,1C SFOx rats compared with the 2K,1C sham-lesioned group (Fig. 5 and Table 2). There was a significant group × lesion interaction on upper plateau (P = 0.0001), range (P = 0.0002), and gain (P = 0.0234) of the reflex response of RSNA.
Fig. 5.
Baroreflex curves of the RSNA response in the 4 groups of rats. Mean baroreflex curves of the RSNA response are shown for sham-clipped, sham-lesioned (•), sham-clipped SFOx (○), 2K,1C sham-lesioned (▾), and 2K,1C SFOx rats (▿). Inset depicts the baroreflex gain in the same groups. Symbols on the curves represent resting values (means ± SE). See Tables 2 and 3 for complete statistics and numbers in each group.
Baroreflex control of HR was not affected by SFO ablation in sham-clipped rats. 2K,1C SFOx rats displayed a significantly reduced range of HR baroreflex (Fig. 4 and Table 2). Similarly to RSNA, the upper plateau of the HR baroreflex was lower in 2K,1C SFOx rats compared with 2K,1C sham-lesioned rats, but this did not achieve significance (P = 0.064). For the HR, there was a significant group × lesion interaction only on the upper plateau (P = 0.0328).
Effect of SFO lesion on plasma ANG II levels.
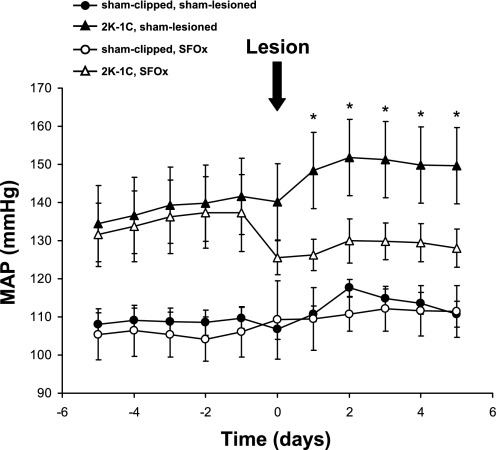
Figure 6 and Table 3 show that rats in all the groups monitored by radiotelemetry tended to have lower arterial pressures compared with those whose hemodynamic parameters were assessed by conventional monitoring (compare with Table 1 and Fig. 2). Notably, the data in freely moving rats confirm that MAP is significantly elevated in the 2K,1C rats compared with sham-clipped rats before SFO lesioning (P < 0.01 for 2K,1C vs. sham-clipped overall). On the day of lesioning (or sham lesioning), average MAP tended to decrease associated with administration of anesthesia. However, MAP remained significantly lower in the 2K,1C SFOx group compared with MAP before the lesion (P < 0.05). After sham lesioning of 2K,1C rats, MAP returned to the higher values and continued to rise slightly, thereby remaining significantly higher compared with either the sham-clipped, sham-lesioned group (P < 0.01) or the sham-clipped SFOx group (P < 0.01). Importantly, MAP remained significantly lower on postlesioning days 1–5 in the 2K,1C SFOx group compared with 2K,1C sham-lesioned rats (P < 0.05, as shown in Fig. 6). Although MAP in the 2K,1C SFOx rats remained ∼7–13 mmHg higher than that in either of the sham-clipped groups, it did not differ statistically from MAP in either of the sham-clipped groups on any of the five postlesion days.
Fig. 6.
MAP averaged over 24 h from telemetry recordings in the 4 groups of rats. MAP was for measured 5 days before and 5 days after SFOx or sham lesioning in 2K,1C or sham-clipped rats. Values are means ± SE; numbers in each group are as given in Table 3. *P < 0.05 vs. 2K,1C SFOx group. See results for other comparisons.
Table 3.
Resting MAP, kidney weights, and plasma ANG II levels in all 4 groups of rats
| Group | n |
MAP, mmHg |
Kidney Weight, g
|
Plasma ANG II, fmol/ml | ||
|---|---|---|---|---|---|---|
| Pre-SFO Lesion | Post-SFO Lesion | Left | Right | |||
| Sham clipped, sham lesioned | 5 | 106±7 | 110±5 | 1.1369 | 1.1452 | 36.7±8.3 |
| Sham clipped, SFOx | 5 | 107±5 | 110±4 | 1.1976 | 1.1758 | 43.9±5.3 |
| 2K-1C, sham lesioned | 7 | 133±6a | 150±9a,c,d | 1.2441 | 1.0641e | 138.4±21a |
| 2K-1C, SFOx | 8 | 135±6b | 123±5d | 1.2527 | 1.0709e | 134.4±26b |
Values are means ± SE; n = no. of animals.
