Abstract
This study examined the genetic basis of hypertension and renal disease in Dahl SS/Mcwi (Dahl Salt-Sensitive) rats using a complete chromosome substitution panel of consomic rats in which each of the 20 autosomes and the X and Y chromosomes were individually transferred from the Brown Norway (BN) rat onto the Dahl SS/Mcwi genetic background. Male and female rats of each of the two parental and 22 consomic strains (10–12 rats/group) were fed a high-salt (8.0% NaCl) diet for 3 wk. Mean arterial blood pressure rose by 60 mmHg and urinary protein and albumin excretion increased 3- and 20-fold, respectively, in male SS/Mcwi rats compared with BN controls. Substitution of chromosomes 1, 5, 7, 8, 13, or 18 from the BN onto the SS/Mcwi background attenuated the development of hypertension, proteinuria, and albuminuria in male rats. In female rats, substitution of chromosomes 1 and 5 also decreased blood pressure, protein excretion, and albumin excretion. These studies also identified several chromosomes in male (6, 11, Y) and female (4, 6, 11, 19, 20) rats that reduced albuminuria without altering blood pressure. These data indicate that genes contributing to salt-sensitive hypertension are found on multiple chromosomes of the Dahl SS/Mcwi rat. Furthermore, this consomic rat panel provides a stable genetic platform that can facilitate further gene mapping by either linkage studies or the breeding of congenic and subcongenic rats.
Keywords: hypertension, kidney disease, rats, consomic
the dahl SS/Mcwi (salt-sensitive) rat strain is a genetic animal model of hypertension and kidney disease that exhibits disease traits similar to those observed in humans (3, 5, 9, 14, 17). This inbred strain exhibits a low-renin, sodium-sensitive form of hypertension that is associated with severe and progressive proteinuria, glomerulosclerosis, and renal interstitial fibrosis (29, 36). To examine the genetic basis of hypertension and renal disease in the SS/Mcwi, 22 chromosome substitution strains of rats were generated in which one chromosome at a time was introgressed from a disease-resistant Brown Norway (BN) rat into the Dahl SS/Mcwi genetic background. With the use of this strategy, phenotypic differences detected between the consomic and the recipient strains indicate that there is a gene or genes present on the substituted chromosome that influences the phenotype of interest. Consomic animals permit repeated phenotyping of replicate animals as well as the rapid generation of congenic strains for narrowing the region of interest along a chromosome to facilitate the positional cloning of candidate genes. It also allows the substitution of multiple chromosomes to study chromosomal interactions. This genetic strategy has already proven useful to understand the genetic basis of a number of complex disease-related phenotypes in rats and mice (4, 6, 25, 32, 33).
In the present study, male and female rats of each of the two parental and consomic strains were fed a high-salt (8.0% NaCl) diet for 3 wk. A standardized phenotyping protocol was performed on each sex of each strain in addition to the SS/Mcwi and BN parental rats to quantify phenotypes related to hypertension (conscious mean arterial blood pressure and heart rate) and renal disease (creatinine clearance, albumin excretion, and protein excretion).
Although the genetic basis of Dahl SS hypertension has been studied extensively, the present approach is complementary and has several distinct differences from previous studies. First, this is the first systematic dissection of hypertension and renal injury in the Dahl SS background on a chromosome-by-chromosome basis using a standardized phenotyping protocol. Second, the normotensive background utilized in the present study is the BN. This study should therefore provide a set of observations unique from those observed in previous crosses of Dahl SS rats with Lewis, Dahl R, or other rat strains. A concordance of the present results with previous studies that utilized the Lewis or Dahl R rats, however, will permit conclusions to be made regarding the contribution of prohypertensive regions of the SS genome. Third, this study examined both male and female rats. Females have been largely ignored in these types of studies, and the present data provide important new information regarding gender differences. Finally, many previous phenotyping studies in the Dahl SS background have been performed on rats maintained on a normal salt (1.0% NaCl) diet with blood pressure phenotyping performed by tail-cuff plethysmography. The present studies were performed in rats in which hypertension and renal disease were induced by feeding an elevated salt (8.0% NaCl) diet, and blood pressure was directly measured with chronically implanted catheters.
