Abstract
Deficiency of nitric oxide (NO) represents a consistent manifestation of endothelial dysfunction (ECD), and the accumulation of asymmetric dimethylarginine occurs early in renal disease. Here, we confirmed in vitro and in vivo the previous finding that a fragment of collagen XVIII, endostatin, was upregulated by chronic inhibition of NO production and sought to support a hypothesis that primary ECD contributes to nephrosclerosis in the absence of other profibrotic factors. To emulate more closely the indolent course of ECD, the study was expanded to an in vivo model with NG-monomethyl-l-arginine(l-NMMA; mimics effects of asymmetric dimethylarginine) administered to mice in the drinking water at subpressor doses of 0.3 and 0.8 mg/ml for 3–6 mo. This resulted in subtle but significant morphological alterations detected in kidneys of mice chronically treated with l-NMMA: 1) consistent perivascular expansion of interstitial matrix components at the inner stripe of the outer medulla and 2) collagen XVIII/endostatin abundance. Ultrastructural abnormalities were detected in l-NMMA-treated mice: 1) increased activity of the interstitial fibroblasts; 2) occasional detachment of endothelial cells from the basement membrane; 3) splitting of the vascular basement membrane; 4) focal fibrosis; and 5) accumulation of lipofuscin by interstitial fibroblasts. Preembedding labeling of microvasculature with anti-CD31 antibodies showed infiltrating leukocytes and agglomerating platelets attaching to the visibly intact or denuded capillaries. Collectively, the data indicate that the mouse model of subpressor chronic administration of l-NMMA is not a robust one (endothelial pathology visible only ultrastructurally), and yet it closely resembles the natural progression of endothelial dysfunction, microvascular abnormalities, and associated tubulointerstitial scarring.
Keywords: endothelial dysfunction, interstitial scarring, l-NMMA, ADMA
traditional risk factors, together with hypertension, oxidative stress, proinflammatory changes, hyperhomocysteinemia, and anemia, have been invoked to explain the unusually accelerated development of endothelial cell dysfunction in chronic renal failure (reviewed in Ref. 18). Perhaps the most powerful independent contributor to the process is asymmetric dimethylarginine (ADMA), which is elevated even before the decrease in its excretion due to the decline of renal function. This guanidino compound, fulfilling many characteristics of uremic toxin, is elevated in the course of renal disease and has been found to be the second most valuable (after the patient's age) predictor of cardiovascular events and mortality in dialysis patients, as well as in the general population (53, 54, 59). This potent inhibitor of nitric oxide synthases (NOS; competing with the substrate l-arginine) is a natural product of degradation of methylarginine residues in various proteins, generating daily >60 mg of ADMA, of which 50 mg are metabolized by dimethylarginine dimethylaminohydrolases and the rest is excreted in the urine (56). Vascular endothelium is extremely sensitive to ADMA: infusion of ADMA elevates blood pressure and peripheral resistance at concentrations equivalent to those seen under pathological conditions (2). Inhibition of endothelial NOS (eNOS) and/or deficiency of bioavailable NO are the most consistent causes of endothelial cell dysfunction, leading to vasculopathy and defective angiogenesis (18, 19, 20, 37).
The indolence of the course with which tubulointerstitial scarring commences and progresses in the majority of renal diseases presents logistical and technical problems to the efforts to conclusively identify the underlying pathogenetic mechanisms of this process. Most existing models of renal disease in laboratory animals display either a reversible or a fulminant course (13), making it difficult to faithfully mimic progression of actual chronic renal disease.
We addressed the question related to the potential role played by endothelial cell dysfunction in the progression of tubulointerstitial disease: i.e., does primary disturbance of endothelial function contribute to the development of nephrosclerosis? An early loss of peritubular capillaries (8) and injury to endothelial cells (29) have been proposed as mechanisms of progressive renal disease. Studies from Johnson's laboratory provide solid experimental evidence in support of the concept that microvascular injury and chronic ischemia are prerequisites to progression of fibrosis in etiologically diverse renal diseases (24–27). These studies also demonstrated decreased proliferation and increased apoptosis of renal microvascular endothelial cells (38, 39) as the structural basis for capillary drop-out.
Based on the body of evidence that insufficient generation or bioavailability of nitric oxide (NO) represents one of the most consistent manifestations of endothelial dysfunction generally and that an accumulation of a potent NOS inhibitor, ADMA, occurs early in renal disease (59), attempts to pharmacologically suppress NO generation while monitoring renal function have been made previously (5). While the pressor doses of NOS inhibitors produced a relatively rapid tubulointerstitial scarring in rats, subpressor doses caused no discernible or a minimal tubulointerstitial disease in mice (41). We have argued that a compound used to inhibit NO production in those experiments, NG-nitro-l-arginine methyl ester (l-NAME), does not fully mimic the action of ADMA in that l-NAME does not uncouple eNOS to generate superoxide anions (1), as the NG-monomethyl-l-arginine (l-NMMA) does. Therefore, in the current series of experiments subpressor doses of l-NMMA, an inhibitor that produces eNOS uncoupling similar to the action of ADMA, were used for chronic administration in mice. We performed a thorough morphological characterization of this model and report here an array of delicate morphological manifestations gradually occurring in mice subjected to such treatment: mild tubulointerstitial scarring in the inner stripe of outer medulla along the vascular bundles, abnormalities in microvascular endothelium, and the appearance of platelet aggregates and invading leukocytes, all consistent with developing endothelial dysfunction.
