Abstract
The fundamental changes which predispose for renal cell carcinoma (RCC) are poorly characterized. It is hypothesized that “cancer stem cells” may be influential in carcinogenesis, and the epithelial side population (SP) is enriched for stemlike cells in other epithelial cancers. In this study, we have isolated and characterized the SP and non-SP (NSP) populations from normal (NK) and malignant (RCC) human kidney tissue. NK specimens were taken from patients undergoing non-renal cancer surgery and paired malignant and macroscopically normal tissue samples were taken from patients undergoing surgery for RCC. The Hoechst 33342 dye efflux technique was used to isolate epithelial SP and NSP from normal and malignant human renal tissue. Cellular subpopulations were phenotyped for lineage, cell cycle, and putative stem cell markers, and functionally characterized using in vitro colony-forming and proliferation assays. The SP constituted 3.8 ± 0.4 and 5.9 ± 0.9% of epithelial cells in NK and RCC, respectively, of which 14.1 ± 3.5 and 13.2 ± 3.6% were shown to be in G0. SP cells demonstrated greater proliferative potential in colony-forming efficiency, long-term culture, and spheroids assays and were shown to be maintained upon tissue culture passage. We have shown that the renal SP is enriched for quiescent cells, with a high proliferative capacity and stemlike properties. The population is, however, heterogeneous, confirming that the terms “SP cell” and “stem cell” cannot be used interchangeably.
Keywords: kidney, renal cancer
renal cell carcinoma (RCC) is one of the commonest malignancies of the genitourinary tract accounting for 38,890 new cases and 12,840 deaths in the United States per annum (17). RCC is thought to originate from renal tubular epithelium, but the fundamental changes predisposing to carcinogenesis are poorly understood. One theory proposed is that the disease arises in renal stem cells (SC) (24). The embryonic metanephric mesenchyme and ureteric bud differentiate into over 26 different types of cell constituting the adult kidney (1). While it has been shown that a single metanephric mesenchymal cell from a developing kidney can generate all the epithelial cell types of the nephron, except for the collecting duct, (1) it is unknown whether these multipotent cells are retained in the mature kidney (26). However, RCC cells differentially express fetal nephron differentiation molecules, suggesting that RCC may arise from cells that have “reexpressed” the differentiation characteristics of the metanephric blastema (11).
Isolation of adult stem cells (ASC) has proven difficult due to the lack of clearly defined ASC markers. Goodell et al. (12) demonstrated that hemopoietic stem cells (HSC) could be isolated by the ability of SCs to efflux Hoechst 33342 and form a side population (SP) on FACS analysis (12). This method of SC isolation has been adapted for solid tissues such as the prostate (4) and, recently, unfractionated normal human renal tissue (15).
In this study we have utilized the Hoechst 33342 dye efflux technique to phenotypically characterize the SP from matched normal and malignant human renal specimens.
MATERIALS AND METHODS
Sigma-Aldridge (Poole, UK) was the source of reagents apart from Matrigel (BD Biosciences), RPMI 1640, and FCS (Cambrex BioSciences, Verviers, Belgium), collagenase type 1 and trypsin (Lorne Laboratories, Twyford, UK), HBSS without phenol red, HEPES, keratinocyte serum-free media (KSFM), DMEM, insulin-transferrin-selenium, and Hoechst 33342 (Invitrogen, Paisley, UK).
Isolation of Primary Renal Cells
Normal kidney specimens from patients undergoing non-renal cancer surgery and paired malignant and macroscopically normal tissue samples were taken from patients undergoing surgery for RCC, with local ethical committee approval and informed consent. Specimens were digested overnight in the presence of collagenase type 1 (200 U/ml in RPMI 1640 medium, 2% FCS) on a shaker at 37°C. Live cells were isolated over metrizamide (1.08 g/ml), and fibroblasts were depleted by differential centrifugation (360 g, 1 min).
Hoechst 33342 Staining
CD45-depleted cells were stained with Hoechst 33342 ± 50 μM verapamil (4). Cells were resuspended in Hoechst buffer (HBSS; 10% FCS, 20 mM HEPES, 1% d-glucose) and stained with 5 μM Hoechst 33342 and CD45-FITC (1:500) for 75 min on a shaking platform at 37°C. Cell cycle status was studied by Hoechst 33342 and pyronin Y (0.5 ng/ml) staining. Cells were washed and resuspended in ice-cold Hoechst buffer before immediate FACS analysis. Flow cytometry was carried out using a Becton Dickinson FACS Vantage SE Flow Cytometer. Hoechst blue was measured with a 424/44 broad-pass (BP) filter and Hoechst red with a 675/20 BP filter.