METHODS
Experimental animals.
Experiments were performed on inbred lines of SS/Mcwi (Dahl salt-sensitive rats), BN/SSNHsdMcwi (BN), and a panel of consomic rats. The consomic rat lines were derived from inbred BN and SS/Mcwi rats as previously described (6, 18, 26). Studies were performed on male and female rats of each of the parental and consomic strains fed a high-salt (8.0% NaCl) diet for 3 wk. A total of 361 male and 277 female rats was studied (average group size for each phenotype was ∼12 for male rats and 10 for female rats). The MCW Institutional Animal Care and Use Committee approved all experimental protocols.
The consomic rat panel consisted of rat strains in which each of the 20 autosomes as well as the X and Y chromosomes from the BN rat were transferred onto the SS/Mcwi genetic background. The consomic strains are formally designated as SS-nBN/Mcwi (SS-nBN throughout the text), where n designates the substituted chromosome (i.e., SS-1BN/Mcwi, SS-2BN/Mcwi, etc). In two of the strains, the entire chromosome was not transferred from the BN to the SS, and the strains are congenic. In these strains, the portion of the BN genome within the indicated genetic markers was transferred to the SS/Mcwi genetic background [i.e., SS.BN-(D8rat163-D8rat81)/Mcwi and SS.BN-(D12arb13-D12rat79)/Mcwi]. Greater than 70% (by size) of BN chromosome 8 was introgressed into the SS/Mcwi background in SS.BN-(D8rat163-D8rat81)/Mcwi, and >60% of BN chromosome 12 was transferred in SS.BN-(D12arb13-D12rat79)/Mcwi.
Phenotyping protocol.
The breeders of each strain were maintained on chow containing 0.4% NaCl obtained from Harlan Teklad (3075S, Madison, WI). At weaning, the rats were placed on a purified AIN-76A rodent diet containing 0.4% NaCl (Dyets, Bethlehem, PA). At ∼9 wk of age, the rats were placed on AIN-76A chow containing 8.0% NaCl; 2 wk later, the rats were anesthetized with ketamine (35 mg/kg ip), xylazine (10 mg/kg ip), and acepromazine (0.5 mg/kg ip), and catheters were implanted in the femoral artery and exteriorized at the back of the neck through a lightweight tethering spring. Both antibiotic (100,000 U/kg penicillin G im) and analgesic (0.1 mg/kg buprenex sc) were administered postsurgically. Following recovery from surgery, the rats were placed in individual stainless steel cages that permit daily measurement of arterial blood pressure and overnight urine collection.
After a 2-day recovery period, blood pressure and heart rate measurements were made from 9:00 a.m. to 12:00 p.m. on postsurgical days 3–5. An overnight urine collection (16 h) was obtained for measurement of urinary excretion of albumin, protein, and creatinine, and a blood sample was obtained for measurement of plasma creatinine concentration and renin activity.
Histological analysis.
Kidneys from male rats of each parental strain and from consomic strains with extreme renal disease phenotypes (SS/Mcwi, BN, SS-1BN, and SS-17BN) fed an 8% salt diet for 3 wk were collected for histological analysis. This analysis was designed to demonstrate the differences that occur in histology in male rats with pronounced differences in albuminuria/proteinuria. It did not assess differences in female rats or effects on glomerular injury that may be independent of increased protein excretion. The kidneys were fixed in a 10% formalin solution, paraffin-embedded, cut in 3-μm sections, and stained with Gomori's One-Step Trichrome (sections from all rats were stained simultaneously). Tissue sections were photographed using a Nikon E-400 fitted with a Spot Insight camera; digital micrographs were obtained at ×40 magnification to visualize and assess glomerular injury as we previously described (18, 19, 20).
Statistical analysis.
Data are presented as means ± 1 SE. A two-way ANOVA with a Holm-Sidak post hoc test was used to assess sex differences for each phenotype within the different consomic and parental rats. A one-way ANOVA with a Holm-Sidak post hoc test was used to evaluate differences between the parental SS/Mcwi and the other strains. P < 0.05 was considered to be significant.
