Abstract
The pathways implicated in the control of epithelial Na+ channel (ENaC)-dependent Na+ transport in renal collecting duct cells share substantial parallels with those implicated in insulin-regulated glucose metabolism. Notably, both are inhibited by wortmannin and LY294002 and signal through phosphatidylinositol-3-kinase (PI3K)-dependent kinases SGK1 and Akt. The inhibitor pattern is thought to reflect dependence on PI3K activity since wortmannin and LY294002 are both effective inhibitors of this kinase. However, these inhibitors block a variety of kinases from different families and lack specificity within the PI3K family. To begin to dissect more precisely the pathways required for signaling and for control of Na+ transport in renal collecting duct cells, we have examined the effect of a set of PI3K inhibitors, which selectively block distinct subsets of PI3K catalytic subunit isoforms. We have found that ENaC-dependent Na+ transport was blocked by inhibitors of the p110-α isoform of PI3K, but not by inhibitors of p110-β, -γ, or -δ. Inhibitors that block Na+ current also blocked SGK1 and Akt phosphorylation. In contrast to insulin-stimulated glucose uptake in muscle cells, p110-β inhibition did not enhance sensitivity to p110-α inhibition. These data support the conclusion that ENaC-dependent Na+ current is controlled exclusively by p110-α, the same isoform that is the principal mediator of insulin effects on glucose metabolism, and lacks any dependence on p110-β. These findings further underscore the extent to which Na+ and glucose regulation are intertwined and provide additional insight into the interconnections between diabetes and hypertension.
Keywords: phosphoinositide, sgk, phosphatidylinositol-3-phosphokinase, insulin, aldosterone
through their effects on kidney tubule sodium transport, insulin and aldosterone have pronounced effects on blood pressure in both physiologic and pathophysiological contexts (25). Aldosterone is a key physiological regulator of Na+ transport, and a tightly regulated endocrine feedback loop controls its blood concentration and functional effects. Aldosterone acts through the mineralocorticoid receptor (MR) to alter the transcription rate of a set of target genes, which include the phosphatidylinositol 3-kinase (PI3K)-dependent serine-threonine kinase SGK1 (10, 24, 33). SGK1, together with other MR target gene products, stimulates the activity and apical membrane localization of the epithelial sodium channel (ENaC), the rate-limiting step in transepithelial Na+ transport in the kidney collecting duct (CD; reviewed in Ref. 22). Insulin also stimulates ENaC-mediated Na+ transport and NaCl retention, resulting in increased total body sodium and frequently elevated blood pressure (28). Insulin's effects, like those of aldosterone, require active PI3K, or a PI3K-like kinase, and are also in part mediated by SGK1 (21). However, unlike those of aldosterone, insulin's effects on Na+ transport and blood pressure are not subject to feedback inhibition. Moreover, in a large subset of prediabetic and diabetic patients, even as resistance to the glucose metabolic actions of insulin develops, sensitivity to its Na+ transport-stimulatory activities is retained (30). Under these conditions of imbalance, hyperinsulinemia can contribute to salt-sensitive hypertension (28).
PI3Ks are a family of lipid kinases, which phosphorylate phosphoinositides at the 3-OH position of the inositol ring and generate a variety of 3-phosphorylated species of phosphatidylinositol (PIPs). A large body of evidence has led to the paradigm that class I PI3Ks mediate most of insulin's effects on glucose metabolism; however, there has been conflicting evidence regarding which class I isoform is primarily responsible. Moreover, recent evidence has implicated other classes of PI3K (15), as well as PI3K-related protein kinases (PIKKs), such as the mammalian target of rapamycin (mTOR), PI4K, and casein kinase II (8, 12, 35, 36), in insulin/IGF1 action. Evidence that class I PI3Ks mediate insulin/IGF1 effects on Na+ transport in renal tubule cells has been based primarily on experiments using inhibitors with poor specificity (e.g., LY294002 and wortmannin), which block a variety of PI3Ks and PIKKs (19, 29, 37).