Colony-Forming Efficiency
Cells were cultured in the presence of an irradiated (50 Gray) STO feeder layer in AR-5 media [50:50 DMEM and Ham's F12 media supplemented with insulin (200 μg/l), transferrin (100 μg/l), selenium (25 nM), hydrocortisone (18.2 μg/l), epidermal growth factor (1 μg/l), 10 mM ethanolamine, 10 mM HEPES, FCS (5%), bovine serum albumin (0.2%), O-phosphorylethanolamine (1.4 mg/l), triiodothyronine (0.68 μg/1), and 0.5 mM sodium pyruvate] at 37°C and 5% CO2. Media was changed twice weekly, and colonies (>32 cells) were counted. Colonies were fixed in 50:50 methanol and acetone and stained with anti-pan cytokeratin.
Long-Term Culture
SP and NSP from RCC and NK were plated in T-25 flasks in AR-5 media at 37°C and 5% CO2. Confluent cells were passaged until senescence. Established cultures from both SP and NSP were restained with Hoechst 33342 and pyronin Y.
Spheroid Culture in 3D Matrigel
Spheroids were cultured as defined for prostate spheroids (18). Briefly, SP and NSP were resuspended in ice-cold AR-5 media and mixed 1:1 with growth factor-reduced Matrigel and rat tail type 1 collagen. Two hundred-fifty-microliter aliquots were plated onto 0.4-μm culture inserts (Becton Dickinson) and set at 37°C for 30 min before transfer to an irradiated STO feeder layer. Media was refreshed every 3 days. Spheroids were viewed using an inverted Olympus BX51 microscope, and images were captured (Soft Imaging Systems). Formalin-fixed, paraffin-embedded spheroids were sectioned and stained.
Immunohistochemistry
Cells adhered to poly-l-lysine-coated slides were fixed in 4% formalin and permeabilized with methanol. Slides were blocked using secondary antibody host serum before being stained with p21cip1/waf1, p27kip1, E-cadherin, N-cadherin, Ki-67 (Dako Cytomation), anti-pan cytokeratin clone 11 (Sigma-Aldridge), CD44, CD29 (Vector Laboratories, Burlingame, CA), CD133, β-catenin, cleaved notch (Miltenyi Biotec), Pax-2 (Zymed Laboratories, South San Francisco, CA), SHH, Notch-1 (Santa Cruz Technologies, Santa Cruz, CA), and Musashi-1 (courtesy of R. Clarke, Manchester, UK) overnight at 4°C. Slides were treated with H2O2 followed by addition of biotinylated secondary antibodies, Vector Elite ABC and 3–3 diaminobenzidine superoxide substrate (DAB), and counterstained with hematoxylin.
Statistics
All values are presented as means ± SE. All assays were compared by use of a two-tailed Student's t-test. A threshold of significance was set at P < 0.05.
RESULTS
Renal Epithelial Hoechst Profile
The Hoechst 33342 staining protocol was standardized for renal epithelial-enriched populations using previously described methods (3, 4), and CD45−ve profiles were generated from both NK (n = 19) and RCC (n = 15) (Fig. 1A). A consistent observation using matched paired specimens (n = 10) was that RCC samples contained a higher percentage of CD45+ve lymphocytes compared with NK (49.6 ± 5.1 vs. 25.2 ± 5.3%, respectively; P < 0.0002).
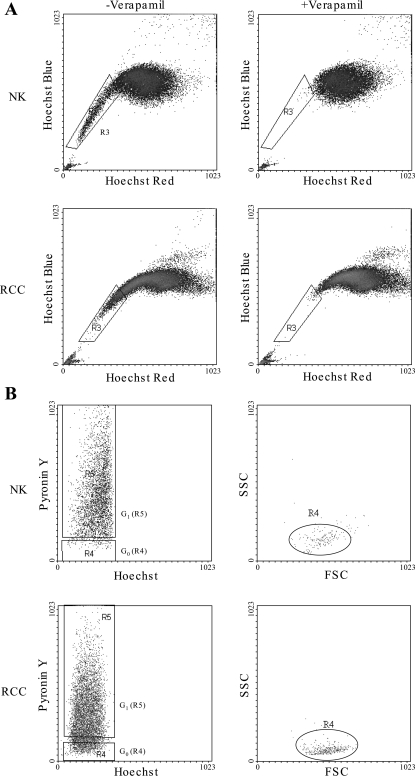
Fig. 1.