MATERIALS AND METHODS
Animals, treatment protocols, and tissue preservation.
Adult 129J mice were obtained from Charles River Laboratories. All procedures were approved in advance by the Berlin Senate (registration no. G0178/03). Animals were cared for in accordance with the American Physiological Society Guiding Principles in the Care and Use of Animals and the German law for animal protection. The animals were housed in the Charité animal facility with free access to standard laboratory diet and tap water. After an adjustment period, the animals were divided into five groups (n = 9 each); in each group, four mice were kept for morphological evaluation and five mice for biochemical evaluation. For treatment, animals received l-NMMA (Axxora; 0.3 or 0.8 mg/ml tap water) for 3 or 6 mo. Control animals received tap water throughout. Blood pressure was measured by tail-cuff plethysmography as previously described (47). Mice randomly assigned for morphological evaluation were treated for 3 or 6 mo and received an intraperitoneal injection of 60 mg/kg pimonidazole (Hypoxyprobe; Chemicon) 30 min before death. Mice were subsequently perfusion-fixed via the abdominal aorta using 3% paraformaldehyde (PFA), and kidney samples were prepared for electron microscopy, as well as paraffin and cryostat sectioning. Mice randomly assigned for biochemical analysis were treated for 3 mo with high- or low-dose l-NMMA or vehicle, respectively. At the end of the treatment period, mice were killed and the kidneys were removed and immediately frozen in liquid nitrogen.
Histochemistry.
Masson trichrome staining was routinely performed on 4-μm-thick paraffin sections. Immunostaining was performed on 5-μm-thick cryostat sections blocked in 5% milk powder dissolved in PBS as described (10). Antibodies were diluted in PBS. The following antibodies and concentrations were applied: rat anti-CD31 (1:50; BD Pharmingen) and goat anti-endostatin (1:200; R&D systems). After overnight incubation at 4°C, sections were washed and further incubated with appropriate horseradish peroxidase-conjugated secondary antibodies. For quantitative evaluation of Masson trichrome-stained sections, four adjacent areas of the renal medulla were photographed and evaluated by counting the number of focal matrix expansion sites. Counts were normalized for examined areas.
Images were quantified using ImageJ, a Java-based image processing program (http://rsb.info.nih.gov/ij/download.html) developed by the National Institutes of Health. Once histochemical images were uploaded onto ImageJ, all images were changed to eight-bit binary images. Next, threshold values were adjusted for all images. Selected threshold values were kept constant for all images to standardize the amount of background included in quantification. Next, we used a routine for particle analysis allowing the selection of the size and shape of brown-stained particles within images to be quantified. The size of particles included in measurements were 0–infinity (pixel^2), and circularity was 0.00–1.00. Using these routines, we obtained the results of the integrated density measurements. Integrated density is the sum of the values of the pixels in an image, or in other words, it is equivalent to the product of area and the mean brown-stained value.
Pimonidazole immunostaining was performed employing a Hypoxyprobe Plus kit (Chemicon) on 5-μm-thick cryostat sections.
Ultrastructure.
For fine structural morphology, kidney slices were postfixed overnight in a solution containing 1.5% glutaraldehyde, 1.5% PFA, and 0.05% picric acid in 0.1 M Na-cacodylate (pH 7.4). After Epon embedding, 1-μm semithin sections were cut and stained with Richardson's solution. For electron microscopic studies, ultrathin sections poststained with uranyl acetate and lead citrate were analyzed in a Zeiss EM 900 electron microscope (Zeiss, Oberkochen, Germany). For preembedding immunoperoxidase labeling, 30-μm-thick cryostat sections were stained with CD31 antibody (dilution 1:20) and processed for electron microscopy as previously described (10).
Cell culture.
Mouse cultured endothelial cells from myocardial microvasculature (MyEnd) cells were used (58). Cells were grown in DMEM supplemented with 4.5 g/l glucose, 10% FCS, and 0.5% penicillin/streptomycin. For immunocytochemistry, cells were grown on coverslips for 3–7 days and treated subsequently for 24 or 48 h with l-NMMA (1 mM final concentration in culture medium) or vehicle. Cells were fixed with 3% PFA in PBS for 10 min, washed in PBS, and incubated with anti-endostatin antibody (1:400). Bound antibody was detected using a Cy3-labeled donkey anti-goat secondary antibody. Nuclei were visualized by 4,6-diamidino-2-phenylindole staining (Abcam).