The class I PI3Ks are activated by receptor tyrosine kinases (class IA) or G protein-coupled receptors (class IB) and generate predominantly phosphatidyl inositol (3,4,5) trisphosphate (PIP3) using phosphatidyl inositol (4,5) bisphosphate (PIP2) as a substrate (reviewed in Ref. 11). They are composed of a catalytic (p110) and regulatory (most commonly p85) subunit, and activation of catalytic activity requires receptor-dependent recruitment of the catalytic subunit to the plasma membrane. Importantly, there are four distinct p110 subunit isoforms, and data addressing which are implicated in signaling to specific functional endpoints have been conflicting. This is a particularly important issue in light of the wide variety of processes impacted by PI3Ks, and the emerging use of PI3K inhibitors as therapeutic agents for an expanding set of medical indications (16). The recent development of selective small-molecule inhibitors has begun to allow more precise dissection of PI3K-dependent processes and has been used in particular to dissect insulin-regulated glucose metabolism in cultured cells and in animals. Data using these inhibitors have strongly supported a predominant role for p110-α in the control of glucose metabolism, but they have also pointed to a secondary role for p110-β, particularly in setting basal PI3K activity in muscle (18). Although the regulation of glucose metabolism and Na+ transport is similar in many ways, there are also clear differences. Most notably, the Na+-retaining effects of insulin are retained, even as resistance to its glucose metabolic effects is developing (28, 30). In the present study, we have examined the effects on ENaC-dependent Na+ transport of a panel of newly developed inhibitors, which inhibit subsets of p110 isoforms (Table 1 and Ref. 18). Our data using these inhibitors provide the first evidence that p110-α is the principal PI3K isoform required for ENaC-dependent Na+ transport, and in contrast to glucose metabolism in cultured L6 mytotubes (17), p110-β does not appear to play any role in the control of Na+ transport in CD cells. Furthermore, p110-α blockade also blocks phosphorylation of PI3K-dependent kinases Akt and SGK1. These observations support the idea that the pathways that mediate glucose and sodium homeostasis are intertwined; however, there may be significant differences that may have implications for the pathogenesis of the insulin resistance syndrome, as well as for the treatment of hypertension.
Table 1.
In vitro potencies of inhibitors used in the present studies
| α | β | δ | γ | |
|---|---|---|---|---|
| PIK-90 | 0.011 | 0.35 | 0.058 | 0.018 |
| PI-103 | 0.008 | 0.088 | 0.048 | 0.15 |
| TGX-221 | 5 | 0.005 | 0.1 | >10 |
| SW-30 | 85 | 0.74 | 0.007 | 1.3 |
| SW-14 | 8.9 | 0.7 | 0.009 | 0.021 |
Shown are IC50 values for p110 isoforms (in μM). In addition to the activities shown, PI-103 has an IC50 of 0.02–0.083 against the mammalian target of rapamycin, and PIK-90 has an IC50 of 0.047–0.064 against phosphatidylinositol-3-kinase (PI3K) C2 and 0.013 against DNA-PK. As described previously (18), these inhibitors had little or no activity against a large panel of other kinases.
MATERIALS AND METHODS
Cell culture and electrophysiological measurements.
mpkCCDc14 (mpkCCD) cells were maintained in plastic tissue culture flasks in modified DMEM/Ham's F12 (1:1) medium (“regular medium”) as described previously (4, 33). For electrophysiological measurements, the cells were seeded on type VI collagen (Sigma, St. Louis, MO)-coated filters (Transwell, pore size 0.4 μm, Corning Costar) until the cell monolayers reached transepithelial resistance >1,000 Ω·cm2. They were then maintained in “plain medium” [modified DMEM/Ham's F12 (1:1), 20 mM HEPES, pH 7.4, 2 mM glutamine, 20 mM d-glucose, 100 U/ml penicillin-streptomycin] supplemented with 2% charcoal dextran-treated, hormone-free FBS (Hyclone, Logan UT) or serum-free medium for at least 24 h before treatment with aldosterone (1 μM), insulin (100 nM), and inhibitors or equal volumes of vehicle as the control for specified periods of time. Following electrophysiological measurements, cells were harvested and processed for immunoblot analysis.
For insulin-only experiments, cell monolayers exhibiting high transepithelial resistance were maintained in plain medium [modified DMEM/Ham's F12 (1:1), 20 mM HEPES, pH 7.4, 2 mM glutamine, 20 mM d-glucose, 100 U/ml penicillin-streptomycin] supplemented with 10% Hyclone Cell Boost 1 supplement (Hyclone) according to the manufacturer's instructions, lacking growth factors, mitogens, insulin, transferrin, dexamethasone, or aldosterone, and then treated with insulin (100 nM, apical and basolateral sides, Sigma) with or without inhibitors, as shown.