Isolation of a side population (SP) and non-SP (NSP) using Hoechst 33342 protocol and cell cycle analysis. A: typical Hoechst 33343 red/blue profiles of normal (NK) and malignant (RCC) human kidney CD45−ve epithelial cells with and without the SP inhibitor verapamil (5 μM). B: cell cycle status of NK and RCC-SP. CD45−ve renal epithelial cells were stained with Hoechst 33342 and pyronin Y (PY). Verapamil-sensitive Hoechst 33342 SP was isolated, and the mRNA content was determined by PY staining. Plot shows a typical cell cycle profile of SP cells. Hoechst blue was used as a marker of DNA content (x-axis) and PY as a marker of RNA content (y-axis). The cells with DNALow/RNALow gated as G0 (R4) and DNALow/RNAHigh as G1 (R5). Back gating the G0 cells on forward cell scatter (FSC) and side cell scatter (SSC) shows that they have minimal scatter compared with the total SP.
A verapamil-sensitive SP was isolated from both NK and RCC CD45-depleted, epithelial-enriched cells (3.8 ± 0.4 and 5.9 ± 0.9%, respectively; P < 0.05), and the cell cycle status was determined by Hoechst/pyronin Y levels. Both NK and RCC contained populations of quiescent G0 cells (14.1 ± 3.5 and 13.2 ± 3.6%; P = 0.9) and cells accumulating RNA as they move into G1 (81.1 ± 4.4 and 77.2 ± 5.7%; P = 0.6). Backgating the G0 cells onto forward cell scatter (FSC) and side cell scatter (SSC) dotplots demonstrates that both NK and RCC G0 cells form discrete populations (R4) with characteristics of small cell size with little granularity or cytoplasm (Fig. 1B).
SP Characterization
Immunohistochemistry.
Phenotyping was performed on matched pairs of NK and RCC-SP and NSP cells (Table 1; n = 4). Epithelial cells of mesenchymal origin were confirmed by pan-cytokeratin and vimentin staining. Differential cadherin expression was observed between the SP and NSP, with SP containing more cells expressing both E- and N-cadherin, although E-cadherin did not achieve significance in RCC (P = 0.16). In a comparison of the differential expression of cadherins between NK and RCC specimens, only SP cells showed a significant difference, with fewer cells expressing E- and N-cadherins in the RCC-SP.
Table 1.
Immunophenotyping of SP and non-SP cells from paired NK and RCC specimens
| Phenotypic Markers | NK SP (±SE) | NK NSP (±SE) | SP vs. NSP P Value | RCC SP (±SE) | RCC NSP (±SE) | SP vs. NSP P Value | NK vs. RCC SP P Value | NK vs. RCC NSP P Value |
|---|---|---|---|---|---|---|---|---|
| Lineage | ||||||||
| Pan-cytokeratin | 82.3 (5.8) | 47.5 (6.9) | 0.01 | 67 (14.4) | 49 (11.2) | 0.02 | 0.36 | 0.91 |
| E-cadherin | 67.3 (1.6) | 37.3 (8.1) | 0.02 | 30 (11.2) | 21.8 (10.9) | 0.16 | 0.01 | 0.31 |
| N-cadherin | 56.8 (1.9) | 28 (9.5) | 0.03 | 34.8 (7.1) | 20.5 (5.9) | 0.05 | 0.02 | 0.54 |
| Cell cycle | ||||||||
| Ki- 67 | 19.5 (3.7) | 13 (5.6) | 0.7 | 27 (8.6) | 35.3 (10.5) | 0.04 | 0.45 | 0.11 |
| p21cip1/waf1 | 36 (10.7) | 10.8 (3.1) | 0.07 | 38.3 (8.4) | 21.3 (5.4) | 0.02 | 0.87 | 0.14 |
| p27kip1 | 66.8 (4.9) | 27.8 (7.7) | 0.001 | 66.8 (6.7) | 30 (7.6) | 0.0008 | 0.99 | 0.84 |
| Stem cell | ||||||||
| β-Catenin | 83.5 (3.6) | 55 (3.9) | 0.006 | 83 (5.9) | 58.5 (9.9) | 0.05 | 0.95 | 0.75 |
| Notch1 | 67.8 (5.5) | 42.3 (2.4) | 0.02 | 68 (7.7) | 42.8 (9.2) | 0.01 | 0.98 | 0.96 |
| Cleaved Notch | 56.5 (6.1) | 26.8 (2.4) | 0.03 | 50.3 (9.9) | 23 (5.5) | 0.04 | 0.61 | 0.55 |
| Sonic HedgeHog | 74.8 (2.6) | 40.3 (5.7) | 0.002 | 72.3 (7.1) | 48.5 (3.9) | 0.01 | 0.75 | 0.28 |
| Musashi-1 | 3 (0.6) | 1 (0.6) | 0.4 | 1.3 (0.5) | 0.3 (0.3) | 0.4 | 0.32 | 0.37 |
| CD133 | 15.3 (3.7) | 1.7 (0.9) | 0.04 | 3.3 (0.7) | 1 (0.6) | 0.02 | 0.03 | 0.56 |
| CD44 | 71 (15.6) | 45.3 (5.5) | 0.16 | 70.3 (15.1) | 60 (11.1) | 0.1 | 0.98 | 0.29 |
| CD29 | 97 (1) | 82.3 (2.3) | 0.01 | 92 (4.1) | 86 (2) | 0.2 | 0.31 | 0.29 |
| PAX2 | 62.3 (1.5) | 34.7 (0.3) | 0.004 | 63.5 (12.5) | 24.5 (9.5) | 0.05 | 0.91 | 0.25 |
Values are mean percentage (±SE) of each population positive for each marker and display the significant difference between normal and malignant population by t-test. Side population (SP) and non-SP (NSP) cells from paired normal kidney (NK) and malignant (RCC) specimens were adhered to poly-l-lysine-coated slides and immunostained with lineage (Pan-Cytokeratin, E-Cadherin, N-Cadherin), cell cycle (Ki- 67, p21cip1/waf1, p27kip1), or putative stem cell markers (β-catenin, Notch1, Cleaved Notch, Sonic HedgeHog, Musashi-1, CD133, CD44, CD29, PAX2).