P < 0.05 vs. sham-clipped, sham-lesioned group.
P < 0.05 vs. sham-clipped SFOx group.
P < 0.05 vs. 2K,1C sham-lesioned group.
P < 0.05 vs. pre-SFO lesion (by paired t-test).
P < 0.05 vs. left kidney.
Table 3 shows MAP values during the 30 min immediately before anesthesia before the SFO lesioning and again 5 days after lesioning, when blood was obtained for ANG II levels. As in Fig. 6, 2K,1C SFOx rats displayed a significantly lower MAP compared with values before the lesion. In the 2K,1C sham-lesioned rats, MAP continued to rise, as is typical during the first 6–7 wk of this model of hypertension (36), and was significantly higher compared with MAP in 2K,1C SFOx rats.
The nonclipped kidney weights were significantly higher compared with the clipped kidney weights in both clipped groups, but left and right kidney weights were comparable in the sham-clipped animals. Plasma ANG II concentrations were significantly elevated in the 2K,1C sham-lesioned group compared with sham-clipped, sham-lesioned rats (P < 0.05). Notably, lesioning the SFO did not change plasma ANG II levels in either the sham-clipped or the 2K,1C rats compared with their nonlesioned counterparts.
Effect of SFO lesion on water balance.
Water intake was ∼40% lower in the sham-clipped SFOx group (Table 4); this was also true of 2K,1C SFOx rats (data not shown). Nonetheless, sham-clipped SFOx rats gained 13 ± 2 g over the next 5 days, which did not differ from the sham-clipped sham-lesioned rats (P = 0.17). Baseline serum Na concentration and osmolality were identical in both groups. Serum osmolality in the 2K,1C SFOx rats was 290 ± 3 mosmol/kgH2O (n = 8) and did not differ from that in 2K,1C sham-lesioned rats of 289 ± 2 mosmol/kgH2O (n = 8; P > 0.05) or in either of the sham-clipped groups. Baseline plasma VP level was higher in the sham-clipped SFOx group, but this did not achieve statistical significance (P = 0.34). Hypertonic saline evoked a significant increase in VP that did not differ between the groups.
Table 4.
Effect of SFO lesion on daily water intake and response to osmotic challenge
| Group |
Water Intake |
Serum [Na+], mM
|
Serum Osmolality, mosmol/kgH2O
|
Plasma VP, pg/ml
|
||||
|---|---|---|---|---|---|---|---|---|
| ml/d | ml·kg−1·day−1 | Baseline | After 5% NaCl | Baseline | After 5% NaCl | Baseline | After 5% NaCl | |
| Sham lesioned | 43±1 | 170±13 | 141±2 | 149±2b | 287±1 | 298±1c | 1.4±0.6 | 36.4±3.8b |
| SFOx | 24±1a | 101±2a | 141±1 | 149±1b | 287±2 | 300±2c | 2.3±0.4 | 49.1±12.4c |
Values are means ± SE; n = 5 animals in each group. VP, vasopressin.
P < 0.0005 vs. sham-lesioned group.
P < 0.05;
P < 0.01 vs. baseline (by paired t-test).
DISCUSSION
Three major findings emerged from this study. First, selective lesioning of the SFO resulted in significantly lower resting arterial pressure in hypertensive 2K,1C rats but left arterial pressure unchanged in normotensive sham-clipped rats. Second, the SFO, an anterior hypothalamic structure, modulated baroreflex function, particularly the control of RSNA and HR, during unloading of the arterial baroreceptors. An important, albeit unexpected finding was that the SFO lesion exerted an opposite influence on baroreflex function in normotensive sham-clipped rats compared with hypertensive 2K,1C rats. In SFO-lesioned sham-clipped rats, the upper plateau and the range of baroreflex control of RSNA increased despite no significant change in resting arterial pressure. In contrast, both the range and upper plateau decreased in SFO-lesioned 2K,1C hypertensive rats. The effect of SFO ablation on the upper plateau was observed in addition to the leftward shift in the baroreflex curve that occurs with other maneuvers that decrease arterial pressure. Thus the SFO exerted a significant but opposite influence on maximum baroreflex sympathetic response in normotensive sham-clipped rats compared with hypertensive 2K,1C rats. Finally, the SFO lesion did not change plasma ANG II concentrations in either sham-clipped or 2K,1C rats. This would support the concept that the effects of ablation of the SFO on the baroreflex function are not caused by circulating ANG II even though plasma ANG II has access to the SFO, which lies outside the blood-brain barrier.