Western blot analysis.
MyEnd cells were grown on gelatin-coated petri dishes until subconfluence, treated for 24 or 48 h with l-NMMA (final concentration 1 mM) or vehicle, and subsequently lysed for 30 min in RIPA buffer (50 mM Tris·HCl, 1% IGPAL, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA) supplemented with protease inhibitor cocktail. Homogenates were centrifuged for 10 min at 1,000 g to remove cell nuclei and detritus. The supernatants were subjected to PAGE, applying 50 μg protein/lane as determined by a BCA protein assay kit (Pierce). After electrophoretic transfer to a polyvinylidene fluoride membrane, equity in protein loading and blotting was verified by membrane staining using 0.1% Ponceau red staining. Membranes were immersed in 5% nonfat dry milk/PBS to block nonspecific protein binding sites and incubated with goat endostatin (dilution 1:400; R&D Systems) and with an antibody to the housekeeping gene, β-actin, using a monoclonal antibody (dilution 1:200; Sigma). After the membranes were rinsed in PBS, bound antibodies were detected using the appropriate horseradish peroxidase-conjugated secondary antibodies. Bands were visualized using ECL Western Blotting Substrate (GE Healthcare). Developed X-ray films were scanned and densitometrically evaluated. VEGF Western blot was performed on whole kidney lysates of l-NMMA-treated mice and controls as described above, using a rabbit VEGF antibody (1:200; Santa Cruz Biotechnology).
Statistics.
Students t-test was used with P < 0.05. Differences in the morphological staining patterns and signal intensities were evaluated in a blinded fashion by two individuals.
RESULTS
Inhibition of eNOS in cultured mouse endothelial cells.
In our previous work, human umbilical vein endothelial cells showed upregulation of collagen XVIII and its antiangiogenic fragment endostatin after treatment with NOS inhibitor l-NAME (41). It is not clear, however, whether this response is common for endothelial cells or is species specific and whether it can be elicited using another NOS inhibitor, l-NMMA, a perfect ADMA mimetic. Therefore, experiments were conducted in immortalized mouse endothelial cells, MyEnd, subjected to l-NMMA. As shown in Figs. 1 and 2, l-NMMA resulted in a gradual increase in endostatin abundance. Representative images (Fig. 1) show that sporadic endothelial cells faintly expressed collagen XVIII and endostatin under baseline conditions: a low-intensity fluorescence signal can be seen mainly in the cytosol. In contrast, 24- to 48-h treatment with l-NMMA resulted in endostatin expression by a majority of endothelial cells, with the predominant localization at the periphery of the cells and the extracellular matrix. Western blotting confirmed these findings, documenting a three- to fourfold increase in the expression of endostatin (Fig. 2). Furthermore, this pure population of endothelial cells devoid of contaminating smooth muscle cells (which is unachievable in primary cultured human umbilical vein endothelial cells) showed transiently enhanced expression of α-SMA, a marker of myofibroblastic transformation of endothelial cells. The similar phenomenon was previously observed in human umbilical vein endothelial cells (41). Hence, the upregulation of collagen XVIII and its antiangiogenic fragment endostatin appear to be consistently detected in macro- (41) and microvascular (this study) endothelial cells obtained from different species and subjected to mechanistically diverse NOS inhibitors, suggesting that the observed phenomenon may represent a part of a general default vascular response to NO deficiency.
Fig. 1.
Immunofluorescence detection of endostatin in cultured MyEnd endothelial cells. Increased endostatin immunoreactive signal is shown in NG-monomethyl-l-arginine (l-NMMA; 1 mM)-treated cells [control (A); l-NMMA (24-h treatment; B) and 48-h treatment (C)]. Magnification ×1,000.
Fig. 2.
Semiquantitative Western blot evaluation of endostatin expression in control and l-NMMA-treated MyEnd cultured endothelial cells. Top: results of Western blotting show elevated expression of endostatin in l-NMMA-treated cells (vehicle control; 1 mM l-NMMA, 24 and 48 h). Representative blots of endostatin and corresponding β-actin are shown in duplicate. Bottom: semiquantitative densitometric evaluation. *P < 0.05 relative to controls.
Morphological studies in mice chronically treated with l-NMMA.
Subpressor doses of l-NMMA (0.8 mg/ml of drinking water) resulted in the maintenance of arterial blood pressure (Fig. 3). This finding provides indirect evidence that, despite the inhibition of constitutive NOS, chronic administration of l-NMMA induced only marginal NO deficiency that did not cause hypertension. Importantly, trichrome staining of the kidneys revealed a consistent perivascular expansion of interstitial matrix components mainly at the inner stripe of the outer medulla (Fig. 4) in mice with chronic administration of l-NMMA. Immunohistochemical staining (Fig. 5) showed that collagen XVIII and endostatin, nearly absent in control kidneys, were abundant in the kidneys of mice treated with a subpressor doses of l-NMMA (0.8 mg/ml of drinking water). Immunohistochemical images of CD31-labeled endothelial cells (Fig. 6) revealed no statistical difference in the microvascular density between control and low-dose medulla, which reached significance between control and high-dose l-NMMA treatment groups.
