Abstract
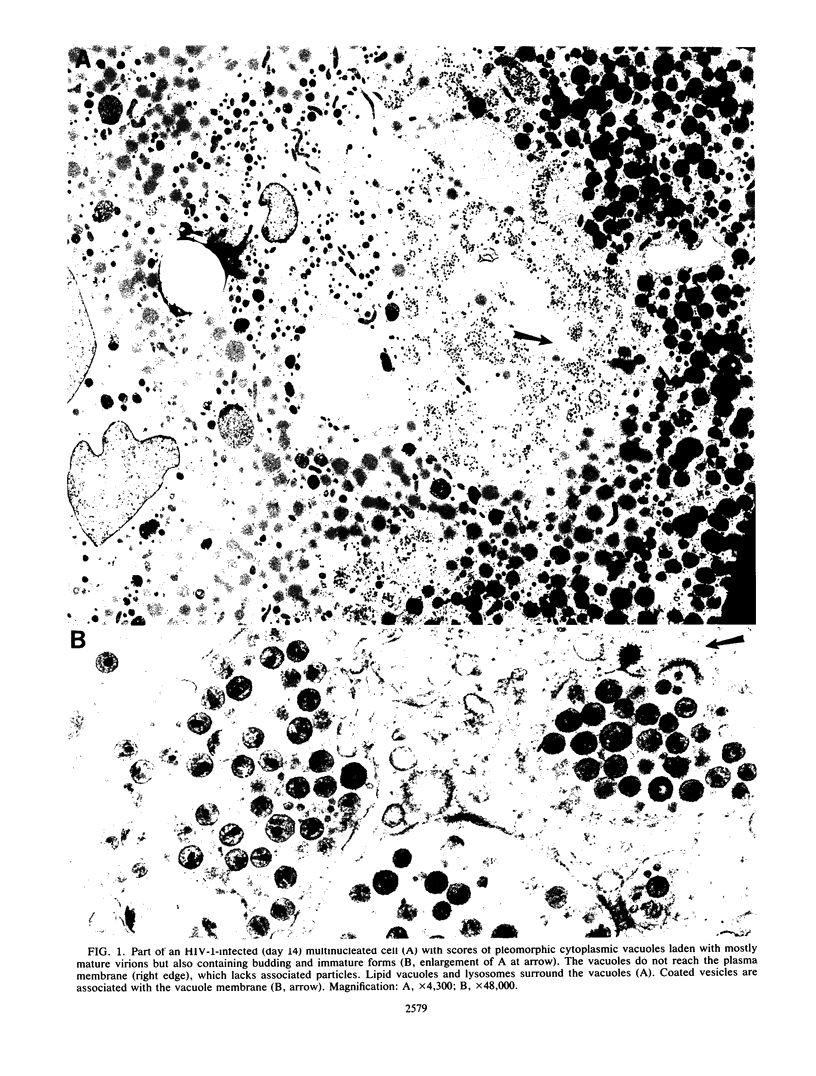
Recombinant human colony-stimulating factor-1-treated human peripheral blood-derived monocytes-macrophages are efficient host cells for recovery of the human immunodeficiency virus (HIV) from blood leukocytes of patients with acquired immunodeficiency syndrome. These cells can be maintained as viable monolayers for intervals exceeding 3 months. Infection with HIV resulted in virus-induced cytopathic effects, accompanied by relatively high levels of released progeny virus, followed by a prolonged low-level release of virus from morphologically normal cells. In both acutely and chronically infected monocytes, viral particles were seen budding into and accumulating within cytoplasmic vacuoles. The number of intravacuolar virions far exceeded those associated with the plasma membrane, especially in the chronic phase, and were concentrated in the perinuclear Golgi zone. In many instances, the vacuoles were identified as Golgi elements. Fusion of virus-laden vacuoles with primary lysosomes were rare. The pattern of cytoplasmic assembly of virus was observed with both HIV types 1 and 2 and in brain macrophages of an individual with acquired immunodeficiency syndrome encephalopathy. Immunoglobulin-coated gold beads added to acutely infected cultures were segregated from the vacuoles containing virus; relatively few beads and viral particles colocalized. The assembly of HIV virions within vacuoles of macrophages is in contrast to the exclusive surface assembly of HIV by T lymphocytes. Intracytoplasmic virus hidden from immune surveillance in monocytes-macrophages may explain, in part, the persistence of HIV in the infected human host.
