Abstract
Bladder suburothelial myofibroblasts may modulate both sensory responses from the bladder wall and spontaneous activity. This study aimed to characterize further these cells in their response to exogenous agents implicated in mediating the above activity. Detrusor strips, with or without mucosa, and isolated suburothelial myofibroblasts were prepared from guinea pig bladders. Isometric tension, intracellular Ca2+, and membrane current were recorded. Cell pairs were formed by pushing two cells together. Tension, intracellular Ca2+, and membrane potential were also recorded from bladder sheets using normal or spinal cord-transected (SCT) rats. Spontaneous contractions were greater in detrusor strips with an intact mucosa and were augmented by 10 μM UTP. ATP, UTP, or reduced extracellular pH elicited Ca2+ transients and inward currents (Erev −30 mV) in isolated cells. Capsaicin (5–30 μM) reduced membrane current (37 ± 12% of control) with minor effects on Ca2+ transients: sodium nitroprusside reduced membrane currents (40 ± 21% of control). Cell pair formation, without an increase in cell capacitance, augmented ATP and pH responses (180 ± 58% of control) and reduced the threshold to ATP and acidosis. Glivec (20–50 μM) reversibly blocked the augmentation and also reduced spontaneous activity in bladder sheets from SCT, but not normal, rats. Glivec also disrupted the spread of Ca2+ waves in SCT sheets, generating patterns similar to normal bladders. Suburothelial myofibroblasts respond to exogenous agents implicated in modulating bladder sensory responses; responses augmented by physical intercellular contact. The action of glivec and its selective suppression of spontaneous activity in SCT rats identifies a possible pathway to attenuate bladder overactivity.
Keywords: suburothelium, spontaneous activity, purinergic signaling
the cellular pathways involved in the sensation of bladder volume are incompletely understood. An important advance was the observation that stretching of the bladder wall, or a change in transmural pressure, generated ATP release (10). Subsequently, in the urinary tract ATP was shown to be released from the urothelium under these circumstances (15). It was hypothesized that ATP would ultimately excite bladder afferents and signal the extent of bladder filling to the central nervous system. This hypothesis was given credence when nerve firing in bladder afferents and the micturition reflex itself were reduced in P2X3 knockout mice (6, 29). Other substances are also released from the urothelium during stretch, including nitric oxide (NO), which may attenuate afferent firing (2). Activation of TRPV channels has also been implicated in mediating bladder sensory responses (20), and indeed modulation of receptor function by vanilloid receptor ligands such as capsaicin and resiniferatoxin has been tested to reduce pathological bladder overactivity (7). A reduction of pH and temperature activates these receptors (4), and their involvement in provoking abnormal sensory activity and pain in the bladder has been proposed.
More recently, a layer of interstitial cells situated immediately below the urothelium, with the characteristics of myofibroblasts, has been identified, and individual cells form close connections to suburothelial nerves (30). These cells form a syncitium, connected by gap junctions (26), and respond to exogenous ATP by generating intracellular Ca2+ transients and a consequent Ca2+-dependent Cl− current (32). They are thus ideally situated, and have the functional characteristics, to mediate in the above-noted sensory process. Similar cells in the muscle layer are innervated by fibers that increase cellular cGMP and may also release NO (25). More recently, NO-dependent cGMP generation has also been measured in these suburothelial cells (11). If these suburothelial myofibroblasts do indeed modulate the sensory process, they may be expected to respond not only to ATP but also to these other chemical mediators. The aims of this study were to investigate further the cellular physiology of isolated suburothelial myofibroblasts and to determine whether their properties were altered when in proximity to neighbors. Finally, interventions were identified using isolated cells that could be used to gain insight into myofibroblast function in the intact bladder by measuring spontaneous waves of Ca2+ and membrane potential (Vm) activity using optical imaging. Guinea pig preparations were used for the isolated cell experiments and rats for optical mapping measurements, as we have previous experience with these respective animal models.
MATERIALS AND METHODS
Cell preparation.
Male guinea pigs (300–400 g) were killed in accordance with the UK Animals (Scientific Procedures) Act 1986, and the bladders were rapidly removed and placed in a Ca-free medium. All protocols were reviewed by an institutional ethical review panel before submission to the national statutory bodies for approval. The urothelium/suburothelium was separated from the underlying detrusor layer by blunt dissection under ×10–20 magnification. Samples were digested at 37°C with a nominally Ca-free collagenase-based medium, described previously for the preparation of isolated human detrusor myocytes (19), with constant stirring for 30 min. After treatment, the tissue was partially disrupted by gentle trituration, and two main cell types could be seen under a light microscope: 1) large, round urothelial cells and 2) a layer of ovoid or spindle-shaped cells, with or without one or more dendrite-like structures. The latter cells were used for experimental recording. In some preparations, the detrusor layer was disrupted similarly to yield isolated smooth muscle cells.
Solutions.