RESULTS
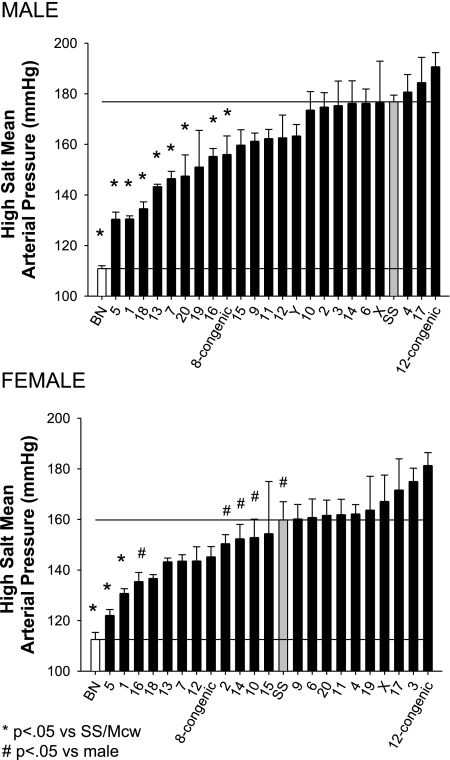
Mean arterial blood pressures (MAP) in the male and female rats fed a high-salt (8.0% NaCl) diet for 3 wk are illustrated in Fig. 1. MAP was significantly greater in the male and female SS/Mcwi (177 ± 3 and 160 ± 7 mmHg, respectively) than observed in the male and female BN (111 ± 1 and 112 ± 3 mmHg, respectively) rats. Similarly, MAP was higher in males than females in some of the consomic strains. Substitution of chromosomes 1, 5, 7, 8, 13, 16, 18, and 20 significantly decreased MAP compared with parental SS/Mcwi in the male rats. Transfer of chromosomes 1 and 5 also significantly lowered MAP in the female rats. Minimal differences were observed in heart rate (data not shown). The only strains in which conscious heart rate was different from the value observed in male SS/Mcwi (400 ± 4 beats/min) were the BN (363 ± 6 beats/min) and the SS-12BN (359 ± 11 beats/min). Heart rate was not different between female SS/Mcwi (416 ± 7 beats/min) and female BN rats or any of the consomic strains.
Fig. 1.
Mean arterial blood pressure in male (top) and female (bottom) SS/Mcwi (SS), Brown Norway (BN), and consomic rat strains (indicated by the substituted chromosome) fed a high-sodium diet (8.0% NaCl). P < 0.05 vs. SS of the same sex (*) and vs. the male rat of the same strain (#).
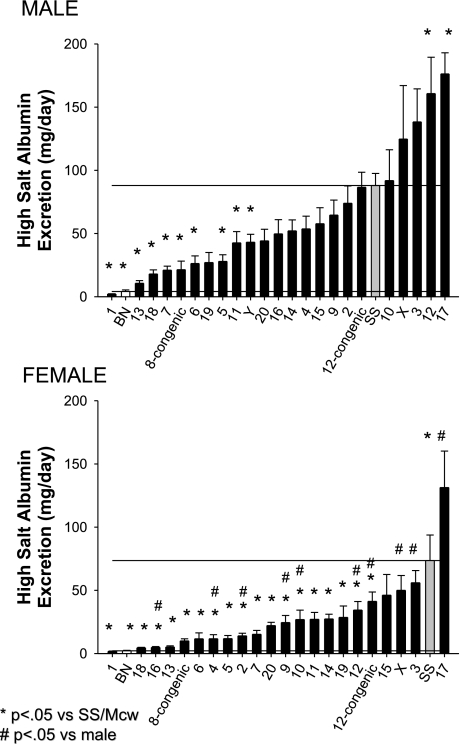
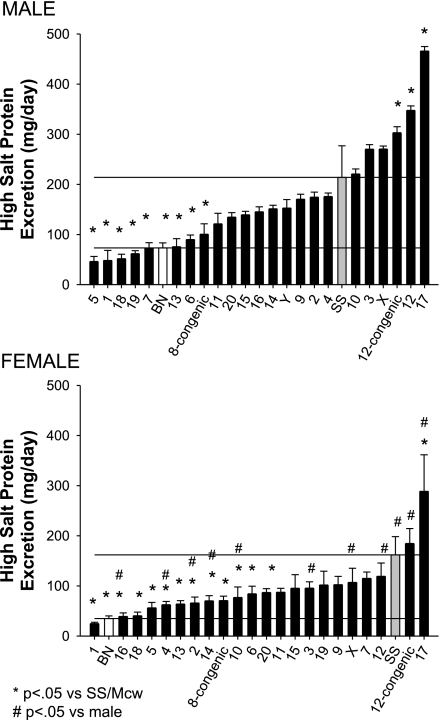
The albumin excretion rate was significantly greater in the SS/Mcwi rats fed a 8% salt diet (88 ± 9 and 73 ± 20 mg/day for males and females, respectively) than in the BN (4.0 ± 1.3 and 2.1 ± 0.4 mg/day for males and females, respectively) rats (Fig. 2). In male rats, the substitution of chromosomes 1, 5, 6, 7, 8, 11, 13, 18, and Y from the BN onto the SS/Mcwi genetic background significantly reduced albumin excretion compared with the SS/Mcwi. Urinary albumin excretion was significantly elevated in the male SS-12BN and SS-17BN compared with the SS/Mcwi. The albumin excretion rate in female consomic rats was significantly lower than that observed in the female SS/Mcwi in all but four