Transepithelial resistance and potential difference across the cell monolayers were measured using a mini-voltohmmeter (MilliCell ERS, Milipore) at specified time periods following treatment. The equivalent short-circuit current (Ieq) was calculated using Ohm's law, as previously described (4, 5, 26). Phenamil (5 μM) added to the medium completely inhibited this current, thereby indicating its ENaC dependence (data not shown).
Preparation of protein lysates.
Following electrophysiological measurements, the mpkCCDc14 cell monolayers were incubated in lysis buffer (50 mM Tris·Cl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) containing 1 mM PMSF (Sigma), 1× Complete Protease-Inhibitor Cocktail (Roche), 1× phosphatase inhibitors Set-1 and Set-2 (Calbiochem), 1 mM benzamidine (Calbiochem), and 2 μM microcystin-LR (Sigma). Lysates were clarified by centrifugation at 10,000 rpm for 15 min at 4°C and used for Western blot analysis.
Western blot analysis.
Equal quantities of protein (100 μg mpkCCDc14 cell lysate) were subjected to electrophoresis in 7.5% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Amersham Biosciences). Immunoblotting was performed as described previously (33) using horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences) and the enhanced chemiluminescence detection system (Amersham Biosciences). The blots were first incubated with rabbit polyclonal antibody raised against rat SGK (a gift of Gary Firestone), as described previously (37). The primary antibody was incubated at 1:2,000 dilution in TBST/0.1% Tween 20 with 5% BSA overnight, washed, and incubated with the secondary antibody. Each blot was subsequently stripped and restained with anti-phospho-Ser473-Akt antibody (1:1,000 dilution, overnight, Cell Signaling), phospho-Thr308-Akt antibody (1:1,000 dilution, overnight, Cell Signaling), and total Akt antibody (1:1,000 dilution, overnight, Cell Signaling). The anti-a-tubulin mouse mAB (1:1,000 dilution, Calbiochem) was used as a loading control.
Semiquantitative RT-PCR.
Total RNA was isolated from 4-h aldosterone- or vehicle-treated cells using standard procedures and made free of residual genomic DNA contamination using a DNase-treatment and Removal kit (Qiagen, Valencia, CA). Purified RNA was reverse transcribed with MultiScribe reverse transcriptase (PE Applied Biosystems, Foster City, CA), and PCR reactions were set up using a Promega PCR Kit (Promega Life Science, Madison, WI) according to the manufacturer's instructions. The following primers were used for PCR: 5′-GTC TAC CCT CCA AAT GTC GAG TC (mouse p110-α), 3′-CTC CTC CAT GGT AGA TAC CTG TTC (mouse p110-α); 5′-CTG AAC TGG CTC AAG GAG TAC AAC (mouse p110-β), 3′-GGA CCT GTA GTC TTT CCG TAC TGT (mouse p110-β); 5′-AGC GTC TGA GTT TCC TTG TCT C (mouse p110-γ), 3′-GCA CCA CTT GGT ACC TGT CAT A (mouse p110-γ); 5′-CAG CCC TAG AGG TAA AAG GAC AGT (mouse p110-δ), 3′-GGG GTA AGG GAC AGA GTC TAC ATA (mouse p110-δ); and 5′-TTT TTT ATC TGC ACT GCC AAG ACT (cyclophilin), 3′-CAC CTT CCC AAA GAC CAC ATG (cyclophilin). All PCR reactions were performed in triplicate using samples obtained from three independent experiments. A “minus-RT” negative control was included with each run.
RESULTS
PI3K isoform dependence of Na+ current.