The Ca-free solution had the following composition (mM): 105.4 NaCl, 22.3 NaHCO3, 3.6 KCl, 0.9 MgCl2, 0.4 NaH2PO4; 19.5 HEPES, 5.4 glucose and 4.5 Na pyruvate, pH 7.1. For experiments, cells were superfused with Tyrode's solution (in mM): 118 NaCl, 4.0 KCl, 24 NaHCO3, 0.4 NaH2PO4, 1.0 MgCl2, 1.8 CaCl2, 6.1 glucose, and 5.0 Na pyruvate, gassed with 5% CO2-95% O2, pH 7.4. For low-pH solutions, NaHCO3 was replaced with 10 mM MES plus 14 mM NaCl and titrated with NaOH to a pH between 4.8 and 5.5. Aliquots from stock solutions of Na3 UTP (in water) and capsaicin (in DMSO) were dissolved in Tyrode's solution to achieve the desired concentrations. Sodium nitroprusside (SNP) was added as a solid to Tyrode's solution. All chemicals were from Sigma, except the fluorescent dyes for optical imaging experiments (Molecular Probes). Glivec (imatinib mesylate) was a gift from Novartis. All experiments were carried out at 37°C.
Tension recording.
Strips of detrusor (<1-mm diameter; 3–4 mm in length) were cut from the dome, without first removal of the mucosa, tied to an isometric force transducer, and superfused with Tyrode's solution, and the preparation was left for ∼30 min to allow spontaneous contractions to develop. Agonists were added to the superfusate to achieve the desired concentrations. After recordings, the muscle was slackened, the mucosa was gently removed by blunt dissection, and the muscle was then stretched to the previous length (measured with a Vernier scale on the micromanipulator holding the force transducer). The preparation was left for an additional 30 min, and recordings again commenced. At the end of the experiment, the muscle length was recorded and the muscle was then weighed. Muscle volume was calculated assuming a tissue density of 1.05, and the mean cross-sectional area was then calculated.
Electrophysiology.
Recordings were made using patch-type pipettes (3–4 MΩ) made from borosilicate glass and filled with a Cs-filling solution (in mM): 20 CsCl, 110 aspartic acid, 5.45 MgCl2, 5.0 Na2ATP, 0.1 Na4GTP, 0.1 EGTA, and 5.0 HEPES, pH to 7.1 with CsOH. An Axopatch 1-D system (Axon Instruments) was used for experiments: data were recorded via an analog-digital converter (Digidata 1200, Axopatch version 9, Axon Instruments) at 4 kHz and filtered with a low-pass filter with a cut-off frequency of 2 kHz. Cells were maintained at a holding potential, Vh, of −60 mV for experiments, as this is close to the average resting potential Vm (32). Cell capacitance, cm, was measured by integrating the current response to a 20-mV step and was calculated automatically in the “membrane test” subroutine. Ionic currents was normalized to cell capacitance as picoamperes per picofarad.
Measurement of intracellular Ca2+ concentration.
Electrophysiological data and intracellular Ca2+ concentration ([Ca2+]i) were recorded simultaneously in some experiments. In this case, 100 μM K5 fura 2 was added to the pipette filling solution; the EGTA concentration was 50 μM. These concentrations of Ca2+ buffers contribute ∼10% to the total Ca2+-buffering power of detrusor smooth muscle cells (31), and a similar proportion was assumed in these experiments. Cells were excited at 340/380 nm at 50 Hz, and the fluorescence intensity was recorded between 410 and 480 nm. The fura 2 signal was calibrated using solutions of varying [Ca2+] in the absence of cells, as described previously (32).
Experiments with whole bladders.
Animals were treated humanely in accordance with the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals employing a University of Pittsburgh Institutional Animal Care and Use Committee-approved protocol. Adult rats (4 mo old) were euthanized with inhalational CO2 after anesthetization with 2.5% halothane, and the bladders were removed. A group of rats underwent spinal (T8/T9) cord transection (SCT) as described previously (16), and bladders were similarly removed. Bladders were cannulated with a 22-gauge needle and loaded intravesically with voltage (10 μM di-4-ANNEPS, 15 min, 25°C)- and Ca2+ (5 μM Rhod-2-AM, 15 min, 37°C)-sensitive dyes. After dye loading, the cannula was removed and the bladder was cut from neck to dome on the ventral surface to produce a sheet (mucosa uppermost). The sheet was fixed to anchored pins at the neck end and tied to an isometric force transducer at the dome end, to record tension transients. The preparation was superfused (0.5 ml/min) with a modified Tyrode's solution (in mM): 113 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 25 NaHCO3, 1.2 KH2PO4, and 11.5 glucose, pH 7.4, bubbled with 95% O2-5% CO2.
Ca2+ and Vm were recorded from the preparation surface using an optical imaging system, as described previously (13). Briefly, broad-spectrum white light was passed through a 540 ± 40-nm excitation filter and focused on the preparation. Fluorescence was directed to a 635-nm-long wave-pass dichroic mirror; light of shorter wavelengths was reflected to the Ca2+ camera, and light of longer wavelengths was transmitted to the voltage camera. Each of the 16 × 16 photodiode array camera elements has a sensing area of 0.95 mm2 and an interpixel spacing of 1.1 mm. Photodiode array outputs were amplified (1–1,000 times), digitized (14 bits), and sampled (4 kHz) for up to 60 s; the signal-to-noise ratio was ∼200:1. Cross-correlation of Ca2+ and Vm waveforms allowed transients with the earliest rise time to act as initiators and determine delays in other transients. Propagating Ca2+ and Vm waves were constructed from isochrones linking points of equal delays at different pixels.
Data presentation and calculations.
Data are means ± SD (n = number of experiments). Significance between data sets was tested using unpaired or paired Student's t-tests; the null hypothesis was rejected at P < 0.05.
RESULTS
Responses of isolated cells, tissue strips, and whole bladders to exogenous nucleotides.