strains. In contrast, albumin excretion was significantly elevated in SS-17BN compared with the parental SS/Mcwi. Similar to the differences observed for albumin excretion, the male and female SS/Mcwi rats (214 ± 15 and 162 ± 36 mg/day for males and females, respectively) excreted significantly more protein than BN (73 ± 12 and 35 ± 5 mg/day for males and females, respectively) (Fig. 3). In male rats, protein excretion rate was significantly lower than observed in the SS/Mcwi in rats in which chromosomes 1, 5, 6, 7, 8, 13, 18, and 19 were substituted. Substitution of chromosome 12 and 17 led to an increase in urine protein excretion in the male rats. In female rats, substitution of chromosomes 1, 2, 4, 5, 6, 8, 10, 13, 14, 16, and 18 significantly decreased the protein excretion rate compared with the parental SS/Mcwi female. In contrast, protein excretion was significantly increased to 289 ± 73 mg/day in the female SS-17BN compared with the SS/Mcwi parental strain.
Fig. 2.
Urinary albumin excretion rate in male (top) and female (bottom) SS/Mcwi (SS), BN, and consomic rats (indicated by the substituted chromosome) fed a high-sodium diet (8.0% NaCl). P < 0.05 vs. SS of the same sex (*) and vs. the male rat of the same strain (#).
Fig. 3.
Urinary protein excretion rate in male (top) and female (bottom) SS/Mcwi (SS), BN, and consomic rats (indicated by the substituted chromosome) fed a high-sodium diet (8.0% NaCl). P < 0.05 vs. SS of the same sex (*) and vs. the male rat of the same strain (#).
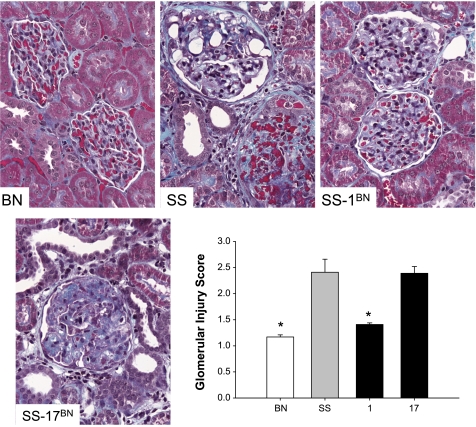
Representative examples of the degree of glomerular injury in the parental strains and consomic strains that exhibited large differences in proteinuria (SS/Mcwi, BN, SS-1BN, and SS-17BN) are presented in Fig. 4. Marked glomerular damage (blue fibrotic tissue with collapsed capillaries) was observed in the glomeruli of SS/Mcwi compared with the BN rats. Substitution of chromosome 1 from the BN to the SS/Mcwi markedly reduced glomerular damage; in contrast, the SS-17BN had a similar degree of glomerular damage as that observed in the SS/Mcwi.
Fig. 4.
