Abstract
Adipose tissue expresses components of the renin-angiotensin system (RAS). Angiotensin converting enzyme (ACE2), a new component of the RAS, catabolizes the vasoconstrictor peptide ANG II to form the vasodilator angiotensin 1-7 [ANG-(1-7)]. We examined whether adipocytes express ACE2 and its regulation by manipulation of the RAS and by high-fat (HF) feeding. ACE2 mRNA expression increased (threefold) during differentiation of 3T3-L1 adipocytes and was not regulated by manipulation of the RAS. Male C57BL/6 mice were fed low- (LF) or high-fat (HF) diets for 1 wk or 4 mo. At 1 wk of HF feeding, adipose expression of angiotensinogen (twofold) and ACE2 (threefold) increased, but systemic angiotensin peptide concentrations and blood pressure were not altered. At 4 mo of HF feeding, adipose mRNA expression of angiotensinogen (twofold) and ACE2 (threefold) continued to be elevated, and liver angiotensinogen expression increased (twofold). However, adipose tissue from HF mice did not exhibit elevated ACE2 protein or activity. Increased expression of ADAM17, a protease responsible for ACE2 shedding, coincided with reductions in ACE2 activity in 3T3-L1 adipocytes, and an ADAM17 inhibitor decreased media ACE2 activity. Moreover, ADAM17 mRNA expression was increased in adipose tissue from 4-mo HF-fed mice, and plasma ACE2 activity increased. However, HF mice exhibited marked increases in plasma angiotensin peptide concentrations (LF: 2,141 ± 253; HF: 6,829 ± 1,075 pg/ml) and elevated blood pressure. These results demonstrate that adipocytes express ACE2 that is dysregulated in HF-fed mice with elevated blood pressure compared with LF controls.
Keywords: angiotensin I-converting enzyme, angiotensin
in 2000, a new component of the renin-angiotensin system (RAS) was described as a monocarboxypeptidase homolog of angiotensin I-converting enzyme (ACE), identified as ACE2 (9, 45). ACE2 exhibits catalytic activity for both ANG I and II; however, its catalytic efficiency for ANG II is ∼400-fold greater than ANG I (47). The product of ACE2 cleavage of ANG II is ANG-(1-7), a peptide of the RAS, which exhibits several effects to decrease blood pressure (14, 15). The ability of ACE2 to catabolize ANG II to ANG-(1-7) has been suggested as the counterbalancing arm of the RAS in blood pressure control, limiting the effects of ANG II and promoting effects of ANG-(1-7) (14). Supporting this hypothesis, recent studies demonstrated that deficiency of ACE2 on a C57BL/6 background results in a modest increase in blood pressure, and a markedly elevated response to acute and chronic ANG II (23).
The tissue distribution of ACE2 was originally thought to be restricted to kidney and heart; however, further studies demonstrated relatively widespread distribution of ACE2 mRNA and enzymatic activity in rodents (18). Interestingly, a comprehensive analysis of ACE2 mRNA and protein in tissues from rats and mice demonstrated ACE2 expression in adipose tissue (18). The physiological relevance of ACE2 expression in kidney, heart, and brain has been investigated, but the role of ACE2 in other tissues, including adipose tissue, is not as well understood. Regulatory mechanisms for ACE2 include differential glycosylation, shedding from the cell membrane (30, 45), and tissue-specific regulation through inhibition of ANG II synthesis or activity (12). Recent studies demonstrate that ADAM17, the metallopeptidase that cleaves TNF-α from cell membranes, can mediate shedding of ACE2 (30). However, the role of ADAM17-mediated ACE2 shedding in diseases associated with an activated RAS is unknown.
Adipocytes express components of the RAS, including angiotensinogen (4, 7), renin-like activity (44), ACE (42, 43), and angiotensin receptors (type 1 and type 2) (6, 35, 37). Interestingly, adipocyte expression of angiotensinogen has been demonstrated to exhibit nutritional regulation by fatty acids and by high-fat (HF) feeding (11, 20, 39). Moreover, angiotensinogen expression in adipose tissue, similar to the liver, is positively regulated by ANG II (34). In addition to components of the RAS, adipocytes express the ACE2-shedding metallopeptidase ADAM17, which has been suggested to contribute to enhanced release of TNF-α from adipocytes with obesity (25, 26). In this study, we investigated whether adipocytes express ACE2 and defined mechanisms for regulation of adipose ACE2 by manipulation of the RAS, or nutritionally by HF feeding.
MATERIALS AND METHODS
Animals.
Male, C57BL/6 mice (2 mo of age; The Jackson Laboratories, Bar Harbor, MA) were housed in a temperature-controlled room with a 12:12-h light-dark cycle. Male, AT1a receptor (AT1aR) −/− mice (5 mo of age; n = 10/group) on a C57BL/6 background and C57BL/6 wild-type controls were obtained from The Jackson Laboratories. ACE2-/y mice (on a C57BL/6 background) (23) were obtained from Dr. Thomas Coffman, Duke University. Mice were given free access to food and water. All experiments involving mice conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Diet-induced obesity.
Male C57BL/6 mice (2 mo of age) were fed a LF (10% kcal as fat; D12450B; Research Diets, New Brunswick, NJ) or a HF diet (60% kcal as fat; D12492; Research Diets) for 1 wk or 4 mo. Diets were matched in protein content (20% kcal), had a similar source of dietary fat (lard), and provided energy at 3.85 or 5.25 kcal/g (LF and HF, respectively). Diets were provided to mice ad libitum, and body weight was recorded weekly.