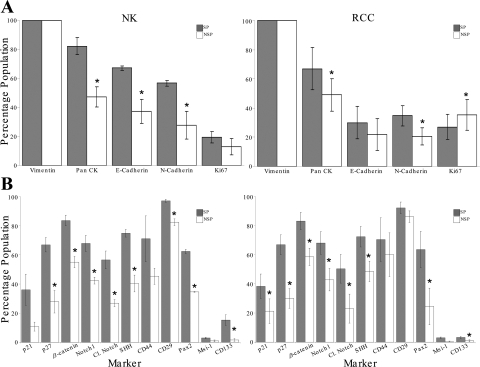
The SP from both NK and RCC were enriched for the cell cycle and putative SC markers p21cip1/waf1 and p27kip1. Both SP populations were also enriched for the putative SC markers β-catenin, Notch-1, Cleaved Notch, SHH, CD133, and the mesenchymal SC marker Pax2. Musashi-1 and the mesenchymal SC markers CD44 and CD29 (for RCC only) were enriched in the SP but did not achieve significance (P ≤ 0.05) (Fig. 2B and Table 1). In a comparison of the NK and RCC-SP marker profile, there was no difference except in relation to CD133, which was expressed to a greater extent in NK-SP (15.3 ± 3.7 vs. 3.3+0.7%; P = 0.03).
Fig. 2.
Phenotype of NK and RCC SP and NSP CD45-depleted renal epithelial cell populations. Bar graph shows the percentage of SP and NSP cells expressing markers of epithelial lineage (A), i.e., pan cytokeratin (Pan CK), mesenchymal (Vimentin), and cadherin [E-cadherin (E-Cadh), N-cadherin (N-Cadh)]; cell cycle markers (B) p21cip1/waf1 and p27kip1; and putative stem cell markers β-catenin, Notch, Cleaved notch, Sonic Hedgehog, CD44, CD29, Pax, Msi-1, and CD133. Comparative statistical analysis was performed using paired and unpaired t-tests. Markers with statistically significant differential expression are marked (*P < 0.05), and SE is shown by the error bars.
Expression of the proliferative marker Ki-67 was low in both the NK subpopulations (SP 19.5 ± 3.7 and NSP 13 ± 5.6%; P = 0.7) compared with that in RCC (SP 27 ± 8.5 and NSP 35.3 ± 10.4%; P = 0.04), although this difference between NK and RCC did not achieve significance (Table 1).
Taken together the marker profiles show that NK and RCC-SP are distinct, with putative stem enrichment in both cancer and non-cancer. However, the two types cannot be readily differentiated by this panel of markers.
Proliferative potential.