Photomicrographs of the renal cortex (×40 original magnification) of male SS/Mcwi (SS), BN, SS-1BN, and SS-17BN consomic strains. Graph depicts the glomerular injury score in these strains. *P < 0.05 vs. SS.
Creatinine clearance was not significantly different between the male SS/Mcwi (0.68 ± 0.04 ml·min−1·100 g body wt−1) and male BN or between the female SS/Mcwi (0.73 ± 0.06 ml·min−1·100 g body wt−1) and female BN. Chromosome substitution did not affect creatinine clearance in males or females. Plasma renin activity was also not different in male SS/Mcwi (0.67 ± 0.12 ng ANG I·ml−1·h−1) and BN rats fed an 8.0% salt diet (1.01 ± 0.16 ng ANG I·ml−1·h−1). PRA was significantly greater in female BN (4.39 ± 1.30 ng ANG I·ml−1·h−1) than female SS/Mcwi (1.17 ± 0.23 ng ANG I·ml−1·h−1), but PRA levels were not different in any of the consomic strains compared with SS/Mcwi.
DISCUSSION
Dahl SS/Mcwi rats fed a high-salt diet for 3 wk had higher levels of arterial blood pressure, elevated urinary excretion of albumin and protein, and greater renal histological damage than BN rats. Substitution of several chromosomes from the disease-resistant BN to the disease-prone SS/Mcwi significantly reduced the development of hypertension and/or proteinuria, indicating that multiple genes, gene products, or gene pathways influence these disease phenotypes. Sodium-sensitive hypertension, albuminuria, and proteinuria were attenuated in the male and female SS-1BN and SS-5BN compared with the SS/Mcwi rats. In addition, substitution of chromosomes 8, 13, and 18 decreased MAP, albuminuria, and proteinuria in male rats and attenuated proteinuria and albuminuria in female rats. Interestingly, albuminuria and proteinuria were diminished in the SS-6BN and enhanced in the SS-17BN in the absence of significant changes in MAP, indicating that genes of renal disease that act independently of blood pressure are found on chromosomes 6 and 17. Finally, the influence of a number of chromosomes was dependent upon the sex of the recipient. Substitution of chromosomes 2, 4, 10, 14, 16, and 20 decreased proteinuria in female rats but had no influence in males; similarly, substitution of chromosomes 7, 8, 13, 16, 18, and 20 significantly decreased blood pressure in male rats compared with the parental SS/Mcwi without having a significant effect on blood pressure in female rats.
The present rat study demonstrates marked effects of substitution of chromosomes 1 and 5 on high-salt MAP in both male and female rats. A number of quantitative trait loci (QTLs) for blood pressure have been previously localized on both chromosomes 1 and 5 in genetic crosses that utilized the Dahl SS as well as other hypertensive rat strains (11, 27, 28, 34, 35). Moreover, we observed that male consomic strains with BN chromosome 7, 8, 13, 16, 18, and 20 on the SS background had a correction of hypertension. It was previously demonstrated that QTLs for blood pressure reside on chromosomes 7, 8, 9, 13, 16, 18, and 19 in crosses with the Dahl SS rat (1, 11, 12, 13, 22, 23, 24, 27, 28, 31, 37). Blood pressure QTLs have also been previously identified on chromosome 20 in genetic crosses with other hypertensive strains (18, 27, 28), but QTLs related to blood pressure have not been detected previously on chromosome 20 in the Dahl SS. Interestingly, in previous studies in the Dahl SS rat, QTLs related to blood pressure were also observed on chromosomes 2, 3, 4, and 14 (7, 8, 12, 35); substitution of these chromosomes did not affect blood pressure in the present study. The present data therefore confirm the existence of several chromosomes (1, 5, 7, 8, 13, 16, and 18) known to harbor large blood pressure QTLs and indicate that chromosome 20 harbors blood pressure QTLs in the Dahl SS rat.