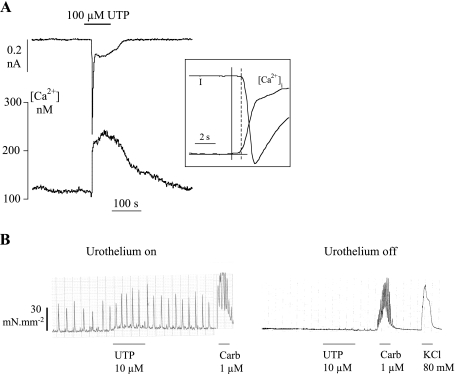
Suburothelial myofibroblasts responded to exogenous nucleotides by generating a transient increase in intracellular Ca2+ and an inward current. Figure 1A shows the responses to 100 μM UTP, similar to those generated with ATP (32), namely a Ca transient immediately followed by an inward current at the holding potential of −60 mV; the temporal relationship between the two variables is seen more clearly in the inset traces.
Fig. 1.
Responses of bladder isolated preparations to UTP. A: effect of 100 μM UTP on an isolated suburothelial myofibroblast (holding potential, Vh, −60 mV). The period of intervention in this and all subsequent figures is shown by the horizontal bar above or below the traces. Inset: initial phase of the Ca transient and current record to illustrate the temporal relationship between the 2 variables The solid vertical line marks when the Ca2+ trace deviated from the baseline, and the dotted line when inward current was initiated. B, left: tension recording from a detrusor strip with intact mucosa showing spontaneous activity before and after exposure to 10 μM UTP. After UTP washout, the preparation was exposed to 1 μM carbachol. Right: the same strip after removal of mucosa. After UTP intervention, the muscle was again exposed to 1 μM carbachol and also 80 mM KCl Tyrode's solution.
Their relevance to functional bladder responses is illustrated in Fig. 1B. Tension was recorded in an unstimulated detrusor strip with an intact mucosa, and after ∼30 min regular spontaneous contractions appeared. Addition of UTP (10 μM) increased contractile activity through an increase in frequency and, in this example, the average amplitude of the contraction. After washout of UTP and brief exposure to carbachol to evoke a muscle response, the mucosa was carefully dissected away from the preparation. Now, spontaneous activity was abolished and UTP failed to evoke a response; similar observations were seen in six preparations. However, exposure of the mucosa-free preparation to carbachol still evoked a similar magnitude contracture, showing that the loss of spontaneous activity was not due to muscle damage during mucosa removal; in the example, the muscle also responded to a high-K solution (80 mM KCl). The frequency and magnitude of contractions were recorded for 3-min periods before and during UTP exposure. With an intact mucosa, UTP increased frequency significantly (paired t-test; frequency 2.1 ± 1.1 vs. 2.8 ± 2.1 min−1, n = 6): on average, mean amplitude was not increased significantly (18.3 ± 12.9 vs. 20.9 ± 16.2 mN·mm−2). However, the product of mean force and frequency, which reflects overall muscle contractile performance, was increased (29.9 ± 23.2 vs. 51.5 ± 35.3 mN·mm−2·min−1, paired t-test).
Responses to low pH.
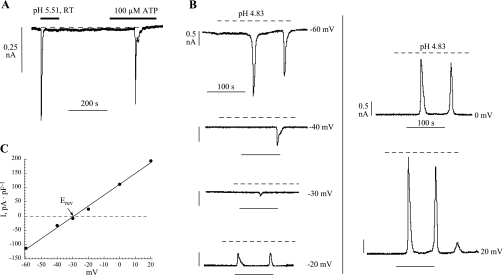
In response to an extracellular acidosis, isolated myofibroblasts generated similar responses to a nucleotide (Fig. 2A); in general, responses were elicited over the range pH 5.5 to 4.5. Figure 2B shows a series of successive low-pH exposures in a single cell, as holding potential was varied between −60 and +20 mV. Current reversed between −30 and −20 mV, and Fig. 2C shows the current-voltage relationship with a reversal potential of −29 mV and a slope conductance of 3.8 nS/pF; similar results were seen in six cells. The similar characteristics of the low-pH and nucleotide-evoked responses (32) imply that they are elicited through similar mechanisms, i.e., an increase in intracellular Ca2+ and generation of a Ca2+-activated current.
Fig. 2.
pH-evoked current in suburothelial myofibroblasts. A: membrane current in response to a low-pH solution and 100 μM ATP (Vh −60 mV). B: membrane current in response to exposure to a superfusate of pH 4.83. In the different panels, the Vh of the cell was held at the various values indicated. C: plot of the peak current as a function of Vh for the data shown in B. Erev, reversal potential.
Modulation of ATP-dependent transients.
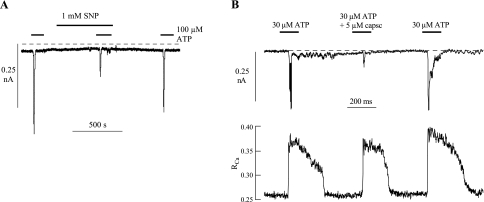
Nitrergic fibers have been hypothesized to influence the activity of suburothelial myofibroblasts (25): thus the effect of NO donors on ATP-induced phenomena was examined. Figure 3A shows that 1 mM SNP attenuated reversibly the ATP-induced inward current; overall, current was reduced to 40 ± 21% (n = 4) of control.
Fig. 3.