Fig. 3.
Blood pressure. Blood pressure was measured by tail-plethysmography. Values were similar in control animals (filled bar) and long term-high-dose l-NMMA-treated mice (open bar).
Fig. 4.
Trichrome staining. Top: Masson-Goldner trichrome staining showing focal matrix accumulations in kidneys of low- and high-dose l-NMMA-treated mice. Perivascular and peritubular areas of matrix accumulation are stained green. A and B: overviews. Vascular bundles of inner stripe as used for the numerical evaluation are shown at the bottom. C and E: interbundle region. Peritubular matrix accumulations are shown as exemplified around a labeled collecting duct (*). D and F: vascular bundle with focal matrix accumulations near single vasa recta profiles as exemplified for 1 vessel (*). Bottom: quantification of histochemical distribution of focal fibrotic areas as shown in A and B. *P < 0.05.
Fig. 5.
Endostatin staining for immunoreactivity. Immunoperoxidase staining of control (A, C, E, and G) and long-term, high dose l-NMMA-treated animals (B, D, F, and H) show an interstitial staining pattern in the cortex around the glomerular capsule (A and B), outer stripe proximal tubule segments (C and D), in the inner stripe interbundle region (E and F), and in the vascular bundles (G and H). Counterstaining was done with hematoxylin. Original magnification ×1,000.
Fig. 6.
CD31 staining for CD31 immunoreactivity. Top: immunoperoxidase staining of the inner medulla of control (A) and low-dose (B) and high-dose (C) l-NMMA-treated animals. Original magnification ×400. Captured images were digitally analyzed using the National Institutes of Health ImageJ program. Bottom: quantification of endothelial cell density as shown in A–C. *P < 0.05.
Immunostaining of pimonidazole adducts (Fig. 7) was used to label areas with Po2 lower than 10 mmHg. Staining intensity showed uneven distribution, with generally weak cortical staining limited to the medullary rays (Fig. 7, A and B). In the outer medulla, immunoreactive pimonidazole was localized to cells of the thick ascending limb in the inner stripe and S3 segments of the proximal tubule in the outer stripe (Fig. 7, C and D). Staining in the central part of the inner medulla showed pimonidazole immunoreactivity localized to epithelial cells of the collecting duct as well as to interstitial cells (Fig. 7, E and F). Comparison of staining distribution and intensity was quantified in a blinded fashion by two of the authors and revealed no significant differences between l-NMMA-treated animals and controls. Considering the fact that pimonidazole staining is not particularly sensitive to tissue hypoxia, we next measured expression of vascular endothelial growth factor (VEGF), a downstream target of hypoxia-inducible factor. As shown in Fig. 8, VEGF expression was increased in mice receiving 0.8 mg/ml l-NMMA. Densitometric analysis of data confirmed an almost twofold increase in the VEGF level.
Fig. 7.
Pimonidazole staining with anti-pimonidazole antibody and immunoperoxidase staining. Bound pimonidazole is shown in cortex of control (A, C, and E) and long-term, high dose l-NMMA-treated animals (B, D, and F) showing positive signals in proximal tubular profiles (A and B) and thick ascending limbs of outer medulla (C and D) and in collecting ducts of inner medulla (E and F). Original magnification ×1,000.
Fig. 8.
VEGF Western blot. Top: Western blot analysis showing VEGF protein expression in whole kidney extracts of animals treated with low- or high-dose l-NMMA for 3 mo compared with controls. Bottom: quantification and normalization for β-actin revealed an increased amount of VEGF in the high-dose-treated animals compared with controls. VEGF expression in low-dose-treated animals was not altered. *P < 0.05.