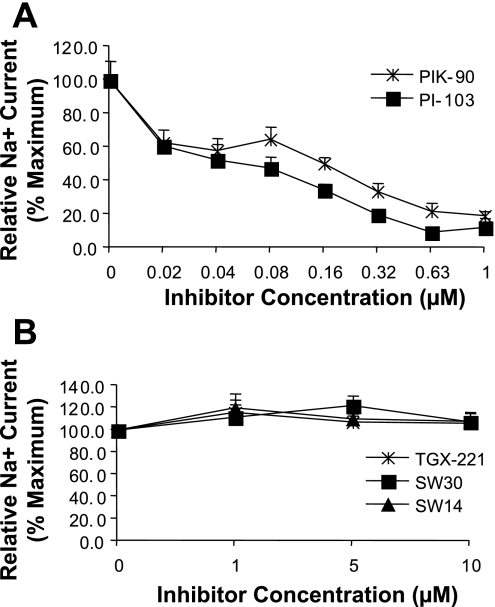
We first tested the concentration dependence of Na+ current inhibition by five newly available compounds, which have distinct inhibitory patterns for the different isoforms of PI3K catalytic (p110) subunits (18). Their in vitro activities against p110 isoforms are shown in Table 1. Notably, two of the inhibitors, PIK-90 and PI-103, are potent inhibitors of p110-α, while SW-30 and SW-14 have virtually no α-inhibitory activity, and TGX-221 inhibits α only at high concentrations. PIK-90 also inhibits p110-δ and -γ, and, at high concentrations, -β. PI-103 inhibits all four p110s at submicromolar concentrations, although it is most potent against α. TGX-221 is largely selective for p110-β, although at intermediate concentrations it inhibits p110-δ. SW-30 is most potent against p110-δ, while SW-14 inhibits both p110-δ and -γ. To begin to test the effects of these inhibitors on ENaC-dependent Na+ current, mpkCCD cells were grown on Transwell filters and treated with aldosterone and insulin for 4 h to activate current, followed by addition of inhibitors at concentrations shown. Current was measured by an Evometer at time points shown (Fig. 1). PI-103 and PIK-90 inhibited Na+ current with IC50 of ∼0.08 and 0.16 μM, respectively (Fig. 1A), while TGX-221, SW-30, and SW-14 had no effect, even at concentrations up to 10 μM. A similar inhibitor profile was found in cells stimulated with either aldosterone or insulin alone (not shown), with the exception that in cells treated with insulin, TGX-221 had a mild inhibitory effect at high concentrations (≥5 μM).
Fig. 1.
Concentration dependence of Na+ current inhibition by isoform-selective phosphatidylinositol-3-kinase (PI3K) inhibitors. mpkCCD cells grown on Transwell filters were pretreated with aldosterone and insulin for 4 h, followed by treatment with inhibitors at concentrations shown for additional 2 h. Equivalent current was determined by measurement of potential difference and resistance across monolayers, as described in materials and methods. A: cells treated with PIK-90 and PI-103. B: cells treated with TGX-221, SW-30, and SW-14.
Time course of Na+ current inhibition.
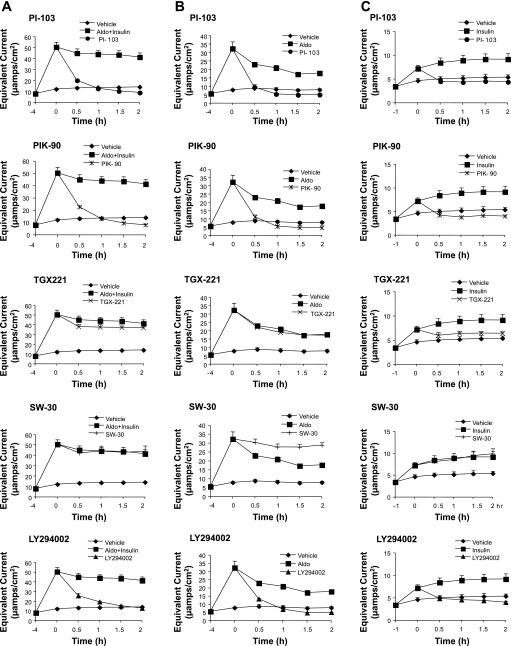
We next examined the time course of inhibitor effects on current (Fig. 2). In cells treated with aldosterone and insulin together, PIK-90 (1 μM) and PI-103 (0.5 μM) inhibited current with a time course comparable to that of the nonspecific PI3K inhibitor LY294002 (Fig. 2A). Onset of inhibition occurred within 30 min, and current had dropped to, or below, that of baseline within 2 h. After withdrawal of the inhibitor, the current returned to preinhibition levels over 24 h (data not shown). TGX-221 and SW-30 had no significant effect on current at any of the times examined (Fig. 2A). Similar inhibitor effects were found for cells treated with aldosterone alone (Fig. 2B). The inhibitor pattern of cells treated with insulin alone was similar, with the exception that TGX-221 inhibited current significantly at 5 μM (Fig. 2C). This inhibition at high concentrations of TGX-221 probably reflects primarily a p110-α-dependent effect (cf. Table 1), although why it occurs only in the context of insulin-stimulated current is unclear. In view of the lack of effect of TGX-221 on the potency of PIK-90 (see Fig. 6, and below), it seems unlikely that the effect of 5 μM TGX-221 is due to combined inhibition of p110-α and -β; however, they leave open the interesting possibility that p110-δ (which is inhibited by TGX-221 at a higher IC50 than p110-β) might play a role selectively in insulin-stimulated Na+ current. Further experiments will be needed to clarify this issue. In any case, the present results strongly support the conclusion that p110-α is the principal PI3K isoform mediating aldosterone- and insulin-induced Na+ current in CD cells.