Several studies have implicated the SFO in ANG II actions on arterial pressure and sympathetic activity (1, 11, 19, 29, 30). Our observation that ablation of the SFO lowered arterial pressure in 2K,1C rats, which have an endogenously activated renin-angiotensin system, is consistent with reports showing that SFO lesions attenuate hypertension due to both endogenous (6) and exogenous ANG II (23, 32). In contrast to findings in the 2K,1C hypertensive rats, lesioning the SFO did not change resting blood pressure in normotensive sham-clipped rats. These findings underscore previous studies showing that SFO ablation does not alter resting arterial pressure in normotensive animals (23, 47). Moreover, c-fos immunoreactivity within the SFO is induced after renal artery occlusion and is reduced after blockade of ANG II formation with enalapril (7). Notably, plasma ANG II levels did not differ in sham-lesioned compared with SFOx rats in either the sham-clipped or the 2K,1C groups, yet arterial pressure declined significantly. Although plasma ANG II values were not reported, SFO ablation was not likely to alter plasma ANG II concentrations with exogenously administered ANG II. Nevertheless, SFO lesioning does decrease MAP in that model (23, 32). Our current data in the 2K,1C model with endogenously elevated ANG II are consistent with their findings. Together with the present study, these findings support the concept that there is a neurogenic component associated with 2K,1C hypertension 5–6 wk after renal artery clipping that requires an intact SFO. Since the secretion of renin, which is rate limiting for the formation of ANG II, is influenced by the renal sympathetic nerves (25), the observation that SFO lesioning did not change circulating ANG II levels supports the interpretation that the neurogenic mechanism associated with the SFO does not exert its major effect by controlling the renin-angiotensin system. Hendel and Collister (23) demonstrated that the SFO is important in the control of chronic sodium homeostasis; however, this was evident only after more than 1 wk post-SFO ablation. Although we did not directly assess sodium balance, the present data show an attenuation in arterial pressure from the first day after SFO lesioning comparable to that at day 5. Thus it is unlikely that sodium balance accounts for the attenuation of the hypertension during the period of study. Our data cannot exclude an effect on renal sodium excretion and overall sodium homeostasis at later time points. Finally, it is possible that this neurogenic mechanism may involve direct action on resistance vessels. The identity of the factor(s) that act at the SFO remain to be identified.
Although the discrete lesion of the SFO decreased water intake, baseline fluid balance as evidenced by weight gain, serum osmolality, and serum sodium concentration was comparable in SFOx and sham-lesioned rats. This is consistent with other reports showing that SFO lesions attenuate dipsogenic behavior but not basal volume balance (33, 45, 47). The primary purpose of measuring VP was to assess whether VP secretion to an osmotic stimulus remained intact after an SFO lesion delivered in our hands. We chose to assess VP only in the sham-clipped groups, since they have comparable blood pressures, whereas the elevated arterial pressure in 2K,1C rats could exert an independent effect to suppress VP via baroreflex mechanisms. The osmotically induced VP secretory response was comparable in our sham-clipped SFOx and sham-clipped, sham-lesioned groups. This contrasts with the findings by McKinley et al. (33) in sheep, where SFO lesions attenuated the VP response to hypertonic saline. Notably, they showed that osmoresponsive neurons were located in the periphery of the SFO, whereas the ANG II-responsive neurons tended to congregate more centrally in the nucleus. Since our SFO lesions were directed to the center of the SFO and spared the dorsal peripheral region of the nucleus (Fig. 1), thereby leaving putative osmoresponsive neurons intact, our sham-clipped SFOx rats were able to release VP in response to the osmotic stimulus. Likewise, the slightly higher baseline circulating plasma VP, although not statistically significant, may have exerted sufficient biological activity to maintain water balance. Furthermore, Hendel and Collister (23) have demonstrated that SFO-lesioned rats do not display significantly different daily or cumulative sodium or water balance during the first 7 days after lesioning. Thus changes in arterial pressure and baroreflex activity in the SFO-lesioned groups are not likely to be due to changes in sympathetic tone in response to alteration in fluid volume status.