Full text
PDF

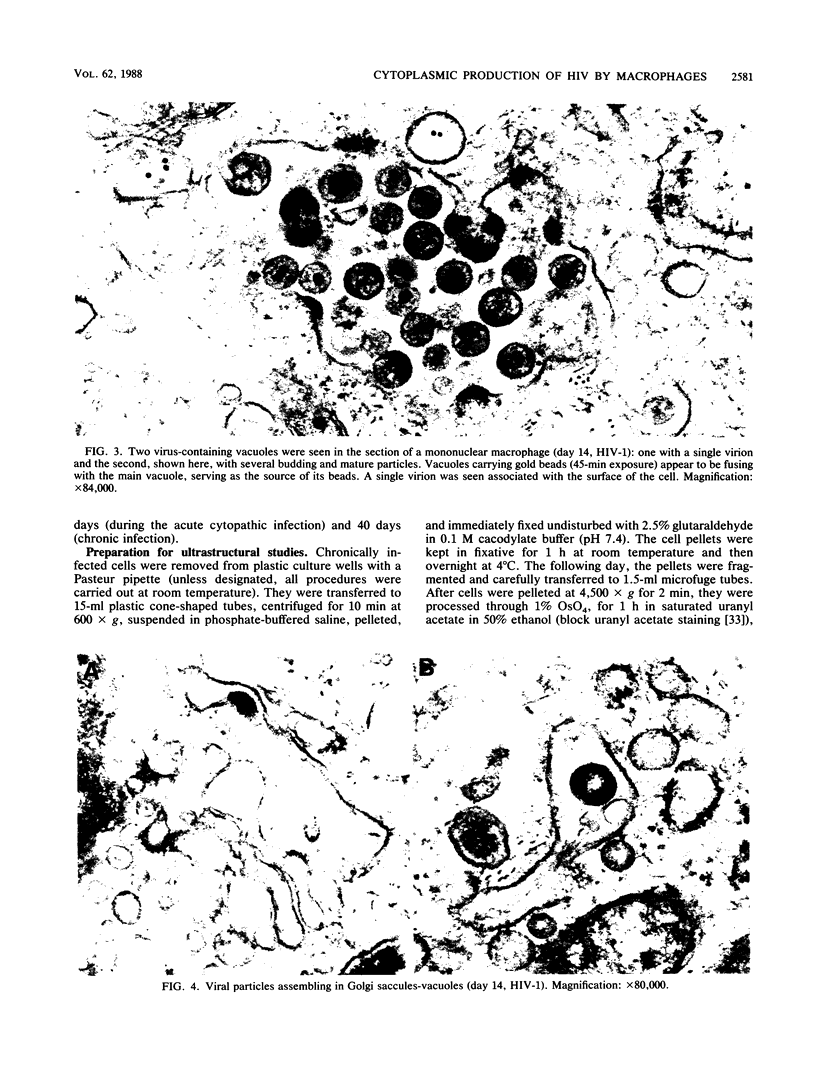
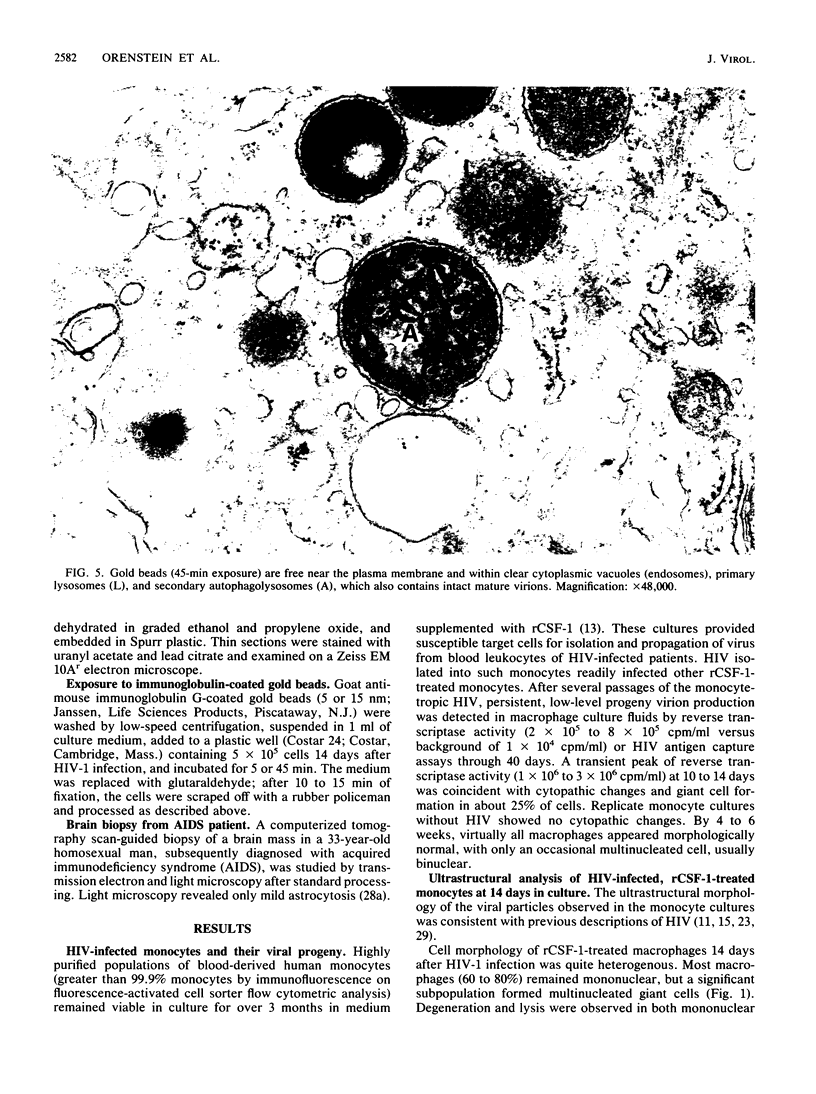
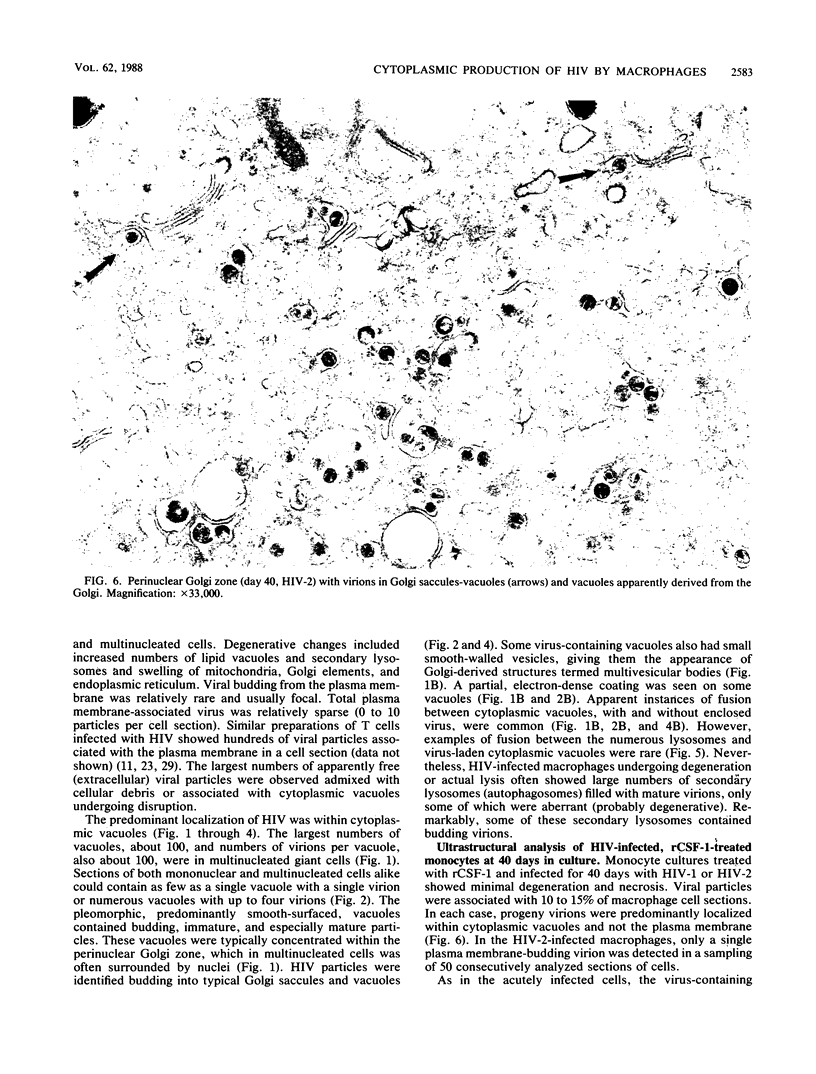
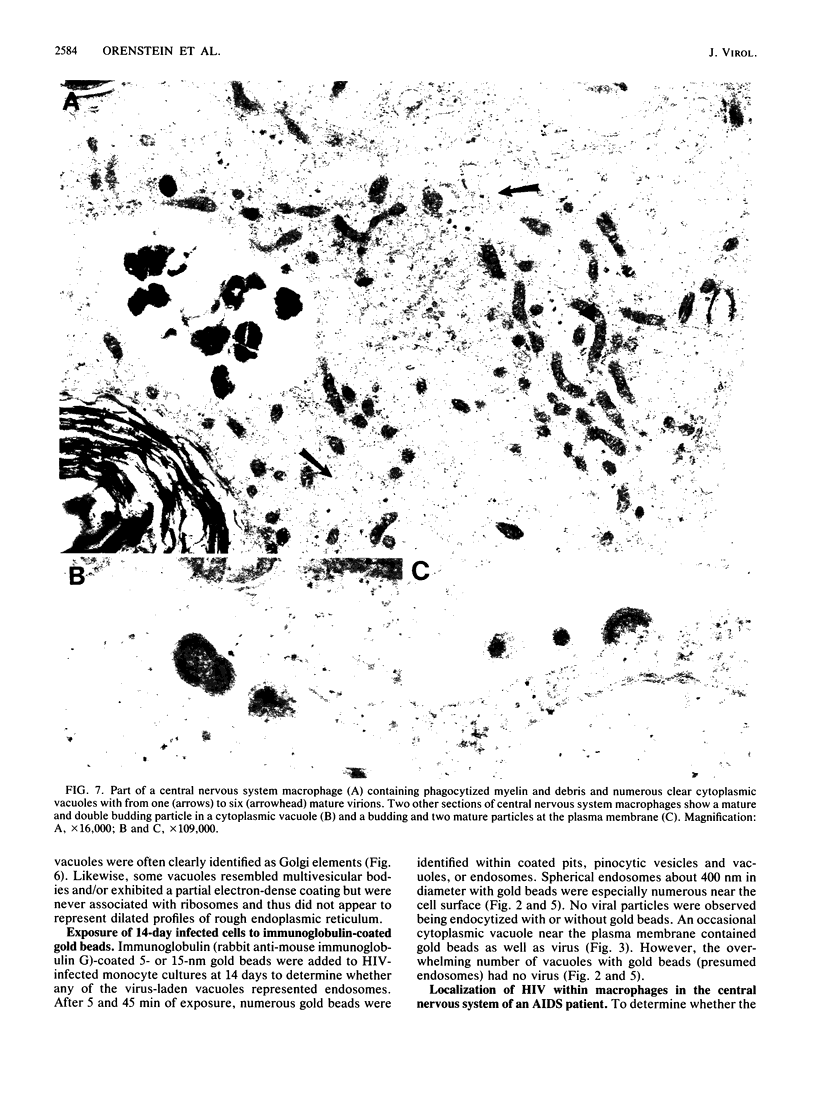


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouillant A. M., Becker S. A. Ultrastructural comparison of Oncovirinae (type C), Spumavirinae, and Lentivirinae: three subfamilies of Retroviridae found in farm animals. J Natl Cancer Inst. 1984 May;72(5):1075–1084. [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Crawford T. B., Adams D. S., Cheevers W. P., Cork L. C. Chronic arthritis in goats caused by a retrovirus. Science. 1980 Feb 29;207(4434):997–999. doi: 10.1126/science.6153243. [DOI] [PubMed] [Google Scholar]
- Crowe S., Mills J., McGrath M. S. Quantitative immunocytofluorographic analysis of CD4 surface antigen expression and HIV infection of human peripheral blood monocyte/macrophages. AIDS Res Hum Retroviruses. 1987 Summer;3(2):135–145. doi: 10.1089/aid.1987.3.135. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Gaskin J. M., Perk K. Morphological and immunological comparison of caprine arthritis encephalitis and ovine progressive pneumonia viruses. J Virol. 1981 Sep;39(3):914–919. doi: 10.1128/jvi.39.3.914-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhurst S., Sakai K., Bresser J., Stevenson M., Evinger-Hodges M. J., Volsky D. J. Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV. J Virol. 1987 Dec;61(12):3774–3782. doi: 10.1128/jvi.61.12.3774-3782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D. S. Cytochemical staining of multivesicular body and golgi vesicles. J Cell Biol. 1969 Apr;41(1):269–279. doi: 10.1083/jcb.41.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Betts R. F., Popovic M. Virus isolation from and identification of HTLV-III/LAV-producing cells in brain tissue from a patient with AIDS. JAMA. 1986 Nov 7;256(17):2365–2371. [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Hausmann E. H., Ozel M., Pauli G., Koch M. A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987 Jan;156(1):171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Martin M. A., Ferrua C., Mitra R., Phipps T., Wahl L. A., Lane H. C., Fauci A. S., Burke D. S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988 Apr 1;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda M. A., Charman H. P., Walker J. L., Coggins L. Scanning and transmission electron microscopic study of equine infectious anemia virus. Am J Vet Res. 1978 May;39(5):731–740. [PubMed] [Google Scholar]
- Gonda M. A., Wong-Staal F., Gallo R. C., Clements J. E., Narayan O., Gilden R. V. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science. 1985 Jan 11;227(4683):173–177. doi: 10.1126/science.2981428. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxie J. A., Haggarty B. S., Rackowski J. L., Pillsbury N., Levy J. A. Persistent noncytopathic infection of normal human T lymphocytes with AIDS-associated retrovirus. Science. 1985 Sep 27;229(4720):1400–1402. doi: 10.1126/science.2994222. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H. Acid-vesicle function, intracellular pathogens, and the action of chloroquine against Plasmodium falciparum. N Engl J Med. 1987 Aug 27;317(9):542–549. doi: 10.1056/NEJM198708273170905. [DOI] [PubMed] [Google Scholar]
- Lairmore M. D., Akita G. Y., Russell H. I., DeMartini J. C. Replication and cytopathic effects of ovine lentivirus strains in alveolar macrophages correlate with in vivo pathogenicity. J Virol. 1987 Dec;61(12):4038–4042. doi: 10.1128/jvi.61.12.4038-4042.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn R. J., Marx P. A., Yamamoto J. K., Gardner M. B. Ultrastructural comparison of the retroviruses associated with human and simian acquired immunodeficiency syndromes. Lab Invest. 1985 Aug;53(2):194–199. [PubMed] [Google Scholar]
- Narayan O., Cork L. C. Lentiviral diseases of sheep and goats: chronic pneumonia leukoencephalomyelitis and arthritis. Rev Infect Dis. 1985 Jan-Feb;7(1):89–98. doi: 10.1093/clinids/7.1.89. [DOI] [PubMed] [Google Scholar]