Measurement of blood pressure.
Systolic blood pressure was measured by tail cuff using the Visitech 2000 system for mice fed the LF or HF diet for 1 wk. Measurements were obtained 4 days/wk beginning 1 wk prior to initiation of the diet and through day 7 (24). Criteria for inclusion of measurements from individual mice were 5 out of 10 successful measurements with a standard deviation <50. For mice fed the LF or HF diets for 4 mo, radiotelemetry was used to measure blood pressure. At month 4, mice were anesthetized (isoflurane), the left carotid artery was isolated, and the telemeter catheter was inserted into the artery and advanced to reach the aortic arch. The telemetry implants (model TA11PA-C10, Data Sciences International, St. Paul, MN) were placed in a subcutaneous pocket on the right flank. Mice were allowed to recover for 1 wk before recordings began (3 consecutive days at 24 h/day). The telemeter signal was processed using a model RPC-1 receiver, a 20-channel data-exchange matrix, APR-1 ambient pressure monitor, and Dataquest ART 2.3 acquisition system (Data Sciences International). The system was programmed to acquire data for 10 s every minute and to calculate 10-min averages of the mean systolic and diastolic blood pressure.
3T3-L1 adipocytes.
3T3-L1 cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in DMEM containing 10% FBS. Cells were grown to confluence, and then differentiation was induced with a cocktail of insulin (0.1 μM, Sigma, St. Louis, MO), dexamethasone (1 μM; Sigma), and isobutyl methyl xanthine (0.5 mM; Sigma). Cells were collected for mRNA analysis every day beginning 1 day prior to addition of cocktail through day 10.
For studies using inhibitors of the RAS, losartan (1 μM), PD123319 (1 μM), ANG-(1-7) (1 μM), ANG II (1 μM), or combinations thereof were added to the media on day 8, and cells were harvested 24 h later (n = 3). As a positive control, cells (day 8) were incubated with rosiglitazone (Rosi; 1 μM) for 24 h. In separate experiments (n = 3), 3T3-L1 adipocytes (day 10) were incubated with vehicle or GM6001 (10 μM) for 1 h prior to measurement of ACE2 activity in media.
mRNA quantification.
Total RNA was extracted from tissues (epididymal fat, EF; subcutaneous fat, SubQ; retroperitoneal fat, RPF; interscapular brown adipose tissue, BAT; kidney, heart, liver, testes) using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA (0.4 μg) was reverse transcribed for 1 h at 55°C with the following components: random decamers, 10× reverse transcription buffer, deoxynucleotide triphosphate mix, ribonuclease inhibitor, and reverse transcriptase (RETROscript; Ambion, Austin, TX). Relative quantification of gene expression was performed with an iCycler (Bio-Rad, Hercules, CA) using the SYBR Green PCR core reagent (Applied Biosystems, Foster City, CA). The reaction mix consisted of SYBR Green mix (1×), MgCl2 (3 mM), dNTP mix (1.25 mM), fluorescein (0.01 μM), primers (0.5 μM), and AmpliTaq gold (2.5 units). The real-time PCR conditions were 5 min at 94°C, 40 cycles for 1 min at 94°C, 1 min at the annealing temperature, 1 min at 72°C, and a final elongation step for 10 min at 72°C. 18S rRNA was used as the endogenous control for normalization. Primer sequences are depicted in Supplemental Table 1 (see the online version of this article).
The abundance of each mRNA transcript was measured using a standard curve method. Briefly, cDNA (10−7 to 10−3 starting concentration, total of five concentrations) from a tissue source known to express each gene of interest was amplified with unknowns. Software on MyiQ Single-Color Real Time PCR Detection System (Bio-Rad) plotted the Ct value for each DNA standard against the starting quantity (SQ) of cDNA (R2 = 0.98 − 1.00), and extrapolated unknowns from the standard curve. cDNA template (SQ) for each gene was normalized to 18S RNA (SQ obtained using the standard curve method) to control for starting amount of DNA, and data are expressed as the ratio of gene/18S RNA.
Measurement of ACE2 enzymatic activity.
ACE2 activity was quantified in 3T3-L1 adipocytes, tissues, and plasma by determining the conversion of [125I]ANG II to [125I]ANG-(1-7) (13). Tissues were homogenized in a Tris buffer (100 mM) containing NaCl (0.3 M), ZnCl2 (10 μM), and Z pro-prolinal (10 μM). Following centrifugation (30,000 g for 20 min, 4°C), pellets were reconstituted in the above buffer containing 0.5% triton-X and incubated overnight at 4°C. Samples were centrifuged, and the supernatant was used for measurement of ACE2 enzyme activity. Membrane (0.05–0.2 mg protein) was added to tubes with buffer (Tris, 100 mM; total volume 250 μl) containing the following inhibitors: thiorphan (0.1 mM), phoshoramidon (0.1 mM), bestatin (100 μM), pepstatin (100 μM), and captopril (10 μM). Initial experiments optimized the ACE2 activity assay for membrane protein, inhibitor cocktail, substrate concentration, and validated loss of ACE2 activity in adipose membranes from ACE2-/y mice (see Supplemental Fig. 1 in the online version of this article) (23). [125I]ANG II (specific activity 2,200 Ci/mmol; 2 × 106 cpm equivalent to 414 fmol) was incubated with samples for 1–30 min, and the reaction was stopped by the addition of 1% phosphoric acid. Samples were centrifuged, filtered, and injected onto a Beckman HPLC system for resolution of [125I]ANG II from [125I]ANG (1-7). Reverse-phase HPLC was used to resolve angiotensins with a linear gradient varying from 15% to 33% acetonitrile (0.5 ml/min). The mobile phase was 25 mM NaPO4, with a retention time of 6.6 min for [125I]ANG (1-7) and 13.6 min for [125I]ANG II (Supplemental Fig. 1). HPLC fractions (1 min) were collected, and radioactivity was quantified by gamma counting. ACE2 activity is expressed as femtomoles per milligram protein per minute, based on the specific activity of [125I]ANG II (2,175 Ci/mmol). Protein was measured using the BCA Assay (Pierce Chemicals, Rockford, IL).