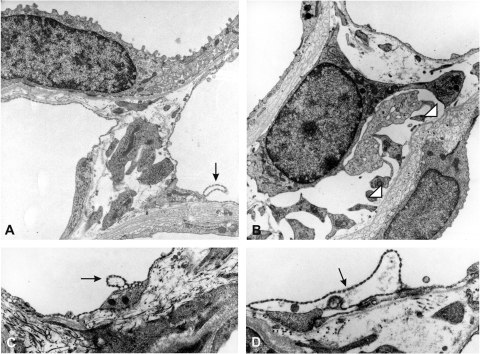
Based on increases in histochemical trichrome staining in interstitial areas of the medulla near the vascular axes under chronic subpressor l-NMMA treatment, high-resolution aspects of these areas were evaluated by electron microscopy. The overall activity of the interstitial fibroblasts appeared to be high, as judged from the high density of organelles and granules involved in endocytic machinery, and polysomes were frequent (Fig. 9, A and B). The spectrum of additional abnormalities detectable in l-NMMA-treated mouse kidneys included occasional detachment of endothelial cells from the basement membrane, forming omega-shaped protrusions and pseudolumina (Fig. 9, C and D). Some vasa recta revealed splitting and an absence of the vascular basement membrane, whereas others had retained normal-looking microvasculature. Focal fibrosis was evident by substantial increases in collagen I fibrils and amorphous matrix material (Fig. 10, A and C) . Focally, basement membrane-like material was massively increased underneath the vascular and tubular profiles of the vascular bundles. Interstitial fibroblasts of these areas appeared to be numerically increased and were containing electron-dense granules of lipofuscin, a marker of senescent cells (Ref. 52 and Fig. 10, D and E). These granules were clearly more frequent in the treated groups than in controls. Preembedding electron microscopic labeling of microvasculature with anti-CD31 antibodies showed substantial staining in the perinuclear and fenestrated areas of arterial and venous vasa recta and branching capillaries with no particular intensity differences in the treated groups, confirming the light microscopic findings (Fig. 11, C and G). The patterns described for infiltrating leukocytes were not uncommon, as were agglomerating platelets attaching to the visibly intact or denuded capillaries. Interestingly, detached portions of endothelial cells were seen attached to platelet aggregates (Fig. 11, H and I). Curiously, areas of platelet adhesion to endothelial cells appeared to be depleted of CD31 and sometimes showed deposits of collagen filaments. Endothelial flaps and omega-shaped protrusions of endothelial cells appeared to be enriched in CD31.
Fig. 9.
Ultrastructural patterns of vasa recta areas, inner stripe. A: control picture showing a thin limb on top and 2 capillary sections from vasa recta area. The capillary on the right shows a single detached endothelial blister. B–D: high-dose l-NMMA. B: centrally positioned fibroblast with dilated rough endoplasmic reticulum. Neighboring thin limb epithelia reveal layered basement membranes. C and D: vasa recta walls showing capillary detachments of fenestrated endothelium of different degrees.
Fig. 10.
Ultrastructure of fibrotic interstitial changes in vasa recta areas of inner stripe in the kidneys of high-dose l-NMMA-treated mice. A: vas rectum (center) with mild interstitial fibrosis and matrix expansion as indicated by insets in higher magnification (B and C). B: accumulations of collagen I and amorphous matrix components between fenestrated capillary endothelium (right) and fibroblast extension. C: similar, but more abundant matrix accumulation. D: extended interstitium between tubular epithelia and a capillary showing a lipofuscin-laden interstitial cell as indicated by the inset in higher magnification (E). E: lipofuscin granules are represented by dense heterolysosomal residual bodies.
Fig. 11.
Fine structural immunocytochemistry of CD31- immunoreactive endothelial labeling in vasa recta areas of inner stripe. A and B: control pictures showing intact capillary sections. Intensive CD31 staining is shown in fenestrated areas and the perinuclear region. C and D: low-dose l-NMMA. C: normal-appearing vas rectum endothelium. D: intensely CD31-positive portions of capillary endothelium showing partial detachment of fenestrated areas. E–I: high-dose l-NMMA. E: overview of a moderately CD31-positive fenestrated capillary. F and G: CD31-positive detachments of flaps from fenestrated endothelia. H: cluster of thrombocytes partially attached to CD31-positive endothelium of a venous vas rectum. I: single leukocyte attached to a CD31-positive endothelium of a venous vas rectum.
DISCUSSION
Physiological actions attributed to NO in the cardiovascular system include regulation of vascular tone, blood flow and blood pressure (4, 52), inhibition of platelet aggregation and adhesion to the blood vessel wall (3, 55), suppression of vascular inflammation (32), inhibition of vascular cell apoptosis (48), and mediation of angiogenesis (6, 30). Importantly, NO insufficiency in blood vessels has been implicated in the genesis and progression of major cardiovascular diseases, such as hypertension (43), atherosclerosis (31, 35), heart failure (37, 57), diabetic vasculopathy (7, 12), and end-stage renal disease (22, 23). It is remarkable that some of these manifestations of NO deficiency were reproducible in our model of subpressor inhibition of NOS by chronic administration of l-NMMA. Endothelial abnormalities, platelet aggregation at the microvascular wall, and occasional leukocyte-endothelial cell interactions were all observed in the kidney, together with the areas of a mild fibrosis and microvascular rarefaction. The studies presented herein provide morphological hallmarks of endothelial dysfunction and support an important viewpoint, namely, that primary chronic disturbances of the vascular endothelium can lead to the development of nephrosclerosis.
Glomerulosclerosis and tubulointerstitial scarring (TIS), the main processes governing the progression of chronic renal diseases, are well studied and described (9, 15, 28, 33, 34, 46). Some pathogenetic assumptions could be made based on therapeutic strategies proposed and used to halt the progression of nephrosclerosis: angiotensin-converting enzyme inhibition, activation of bradykinin B2 receptors, l-arginine supplementation, and a combination of lisinopril and l-arginine (14, 17, 49), which are all agonists of eNOS. Indeed, eNOS function serves as a good predictor of individual susceptibility to renal damage in subtotally nephrectomized rats, and its maintained function protects Wistar-Furth rats from chronic renal injury (45, 60).