A: effect of 1 mM sodium nitroprusside (SNP) on membrane currents elicited by 100 μM ATP. B: effect of 5 μM capsaicin on membrane currents and intracellular Ca2+ transients elicited by 30 μM ATP. Vh, −60 mV in both traces.
Capsaicin and its derivatives eventually suppress overactive bladder behavior when infused into the lumen (8, 28). It was therefore of interest to determine whether capsaicin would influence myofibroblast function. Figure 3B shows simultaneous recordings of intracellular [Ca2+] and membrane current in an isolated cell. Capsaicin (5 μM) had only a small effect on the magnitude of the Ca2+ transient, but attenuated profoundly the inward current (peak current was reduced to 24.8% control); similar observations were made in four further experiments with 30 μM capsaicin. In control observations, the magnitude of the ATP-evoked inward current was dependent on the rate of rise of the Ca2+ transient; however, in these paired experiments, the rise time of the Ca2+ transient (10–90% of peak value) was similar in the absence and presence of capsaicin (0.4 ± 0.1 vs. 0.5 ± 0.2 s, respectively). On average, capsaicin reduced peak current to 37 ± 12% of control, while the Ca2+ transient was reduced to only 87 ± 7% of control (n = 5). The total net charge carried by the inward current transient was also estimated by integrating the current trace over a 50-s interval. There was a similar conclusion; capsaicin reduced net charge to 35 ± 13% of control. Thus capsaicin reduced ATP-generated inward current magnitude independently of the Ca2+ transient.
Augmentation of responses in cell pairs.
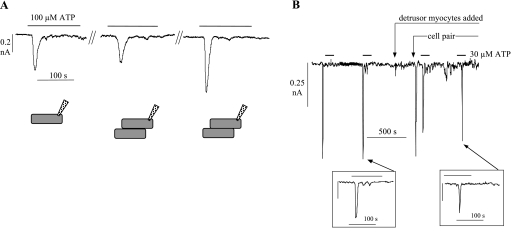
The response of an isolated interstitial cell to exogenous activators was altered when the cell made physical contact with a second cell. Figure 4A shows an experiment whereby an ATP response was elicited initially from a myofibroblast. The cell was then moved with the patch electrode, but with the seal remaining in place, until it made gentle contact with a second cell, and an ATP response was again elicited; this was slightly smaller, but similar to the initial response, and represents the run-down that was seen in many isolated cells upon repeated exposure to ATP (see Action of glivec on augmentation of responses in cell pairs). The two cells were then forced to make closer contact, after which by contrast a much larger ATP-dependent transient current was evoked. Figure 4B shows a continuous recording of a similar experiment when a myofibroblast was pushed this time against a detrusor smooth muscle cell. Initially, only myofibroblasts were in the perfusion bath, and in one cell the responses to two exposures of 30 μM ATP were recorded, both of similar size. At the point indicated, a drop of superfusate containing detrusor myocytes was added to the bath, and when these had settled on the chamber base the interstitial cell was pushed to make close contact with a myocyte (labeled cell pair). At the time of initial contact, a large spontaneous current transient was recorded. However, in contrast to Fig. 4A, successive exposures to the same concentration of ATP did not generate an augmented response compared with that from the isolated cell. The insets show, on an expanded time base similar to Fig. 4A, two transients, before and after touching the detrusor myocyte, demonstrating a similar morphology of the responses. Thus the augmentation of the response to ATP by cell pair formation is specific to myofibroblasts.
Fig. 4.
A: membrane currents elicited by 100 μM ATP in an isolated cell (left), when touching loosely on a second cell (middle), and more firmly on the second cell (right). Bottom: drawing of the experimental system. B: continuous tracing of membrane current showing inward transients elicited by repeated exposures to 30 μM ATP. At the first arrow, isolated detrusor myocytes were also added to the cell chamber; at the second arrow, the myofibroblast from which recordings were made was pressed firmly against a smooth muscle cell. Vh, −60 mV. Insets: second and fourth ATP-evoked current transient on a faster time base.
Cell capacitance, ATP responses, and cell pairs.
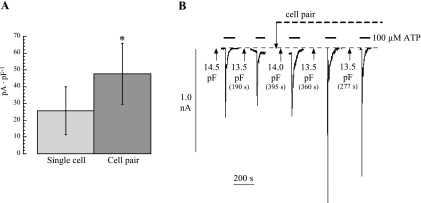
Figure 5A plots results from 29 experiments showing the inward current transient magnitudes elicited immediately before and after cell pair formation; currents were normalized to unit cell capacitance and were significantly increased after cell pair formation. The percentage increase in current was independent of cell capacitance (r2 = 0.032, P > 0.05) and had a mean value of 180 ± 58% of control (n = 29).
Fig. 5.
A: magnitude of peak inward current induced by 100 μM ATP from isolated cells or cell pairs. Values were normalized to unit cell capacitance (Vh = −60 mV, *P < 0.05). B: continuous recording of membrane current (Vh, −60 mV) in response to repeated exposures of 100 μM ATP. After the second exposure, a cell pair was formed. The breaks in the record correspond to times when membrane capacitance was estimated. Values are indicated at the arrows.
One explanation for the enhanced response in the cell pair is that gap junctions form between the two cells so that the patch electrode records from a larger cell membrane area. Figure 5B shows a sample experiment where ATP-dependent inward currents were recorded before and after cell pair formation. The breaks in the current trace represent times when cell capacitance (and hence membrane area) was estimated and show that the value remained constant throughout the experiment. The average cell capacitance in seven cells was 11.6 ± 2.7 before and 11.0 ± 2.8 pF after cell pair formation. From these measurements, there was no evidence of gap junction formation during establishment of cell pairs, and thus it was concluded that the enhanced current was elicited from the same membrane area.