Although SFO lesioning significantly lowered MAP in 2K,1C rats, whether compared with prelesion MAP in the same animal or with MAP in the sham-lesioned 2K,1C group, MAP still remained significantly higher than in sham-lesioned, sham-clipped normotensive rats. Nonetheless, the present findings together with earlier studies indicate that the SFO plays a critical role in the full manifestation of the hypertensive response. Undoubtedly, the hypertensive effect of ANG II cannot be explained solely by its actions on SFO. In addition to direct effects on cardiovascular sites, ANG II actions on other circumventricular organs such as the area postrema may also contribute to the hypertensive response (12). Even so, our data support the concept that forebrain cardiovascular centers play an important role in the mechanisms of renin-dependent hypertension (21). It is reasonable to conclude that the hypotensive effect of SFO ablation in 2K,1C rats results from eliminating a central action on SFO neurons, either directly or indirectly, by angiotensin peptides (19). This, in turn, would decrease the activity of sympathoexcitatory neurons in the PVN that receive input from the SFO (1), project to the medullary and spinal sympathetic neurons (43), and have been shown to be activated in the 2K,1C model (24). More conclusive evidence regarding the effect of angiotensin peptides or other neurotransmitters on the SFO in this model will require testing with direct microinjection of selective antagonists into the SFO.
In sham-lesioned rats, the parameters of the baroreflex curve of RSNA were similar between sham-clipped and 2K,1C rats with the parallel shift of the MAP-RSNA relationship toward higher pressure levels in the 2K,1C hypertensive rats. At the same time, the range of baroreflex control of HR in the 2K,1C group was markedly reduced due to the attenuated bradycardic response to an increase in arterial pressure. Our results are in line with previous evidence that baroreflex regulation of sympathetic nerve activity is preserved, whereas that of HR is impaired in different models of hypertension (16, 18, 22, 48).
After SFO ablation, baroreflex function was altered in both sham-clipped and 2K,1C rats. The most pronounced effect of SFO lesioning was on the response of RSNA to baroreceptor unloading. This finding is in agreement with studies indicating that hydralazine- or nitroprusside-induced hypotension or hemorrhage evokes a large number of c-fos-stained neurons in the SFO but little to no c-fos expression in the SFO during phenylephrine-induced hypertension (10, 15). Interestingly, ablation of the SFO resulted in not only a significant but also a diametrically opposite effect on the baroreflex responses in sham-clipped and 2K,1C rats. Specifically, in sham-clipped rats with an SFO lesion, the maximum RSNA response to baroreceptor unloading was greater than in animals with an intact SFO with a concurrent increase in the range of the baroreflex of RSNA. This would suggest that the SFO exerts a restraint on the sympathetic response to a hypotensive challenge in sham-clipped rats. In contrast, SFO ablation in 2K,1C rats resulted in a lowering of the upper plateau and decreased the range of the RSNA baroreflex curve. Thus, although the SFO clearly modulates baroreflex control of RSNA, the mechanisms involved in normotensive conditions may likely differ compared with those during hypertension associated with an activated renin-angiotensin system.
A change in plasma ANG II could have been one possible explanation for the apparent opposite effect of the SFO lesion on baroreflex in normotensive compared with 2K,1C hypertensive rats. As expected, plasma ANG II concentrations in the 2K,1C rats were at least threefold higher than in the sham-clipped rats. Importantly, lesioning the SFO did not change circulating plasma ANG II concentrations. Thus the qualitatively different response of the upper plateau of the baroreflex of RSNA cannot be ascribed to changes in circulating ANG II after SFO ablation. However, the present data do not exclude the possibility that the number or subtypes of angiotensin receptors within the SFO may differ or that local neurotransmitters may differ between 2K,1C and sham-clipped rats, as reported in SFO in spontaneously hypertensive rats compared with control normotensive Wistar-Kyoto rats (40). Furthermore, lesioning the SFO removes the influence of the SFO but leaves intact modulation of the baroreflex through other brain regions outside the blood-brain barrier, such as the area postrema, that are endowed with angiotensin receptors (41, 42). It may well be that under conditions where SFO signaling is removed, regulation of the baroreflex via area postrema may differ in normotensive and 2K,1C rats.