Fig. 2.
Time course of PI3K inhibitor effects on epithelial Na+ channel (ENaC)-dependent Na+ current in mpkCCD cells treated with aldosterone and insulin together (A), aldosterone alone (B), or insulin alone (C). mpkCCD cells were grown on Transwell filters, as in Fig. 1, and pretreated with hormones, as described in materials and methods, and inhibitors then were added and equivalent current was measured at times shown. Inhibitor concentrations were as follows: LY294002, 20 μM; PI-103, 0.5 μM; PIK-90, 1 μM; TGX-221, 5 μM; and SW-30, 5 μM.
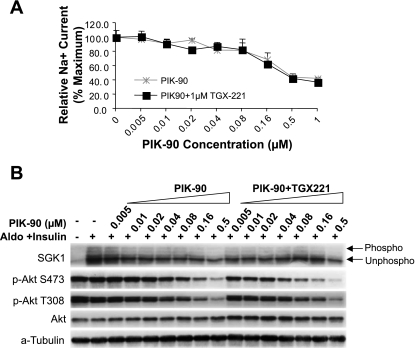
Fig. 6.
p110-β inhibition does not influence Na+ current (A) or phosphorylation of either Akt or SGK1 (B). mpkCCD cells were grown on Transwell filters and pretreated with aldosterone and insulin or vehicle, as in Fig. 2. Cells were then treated with PIK-90 at concentrations shown, in the presence or absence of 1 μM TGX-221. After measurement of equivalent current, lysates were prepared and subjected to Western blotting as in Fig. 3.
PI3K isoform dependence of SGK1 and Akt expression and phosphorylation.
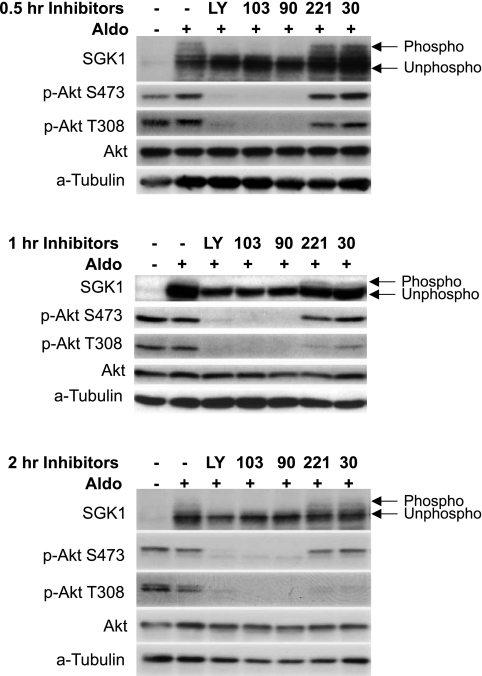
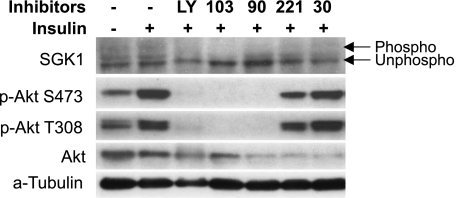
Both SGK1 and Akt are PI3K-dependent kinases, which recognize a common substrate sequence present in several target proteins, which include the ENaC inhibitor Nedd4-2 (7, 13, 24). Both kinases undergo insulin-stimulated, PI3K-dependent phosphorylation at two sites (Thr308 and Ser473 in Akt, and Thr256 and Ser-422 in SGK1, respectively) (20, 24). SGK1 is a well-established mediator of aldosterone action (22), and there is also evidence that SGK1 and Akt together mediate insulin stimulation of Na+ current (3, 14, 21). The abundance of SGK1, but not of Akt, is markedly increased by aldosterone, and there is substantial basal phosphorylation of both kinases in the absence of insulin or other hormonal activators of PI3K (3, 21, 37). With these observations in mind, we next examined the effect of the inhibitor panel on the expression and phosphorylation status of SGK1 and Akt in the presence of aldosterone and insulin together (Fig. 3), or the two hormones separately (Figs. 4 and 5). As shown previously (37), SGK1 expression was strongly stimulated by aldosterone (Figs. 3 and 4), and the phosphorylated forms were enhanced by insulin (Fig. 3A). Akt expression, on the other hand, was unaffected (or slightly decreased) by aldosterone (Figs. 3 and 4), while its phosphorylation at both Thr308 and Ser473 was strongly stimulated by insulin (Figs. 3 and 5), as previously established (2).