Cell pairs and threshold of activation.
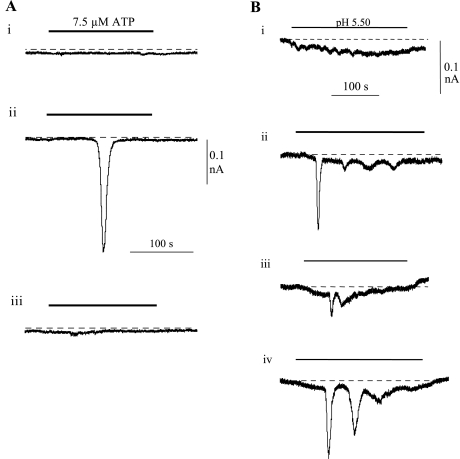
The above data showed that responses to agonists such as ATP and low pH were augmented by close contact with a neighbor. In addition, the threshold concentration required to elicit a response was also reduced in cell pairs. Figure 6A shows an example where 7.5 μM ATP failed to elicit an inward current in an isolated cell. However, when touched onto a second cell, a large response was elicited, which was again absent when the cells were separated. In six separate experiments, an ATP concentration >5 μM was required to elicit a response, whereas with cell pairs the threshold was ∼1 μM. A similar phenomenon was observed with responses to low pH (Fig. 6B, n = 6), when a single cell failed to respond to a solution of pH 5.50 but a response was elicited when a cell pair was formed. With this experiment, the process could be repeated as the response was almost abolished when the cell pair was broken, by withdrawing the recording cell, but reestablished when it was again touched to the second cell.
Fig. 6.
Cell pairs reduce the threshold to ATP and low pH. A: continuous recording of membrane current from an isolated myofibroblast when exposed repeatedly to 7.5 μM ATP. Exposures were made when the cell was isolated (i), when a cell pair was formed (ii), and when the cell was again isolated (iii). The intervals between records (i) and (ii) and between (ii) and (iii) were 1,078 and 2,251 s, respectively. B: similar protocol when an isolated myofibroblast was exposed repeatedly to low-pH solution (pH 5.50). Exposures were made when the cell was isolated (i), when a cell pair was formed (ii), when the cell was again isolated (iii), the same cell pair was re-formed (iv). The intervals between the exposures were 710, 719, and 1,362 s, respectively. Vh, −60 mV in both experiments.
Action of glivec on augmentation of responses in cell pairs.
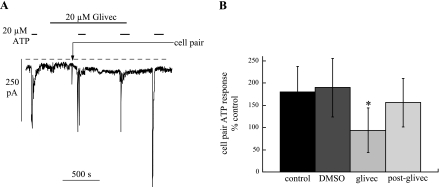
The above data suggest that augmentation of agonist responses in cell pairs is not due to gap junction formation but may involve other coupling mechanisms, such as adherens junctions. These junctions interact with the cytoskeleton and augment intracellular pathways that involve, for example, tyrosine-receptor kinases (3). Glivec is an agent that inhibits such receptors and also reduces bladder spontaneous activity (1). Thus the effect of glivec on augmented nucleotide responses after formation of cell pairs was measured. Figure 7A shows an experiment when an ATP response was elicited initially in a single cell; the cell was then exposed to glivec, which generated no response per se. A cell pair was formed with further exposures to ATP: augmentation of the ATP response was now absent [94 ± 50% of control (n = 24)]. In parallel experiments, the glivec solvent alone (DMSO; 1:1,000 vol/vol) was added before cell pair formation (Fig. 7B): ATP responses were augmented (190 ± 66% of control, n = 8) to a similar degree as in the absence of DMSO.
Fig. 7.
Effect of glivec on ATP-induced inward current. A: continuous recording of membrane current with repeated exposures to 20 μM ATP. The preparation was exposed to 20 μM glivec during the second and third exposures to ATP. Before the second exposure to ATP a cell pair was formed at the arrow. Vh, −60 mV. B: percent increase in membrane currents to 100 μM ATP on formation of cell pairs under control conditions, during exposure to DMSO (0.1% vol/vol), during exposure to glivec (5–30 μM), and after removal of glivec. The final data set was corrected for run-down of the response (see text for details). *P < 0.05 with respect to control.
In some experiments, ATP responses were also elicited in cell pairs after removal of glivec (Fig. 7A) to measure any recovery from the agent. Analysis was complicated, in some preparations, by natural run-down of the response with a mean rate constant of 9.2 ± 2.5 × 10−4/s (τ = 1,087 s; n = 12). When correction was made for run-down, the post-glivec ATP response in cell pairs was 156 ± 55% of control (n = 12). This value was significantly (P < 0.05) greater than in the presence of glivec, but not different from the DMSO response: the data for the effects of glivec are summarized in Fig. 7B.
Action of glivec in isolated rat bladders.