Likewise, the decrease in the range of the baroreflex response of HR after SFO ablation in the 2K,1C rats suggests that the SFO also modulates the baroreflex control of HR in this model. In contrast to RSNA, there was no effect of SFO lesioning in sham-clipped rats. The influence of the SFO on HR may be tempered by the fact that the heart receives parasympathetic as well as sympathetic inputs and/or that efferent sympathetic tone may differ between the heart and the kidney.
The observation that SFO ablation attenuated the maximum RSNA and HR response to lowering arterial pressure in 2K,1C hypertensive rats is reminiscent of the baroreflex responses of area postrema-lesioned, sodium-deprived rats (49). In that study, maximal reflex lumbar sympathetic nerve activity was reduced compared with sham-lesioned rats at low MAP levels, an effect that was attenuated by blockade of AT1 receptors. In both models, the renin-angiotensin system is activated. It seems likely therefore that elevated circulating ANG II acts tonically at both the SFO and area postrema to maintain sympathetic activity at low arterial pressures. Nevertheless, it is important to emphasize that lesioning the SFO eliminates not only AT1 receptors that are abundantly present in this nucleus but also other important receptors, such as endothelin or vasopressin, that may be differentially activated in the 2K,1C model. Further studies are warranted to identify these mechanisms.
Contrary to the enhanced sympathetic tone associated with 2K,1C hypertension (36), in the normotensive state other inputs from the SFO may tonically attenuate maximum RSNA. Lesioning the SFO would then remove these inhibitory inputs and lead to a greater increase in RSNA as arterial pressure declines. There is evidence for an inhibitory pathway emerging from the SFO to sympathoexcitatory neurons within the paraventricular nucleus in other species (38). Such a pathway has yet to be reported in the rat, but the present observations are consistent with its existence.
The relative contribution of other brain loci, neural pathways, and/or neurotransmitters to baroreflex control of RSNA and HR may differ in normotensive rats with low or normal endogenous angiotensins compared with hypertensive rats having an activated renin-angiotensin system. This has been shown for other brain nuclei in other models of hypertension (26, 31). In addition, the SFO in normal and hypertensive rats may differ in the densities of receptors for different neurotransmitters and also in the electrophysiological properties of SFO neurons projecting to the PVN (35). Finally, these processes are not necessarily exclusive of each other. Further studies are warranted to identify the pathways and mechanism(s) involved.
Perspectives and Significance
In summary, the present study provides evidence that electrolytic lesioning of the SFO significantly decreases baseline arterial pressure in 2K,1C rats, a model with an activated endogenous renin-angiotensin system. Furthermore, the SFO modulates arterial baroreflex control of sympathetic activity in both hypertensive 2K,1C and normotensive sham-clipped rats. The role of the SFO is most prominent during unloading of the baroreceptors. Importantly, the SFO differentially modulates the baroreflex response in normotensive sham-clipped rats compared with hypertensive 2K,1C rats. This effect is not a result of changes in circulating ANG II. Together, these results suggest that in the basal state, the SFO inhibits the baroreflex sympathoexcitatory response to hypotension, whereas when the renin-angiotensin system is activated as in 2K,1C rats, the role of the SFO is to maintain or even to augment the sympathetic response to hypotension. These findings support a pivotal role for the SFO in the sympathoexcitation that occurs in renovascular hypertension and in baroreflex regulation of sympathetic activity in both normal and selected hypertensive states.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-079102 and Department of Veterans Affairs Merit Award.