Measurement of ACE2 protein.
Tissues were homogenized on ice in M-PER reagent (Pierce), sonicated (2 min), and lysates were pelleted by centrifugation. Protein (25 μg) from adipose tissue (EF) was electrophoresed on a 7.5% SDS-PAGE gel under reducing conditions. Proteins were blotted onto a PVDF membrane (GE Healthcare, Piscataway, NJ), blocked overnight (5% nonfat milk, 4°C) and incubated with anti-goat ACE2 antibody (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h (22°C). Goat IgG-conjugated horseradish peroxidase antibody (1:5,000 dilution, Santa Cruz Biotechnology) was used for chemiluminescent detection. Blots were stripped and reprobed with anti-mouse β-actin antibody (1:1,000 dilution, clone AC-15; Sigma) for normalization of protein loading. Controls included incubation with a blocking peptide for ACE2 (1:1 peptide/primary antibody; SC-21834; Santa Cruz Biotechnology), which eliminated all immunoreactivity from ACE2 primary antibody. Images were collected on a Kodak Image Station 440CF and analyzed using Kodak 1D analysis system software (ver. 3.6.4, Kodak, New Haven, CT).
Measurement of angiotensin peptides.
Angiotensin peptides were measured in mouse plasma (150 μl) using reverse-phase HPLC followed by radioimmunoassay as previously described (8).
Statistical analysis.
Data are expressed as means ± SE. Data were tested for normality and equal variance. For in vitro studies examining mRNA expression during adipocyte differentiation and effects of angiotensin receptor antagonists, data were analyzed by one-way ANOVA. For in vivo studies, data were analyzed by two-way ANOVA, with time of diet feeding and diet as between-group factors. For post hoc analysis, data were analyzed using Tukey's test, with significance at P < 0.05.
RESULTS
Expression and regulation of ACE2 in 3T3-L1 murine adipocytes and in mouse adipose tissue.
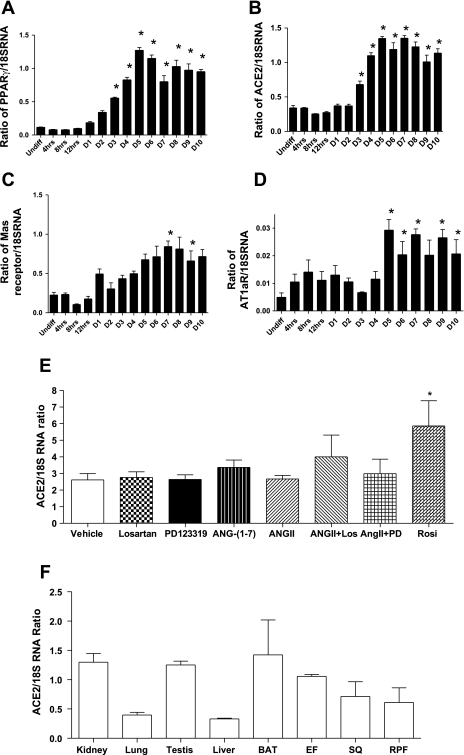
ACE2 mRNA expression in differentiating murine 3T3-L1 adipocytes was contrasted to other components of the RAS and to peroxisome proliferator-activated receptor γ (PPARγ) as an index of adipocyte differentiation (Fig. 1). PPARγ mRNA abundance increased beginning on day 3 compared with undifferentiated (Undiff) preadipocytes (Fig. 1A, P < 0.001). ACE2 mRNA expression also increased on day 3 and remained elevated (Fig. 1B, P < 0.001). By comparison, expression of mas and AT1a receptors increased at later stages (days 5 to 8) of adipocyte differentiation (Fig. 1, C and D; P < 0.05). Manipulation of the RAS using an AT1 receptor antagonist (losartan), AT2 receptor antagonist (PD123319), ANG-(1-7), ANG II, or combinations thereof, did not alter ACE2 expression in 3T3-L1 adipocytes (Fig. 1E). In contrast, incubation with rosiglitazone, a PPARγ agonist, increased ACE2 mRNA expression in 3T3-L1 adipocytes.
Fig. 1.
Relative expression of peroxisome proliferator-activated receptor y (PPARγ) (A), angiotensin I-converting enzyme 2 (ACE2) (B), mas receptor (C) and AT1a receptor (D) in differentiating 3T3-L1 adipocytes. *Significantly different from Undiff, P < 0.05. E: effect of ANG II, losartan, PD1233319, ANG-(1-7) or combinations thereof on ACE2 mRNA expression in differentiating 3T3-L1 adipocytes. Rosiglitazone (Rosi) was included as a positive control to increase ACE2 mRNA expression (P < 0.05). F: relative expression of ACE2 mRNA in tissues from C57BL/6 male mice. Data are expressed as means ± SE from n = 3 experiments.