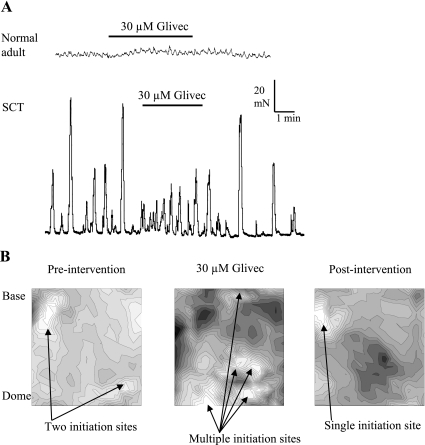
The functional significance of the above responses to nucleotides and other stimuli and their modulation in cell pairs, especially in regard to the development of spontaneous activity, were examined. This was done by measuring the effect of glivec on responses from whole bladder sheets. We showed previously that in spinal cord-transected (SCT) rats not only was there an increase in the magnitude and coordination of spontaneous whole-bladder activity, but also the number of Cx43-labeling suburothelial myofibroblasts was raised (12). Spontaneous pressure transients were accompanied by propagating Ca2+ and Vm waves across the bladder surface, which in the SCT-derived bladders were equally coordinated (12, 13). We therefore tested the effect of glivec on these spontaneous activities in normal and SCT rats. Figure 8A shows an example of the effect of 30 μM glivec on spontaneous contractile activity in sheets of whole bladders from normal and SCT animals. In normal animals (top trace), 30 μM glivec had no significant effect on the frequency or amplitude of the low-level spontaneous activity. However, in SCT rats (bottom trace) glivec reduced the amplitude of these contractions, and in this example increased the frequency, suggesting a loss of coordination in the production of such activity; on average, the amplitude of contractions was reduced by 52 ± 16% (n = 6), when time-averaged measurements over 5-min intervals were calculated.
Fig. 8.
Effect of glivec on whole rat bladder sheets. A: effect of 30 μM glivec on spontaneous activity in bladder sheets from a normal adult rat (top) and a spinal cord-transected (SCT) rat (bottom). The calibration bars are relevant to both traces. B: Ca2+ isochronal maps from an SCT rat bladder sheet (i), an SCT rat bladder sheet in the presence of glivec (30 μM; ii), and after washout of glivec (iii). Isochrones represent successive conduction delays of 44 ms.
Optical imaging of bladder sheets measured fluorescence transients from Ca2+-sensitive dyes simultaneously from 252 sites. Maps of isochronal activity were constructed from these transients using the earliest transient as an initiation point: the colors, shaded from light to dark, show isochrones of successively greater delay. Figure 8B shows three Ca2+ isochronal maps of spontaneous activity from the mucosal surface of a spinal cord-injured rat. The first shows the pattern characteristic of these preparations under control conditions, showing at most two initiation sites dominating the surface. Exposure to glivec altered the pattern, so that multiple initiation sites became evident and reminiscent of the uncoordinated pattern observed in bladder sheets from normal rat bladders (12). Washout of glivec restored the control pattern. Equivalent experiments with control rats (not shown) demonstrated no effect of glivec. To quantify the observations, the conduction velocities of the Ca2+ waves were calculated as an average velocity within a small radius (<1.5 mm) from an initiation point; the small radius avoided signal contamination from other focal points. Conduction velocities of Ca2+ waves in normal and in SCT rat bladders were 2.0 ± 0.8 and 7.0 ± 5.6 mm/s, respectively (n = 7, both groups). Glivec reduced significantly the value in SCT bladders to 1.2 ± 1.0 mm/s (n = 6), a value similar to that for normal adult rat bladders.
A summary of the different experiments and a qualitative description of the outcomes are shown in Tables 1 and 2.
Table 1.
Protocol of different measured variables obtained from guinea pig preparations used in the current study, as a well as a qualitative description of the results
|
Guinea Pig (Normal) |
||||
|---|---|---|---|---|
| Isolated Myofibroblasts, Intracellular Ca2+/Membrane Current Transients | Cell Pairs, Intracellular Ca2+/Membrane Current Transients | Muscle Strip with Mucosa, Isometric Tension | Muscle Strip No. Mucosa, Isometric Tension | |
| Control | Stable cells (n = 90) | Stable cells (n = 47) | SA (n=6) | Reduced SA (n = 6) |
| ATP/UTP | Transients generated (n = 75) | Transients augmented (n = 29) | SA (n=6) | No increase in SA (n = 6) |
| Low pH | Transients generated (n = 6) | Transients augmented (n = 6) | ||
| NO donors | Transients reduced (n = 4) | |||
| Capsaicin | Current transient reduced (n = 5) | |||
| Glivec | Augmentation abolished (n = 12) | |||
n, No. of repeat observations from separate preparations for each experiment; SA, spontaneous activity; NO, nitric oxide.
Table 2.
Protocol of different measured variables obtained from rat preparations
|
Rat (Normal) |
Rat (Spinal Cord Injured)
|
|||
|---|---|---|---|---|
| Bladder Sheets, Isometric Tension | Bladder Sheets, Optical Imaging, Ca/Vm Waves | Bladder Sheets, Isometric Tension | Bladder Sheets, Imaging, Ca/Vm Waves | |
| Control | Small, chaotic SA (n = 6) | Multiple initiation sites (n = 7) | Organized, large SA (n = 6) | One/few initiation site (n = 7) |
| Glivec | No effect (n = 6) | No effect (n = 6) | Reduced amplitude (n = 6) | Reverted to multiple initiation sites (n = 6) |
n, No. of repeat observations from separate preparations for each experiment.