The development of renal disease in the male and female rats, as assessed by urinary albumin and protein excretion, was attenuated significantly by substitution of chromosomes 1, 5, 6, 8, 13, and 18 from the BN on the SS/Mcwi genetic background in male and female rats. A histological analysis of the SS-1BN consomic strain revealed significantly less glomerular damage than observed in the SS/Mcwi; the histology therefore confirmed the proteinuria and albuminuria data. In addition, substitution of chromosomes 2, 4, 10, 14, 16, and 20 corrected proteinuria and albuminuria in the female rats. A previous F2 intercross between SS/Mcwi and BN rats revealed QTLs for proteinuria on chromosomes 8 and 18 (35); a separate F1 backcross between an F1 [spontaneously hypertensive rat (SHR) × SS] and an SS parent identified alleles from the SS rat that increased albuminuria or proteinuria on chromosomes 1, 2, 6, 8, 9, 10, 11, 13, and 19 (10). Similar QTLs for albuminuria were observed in chromosomes 2, 6, 8, 9, 10, 11, and 19 in an F2 cross between SS and SHR (21), whereas a consomic strain in which SHR chromosome 19 was introgressed on the SS background led to attenuation of renal damage (37).
The current set of studies reveals important effects of QTLs on a number of other chromosomes for this renal disease phenotype in the SS/Mcwi (i.e., 4, 5, 14, and 20). Previous studies have revealed QTLs related to renal disease on chromosomes 1 and 14 in genetic crosses between the FHH rat and normal controls (2, 30). The other chromosomes identified as important in albuminuria in the consomic panel represent chromosomes with potential new genes of renal disease in the SS/Mcwi rat. Although albuminuria and proteinuria were simultaneously altered in most strains, these two phenotypes were altered separately in a few of the consomics. The female SS-7BN, SS-9BN, and SS-11BN all had significantly reduced albumin excretion with no changes in proteinuria compared with the female SS/Mcwi. Similarly, proteinuria was significantly increased, but albumin excretion was unaltered in male SS.BN-(D12arb13-D12rat79). In the female rats of that strain, albumin excretion was decreased, but protein excretion was not changed. The results from these strains may indicate important genetic differences in the susceptibility to albuminuria and proteinuria.
The consomic approach represents a completely different mapping strategy that has some advantages over traditional linkage mapping. Compared with a traditional linkage analysis in which every animal has a unique genetic background, studies in a consomic panel greatly reduce genetic heterogeneity, since all of the strains share an identical genetic background. Because each of the consomic strains is inbred, these rats provide the ability to replicate and validate the mapped phenotypes, enable sex-related traits and different age groups to be studied, and permit more extensive mechanistic studies of interesting candidate regions, genes, and phenotypes. In addition, the inbred nature of consomic strains enables the rapid generation of congenic strains in only two generations to narrow the region of interest (4). In contrast, it generally takes six generations to develop a congenic strain following a QTLs mapping study. The time and expense required to produce congenic animals is the primary reason that many of the previously identified QTLs have not been extensively followed up except by the most dedicated and persistent groups. Moreover, the consomic strains can be intercrossed to develop strains in which multiple QTLs and different chromosomes are captured to study chromosome interactions.
The present studies represent a complete chromosomal mapping of the SS genome for hypertension and kidney damage. More than six chromosomes were identified in which substitution of the BN chromosome in the Dahl SS strain provided significant protection of hypertension on a high-salt diet. The degree of protection of nearly the same magnitude by different chromosome substitutions represents the first direct quantitative identification and evidence (not just statistically implied by linkage analysis) that these regions interact epistatically and influence blood pressure. We propose that there are multiple genes on a number of different chromosomes that by themselves can significantly influence the hypertensive and renal disease phenotypes examined in this study. For example, the effects of individual substitution of a BN chromosome 1 or 5 were sufficient to revert the blood pressure phenotypes to the levels observed in the BN (i.e., MAP was not significantly different between the male BN and the male SS-1BN or SS-5BN). The predicted sum of the individual QTLs on different chromosomes is therefore significantly greater than the phenotypic difference observed between the two parental strains. This likely reflects the nonlinearity of the physiological control systems and the ability of these systems to maintain homeostasis through multiple positive and negative feedback control systems (15, 16). Potential feedback and interactions may occur between genes, gene products, and pathways to affect the final phenotype.