ACE2 mRNA expression in adipose tissue from C57BL/6 mice was contrasted to expression levels in other tissues and was comparable to kidney and testis, but greater than levels in lung and liver (Fig. 1F). Similar to findings in 3T3-L1 adipocytes, ACE2 mRNA expression in EF was not significantly different between age-matched male AT1a receptor−/− and C57BL/6 controls (controls, 0.30 ± 0.04; −/−, 0.19 ± 0.05 ACE2/18SRNA ratio; P = 0.126).
The temporal effect of HF feeding on adipose ACE2 mRNA expression and enzymatic activity.
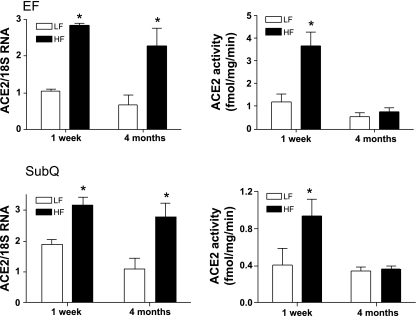
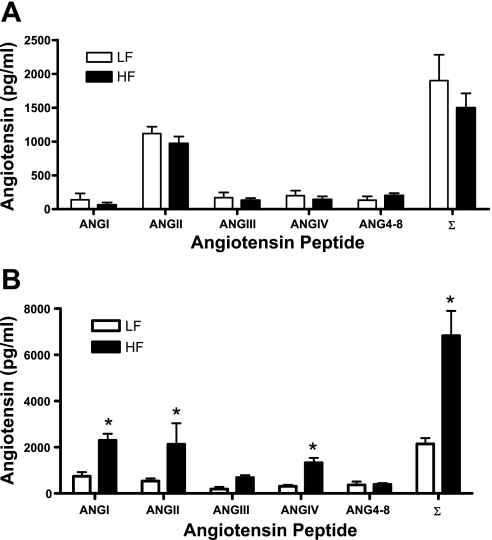
We determined whether ACE2 was nutritionally regulated by short-term and chronic HF feeding. Body weight and EF mass were modestly increased in HF mice at 1 wk (Table 1, P < 0.05 compared with LF). Blood glucose concentrations were increased at 1 wk of HF feeding (Table 1, P < 0.05 compared with LF). At 1 wk of HF feeding, expression of angiotensinogen was increased in adipose tissue, but not liver, of HF-fed mice (Table 1). ACE2 mRNA expression (Fig. 2), activity (Fig. 2) and protein (see Supplemental Fig. 2 posted to the online version of this article) were increased in adipose tissue (EF, SubQ) of HF compared with LF-fed mice. In contrast, ACE2 mRNA expression in heart (LF, 0.97 ± 0.07; HF, 1.17 ± 0.19 ACE2/18S RNA ratio; P > 0.05), kidney (LF, 1.48 ± 0.36; HF, 1.30 ± 0.36; P > 0.05), liver (LF, 0.33 ± 0.01; HF, 0.27 ± 0.05; P > 0.05) or BAT (LF, 3.00 ± 1.6; HF, 3.24 ± 0.53; P > 0.05) were not influenced by 1 wk of HF feeding. Plasma ACE2 activity was modestly, but not significantly, increased by 1 wk of HF feeding (LF, 0.004 ± 0.001; HF, 0.009 ± 0.001 fmol·ml−1·min−1; P = 0.123). Moreover, plasma concentrations of angiotensin peptides (Fig. 3) and systolic blood pressure (LF, 120 ± 5; HF, 120 ± 4 mmHg; P = 0.294) were not altered by 1 wk of HF feeding.
Table 1.
Characteristics of LF- and HF-fed C57BL/6 mice
| Parameter |
LF |
HF
|
||
|---|---|---|---|---|
| 1 wk | 4 mo | 1 wk | 4 mo | |
| Body weight, g | 25.2±0.9 | 29.9±0.3 | 27.8±0.5* | 48.0±1.6* |
| Blood glucose, mg/dl | 130±8 | 132±5 | 155±8* | 161±7* |
| EF, g | 0.43±0.03 | 0.62±0.1 | 0.74±0.06* | 2.19±0.15* |
| Liver angiotensinogen mRNA | 0.85±0.33 | 0.84±0.09 | 0.90±0.24 | 1.62±0.14† |
| EF angiotensinogen mRNA | 0.11±0.01 | 0.25±0.01* | 0.42±0.08*† | 0.58±0.01*† |
EF, epididymal fat.
P < 0.05 compared to LF within time point.
P < 0.05 compared to 1 wk within diet group.
Fig. 2.
The effect of 1 wk and 4 mo of high fat (HF) feeding on ACE2 mRNA expression (left) and activity (right) in epididymal (EF; top) and subcutaneous (SubQ; bottom) adipose tissues. Top, left: ACE2 mRNA expression in EF increased at 1 wk and 4 mo of HF feeding compared with low fat (LF). Top, right: ACE2 activity in epididymal fat (EF) increased at 1 wk, but not at 4 mo of HF feeding compared with LF. Bottom, left: ACE2 mRNA in SubQ adipose tissue increased at 1 wk and 4 mo of HF feeding compared with LF. Bottom, right: ACE2 activity in SubQ adipose tissue was increased at 1 wk, but not at 4 mo of HF feeding compared with LF. Data are expressed as means ± SE from n = 10 mice/time point/diet. *Significantly different from LF, P < 0.05.
Fig. 3.