Acknowledgments
We thank Dr. James Hatfield for assistance with histological evaluation.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol Regul Integr Comp Physiol 268: R625–R633, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM, Malpas SC. What sets the long-term level of renal sympathetic nerve activity: a role for angiotensin II and baroreflexes? Circ Res 92: 1330–1336, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Braam B, Mitchell KD, Fox J, Navar G. Proximal tubular secretion of angiotensin II in rats. Am J Physiol Renal Fluid Electrolyte Physiol 264: F891–F898, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Brooks VL, Ell KR, Wright RW. Pressure-independent baroreflex resetting produced by chronic infusion of angiotensin II in rabbits. Am J Physiol Heart Circ Physiol 265: H1275–H1282, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Brown AJ, Casals-Stenzel J, Gifford J, Lever AF, Morten JJ. Comparison of fast and slow pressor effects of angiotensin II in conscious rat. Am J Physiol Heart Circ Physiol 241: H381–H388, 1981. [DOI] [PubMed] [Google Scholar]
- 6.Buggy J, Huot S, Pamnani M, Haddy F. Periventricular forebrain mechanisms for blood pressure regulation. Fed Proc 43: 25–31, 1984. [PubMed] [Google Scholar]
- 7.Ciriello J, Rosas-Arellano MP, Solano-Flores LP. Induction of c-fos in forebrain circumventricular organs after renal artery stenosis. Brain Res 995: 109–117, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Collister JP, Hendel MD. Role of the subfornical organ in the chronic hypotensive response to losartan in normal rats. Hypertension 41: 576–582, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Cowley JAW, Krieger JE. Role of fluid retention in angiotensin II salt-dependent hypertension. Acta Physiol Scand 139, Suppl 591: 100–106, 1990. [PubMed] [Google Scholar]
- 10.Dun NJ, Dun SL, Shen E, Tang H, Huang R, Chiu TH. c-fos expression as a marker of central cardiovascular neurons. Biol Signals 4: 117–123, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin Exp Pharmacol Physiol 24: 96–101, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Fink GD, Bruner CA, Mangiapane ML. Area postrema is critical for angiotensin-induced hypertension in rats. Hypertension 9: 355–361, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Fitts DA, Freece JA, Ban Bebber JE, Zierath DK, Bassett JE. Effects of forebrain circumventricular organ ablation on drinking or salt appetite after sodium depletion or hypernatremia. Am J Physiol Regul Integr Comp Physiol 287: R1325–R1335, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Gorbea-Oppliger VJ, Melaragno MG, Potter GS, Petit RL, Fink GD. Time course of losartan blockade of angiotensin II hypertension versus blockade of angiotensin II fast pressor effects. J Pharmacol Exp Ther 271: 804–810, 1994. [PubMed] [Google Scholar]
- 15.Graham JC, Hoffman GE, Sved AF. c-Fos expression in brain in response to hypotension and hypertension in conscious rats. J Auton Nerv Syst 55: 92–104, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Grassi F, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension 31: 68–72, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Griffin SA, Brown WCB, MacPherson F, McGrath JC, Wilson VG, Korsgaard N, Mulvany M, Lever AF. Angiotensin causes vascular hypertrophy in part by a non-pressor mechanism. Hypertension 17: 626–635, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Guo GB, Thames MD, Abboud FM. Arterial baroreflexes in renal hypertensive rabbits. Selectivity and redundancy of baroreceptor influence on heart rate, vascular resistance, and lumbar sympathetic nerve activity. Circ Res 53: 223–234, 1983. [DOI] [PubMed] [Google Scholar]
- 19.Gutman MB, Ciriello J, Mogenson GJ. Effect of plasma angiotensin II and hypernatremia on subfornical organ neurons. Am J Physiol Regul Integr Comp Physiol 254: R746–R754, 1988. [DOI] [PubMed] [Google Scholar]
- 20.Hall JE Control of sodium excretion by angiotensin II: intrarenal mechanism and blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 250: R960–R972, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Hartle DK, Brody MJ. The angiotensin pressor system of the rat forebrain. Circ Res 54: 355–366, 1984. [DOI] [PubMed] [Google Scholar]
- 22.Head GA Baroreflexes and cardiovascular regulation in hypertension. J Cardiovasc Pharmacol 26, Suppl 2: S7–S16, 1995. [PubMed] [Google Scholar]
- 23.Hendel MD, Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 288: H680–H685, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Jung JY, Lee JU, Kim WL. Enhanced activity of central adrenergic neurons in two-kidney one clip hypertension in Sprague-Dawley rats. Neurosci Lett 369: 14–18, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Kopp U, DiBona GF. Interaction between neural and non-neural mechanisms controlling renin secretion rate. Am J Physiol Renal Fluid Electrolyte Physiol 246: F620–F626, 1984. [DOI] [PubMed] [Google Scholar]