The present study confirmed, using a panel of SS-BN consomic strains, the existence of major QTLs on chromosomes 1, 5, 7, 8, 13, 16 and 18 that oppose the development of hypertension and identified chromosome 20 as a region of interest for hypertension. These experiments also provide new information that chromosomes 1 and 5 are important for the development of hypertension and renal disease in female SS/Mcwi rats. This consomic panel of rats therefore captured most of the previously identified blood pressure QTLs and identified several others. This new genetic resource now provides a stable platform for breeding of congenic strains for the positional cloning of QTLs for renal disease and hypertension.
GRANTS
The work in this manuscript was partially supported by National Institute of Health Grants HL-66579, HL-54998, and DK-62803.
Acknowledgments
We thank the PhysGen phenotyping staff for technical assistance with these studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ariyarajaha A, Palijana A, Dutila J, Prithiviraja K, Dengb Y, Deng AY. Dissecting quantitative trait loci into opposite blood pressure effects on Dahl rat chromosome 8 by congenic strains. J Hypertens 22: 1495–1502, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Provoost AP, Daly MJ, Lander ES, Jacob HJ. Renal disease susceptibility and hypertension are under independent genetic control in the fawn-hooded rat. Nat Genet 12: 44–51, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Campese VM Salt sensitivity in hypertension. Hypertension 23: 531–550, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Cowley AW, Liang M, Roman RJ, Greene AS, Jacob HJ. Consomic rat model systems for physiological genomics. Acta Physiol Scand 181: 585–592, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Cowley AW, Roman RJ. The role of the kidney in hypertension. JAMA 275: 1581–1589, 1996. [PubMed] [Google Scholar]
- 6.Cowley AW, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Deng AY, Dene H, Rapp JP. Congenic strains for the blood pressure quantitative trait locus on rat chromosome 2. Hypertension 30: 199–202, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Dutil J, Eliopoulos V, Tremblay J, Hamet P, Charron S, Deng AY. Multiple quantitative trait loci for blood pressure interacting epistatically and additively on Dahl rat chromosome 2. Hypertension 45: 557–564, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Feldman HI, Klag MJ, Chiapella AP, Whelton PK. End-stage renal disease in US minority groups. Am J Kid Dis 19: 397–410, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Garrett MR, Dene H, Rapp JP. Time-course genetic analysis of albuminuria in Dahl salt-sensitive rats on low-salt diet. J Am Soc Nephrol 14: 1175–1187, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Garrett MR, Dene H, Walder R, Zhang QY, Cicila GT, Assadnia S, Deng AY, Rapp JP. Genome scan and congenic strains for blood pressure QTL using Dahl salt-sensitive rats. Genome Res 8: 711–723, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Garrett MR, Joe B, Dene H, Rapp JP. Identification of blood pressure quantitative trait loci that differentiate two hypertensive strains. J Hypertens 20: 2399–2408, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Garrett MR, Rapp JP. Defining the blood pressure QTL on Chromosome 7 in Dahl rats by a 177-kb congenic segment containing Cyp11b1. Mammalian Genome 14: 268–273, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Grim CE, Wilson TW, Nicholson GD, Hassell TA, Fraser HS, Grim CM, Wilson DM. Blood pressure in blacks. Hypertension 15: 803–809, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Guyton AC, Coleman TG, Cowley AW Jr, Manning RD Jr, Norman RA Jr, Ferguson JD. A systems analysis approach to understanding long-range arterial blood pressure control and hypertension. Circ Res 35: 159–176, 1974. [Google Scholar]
- 16.Guyton AC, Coleman TG, Granger HJ. Circulation: overall regulation. Ann Rev Physiol 34: 13–46, 1972. [DOI] [PubMed] [Google Scholar]
- 17.Lackland DT, Keil JE. Epidemiology of hypertension in African Americans. Sem Nephrol 16: 63–70, 1996. [PubMed] [Google Scholar]