Plasma angiotensin peptide concentrations in 1 wk (A) and 4 mo (B) LF and HF-fed mice. Plasma concentrations of ANG I, ANG II, ANG III, ANG IV, ANG 4-8, and the sum of all peptides (∑). Data are means ± SE from n = 10 mice/time point/diet. *Significantly different from LF, P < 0.05.
At 4 mo of HF feeding, body weight and adipose mass were markedly increased compared with LF (Table 1, P < 0.05). Moreover, blood glucose concentrations were increased in HF-fed mice (Table 1, P < 0.05 compared with LF). Expression of angiotensinogen in adipose tissue (EF) increased with age in both LF and HF-fed mice (Table 1, P < 0.05, 1 wk compared with 4 mo within diet group). Moreover, liver and adipose angiotensinogen mRNA expression was increased in 4 mo HF compared with LF mice (Table 1, P < 0.05). In adipose tissues, ACE2 mRNA expression remained elevated in HF compared with LF-fed mice (Fig. 2, P < 0.05). Moreover, ACE2 mRNA expression was significantly increased in kidney (LF, 2.58 ± 0.21; HF, 3.84 ± 0.29 ACE2/18S RNA ratio; P < 0.05) and liver (LF, 1.16 ± 0.11; HF, 1.55 ± 0.06; P < 0.05), but not in heart (LF, 2.88 ± 0.40; HF, 3.26 ± 0.31; P > 0.05) from 4 mo HF compared with LF mice. Elevations in ACE2 mRNA expression with age resulted in increased ACE2 activity in kidney of both LF- and HF-fed mice (1 wk: LF, 9.5 ± 0.2; HF, 9.9 ± 0.2; 4 mo: LF, 17.7 ± 3; HF, 18.7 ± 2.2 fmol·mg−1·min−1). However, ACE2 activity in heart was not influenced by HF feeding for 4 mo (data not shown). Surprisingly, elevations in ACE2 mRNA expression did not result in an increase in enzymatic activity (Fig. 2) or protein abundance (Supplemental Fig. 2) in adipose tissue from 4-mo HF-fed mice.
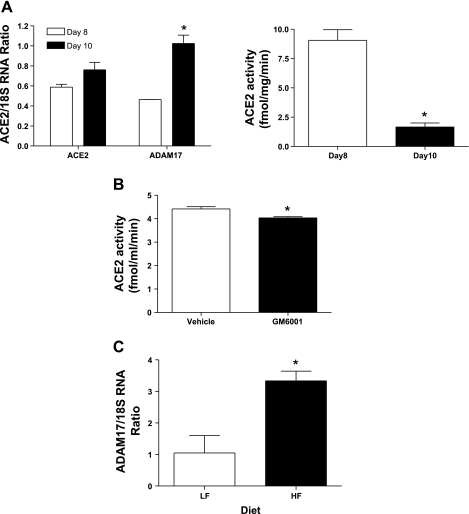
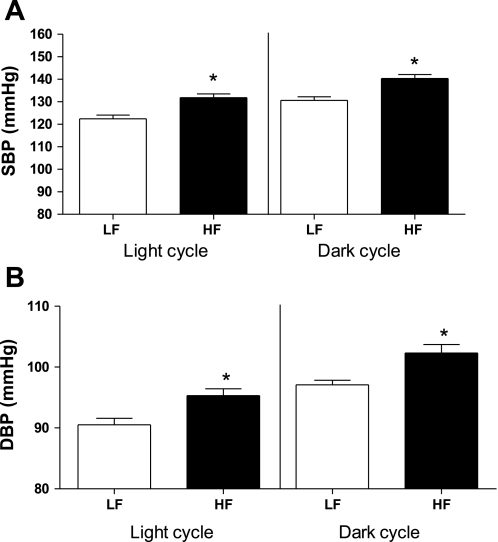
Because ADAM17 has been demonstrated to shed ACE2 from cell membranes (30), we contrasted ACE2 and ADAM17 mRNA expression and ACE2 enzymatic activity in 3T3-L1 adipocytes at day 8 and 10 of differentiation (Fig. 4). ACE2 mRNA expression was not different between day 8 and 10, but ADAM17 mRNA expression increased over this same time course (Fig. 4A) and was associated with reductions in membrane ACE2 activity. ACE2 activity was also detected in the media of 3T3-L1 adipocytes, and levels were reduced by an ADAM17 inhibitor (Fig. 4B). Adipose tissue from 4 mo HF-fed mice exhibited an increase in ADAM17 mRNA expression (Fig. 4C; P < 0.05), and plasma ACE2 activity increased (LF, 1.8 ± 0.3; HF, 3.1 ± 0.1 fmol·mg−1·min−1, P < 0.05). However, plasma concentrations of ANG II, ANG IV, and ANG I were greater in HF compared with LF mice (Fig. 3). Moreover, systolic and diastolic pressures were significantly increased during both the light and dark cycle of 4-mo HF-fed compared with LF-fed mice (Fig. 5, P < 0.05), contributing to an elevation in mean arterial pressure (24-h data: LF, 127 ± 1; HF, 136 ± 2 mmHg; P < 0.05).
Fig. 4.