DISCUSSION
These experiments have shown that the nucleotide responses elicited by suburothelial myofibroblasts from the bladder wall may be duplicated or modified by several agents and conditions that have been proposed to modify sensory responses from the bladder. In addition, these responses were augmented when cells touched each other. This is a state in which these cells will exist in the bladder wall itself, especially under some pathological conditions when bladder activity is increased (12). This augmentation in turn was abolished by glivec, a c-kit inhibitor. Glivec also reduced spontaneous contractile activity in isolated bladders, as well as associated waves of Ca2+ that spread over the bladder surface. Taken together, the data are consistent with the hypothesis that myofibroblasts can affect activity in the bladder and play a role in the perception of sensations associated with bladder filling.
Myofibroblast functional receptors.
Previous studies showed that exogenous ATP evoked an intracellular Ca2+ transient followed by generation of a Cl− current (32). Because the Ca2+ transient occurred independently of Vm, it was hypothesized that the functional receptor was a P2Y subtype. Subsequently, fluorescent immunolabeling demonstrated strong binding for P2Y6 antibodies, with weaker binding for P2Y2 and P2Y4, but with none for P2Y1 (27). The lack of P2Y1 binding was consistent with a lack of effect of P2Y1 antagonists on evoked responses (32). However, the fact that UTP generates equivalent responses to ATP is not entirely consistent with a predominant P2Y6 subtype (22). This might suggest that heterogeneous P2Y receptors are functionally active, or that the intensity of fluorescent signals is no indication of the functional importance of a receptor subtype.
A reduction of extracellular pH also generated Ca2+ and Vm transients: the current reversed at about −30 mV consistent with it being a Cl− current with the extracellular and pipette-filling solutions used here; thus the responses mirror those evoked by nucleotides. Vanilloid receptors have been located on suburothelial bladder myofibroblasts (21); however, capsaicin had differential effects on the Ca2+ and current transients, attenuating the latter to a much greater extent. If capsaicin is acting via a TRPV channel, it may be hypothesized that its activation may modulate the nucleotide-dependent excitation of the cell. The differences in response to low-pH and capsaicin interventions also suggest that they act through different mechanisms and that the former may activate the pathway that is stimulated by P2Y receptor activation. The NO donor SNP also attenuated Ca2+ and current responses to ATP. The mechanism is not known but may represent modulation of this central pathway by cGMP. Overall, these experiments suggest that an increase in intracellular Ca2+ evoked by various exogenous activators is the primary response of the cell, which generates secondary electrical activity. Each of these processes may be separately modulated.
Augmentation of cellular responses by physical contact.
A consistent observation was that, when two myofibroblasts were physically pushed together, the response to exogenous nucleotides or low-pH solutions was significantly increased. Moreover, there was a reduction of the threshold concentration required to evoke a response. The effect was specific between two similar cells as it was absent when a myofibroblast was pushed against a detrusor muscle cell, for example. The simplest explanation was that gap junctions would form between the two cells and thus increase the effective membrane area under voltage clamp. However, no evidence for gap junction formation was found as the membrane capacitance of the isolated cell did not increase, indicating that the augmented response came from the same membrane area, and hence presumably from the same single cell. However, no direct attempt to measure any cell-to-cell conductance, by passing current between two electrodes patched on to each cell in the pair, was carried out. Glivec, a c-kit receptor antagonist, however, abolished this augmentation. This was not mere run-down of the response as, when the agent was removed, an enhanced response was recorded.
Cadherin proteins are vital components of adherens junctions, and recently E-cadherins have been identified in suburothelial myofibroblasts (18). Cadherins are not just structural proteins but regulate intracellular signaling pathways in many cells, in particular through modulation of PLC subtypes, such as PLCγ, and inositol phospholipid pathways (5, 7, 14). Equally, c-kit is a receptor-protein tyrosine kinase that can regulate several intracellular pathways, including those described above (23, 24). The mechanism of interaction between these two converging pathways in these myofibroblasts is unclear, but they cannot be two linearly independent pathways, as glivec alone had no depressant effect on ATP-induced signals but did abolish the augmentation generated by cell pair formation.
Experiments with whole bladder sheets.
Because glivec had such a profound effect on ATP-generated responses in myofibroblasts, it might be expected to alter whole bladder responses if these cells have a significant role. Previous studies showed that glivec abolished spontaneous contractile and electrical activity in isolated detrusor preparations (1, 17). Our experiments found no effect in isolated bladders from normal adult rats, but glivec did have a significant effect in those from SCT animals that show greatly enhanced spontaneous contractions. SCT animals also have a significant increase in suburothelial myofibroblasts as evidenced by an increase in connexin-43 immunolabeling (12). Thus, if myofibroblasts were important in determining the generation of coordinated spontaneous contractions, a rise in their number would indeed increase such activity, and their inhibition by glivec would reduce this effect. It must be stressed that c-kit myofibroblasts are found at other locations in the bladder, in particular between detrusor cells and muscle bundles (9), and these also are upregulated in SCT rats (12). Thus these experiments do not provide unequivocal evidence of a role for suburothelial myofibroblasts.
In addition to reducing spontaneous contractile activity in SCT rat bladders, glivec also reduced the extent and coordination of Ca2+ wave activity in the bladder. Most significantly, activity was altered from there being a single focal source of activity to one in which it was more reminiscent of normal adult bladders, which consisted of multiple focal points that failed to generate coordinated large spontaneous bladder contractions. We have proposed previously that myofibroblasts provide the focus for the generation of spontaneous activity (13) and that an increase in their number would enhance this coordination. The absence of an effect of glivec on Vm waves might imply that the effect is not mediated by gap junction modulation, a conclusion mirrored in the absence of gap junction coupling in isolated pairs of myofibroblasts.