- 18.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Cowley AW Jr, Jacob HJ. Chromosomal mapping of the genetic basis of hypertension and renal disease in FHH rats. Am J Physiol Renal Physiol 293: F1905–F1914, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW Jr. Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiol Genomics 16: 194–203, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Mattson DL, Kunert MP, Roman RJ, Jacob HJ, Cowley AW Jr. Substitution of chromosome 1 ameliorates l-NAME-hypertension and renal disease in the fawn hooded hypertensive rat. Am J Physiol Renal Physiol 288: F1015–F1022, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Mehr AP, Siegel AJ, Kossmehl P, Schulz A, Plehm R, de Bruijn JA, deHeer E, Kreutz R. Early onset albuminuria in Dahl rats is a polygenetic trait that is independent from salt loading. Physiol Genomics 14: 209–216, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Moreno C, Dumas P, Kaldunski ML, Tonellato PJ, Greene AS, Roman RJ, Cheng O, Wang Z, Jacob HJ, Cowley AW Jr. Genomic map of cardiovascular phenotypes of hypertension in female Dahl S rats. Physiol Genomics 15: 243–257, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Moreno C, Kaldunski ML, Wang T, Roman RJ, Greene AS, Lazar J, Jacob HJ, Cowley AW Jr. Multiple blood pressure loci on rat chromosome 13 attenuate development of hypertension in the Dahl S hypertensive rat. Physiol Genomics 31: 228–235, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Moujahidine M, Dutil J, Hamet P, Deng AY. Congenic mapping of a blood pressure QTL on Chromosome 16 of Dahl rats. Mammalian Genome 13: 153–156, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Nadeau JH, Singer JB, Matin A, Lander ES. Analyzing complex genetic traits with chromosome substititution strains. Nature Genetics 24: 221–225, 2000. [DOI] [PubMed] [Google Scholar]
- 26.PhysGen. Program for genomic applications: physiogenomics of stressors in derived consomic rats [Online]. Milwaukee, WI: The Medical College of Wisconsin. http://pga.mcw.edu/.
- 27.Rapp JP Genetic analysis of inherited hypertension in the rat. Physiol Rev 80: 135–172, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Rat Genome Data. Rat Genome Database Web Site [Online], Milwaukee, WI: The Medical College of Wisconsin. http://rgd.mcw.edu/[October, 2007].
- 29.Rostand GS, Kirk KA, Rutsky EA, Pate BA. Racial differences in the incidence of treatment for end-stage renal disease. N Engl J Med 306: 1276–1279, 1982. [DOI] [PubMed] [Google Scholar]
- 30.Shiozawa M, Provoost AP, van Dokkum RPE, Majewski RR, Jacob HJ. Evidence of gene-gene interactions in the genetic susceptibility to renal impairment after unilateral nephrectomy. J Am Soc Nephrol 11: 2068–2078, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Siegel AK, Planert M, Rademacher S, Mehr AP, Kossmehl P, Wehland M, Stoll M, Kreutz R. Genetic loci contribute to the progression of vascular and cardiac hypertrophy in salt-senstivie spontaneous hypertension. Arterioscler Thromb Vasc Biol 23: 1211–1217, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, Justice M, O'Brien WE, Conti DV, Witte JS, Lander ES, Nadeau JH. Genetic dissection of complex traits with chromosome substitution strains of mice. Science 304: 445–448, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Singer JB, Hill AE, Nadeau JH, Lander ES. Mapping quantitative trait loci for anxiety in chromosome substitution strains of mice. Genetics 169: 855–862, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stec DE, Deng AE, Rapp JP, Roman RJ. Cytochrome P4504A genotype cosegregates with hypertension in Dahl S rats. Hypertension 27: 564–568, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Stoll M, Cowley AW Jr, Tonellato PJ, Greene AS, Kaldunski ML, Roman RJ, Dumas P, Schork NJ, Wang Z, Jacob HJ. A genomic-systems biology map for cardiovascular function. Science 294: 1723–1726, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Tobian L, Lange J, Iwai J, Hiller K, Johnson MA, Goossens P. Prevention with thiazide of NaCl-induced hypertension in Dahl S rats. Hypertension 1: 316–323, 1979. [DOI] [PubMed] [Google Scholar]
- 37.Wendt N, Schulz A, Siegel AK, Weiss J, Wehland M, Sietmann A, Kossmehl P, Grimm D, Stoll M, Kreutz R. Rat chromosome 19 transfer from SHR ameliorates hypertension, salt-sensitivity, cardiovascular and renal organ damage in salt-sensitive Dahl rats. J Hypertens 25: 95–102, 2007. [DOI] [PubMed] [Google Scholar]