ACE2/ADAM17 mRNA expression in 3T3-L1 adipocytes (A), effect of ADAM17 inhibition on ACE2 activity (B), and ADAM17 mRNA expression in adipose tissue from 4-mo HF-fed mice (C). A, left: comparison of ACE2 and ADAM17 mRNA expression in 3T3-L1 adipocytes on days 8 and 10 of differentiation (n = 3). Right: ACE2 activity decreased from day 8 to 10 of differentiation. B: GM6001, an ADAM17 inhibitor, decreased ACE2 activity in media (n = 3). C: ADAM17 mRNA expression increased in EF from HF compared with LF mice. Data are expressed as means ± SE from n = 5 mice/group. *Significantly different from day 8 (A), from vehicle (B) or from LF (C); P < 0.05.
Fig. 5.
Systolic (A) and diastolic (B) blood pressure of 4 mo LF and HF-fed mice. Blood pressure was measured using radiotelemetry during the light and dark cycle in the final week of month 4. Systolic (SBP) and diastolic (DBP) blood pressure increased in 4 mo HF compared with LF mice. Data are expressed as means ± SE from n = 5 mice/group. *Significantly different from LF, P < 0.05.
DISCUSSION
This study examined whether adipocytes express ACE2 and its regulation by manipulation of the RAS or by HF feeding. Results demonstrate expression of ACE2 mRNA, protein, and enzymatic activity in 3T3-L1 adipocytes and in mouse adipose tissue, and regulation of adipose ACE2 during HF feeding, but not by manipulation of the RAS. With short-term HF feeding of C57BL/6 mice, ACE2 mRNA expression and enzymatic activity were stimulated in adipose tissue, and blood pressure was not altered. With chronic HF feeding, ACE2 mRNA expression in adipose tissue continued to be elevated, but protein and enzymatic activity did not increase. Expression of ADAM17, a protease that can shed ACE2 from membranes, increased with differentiation of 3T3-L1 adipocytes and in adipose tissues from chronic HF-fed mice. With chronic HF feeding, expression of angiotensinogen increased in both adipose tissue and liver, plasma concentrations of angiotensin peptides were markedly elevated, and HF-fed mice exhibited higher blood pressure compared with LF-fed controls. These results demonstrate that adipocytes express ACE2 and that ACE2 is nutritionally regulated by HF feeding.
The monocarboxypeptidase ACE2 was identified by 5′ sequencing of a human heart ventricle or lymphoma cDNA library (9, 45). Expression of ACE2 was originally suggested to be restricted to human heart, kidney, and testis (9); however, recent studies have demonstrated more widespread distribution of ACE2 (18). In a comparison of tissues from mice and rats, Gembardt et al. (18) reported ACE2 expression in adipose tissue, and recent studies extend ACE2 expression to both brown and white adipose tissue from rats (17). Similarly, ACE2 mRNA was detected in human adipose tissue, with greater ACE2 expression in visceral compared with subcutaneous adipose tissue (32, 48). However, the relative expression of ACE2 in adipocyte vs. nonadipocyte fractions of adipose tissue has not been defined. 3T3-L1 cells are a fibroblast-like cell line from the Swiss mouse embryo that differentiate to mature white adipocytes upon exposure to a differentiating cocktail (22). This system has been used previously to define adipocyte expression of RAS components, including angiotensinogen (41) and AT1 receptors (35). Our results confirm previous findings of AT1 receptor expression in differentiating 3T3-L1 adipocytes (35) and extend these findings by demonstrating that mRNA expression of ACE2 and the mas receptor increases upon differentiation of preadipocyte to adipocyte (25, 26).
Previous investigators have demonstrated tissue-specific regulation of ACE2 by ACE inhibitors or AT1 receptor antagonists (12, 27). In heart, administration of an ACE inhibitor or an AT1 receptor antagonist increased cardiac ACE2 mRNA and activity (12). While administration of an AT1 receptor antagonist regulated ACE2 mRNA in aorta, ACE2 expression was not altered in carotid arteries from spontaenously hypertensive rats (27). Our results do not support a role for ANG II in the regulation of adipose ACE2. Moreover, ANG-(1-7) did not influence adipocyte ACE2 expression despite expression of the mas receptor on adipocytes. The functional relevance of mas receptor expression on adipocytes was not defined in the current study. Interestingly, recent studies demonstrate that mas receptor-deficient mice exhibit a phenotype characteristic of the metabolic syndrome, with increased abdominal adipose mass, dyslipidemia, hyperinsulinemia, and leptinemia, and glucose intolerance (40). Moreover, in agreement with our findings in 3T3-L1 adipocytes, mas receptor expression was detected in mouse adipose tissue (40). Our results extend recent findings by demonstrating that mas receptors in 3T3-L1 adipocytes do not regulate ACE2 expression.
Altered expression of RAS components in adipose tissue has been linked to obesity-related hypertension in experimental models and humans (2, 5, 6, 11, 19, 36, 38, 46). In rats with diet-induced obesity and hypertension, expression of angiotensinogen in visceral adipose tissue increased and was associated with elevated concentrations of angiotensin peptides (2). Administration of an AT1 receptor antagonist to rats with obesity-hypertension decreased blood pressure (3). Similarly, in mice overexpressing human angiotensinogen under the control of its own promoter, a HF diet resulted in an increase in angiotensinogen expression in visceral adipose tissue (38). However, blood pressure was not examined. In human obesity-hypertension, plasma concentrations of angiotensinogen, renin, aldosterone, and ACE were increased compared with lean controls, and weight loss resulted in reductions in these RAS components (11). Our results extend previous findings by demonstrating that while short-term HF feeding does not activate the systemic RAS, chronic HF-feeding is associated with a stimulated RAS (angiotensinogen and ANG II) at a time when blood pressure is increased.