Based on the observations made by many investigators, we have previously screened the effect of eNOS inhibition on the expression of “cardiovascular-relevant” genes in human umbilical vein endothelial cells (50, 51). An unexpected finding of upregulation of mRNA encoding several profibrotic proteins has been pursued, thus leading to the more profound in vitro and in vivo study of NOS inhibition. Indeed, previous observations in experimental animals receiving NOS inhibitors showed the development of nephrosclerosis and chronic renal insufficiency (16, 21). Since hypertension accompanying NOS inhibition could have contributed to nephrosclerosis, we generated a model of nonhypertensive nephrosclerosis, which showed regression of renal microvasculature, as previously detailed (41). The findings of increased endothelial synthesis of collagen XVIII and excessive production of its antiangiogenic fragment endostatin (41) lend additional support to the notion of the premier role played by the renal microvasculature in the progression of disease. Indeed, our data obtained in cultured microvascular endothelial MyEnd cells and in kidneys of mice chronically treated with subpressor doses of l-NMMA further this line of evidence.
Studies from Folkman's laboratory (40) have identified endostatin as a potent inhibitor of angiogenesis. It has been demonstrated that endostatin causes regression of the vasculature by inducing lysosomal dysfunction followed by autophagy (11). This is therefore remarkable in view of our TEM findings of lipofuscin accumulation, as this marker of cell senescence is intricately linked to the progression of lysosomal dysfunction and aberrant autophagy in endothelial cells subjected to conditions limiting the bioavalability of NO (44).
In view of the changes in peritubular capillary endothelium described above, it seems remarkable that pimonidazole adducts were undetectable in l-NMMA-treated kidneys. It should be kept in mind, however, that positive staining is associated with the profound reduction in tissue oxygenation. Consequently, it is reasonable to surmise that the observed endothelial abnormalities and platelet adhesion and aggregation are of a modest degree, so that the rich peritubular capillary network is capable of compensating for these abnormalities. Indeed, the data also indicate that the mouse model of subpressor chronic administration of l-NMMA is not a robust one, and yet it closely resembles the natural progression of endothelial dysfunction, microvascular abnormalities, and associated tubulointerstitial scarring. In this context, it would be of interest to explore in the future the impact of this background on the progression of renal disease in a “double-hit” model, when mild endothelial dysfunction is superimposed on renal disease.
GRANTS
These studies were supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-54602 and DK-45462 (M. S. Goligorsky) as well as SFB Grant 688, TP A4 (J. Waschke).
Acknowledgments
We thank Prof. Dr. D. Drenckhahn (Dept. of Anatomy, Würzburg University) for permission to work with MyEnd cells and Kerstin Riskowsky, Martina Gutsmann, and Petra Schrade (Dept. of Anatomy, Charité Berlin) for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abu-Soud H, Feldman P, Clark P, Stuehr D. Electron transfer in the nitric oxide synthases. Characterization of l-arginine analogs that block heme iron reduction. J Biol Chem 269: 32318–32326, 1994. [PubMed] [Google Scholar]
- 2.Achan V, Broadhead M, Malaki M, Leiper J, MacAllister R, Vallance P. ADMA causes hypertension and cardiac dysfunction in humans and is actively metabolized by DDAH. Arterioscler Thromb Vasc Biol 23: 1455–1459, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Aisaka K, Gross F, Griffith OW, Levi R. l-Arginine availability determines the duration of acetylcholine-induced systemic vasodilation in vivo. Biochem Biophys Res Commun 163: 710–717, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Aisaka K, Gross F, Griffith OW, Levi R. NG-methylarginine, an inhibitor of endothelium-derived nitric oxide synthesis, is a potent pressor agent in the guinea pig: does nitric oxide regulate blood pressure in vivo? Biochem Biophys Res Commun 160: 881–886, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest 90: 278–281, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berk BC, Abe JI, Min W, Surapisitchat J, Yan C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann NY Acad Sci 947: 93–109, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Birks EJ, Yacoub MH. The role of nitric oxide and cytokines in heart failure. Coron Artery Dis 8: 389–402, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Bohle A, Mackensen-Haen S, von Gise H. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney. Am J Nephrol 7: 421–433, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Brenner B Remission of renal disease: recounting the challenge, acquiring the goal. J Clin Invest 110: 1753–1758, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Câmpean V, Theilig F, Paliege A, Breyer M, Bachmann S. Key enzymes for renal prostaglandin synthesis: site-specific expression in rodent kidney (rat, mouse). Am J Physiol Renal Physiol 285: F19–F32, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Chau Y, Lin S, Chen J, Tai M. Endostatin induces autophagic cell death in Eahy926 human endothelial cells. Histol Histopathol 18: 715–726, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Drexler H, Kästner S, Strobel A, Studer R, Brodder OE, Hasenfuss F. Expression, activity and functional significance of inducible nitric oxide synthase in the failing human heart. J Am Coll Cardiol 32: 955–963, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Durvasula R, Shankland S. Models of glomerulonephritis. In: Renal Disease Techniques and Protocols, edited by Goligorsky MS. Totowa, NJ: Humana, 2003, p. 47–66.