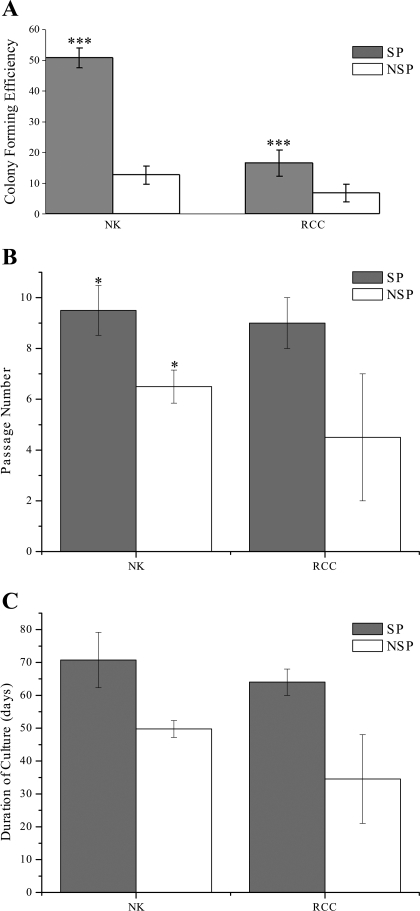
Both NK and RCC-SP and NSP (n = 4) formed well-defined, closely compacted colonies of epithelial cells, as confirmed by positive pan-cytokeratin staining (data not shown). Figure 3A shows that SP cells from both NK and RCC-SP had significantly higher CFE than the NSP (50.8 ± 3.3 vs. 12.6 ± 2.9%; P < 0.0001 and 16.5 ± 4.4 vs. 6.8 ± 2.8%; P < 0.002 for NK and RCC, respectively) and that the NK-SP had a significantly greater CFE compared with RCC-SP (P < 0.0001). However, the SP had a greater proliferative potential when clonal expansion was measured. This was especially marked in the RCC populations, as demonstrated in LTC. Although SP and NSP from both NK (n = 4) and RCC (n = 3) achieved confluency at the same rate (7.7 ± 0.31 days; P = 0.539), both normal and malignant SPs (9.5 ± 0.98 and 9 ± 1 for NK and RCC, respectively; P = 0.764) underwent a significantly (P = 0.01057) greater number of passages than their corresponding NSPs (6.5 ± 0.65 and 4.5 ± 2.5 for NK and RCC, respectively; P = 0.332) (Fig. 3, B and C). This resulted in the NK-SP having greater cell fold-expansion (99.25×) and a massive expansion for RCC-SP cells (823.5×), SP compared with their respective NSP. It is important to note that the overall cellular expansion in RCC was considerably higher than that measured in NK (4.42× and 5.05× for SP and NSP, respectively).
Fig. 3.
SP cells are highly proliferative compared with NSP cells. A: colony-forming efficiency (CFE). SP and NSP from NK and RCC were plated in limiting dilutions on irradiated STOs in AR-5 media, and colonies >32 cells in size were counted. The statistically significant (P < 0.05) higher proliferative potential of SP compared with NSP was demonstrated both in NK (*) and RCC (*). Comparison between NK and RCC showing significantly (P < 0.05) higher CFE in NK-SP compared with RCC-SP (**). B: long-term culture. Bar graph shows the number of passages, in AR-5 media, that SP and NSP from NK and RCC underwent before achieving senescence. Confluent cells were passaged until senescence, and the number of passages was recorded. *P < 0.05. C: bar chart showing the duration of culture, in AR-5 media, of SP and NSP from NK and RCC before achieving senescence. Confluent cells were passaged until senescence.
Retention of SP upon culture.
To determine whether the SP phenotype was retained upon culture or could be isolated from NSP cells postculture, passaged CD45−ve SP and NSP renal epithelial cell Hoechst 33342 dye efflux and cell cycle status were determined.
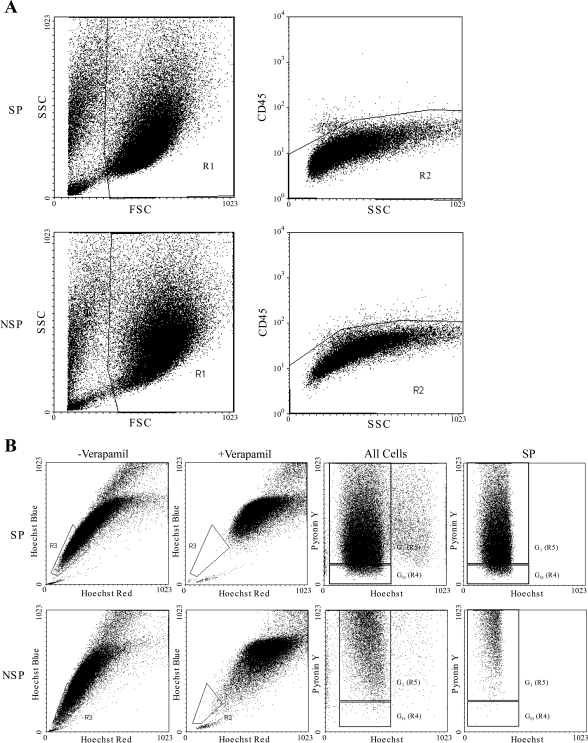
Live cells were CD45−ve, showing that the original FACs sorts were not contaminated with lymphocytes (Fig. 4A). Both NK-SP and NSP cultures gave rise to a verapamil-sensitive SP (R3) on staining with Hoechst 33342 (30.03 ± 2.7 and 26.7 ± 8.3%, respectively). This percentage was significantly greater than the SP percentage isolated from primary NK tissue (4.4 ± 0.6%; P < 0.007) (Fig. 4B). Cell cycle analysis revealed differences in the SPs' and NSPs' SP with quiescence (R4) maintained at a similar level to that seen in the original SP G0 population (13.8 ± 2.02 vs. 14.1 ± 3.5%; P = 0.95) only in cells cultured from the SP fraction.