Since other RAS components in adipocytes exhibit nutritional regulation (1, 10, 16, 28, 31, 39), we examined effects of HF feeding on adipose ACE2 expression. Results from this study are the first to demonstrate that short-term HF feeding increases ACE2 expression and enzymatic activity in adipose tissue. Potential mechanisms contributing to this effect include diet-induced elevations in fatty acid concentrations. Fatty acids have been reported to exhibit several effects on gene expression, potentially related to activation of PPARγ (21, 29). Interestingly, adipose tissue, with a high abundance of PPARγ expression, exhibited robust increases in ACE2 mRNA expression with HF feeding. Moreover, while manipulation of the RAS had no effect on adipocyte ACE2 mRNA expression, stimulation of PPARγ with a thiazolidinedione increased ACE2 gene expression in 3T3-L1 adipocytes. Future studies should address mechanisms for fatty acid and/or PPARγ-induced regulation of ACE2 mRNA expression in adipocytes.
The human ACE2 protein is a type I integral membrane glycoprotein (9, 45), but the enzyme can be shed from the cell surface through proteolytic cleavage of its extracellular domain by tumor necrosis factor-α convertase (ADAM17) (30). Mechanisms for regulation of ACE2 at the mRNA or protein level have not been well described; however, differential glycosylation of the enzyme and shedding from the cell surface have been suggested to regulate cellular ACE2 activity (30, 45). Shedding of ACE2 may impact the RAS, as well as the infectivity of the SARS coronavirus since ACE2 serves as the virus receptor (33). Moreover, phorbol ester-induced shedding of ACE2 in HEK293 cells overexpressing the enzyme was suggested to result in the release of a soluble form of catalytically active enzyme (30). Our results demonstrate that ACE2 activity can be detected in media from adipocytes and activity manipulated by an ADAM17 inhibitor. These results suggest that elevated ADAM17 mRNA expression in adipose tissue from chronic HF-fed mice may contribute to shedding of ACE2 from adipocyte membranes. These findings are in agreement with previous results demonstrating that adipocyte hypertrophy with obesity results in elevated expression and release of another adipocytokine shed by ADAM17, TNF-α (25, 26). Further studies are required to determine whether ADAM17 mediates enhanced shedding of ACE2 from adipocytes with chronic HF feeding, and the implication of these findings on the systemic RAS.
Perspectives and Significance
Results from this study demonstrate that ACE2 is expressed in adipose tissue and nutritionally regulated by HF feeding. Adipose tissue has been demonstrated to possess a local RAS. Our results extend the adipocyte RAS to ACE2 and demonstrate novel mechanisms for regulation of ACE2 by HF feeding. During short-term HF feeding, ACE2 expression in adipose tissue increased; however, chronic HF feeding, while continuing to promote ACE2 mRNA expression, did not increase ACE2 protein or enzymatic activity. Interestingly, elevated ADAM17-mediated shedding of TNF-α has been implicated as a link between obesity and type 2 diabetes. Similarly, our results suggest that shedding of ACE2 from adipocyte cell membranes may impact local ANG II concentrations and thereby contribute to obesity-associated diseases. Importantly, the identification that ACE2 can be shed from adipocytes may serve as a mechanism for discrepancies between ACE2 mRNA expression and protein abundance. An important finding of this study is that despite HF-induced elevations in ACE2 mRNA, the systemic RAS is activated with chronic HF feeding. Thus, either ACE2 concentrations systemically are not sufficient to blunt activation of the RAS, or elevations in synthesis of ANG II overwhelm effects of ACE2. Future studies should define the role of dysregulated ACE2 with HF feeding in obesity-hypertension through the use of ACE2-deficient mice.
GRANTS
This work was supported by the National Institutes of Health Grant R01 HL-73085 to L. A. Cassis.
Supplementary Material
Acknowledgments
We acknowledge the technical assistance of Vicki English for measurement of angiotensin peptide concentrations.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bertile F, Raclot T. Differences in mRNA expression of adipocyte-derived factors in response to fasting, refeeding and leptin. Biochim Biophys Acta 1683: 101–109, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol 287: R943–R949, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Boustany CM, Brown DR, Randall DC, Cassis LA. AT1-receptor antagonism reverses the blood pressure elevation associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 289: R181–R186, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Campbell DJ, Habener DF. Cellular localization of angiotensinogen gene expression in brown adipose tissue and mesentery: quantification of messenger ribonucleic acid abundance using hybridization in situ. Endocrinology 121: 1616–1626, 1987. [DOI] [PubMed] [Google Scholar]
- 5.Cassis LA Angiotensin II in brown adipose tissue from young and adult Zucker obese and lean rats. Am J Physiol Endocrinol Metab 266: E453–E458, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Cassis LA, Fettinger MJ, Roe AL, Shenoy UR, Howard G. Characterization and regulation of angiotensin II receptors in rat adipose tissue. Angiotensin receptors in adipose tissue. Adv Exp Med Biol 396: 39–47, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Cassis LA, Saye J, Peach MJ. Location and regulation of rat angiotensinogen messenger RNA. Hypertension 11: 591–596, 1988. [DOI] [PubMed] [Google Scholar]