- 14.Eddy A Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol 7: 2495–2508, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Eitner F, Floege J. Novel insights into renal fibrosis. Curr Opinion Nephrol Hypertens 12: 227–232, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Erdely A, Wagner L, Muller V, Szabo A, Baylis C. Protection of Wistar-Furth rat from chronic renal disease is associated with maintained renal nitric oxide synthase. J Am Soc Nephrol 14: 2526–2533, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fogo A Renal fibrosis and the renin-angiotensin system. Adv Nephrol Necker Hosp 31: 69–87, 2001. [PubMed] [Google Scholar]
- 18.Goligorsky MS Endothelial cell dysfunction and nitric oxide synthase. Kidney Int 58: 1360–1376, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Goligorsky MS, Abedi H, Noiri E, Tachtajan A, Lense S, Romanov VI, Zachary I. Nitric oxide modulation of focal adhesions in endothelial cells. Am J Physiol Cell Physiol 276: C1271–C1281, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Goligorsky MS, Noiri E, Tsukahara H, Budzikowski A, Li H. A pivotal role of nitric oxide in endothelial cell dysfunction. Acta Physiol Scand 168: 33–41, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Gschwend S, Buikema H, Navis G, Henning R, DeZeeuw D, van Dokkum R. Endothelial dilatory function predicts individual susceptibility to renal damage in 5/6 nephrectomized rat. J Am Soc Nephrol 13: 2909–2915, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Hogan M, Cerami A, Bucala R. Advanced glycosylation endproducts block the antiproliferative effect of nitric oxide. Role in the vascular and renal complications of diabetes mellitus. J Clin Invest 90: 1110–1115, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igarashi J, Michel T. More sweetness than light? A search for the causes of diabetic vasculopathy. J Clin Invest 108: 1425–1427, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang D, Joly A, Oh S, Johnson R. Impaired angiogenesis in the remnant kidney model. J Am Soc Nephrol 12: 1434–1447, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Kang D, Joly A, Mazali M, Johnson R. Impaired angiogenesis in the remnant kidney model. J Am Soc Nephrol 12: 1448–1457, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Kang D, Kanellis J, Hugo C, Johnson R. Role of microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Kang D, Nakagawa T, Feng L, Johnson R. Nitric oxide modulates vascular disease in the remnant kidney model. Am J Pathol 161: 239–248, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lan HY Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opinion Nephrol Hypertens 12: 25–29, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Lee L, Meyer T, Pollock A, Lovett D. Endothelial cell injury initiates glomerular sclerosis in the rat remnant kidney. J Clin Invest 96: 953–964, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Bombeck CA, Yang S, Kim YM, Billiar TR. Nitric oxide suppresses apoptosis via interrupting caspase activation and mitochondrial dysfunction in cultured hepatocytes. J Biol Chem 274: 17325–17333, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Morbidelli L, Donnini S, Ziche M. Role of nitric oxide in the modulation of angiogenesis. Curr Pharm Des 9: 521–530, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Mordvintsev PI, Rudneva VG, Vanin AF, Shimkevich LL, Khodorov BI. Inhibition of platelet aggregation by dinitrosyl iron complexes with low molecular weight ligands. Biokhimiia 51: 1851–1857, 1986. [PubMed] [Google Scholar]
- 33.Morris ST, McMurray JJ, Spiers A, Jardine AG. Impaired endothelial function in isolated human uremic resistance arteries. Kidney Int 60: 1077–1082, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa T, Kang D, Ohashi R, Suga S, Herrera-Acosta J, Rodriguez-Iturbe B, Johnson R. Tubulointerstitial disease: role of ischemia and microvascular disease. Curr Opinion Nephrol Hypertens 12: 233–241, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Nava E, Farré AL, Moreno C, Casado S, Mareau P, Cosentino F, Lüscher TF. Alterations to the nitric oxide pathway in the spontaneously hypertensive rat. J Hypertens 16: 609–615, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Noiri E, Lee E, Testa J, Quigley J, Colflesh D, Keese C, Giaever I, Goligorsky MS. Podokinesis in endothelial cell migration: role of NO. Am J Physiol Cell Physiol 274: C236–C244, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Oemar BS, Tschudi MR, Godoy N, Brovkovich V, Malinski T, Lüscher TF. Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation 97: 2494–2498, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Ohashi R, Kitamura H, Yamanaka N, Johnson R. Peritubular capillary injury during progression of experimental glomerulonephritis in rats. J Am Soc Nephrol 11: 47–56, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Ohashi R, Shimizu A, Masuda Y, Johnson R. Peritubular capillary regression during progression of experimental obstructive nephropathy. J Am Soc Nephrol 13: 1795–1805, 2002. [DOI] [PubMed] [Google Scholar]