Fig. 4.
SP phenotype is retained on passage. A: FSC and SSC profile of cultured SP and NSP cells from NK. Live cells were gated on R1 and CD45 status of the cells in R1 shown on a CD45 SSC dot blot. Dot plot demonstrates minimal positive CD45 staining, confirming CD45+ve lymphocytic depletion at the initial sort. B: typical Hoechst 33342 profile generated from in vitro cultured SP cells and NSP cells and cell cycle analysis by PY staining. Both SP and NSP cultures retained a verapamil-sensitive SP as shown by Hoechst 33342 red/blue profiles, but only cultured SP retained cells in G0.
Spheroid culture in 3D Matrigel.
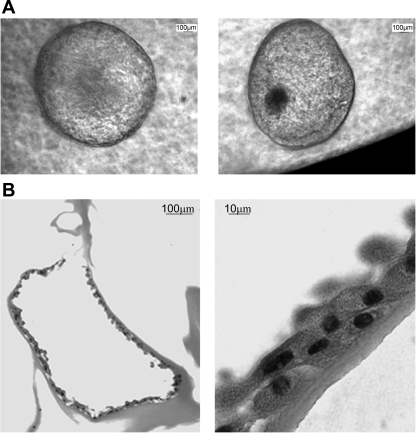
To further characterize the proliferative functionality of both the SP and NSP populations, we assessed their ability to generate complex spheroids in 3D culture, a known feature of stemlike cells. Only SPs from both NK and RCC were able to give rise to 3D spheroids efficiently, with a spheroid-forming efficiency of 0.2% compared with a spheroid-forming efficiency of 0.004% from the NSP. Sections of macroscopically visible (∼4 mm) spheroids (Fig. 5A) revealed a central lumen (Fig. 5B) surrounded by multiple layers of cells.
Fig. 5.
Renal SP cells can form hollow “spheroids” in 3D culture. SP and NSP renal cells from NK and RCC were cultured suspended in Matrigel in the presence of an irradiated STO layer. A: 5-wk-old spheroid grown from a single SP cell in 3D culture. B: spheroids were fixed overnight in 4% formalin before paraffin embedding and sectioning. Micrographs show hematoxylin and eosin staining of spheroid sections displaying a discrete lumen surrounded by multiple layers of cells
DISCUSSION
Inowa et al. (15) recently reported the presence of an SP in a limited number of unfractionated normal human renal specimens which were uncharacterized due to the lack of tissue. Here, we present phenotypic and functional characterization of both normal and malignant human renal epithelial SP.
In this study, the overall cellular population was enriched for epithelial cells before SP analysis. This resulted in the isolation of a larger SP (3.8 ± 0.4 and 5.9 ± 0.9% for NK and RCC, respectively) than previously identified (15). This is in keeping with rodent studies in which embryonic and adult renal SP account for between 0.03 and 5.1% of the cells (14, 16). This variability could be explained by the differences in the isolation techniques and the well-known variability of SP due to different FACS conditions (20). The increased SP in malignant tissue is higher than that of other solid tumors such as prostate cancer, which shows a 2- to 10-fold reduction of SP cells in malignant tissue (4).
The SP phenotype is enriched for cells with SC-like properties, such as quiescence (G0), elaboration of stem markers, augmented growth, and the ability to form differentiated structures. These data combined with data regarding the percentage of SP cells in G1 are in keeping with SP isolated from prostatic tissue (3). The low expression of the proliferative marker Ki-67 in both NK-SP and NSP is consistent with the finding that in randomly selected kidney sections ∼1:1,000 cells exhibit mitotic figures (23). The higher expression of Ki-67 in RCC-NSP compared with SP (although the difference did not achieve significance) runs counter to the suggestion that Ki-67 may be used as a marker of SP cells in malignancy (7). Back gating the SP G0 fraction onto FSC and SSC showed that these cells are small, with little cytoplasm, a finding commensurate with the fact that SCs are smaller than differentiated cells. Cell cycle status was further studied using p21cip1/waf1 and p27kip1 (9, 10). These cell cycle markers were expressed more highly in SP than NSP cells. p27kip1 expression was higher than for p21cip1/waf1 in both the NK and RCC subpopulations. This finding would corroborate with cell cycle analysis whereby a much greater proportion of the SP cells was noted in G0. However, 80% of the SP were in G1, suggesting that the renal SP, like other tissue SPs, is still heterogeneous and will need to be enriched further to isolate this SC-like population (7).