- Narayan O., Kennedy-Stoskopf S., Sheffer D., Griffin D. E., Clements J. E. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun. 1983 Jul;41(1):67–73. doi: 10.1128/iai.41.1.67-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Navia B. A., Price R. W. The acquired immunodeficiency syndrome dementia complex as the presenting or sole manifestation of human immunodeficiency virus infection. Arch Neurol. 1987 Jan;44(1):65–69. doi: 10.1001/archneur.1987.00520130051017. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Cross G. D., Callaway C. S., McDougal J. S. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J Immunol. 1986 Jul 1;137(1):323–329. [PubMed] [Google Scholar]
- Orenstein J. M., Jannotta F. Human immunodeficiency virus and papovavirus infections in acquired immunodeficiency syndrome: an ultrastructural study of three cases. Hum Pathol. 1988 Mar;19(3):350–361. doi: 10.1016/s0046-8177(88)80531-8. [DOI] [PubMed] [Google Scholar]
- Palmer E., Sporborg C., Harrison A., Martin M. L., Feorino P. Morphology and immunoelectron microscopy of AIDS virus. Arch Virol. 1985;85(3-4):189–196. doi: 10.1007/BF01314230. [DOI] [PubMed] [Google Scholar]
- Peluso R., Haase A., Stowring L., Edwards M., Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985 Nov;147(1):231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Popovic M., Gartner S. Isolation of HIV-1 from monocytes but not T lymphocytes. Lancet. 1987 Oct 17;2(8564):916–916. doi: 10.1016/s0140-6736(87)91403-6. [DOI] [PubMed] [Google Scholar]