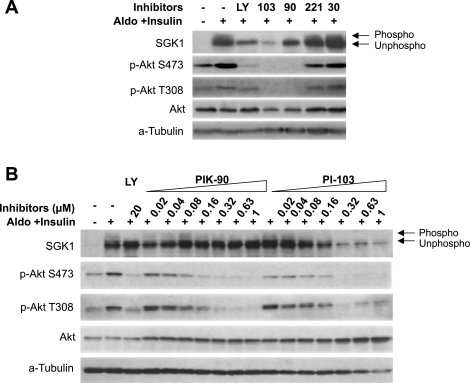
Fig. 3.
Effect of isoform-selective PI3K inhibitors on SGK1 and Akt phosphorylation in mpkCCD cells treated with aldosterone and insulin. A: mpkCCD cells were grown on Transwell filters and treated for 4 h with aldosterone plus insulin, and inhibitors were added for 1 h additionally, at the same concentrations used in Fig. 2. Cells were harvested and subjected to Western blotting for SGK1, phospho-Akt S473, phospho-Akt T308, and total Akt, as shown. Phosphorylated (phospho) forms of SGK1 are distinguished from unphosphorylated (unphospho) forms by mobility shift. α-Tubulin is shown as a loading control. B: titration of PIK-90 and PI-103. Lysates were prepared from mpkCCD cells used for Na+ current measurements (Fig. 1A) and were subjected to Western blotting as in A.
Fig. 4.
Inhibition of p110-α, but not of -β, -γ, or -δ, blocks SGK1 and Akt phosphorylation in aldosterone-treated mpkCCD cells. Cells were pretreated with 1 μM aldosterone for 4 h, and then inhibitors (at concentrations as in Figs. 2 and 3A) were added and cells were harvested for Western blotting at 0.5, 1, or 2 h, as shown.
Fig. 5.
p110-α inhibition blocks SGK1 and Akt phosphorylation in insulin-treated mpkCCD cells. Experiments were performed as in Figs. 3 and 4, using lysates from cells treated with insulin in the absence of aldosterone. The cells were pretreated with 100 nM insulin for 1 h and then incubated with inhibitors for an additional 1 h before harvesting of protein for Western blotting. Inhibitor concentrations were as in Fig. 2.
The pattern of inhibitor effects on phosphorylation of both SGK1 and Akt paralleled the pattern of effects on Na+ current in that LY294002, PI-103, and PIK-90, but not SW-30 or TGX-221, were strongly inhibitory (Figs. 3A, 4, and 5). The potency of PI-103 and PIK-90 for blockade of SGK1 and Akt phosphorylation correlated closely with the concentrations required for blockade of Na+ current. This was true whether Na+ current was stimulated with aldosterone and insulin together (compare Figs. 3B and Fig. 1A), or with the two hormones separately (not shown). It is also interesting to note that PI-103, but not PIK-90, substantially decreased SGK1, but not Akt-protein abundance (Figs. 3, A and B). The mechanism for this effect on protein abundance is uncertain; however, it is notable that although PIK-90 and PI-103 inhibit p110-α with comparable in vitro IC50, PI-103 is a fourfold more potent inhibitor of p110-β (Table 1), which could play a role in SGK1 expression.
The effects of the inhibitors in cells treated with aldosterone alone were similar to those seen in cells treated with aldosterone and insulin together (Fig. 4), although there was somewhat less effect of PI-103 on SGK1 expression (compare Figs. 3A and 4). The reason for this difference is uncertain, but suggests the participation of another kinase, which is more sensitive to PI-103 than PIK-90, for example, mTOR. Importantly, the time course of inhibitor effects on SGK1 and Akt phosphorylation in aldosterone-treated cells was also consistent with p110-α being mechanistically implicated in the control of Na+ current: PIK-90 and PI-103, but not TGX-221 or SW-30, markedly blunted both SGK1 and Akt phosphorylation within 0.5 h, similar to the time course seen for Na+ current inhibition (compare Figs. 4 and 2). This inhibitory effect on SGK1 and Akt phosphorylation persisted for at least 2 h. A similar temporal pattern of inhibitor response was seen in cells treated with aldosterone and insulin together or with insulin alone (data not shown).