Evidence for SP enrichment for cells with stemlike functional properties is supported by the in vitro growth characteristics. CFE and LTC assays showed that both NK and RCC-SP contained cells with a higher proliferative potential than their respective NSP and were able to give rise to differentiated spheroids, unlike NSP cells. A particularly interesting feature was that RCC-SP had a much greater rate of clonal expansion than NK-SP in the absence of an accelerated cycling rate. The reasons for this remain to be elucidated but may include the inhibition of apoptosis, something which was not measured in this study. LTC also showed that the SP G0 population was maintained and expanded upon passage. This suggests that the SP was able to undergo asymmetric cell division, a key characteristic of SCs, which has been observed in neuroblastoma cell line SP subpopulations (13). The increase in SP with culture has also been noted with primary human keratinocyte SP, where the number of SP cells isolated increased 250-fold upon passage (19).
Phenotypic characterization revealed that the SP isolated using Hoechst 33342 is enriched for cells with stemlike characteristics but it is still heterogeneous; as such, determination of a definitive marker profile of a renal SC has not been possible. However, the SP cadherin expression profile suggests that the SP may reside within either the distal or proximal tubules. Cadherins are known to play important roles in the development and maintenance of tissue architecture and in carcinogenesis (21, 28). In the normal kidney, the proximal tubular cells mainly express N-cadherin (25), whilst E-cadherin expression is restricted to distal tubules (2). There was predominant expression of N- and E-cadherins in the NK-SP subpopulation, which decreased in RCC-SP (Fig. 2A). This decreased expression may in part explain the loss of cell-cell adhesion, leading to metastasis, a finding also seen in prostate cancer carcinogenesis (5). LTC experiments showed that both SP monolayers underwent a similar number of passages but the cell fold-expansion of RCC-SP, compared with NSP, was higher, at 823.5-fold compared with 99.25-fold for NK-SP. By combining the cadherin expression with the proliferative data, it can be hypothesized that the upregulation of RCC-SP proliferation combined with the reduced CFE, due to downregulated cell-cell adherence, may be the reason for the aggressive metastatic tendency observed clinically in renal cancer.
SCs, which have a prolonged lifespan, are more prone to accumulation of mutations (23) and the dysregulation of signaling pathways regulating SC self-renewal. This has led to the hypothesis for the existence of cancer SC (24). The canonical Wnt signaling cascade, Sonic HedgeHog (SHH) and Notch pathways, are key for maintaining SC self-renewal and, in the case of HSC, multipotentiality (24, 27). Loss of SHH has been associated with renal defects like hydroureter, suggesting a role in epithelial-mesenchymal interaction in renal embryogenesis (22), and Notch signaling has a crucial role in cell fate determination in proximal tubule cells and podocytes during S-shaped body formation (8). β-Catenin, a downstream activator of Wnt signaling, SHH, and both cleaved Notch and Notch-1 were expressed more widely in SP compared with NSP, without any differences in expression between NK and RCC. The increased expression of Notch-1 in SP correlates with the increased expression of Notch signaling pathway members in a mouse renal SP gene expression profile (7).
CD133 has been used as a marker of SCs in many tissues, including the kidney (6). It has been demonstrated that CD133+ve cells, which comprise 0.8–1.2% of the cells from the renal cortex, were able to proliferate and self-renew and could differentiate into epithelial or endothelial cells both in vitro and in vivo. They expressed the mesenchymal SC markers CD44 and CD29 and the embryonal renal marker PAX-2. The NK-SP isolated in this study was enriched for cells expressing CD133, CD29, CD44, and PAX-2. The higher proportion of CD133+ve cells in our study, 15.3 ± 3.7%, compared with 0.8–1.2% noted by Bussolati et al. (6), can be explained by our method of enrichment using FSC and SSC live cell gating and CD45 depletion. As evident from the very low numbers of CD133+ve cells in NK-NSP and both the RCC subpopulations, the loss of CD133 might be a very early event in SC differentiation and possibly in malignant transformation. This finding runs counter to the notion that CD133 is a definitive SC marker in solid tumors.
In summary, the SP is enriched for cells with a high proliferative capacity, expressing putative SC markers and the ability to produce differentiated spheroids. The SP is maintained on passage, but SP cell cycle status combined with phenotypic characteristics suggest that the SP is a heterogeneous population. This confirms the notion that the terms “side population cell” and “stem cell” cannot be used interchangeably (7). However, further enrichment of this population will enable detailed future study of unexpanded cancer stem cells.
Conclusion
Both normal and malignant renal epithelial cells contain SP which are enriched for cells with SC-like properties. The population is however, heterogeneous, confirming that the terms side population cell and stem cell cannot be used interchangeably.