- 40.O'Reiley M, Boehm T, Shing Y, Fukai N, Vasios G, Lane W, Flynn E, Birkhead J, Olsen B, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88: 277–285, 1997. [DOI] [PubMed] [Google Scholar]
- 41.O'Riordan E, Mendelev N, Patschan S, Patschan D, Eskander J, Cohen-Gould L, Chander P, Goligorsky MS. Chronic NOS inhibition actuates endothelial-mesenchymal transformation. Am J Physiol Heart Circ Physiol 292: H285–H294, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Paliege A, Pasumarthy A, Mizel D, Yang T, Schnerrmann J, Bachmann S. Effect of apocynin treatment on renal expression of COX-2, NOS1, and renin in Wistar-Kyoto and spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 290: R694–R700, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Papapetropoulos A, Garcia-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 100: 3131–3139, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patschan S, Chen J, Polotskaia A, Mendelev N, Cheng J, Patschan D, Goligorsky MS. Lipid mediators of autophagy in stress-induced premature senescence of endothelial cells. Am J Physiol Heart Circ Physiol 294: H1119–H1129, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Peters H, Daig U, Martini S, Ruckert M, Schaper F, Liefeldt L, Kramer S, Neumayer H. NO mediates antifibrotic actions of l-arginine supplementation following induction of anti-thy1 glomerulonephritis. Kidney Int 64: 509–518, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Prabhakar SS l-Arginine-nitric oxide pathway in end-stage renal disease. Am J Kidney Dis 39: 195–198, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Quaschning T, Voss F, Relle K, Kalk P, Vignon-Zellweger N, Pfab T, Bauer C, Theilig F, Bachmann S, Kraemer-Guth A, Wanner C, Theuring F, Galle F, Hocher B. Lack of endothelial nitric oxide synthase promotes endothelin-induced hypertension: lessons from endothelin-1 transgenic/endothelial nitric oxide synthase knockout mice. J Am Soc Nephrol 18: 730–740, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 2: 1057–1058, 1987. [DOI] [PubMed] [Google Scholar]
- 49.Schanstra J, Neau E, Drogoz P, Gomez M, Lopez-Novoa J, Calise D, Pecher C, Bader M, Girolami J, Bascands J. In vivo bradikinin B2 receptor activation reduces renal fibrosis. J Clin Invest 110: 371–379, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smirnova I, Kajstura M, Sawamura T, Goligorsky MS. Asymmetric dimethylarginine upregulates LOX-1 in activated macrophages: the role in foam cell formation. Am J Physiol Heart Circ Physiol 287: H782–H790, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Smirnova I, Sawamura T, Goligorsky MS. Upregulation of lectin-like oxidized LDL receptor-1 (LOX-1) in endothelial cells by NO deficiency. Am J Physiol Renal Physiol 287: F25–F32, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Stroikin Y, Dalen H, Loof S, Terman A. Inhibition of autophagy with 3-methyladenine results in impaired turnover of lysosomes and accumulation of lipofuscin-like material. Eur J Cell Biol 83: 583–590, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Valkonen V, Palva H, Salonen J, Lakka T. Risk of acute coronary events and serum concentration of ADMA. Lancet 358: 2127–2128, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Vallance P Importance of ADMA in cardiovascular risk. Lancet 358: 2096–2097, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Vallance P, Collier J, Moncada S. Nitric oxide synthesised from l-arginine mediates endothelium dependent dilatation in human veins in vivo. Cardiovasc Res 23: 1053–1057, 1989. [DOI] [PubMed] [Google Scholar]
- 56.Vallance P, Leiper J. Cardiovascular biology of ADMA: DDAH pathway. Arterioscler Thromb Vasc Biol 24: 1023–1030, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Wang BY, Ho HK, Lin PS, Schwarzacher SP, Pollmann MJ, Gibbons GH, Tsao PS, Cooke JP. Regression of atherosclerosis: role of nitric oxide and apoptosis. Circulation 99: 1236–1241, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Waschke J, Burger S, Curry F, Drenckhahn D, Adamson RH. Activation of Rac-1 and Cdc42 stabilizes the microvascular endothelial barrier. Histochem Cell Biol 125: 397–406, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Zoccali C, Bode-Boger S, Mallamaci F, Boger R. Plasma concentration of ADMA and mortality in patients with end-stage renal disease: a prospective study. Lancet 358: 2113–2117, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Zoja C, Benigni A, Camozzi D, Corna D, Longaretti L, Todeschini M, Remuzzi G. Combining lisinopril and l-arginine slows disease progression and reduces endothelin-1 in passive Heymann nephritis. Kidney Int 64: 857–863, 2003. [DOI] [PubMed] [Google Scholar]