Next, we examined the effect of the inhibitor panel on SGK1 and Akt phosphorylation in cells treated with insulin alone (Fig. 5). Under these conditions (in the complete absence of aldosterone), SGK1 levels are low (compare lanes 1 and 2 in Fig. 4), and although both the phosphorylated and unphosphorylated forms of SGK1 could be detected, sensitivity of the assay (which relies on phosphorylation-induced mobility shift) was not sufficient to assess the stimulatory effect of insulin. A consistent effect of PI-103 and LY294002, but not PIK-90, to inhibit SGK1 phosphorylation was detected. In this way, insulin-treated cells were different from those treated with aldosterone or aldosterone plus insulin. As in Fig. 3, Akt was readily detectable and its phosphorylation at both Thr308 and Ser473 was substantially increased by insulin and blocked by LY294002, PI-103, and PIK-90. In some experiments, PIK-90, TGX-221, and SW-30 appeared to modestly decrease total Akt expression; however, this effect was inconsistent.
Effect of combined inhibition of p110-α, -β, and -δ.
The inhibitor response profile of glucose uptake and Akt phosphorylation in L6 myotubes and L1 adipocytes also demonstrates a predominant dependence on p110-α (18). However, myotubes and adipocytes differ from each other in their dependence on p110-β: in myotubes, but not adipocytes, the sensitivity of Akt phosphorylation to p110-α inhibition is potentiated by inhibition of p110-β. To determine whether Na+ current or phosphorylation of either Akt or SGK1 exhibited a similar secondary dependence on p110-β or -δ, the effect of PIK-90 at concentrations ranging from 0.005 to 1 μM was determined in the presence and absence of 1 μM TGX-221, which inhibits p110-β and -δ, but not -α (Table 1, Ref. 17). As shown in Fig. 6A, TGX221 did not have any effect on the ability of PIK-90 to inhibit Na+ current. Similarly, neither SGK1 nor Akt phosphorylation (at either Thr308 or Ser473) showed a secondary dependence on p110-β or -δ (Fig. 6B). Together, these results suggest that CCD cells behave more like adipocytes than myotubes in their PI3K isoform dependence.
PI3K isoform expression in mpkCCD cells.
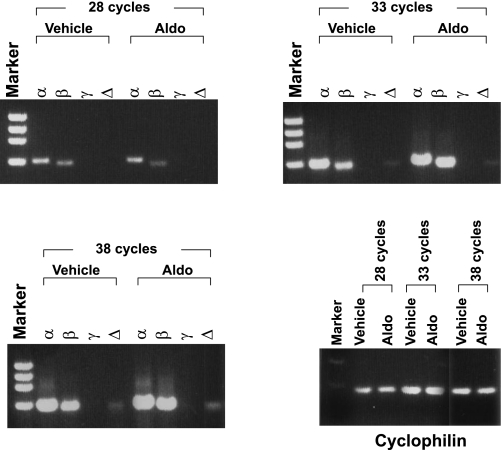
Finally, we examined the expression of p110 isoforms in mpkCCD cells by semiquantitative RT-PCR using RNA isolated from vehicle- or aldosterone-treated mpkCCD cells (Fig. 7). Interestingly, p110-α and -β were comparably expressed, and p110-δ was readily detectable, although at a lower level than α or β. The PI3K IB isoform, p110-γ, was not detected, consistent with its selective expression in leukocytes (32). These results strongly suggest that the mechanism underlying selective use of p110-α in these cells does not reflect a lack of expression of p110-β or -δ.
Fig. 7.
mpkCCD cells express p110-α, -β, and -δ but not -γ. mRNA was harvested from mpkCCD cells treated for 4 h with 1 μM aldosterone (or vehicle) and subjected to PCR with primers selective for p110-α, -β, -γ, or -δ for 28, 33, or 38 cycles, as described in materials and methods. p110-γ was not detected, even with additional PCR cycles (up to 43 cycles; not shown). Cyclophilin products at 28, 33, and 38 cycles are shown as control for amplification and loading.