GRANTS
This work was supported by British Urological Foundation Blackwell Publishing/Sackler Foundation Scholarship 2005–2006.
Acknowledgments
We thank the urological surgeons of the Christie, Salford, and South Manchester University Hospital NHS Trusts for renal tissue collection, the Paterson Institute Flow Cytometry, Advanced Imaging, and Histology Facilities for technical assistance, and the British Urological Foundation for financial support.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Al-Awqati Q, Oliver JA. Stem cells in the kidney. Kidney Int 61: 387–395, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Baer PC, Bereiter-Hahn J, Schubert R, Geiger H. Differentiation status of human renal proximal and distal tubular epithelial cells in vitro: differential expression of characteristic markers. Cells Tissues Organs 184: 16–22, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt RI, Brown MD, Hart CA, Gilmore P, Ramani VA, George NJ, Clarke NW. Novel method for the isolation and characterisation of the putative prostatic stem cell. Cytometry 54: 89–99, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Brown MD, Gilmore PE, Hart CA, Samuel JD, Ramani VA, George NJ, Clarke NW. Characterization of benign and malignant prostate epithelial Hoechst 33342 side populations. Prostate 67: 1384–1396, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bryden AA, Freemont AJ, Clarke NW, George NJ. Paradoxical expression of E-cadherin in prostatic bone metastases. BJU Int 84: 1032–1034, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol 166: 545–555, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells 24: 3–12, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cheng HT, Kopan R. The role of Notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney Int 68: 1951–1952, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287: 1804–1808, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 272: 877–880, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Droz D, Zachar D, Charbit L, Gogusev J, Chretein Y, Iris L. Expression of the human nephron differentiation molecules in renal cell carcinomas. Am J Pathol 137: 895–905, 1990. [PMC free article] [PubMed] [Google Scholar]
- 12.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183: 1797–1806, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA 101: 14228–14233, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hishikawa K, Marumo T, Miura S, Nakanishi A, Matsuzaki Y, Shibata K, Ichiyanagi T, Kohike H, Komori T, Takahashi I, Takase O, Imai N, Yoshikawa M, Inowa T, Hayashi M, Nakaki T, Nakauchi H, Okano H, Fujita T. Musculin/MyoR is expressed in kidney side population cells and can regulate their function. J Cell Biol 169: 921–928, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inowa T, Hishikawa K, Takeuchi T, Kitamura T, Fujita T. Isolation and potential existence of side population cells in adult human kidney. Int J Urol 15: 272–274, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Iwatani H, Ito T, Imai E, Matsuzaki Y, Suzuki A, Yamato M, Okabe M, Hori M. Hematopoietic and nonhematopoietic potentials of Hoechst(low)/side population cells isolated from adult rat kidney. Kidney Int 65: 1604–1614, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin 56: 106–130, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Lang SH, Stark M, Collins A, Paul AB, Stower MJ, Maitland NJ. Experimental prostate epithelial morphogenesis in response to stroma and three-dimensional matrigel culture. Cell Growth Differ 12: 631–640, 2001. [PubMed] [Google Scholar]
- 19.Larderet G, Fortunel NO, Vaigot P, Cegalerba M, Maltere P, Zobiri O, Gidrol X, Waksman G, Martin MT. Human side population keratinocytes exhibit long-term proliferative potential and a specific gene expression profile and can form a pluristratified epidermis. Stem Cells 24: 965–974, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Montanaro F, Liadaki K, Schienda J, Flint A, Gussoni E, Kunkel LM. Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Exp Cell Res 298: 144–154, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Nollet F, Berx G, van Roy F. The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Mol Cell Biol Res Commun 2: 77–85, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Nowaczyk MJ, Huggins MJ, Tomkins DJ, Rossi E, Ramsay JA, Woulfe J, Scherer SW, Belloni E. Holoprosencephaly, sacral anomalies, and situs ambiguus in an infant with partial monosomy 7q/trisomy 2p and SHH and HLXB9 haploinsufficiency. Clin Genet 57: 388–393, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114: 795–804, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 414: 105–111, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Shimazui T, Kojima T, Onozawa M, Suzuki M, Asano T, Akaza H. Expression profile of N-cadherin differs from other classical cadherins as a prognostic marker in renal cell carcinoma. Oncol Rep 15: 1181–1184, 2006. [PubMed] [Google Scholar]
- 26.Steer DL, Nigam SK. Developmental approaches to kidney tissue engineering. Am J Physiol Renal Physiol 286: F1–F7, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature 411: 349–354, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Umbas R, Isaacs WB, Bringuier PP, Schaafsma HE, Karthaus HF, Oosterhof GO, Debruyne FM, Schalken JA. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res 54: 3929–3933, 1994. [PubMed] [Google Scholar]