In conclusion, we have shown that suburothelial myofibroblasts generate intracellular Ca2+ and electrical responses to several physiological and artificial interventions that are known to influence bladder activity either directly or through activation of sensory mechanisms. These response are significantly increased by physical connection between adjacent cells, as would occur in their natural setting. Such augmentation may depend on the formation of adherens junctions, rather than gap junctions, but such gain of function is reduced by blockade of receptors that have intracellular convergence on second messenger/Ca2+ signaling pathways. Because an increase in myofibroblast number is associated with increased spontaneous activity in bladders, downregulation of this mechanism, as with glivec, for example, can offer a novel route to attenuate pathological contractile function, such as that associated with the overactive bladder in humans.
GRANTS
We thank the Wellcome Trust, St. Peter's Trust, and Research into Ageing for financial support; the Deutschesforschungsgemeindshaft for a fellowship to A. Roosen; and the National Institutes of Health for Grant DK-064280 to A. Kanai.
Present address of C. H. Fry, C. Wu, G. P. Sui: Postgraduate Medical School, University of Surrey, Guildford GU2 7WG, UK.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Biers SM, Reynard JM, Doore T, Brading AF. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int 97: 612–616, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Birder LA, Apodaca G, De Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol Renal Physiol 275: F226–F229, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Brunton VG, MacPherson IR, Frame MC. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim Biophys Acta 1692: 121–144, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Caterina M, Schumacher M, Tominaga M, Rosen T, Levine J, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Cavallaro U, Niedermeyer J, Fuxa M, Christofori G. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol 3: 650–657 2001. [DOI] [PubMed] [Google Scholar]
- 6.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407: 1011–1015, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Contreras RG, Shoshani L, Flores-Maldonado C, Lazaro A, Monroy AO, Roldan ML, Fiorentino R, Cereijido M. E-Cadherin and tight junctions between epithelial cells of different animal species. Pflügers Arch 444: 467–475, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Cruz F Mechanisms involved in new therapies for overactive bladder. Urology 63, Suppl 1: 65–73, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol 173: 1385–1390, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol 505: 503–511, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillespie JI, Ittersum MMV, De Vente J. Endogenous nitric oxide/cGMP signalling in the guinea pig bladder: evidence for distinct populations of sub-urothelial interstitial cells. Cell Tissue Res 325: 325–332. 2006. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda Y, Fry CH, Hayashi F, Stolz DB, Griffiths D, Kanai AJ. The role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol 293: F1018–F1025, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, Griffiths D, de Groat W, Fry C. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol 292: F1065–F1072, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kipmen-Korgun D, Osibow K, Zoratti C, Schraml E, Greilberger J, Kostner GM, Jurgens G, Graier WF. T-cadherin mediates low-density lipoprotein-initiated cell proliferation via the Ca2+-tyrosine kinase-Erk1/2 pathway. J Cardiovasc Pharmacol 45: 418–430, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Knight GE, Bodin P, De Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol 282: F281–F288, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Kruse MN, Bray LA, de Groat WC. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J Autonom Nerv Syst 54: 215–224, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Kubota Y, Biers SM, Kohri K, Brading AF. Effects of imatinib mesylate (Glivec) as a c-kit tyr-osine kinase inhibitor in the guinea-pig urinary bladder. Neurourol Urodyn 25: 205–210, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Kuijpers KA, Heesakkers JP, Jansen CF, Schalken JA. Cadherin-11 is expressed in detrusor smooth muscle cells and myofibroblasts of normal human bladder. Eur Urol 52: 1213–1221, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery BS, Fry CH. The action potential and net membrane currents in isolated human detrusor smooth muscle cells. J Urol 147: 176–184, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Mukerji G, Yiangou Y, Agarwal SK, Anand P. Transient receptor potential vanilloid receptor subtype 1 in painful bladder syndrome and its correlation with pain. J Urol 176: 797–801, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Ost D, Roskams T, Van Der Aa F, De Ridder D. Topography of the vanilloid receptor in the human bladder: more than just the nerve fibers. J Urol 168: 293–297, 2002. [PubMed] [Google Scholar]
- 22.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998. [PubMed] [Google Scholar]
- 23.Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol 533: 327–340, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Roskoski R Signaling by Kit protein-tyrosine kinase–the stem cell factor receptor. Biochem Biophys Res Commun; 337: 1–13, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Smet PJ, Jonavicius J, Marshall VR, De Vente J. Distribution of nitric oxide synthase-immunoreactive nerves, and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience 71: 337–348, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int 90: 118–129, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Sui GP, Wu C, Fry CH. Characterization of the purinergic receptor subtype on guinea-pig suburothelial myofibroblasts. BJU Int 97: 1327–1331, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Szallasi A, Fowler CJ. After a decade of intravesical vanilloid therapy: still more questions than answers. Lancet Neurol 1: 167–172, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21: 5670–5677, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int 91: 89–93, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Wu C, Fry CH. Na+/Ca2+ exchange and its role in intracellular Ca2+ regulation in guinea pig detrusor smooth muscle. Am J Physiol Cell Physiol 280: C1090–C1096, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Wu C, Sui GP, Fry CH. Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol 559: 231–243, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]