- 8.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation 110: 3849–3857, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 87: E1–E9, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Einstein FH, Atzmon G, Yang XM, Ma XH, Rincon M, Rudin E, Muzumdar R, Barzilai N. Differential responses of visceral and subcutaneous fat depots to nutrients. Diabetes 54: 672–678, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension 45: 356–362, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111: 2605–10, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Smith D R, Chappell MC. Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int 68: 2189–2196, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol 289: H2281–H2290, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira AJ, Jacoby BA, Araujo CA, Macedo FA, Silva GA, Almeida AP, Caliari MV, Santos RA. The nonpeptide angiotensin-(1-7) receptor Mas agonist AVE-0991 attenuates heart failure induced by myocardial infarction. Am J Physiol Heart Circ Physiol 292: H1113–H1119, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Frederich RC, Kahn BB, Peach MJ, Flier JS. Tissue-specific nutritional regulation of angiotensinogen in adipose tissue. Hypertension 19: 339–344, 1992. [DOI] [PubMed] [Google Scholar]
- 17.Galvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, Ruiz-Gayo M, Huber M, Wehland M, Kreutz R, Fernandez-Alfonso MS. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol 197: 55–64, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss HP, Siems WE, Walther T. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides 26: 1270–1277, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giacchetti G, Faloia E, Sardu C, Camilloni MA, Mariniello B, Gatti C, Garrapa GG, Guerrieri M, Mantero F. Gene expression of angiotensinogen in adipose tissue of obese patients. Int J Obes Relat Metab Disord 24 Suppl 2: S142–S143, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Gorzelniak K, Engeli S, Janke J, Luft FC, Sharma AM. Hormonal regulation of the human adipose-tissue renin-angiotensin system: relationship to obesity and hypertension. J Hypertens 20: 965–973, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Gottlicher M, Demoz A, Svensson D, Tollet P, Berge RK, Gustafsson JA. Structural and metabolic requirements for activators of the peroxisome proliferator-activated receptor. Biochem Pharmacol 46: 2177–2184, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5: 19–27, 1975. [DOI] [PubMed] [Google Scholar]
- 23.Gurley SB, Allred A, Le TH, Griffiths R, Mao L, Philip N, Haystead TA, Donoghue M, Breitbart RE, Acton SL, Rockman HA, Coffman TM. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest 116: 2218–2225, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henriques TA, Huang J, D'Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology 145: 3866–3872, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95: 2409–2415, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM. Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1-7) expression in the aorta of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 289: H1013–H1019, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Jones BH, Standridge MK, Taylor JW, Moustaid N. Angiotensinogen gene expression in adipose tissue: analysis of obese models and hormonal and nutritional control. Am J Physiol Regul Integr Comp Physiol 273: R236–R242, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA 94: 4318–4323, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 280: 30113–30119, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Lay S, Krief S, Farnier C, Lefrere I, Le Liepvre X, Bazin R, Ferre P, Dugail I. Cholesterol, a cell size-dependent signal that regulates glucose metabolism and gene expression in adipocytes. J Biol Chem 276: 16904–16910, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Gao J, Xu YP, Zhou TL, Jin YY, Lou JN. [Expression of severe acute respiratory syndrome coronavirus receptors, ACE2 and CD209L in different organ-derived microvascular endothelial cells]. Zhonghua Yi Xue Za Zhi 87: 833–837, 2007. [PubMed] [Google Scholar]
- 33.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu H, Boustany-Kari CM, Daugherty A, Cassis LA. Angiotensin II increases adipose angiotensinogen expression. Am J Physiol Endocrinol Metab 292: E1280–E1287, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Mallow H, Trindl A, Loffler G. Production of angiotensin II receptors type one (AT1) and type two (AT2) during the differentiation of 3T3-L1 preadipocytes. Horm Metab Res 32: 500–503, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J 15: 2727–2729, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Matsushita K, Wu Y, Okamoto Y, Pratt RE, Dzau VJ. Local renin angiotensin expression regulates human mesenchymal stem cell differentiation to adipocytes. Hypertension 48: 1095–1102, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Rahmouni K, Mark AL, Haynes WG, Sigmund CD. Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. Am J Physiol Endocrinol Metab 286: E891–E895, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Safonova I, Aubert J, Negrel R, Ailhaud G. Regulation by fatty acids of angiotensinogen gene expression in preadipose cells. Biochem J 322: 235–239, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos SH, Fernandes LR, Mario EG, Ferreira AV, Porto LC, Alvarez-Leite JI, Botion LM L, Bader M, Alenina N, Santos RA. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes 57: 340–347, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Saye JA, Cassis LA, Sturgill TW, Lynch KR, Peach MJ. Angiotensinogen gene expression in 3T3-L1 cells. Am J Physiol Cell Physiol 256: C448–C451, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Saye JA, Ragsdale NV, Carey RM, Peach MJ. Localization of angiotensin peptide-forming enzymes of 3T3-F442A adipocytes. Am J Physiol Cell Physiol 264: C1570–C1576, 1993. [DOI] [PubMed] [Google Scholar]
- 43.Schling P, Mallow H, Trindl A, Loffler G. Evidence for a local renin angiotensin system in primary cultured human preadipocytes. Int J Obes Relat Metab Disord 23: 336–341, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Shenoy U, Cassis L. Characterization of renin activity in brown adipose tissue. Am J Physiol Cell Physiol 272: C989–C999, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Van Harmelen V, Ariapart P, Hoffstedt J, Lundkvist I, Bringman S, Arner P. Increased adipose angiotensinogen gene expression in human obesity. Obes Res 8: 337–341, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277: 14838–14843, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Zhang XH, Zeng ZP, Li HZ, Zhou YR, Zhang J, Tong AL, Yan ZL. [Expression of renin-angiotensin-aldosterone system in human adipose tissues]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 28: 766–769, 2006. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.