- Quinn T. C., Mann J. M., Curran J. W., Piot P. AIDS in Africa: an epidemiologic paradigm. Science. 1986 Nov 21;234(4779):955–963. doi: 10.1126/science.3022379. [DOI] [PubMed] [Google Scholar]
- Serwadda D., Mugerwa R. D., Sewankambo N. K., Lwegaba A., Carswell J. W., Kirya G. B., Bayley A. C., Downing R. G., Tedder R. S., Clayden S. A. Slim disease: a new disease in Uganda and its association with HTLV-III infection. Lancet. 1985 Oct 19;2(8460):849–852. doi: 10.1016/S0140-6736(85)90122-9. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima M., Nakajima H., Ito Y. Electron microscopy of equine infectious anemia virus. J Virol. 1969 Oct;4(4):521–527. doi: 10.1128/jvi.4.4.521-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschachler E., Groh V., Popovic M., Mann D. L., Konrad K., Safai B., Eron L., diMarzo Veronese F., Wolff K., Stingl G. Epidermal Langerhans cells--a target for HTLV-III/LAV infection. J Invest Dermatol. 1987 Feb;88(2):233–237. doi: 10.1111/1523-1747.ep12525402. [DOI] [PubMed] [Google Scholar]
- Wahl L. M., Katona I. M., Wilder R. L., Winter C. C., Haraoui B., Scher I., Wahl S. M. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions by flow cytometric analysis. Cell Immunol. 1984 May;85(2):373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]