- 26.LaGrange LP, Toney GM, Bishop VS. Effect of intravenous angiotensin II infusion on responses to hypothalamic PVN injection of bicuculline. Hypertension 42: 1124–1129, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol 18: 383–439, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Maliszewska-Scislo M, Scislo T, Rossi NF. Effect of endogenous angiotensin on baroreflexes in conscious streptozotocin diabetic rats. Am J Physiol Heart Circ Physiol 284: H1601–H1611, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Mangiapane ML, Simpson JB. Subfornical organ: fore-brain site of pressor and drinking actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol 239: R382–R389, 1980. [DOI] [PubMed] [Google Scholar]
- 30.Mangiapane ML, Simpson JB. Subfornical organ lesions reduce the pressor effect of intravenous angiotensin. Neuroendocrinology 31: 380–384, 1980. [DOI] [PubMed] [Google Scholar]
- 31.Martin DS, Haywood JR. Reduced GABA inhibition of sympathetic function in renal-wrapped hypertensive rats. Am J Physiol Regul Integr Comp Physiol 275: R1523–R1529, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Matsukawa S, Reid IA. Role of the area postrema in the modulation of the baroreflex control of heart rate by angiotensin II. Circ Res 67: 1462–1473, 1990. [DOI] [PubMed] [Google Scholar]
- 33.McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL, Vivas L, Wade JD, Oldfield BJ. Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J Neuroendocrinol 16: 340–347, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Mendelsohn FA, Quiron R, Saavedra JM, Aguilera G, Catt KJ. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci USA 81: 1575–1579, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyakubo H, Hayashi Y, Tanaka J. Enhanced response of subfornical organ neurons projecting to the hypothalamic paraventricular nucleus to angiotensin II in spontaneously hypertensive rats. Auton Neurosci 95: 131–136, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Oparil S The sympathetic nervous system in clinical and experimental hypertension. Kidney Int 30: 437–452, 1986. [DOI] [PubMed] [Google Scholar]
- 37.Ortiz RM, Graciano ML, Seth D, Awayda MS, Navar G. Aldosterone receptor antagonism exacerbates intrarenal angiotensin II augmentation in ANG II-dependent hypertension. Am J Physiol Renal Physiol 293: F139–F147, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Osaka T, Yamashita H, Koizumi K. Inhibition of paraventricular neurons by subfornical organ and AV3V in cats. Am J Physiol Regul Integr Comp Physiol 255: R961–R967, 1988. [DOI] [PubMed] [Google Scholar]
- 38a.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed.). San Diego, CA: Academic, 1998.
- 39.Rossi NF Effect of endothelin-3 on vasopressin release in vitro and water excretion in vivo in Long Evans rats. J Physiol 461: 501–511, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saaveddra JM, Correa FM, Kuthara M, Shigematsu K. Increased number of angiotensin II receptors in the subfornical organ of spontaneously hypertensive rats. J Hypertens Suppl 4: S27–S30, 1986. [PubMed] [Google Scholar]
- 41.Sanderford MG, Bishop VS. Angiotensin II acutely attenuates range of arterial baroreflex control of renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol 279: H1804–H1812, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Sanderford M, Bishop VS. Central mechanisms of acute ANG II modulation of arterial baroreflex control of renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol 282: H1592–H1602, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Shioya M, Tanaka J. Inputs from the nucleus of the solitary tract to subfornical organ neurons projecting to the paraventricular nucleus in the rat. Brain Res 483: 192–195, 1989. [DOI] [PubMed] [Google Scholar]
- 45.Starbuck EM, Fitts DA. Influence of salt intake, ANG II synthesis and SFO lesion on thirst and blood pressure during sodium depletion. Appetite 31: 309–331, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka J, Kaba H, Saito H, Seto K. Efferent pathways from the region of the subfornical organ to hypothalamic paraventricular nucleus: an electrophysiological study in the rat. Exp Brain Res 62: 509–514, 1986. [DOI] [PubMed] [Google Scholar]
- 47.Thunhorst RL, Beltz TG, Johnson AK. Effects of subfornical organ lesions on acute thirst and salt appetite. Am J Physiol Regul Integr Comp Physiol 277: R56–R65, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Vitela M, Herrera-Rosales M, Haywood JR, Mifflin SW. Baroreflex regulation of renal sympathetic nerve activity and heart rate in renal wrap hypertensive rats. Am J Physiol Regul Integr Comp Physiol 288: R856–R862, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Xu L, Collister JP, Osborn JW, Brooks VL. Endogenous ANG II supports lumbar sympathetic activity in conscious sodium-deprived rats: role of area postrema. Am J Physiol Regul Integr Comp Physiol 275: R46–R55, 1998. [DOI] [PubMed] [Google Scholar]