DISCUSSION
Our present experiments strongly support the conclusion that Na+ transport in CD cells is dependent on p110-α and has no significant dependence on p110-β, -γ, or -δ. PIK-90 and PI-103, both of which are potent inhibitors of p110-α in vitro, strongly inhibited Na+ current at concentrations comparable to those that inhibit glucose uptake in adipocytes (Fig. 1A and Ref. 17). In contrast, TGX-221 (at concentrations that inhibit p110-β and -δ), SW-30 (at concentrations that inhibit only p110-γ), and SW-14 (at concentrations that inhibit p110-δ and -γ) had no effect on current (Fig. 1B). TGX-221 did not alter the sensitivity of Na+ current to PIK-90, suggesting that p110-β does not play a threshold or partially redundant role in CD cells, as it does in L6 myotubes (17). The time course of inhibition by PIK-90 and PI-103 is rapid, reducing current, as well as SGK1 and Akt phosphorylation, to near-baseline within 30 min (Fig. 2). This time course, which is similar to that of LY294002, a nonspecific inhibitor of class I PI3Ks, and several other PI3K-like kinases, including mTOR (17), is consistent with the mechanistic role for PI3K-α, as well as for SGK1 and Akt in hormone-stimulated Na+ current.
CD cells have baseline PI3K activity, which is further activated by insulin (29, 37); however, evidence is conflicting as to whether there is any effect of aldosterone on PI3K per se (1, 6, 21, 29, 34, 37). Our present data indicate that in mpkCCD cells, there is basal p110-α-dependent phosphorylation of Akt and of SGK1, which is blocked to near-undetectable levels by LY294002, PIK-90, and PI-103. This basal phosphorylation is further stimulated by insulin, but not by aldosterone (Figs. 3–5), consistent with earlier reports suggesting that Akt and SGK1 together mediate insulin's effects on Na+ current (21, 37). The SGK1 level is highly dependent on aldosterone, and although it is difficult to assess its phosphorylation state in the absence of aldosterone, our data are consistent with the idea that the relative proportion of phosphorylated and unphosphorylated SGK1 is not changed by this hormone. Further studies are needed to clarify this issue.
It appears that SGK1 expression is dependent on the activity of a PI-103-inhibitable kinase. The identity of this kinase is uncertain at this time, and, moreover, whether it influences synthesis and/or degradation remains to be determined. The pattern of inhibition in the presence of aldosterone (see particularly Figs. 3A and 4) is consistent with a combined effect of mTOR and p110-α; however, further work is needed to clarify this conjecture. With regard to the action of insulin by itself, it is notable that in the absence of aldosterone, Akt levels are substantially higher than those of SGK1, and although Akt is a weak activator of Na+ current (3), it may be the principal one in the complete absence of aldosterone (21). The extent to which the PI3K-dependent effects are going through either SGK1 and/or Akt vs. an alternate pathway, including direct effects of PIP3 (27), still remains to be determined, as does the relative contribution of Po vs. N. In any case, our data support the idea that the generation of ENaC-stimulatory PIP3 is mediated by p110-α, with no contribution from β or δ, both of which are expressed in these cells (Fig. 7). It is also important to note that comparison of the inhibitor pattern seen in the present study with their in vitro activities (18) does not support a central role for hsVPS34 in this process, although this does not rule out a role for class II or III PI3Ks in ENaC trafficking (9). The baseline and insulin-stimulated PI3K activity, as reflected in ENaC-dependent Na+ current, as well as in SGK1 and Akt phosphorylation, appears to be due to p110-α (Fig. 2, A–C, and data not shown). Thus inhibition of p110-α but not of -β, -δ, or -γ, rapidly and robustly inhibited Na+ current and phosphorylation of both SGK1 and Akt. The concentration profiles, and time courses, for inhibition of SGK1 and Akt phosphorylation were similar to the profiles for inhibition of Na+ current. However, PI-103 at higher concentrations also inhibited SGK1-but not Akt-protein expression (Fig. 3B). The underlying basis for this effect is uncertain, but likely reflects an effect of PI-103 on another mediator, for example mTOR, which is inhibited by PI-103, but not by PIK-90 (17). It is unlikely that p110-β is implicated in SGK1 expression since TGX-221 had no effect on expression, in the presence or absence of PIK-90 (Fig. 6B).
TGX-221, at concentrations that selectively block p110-β or -δ, but not -α, failed to inhibit aldosterone-stimulated Na+ current, even in the presence of PIK-90 (Figs. 2 and 6A). However, TGX-221 did inhibit insulin-stimulated current at high concentration (5 μM). This may simply reflect a p110-α-mediated effect (TGX partially inhibits α at very high concentrations), but the differential effect of this inhibitor on insulin- vs. aldosterone-treated cells warrants note. Akt and SGK phosphorylation in mpkCCD cells similarly were unaffected by TGX, and it is interesting to speculate that p110-δ mediates an effect of PI3K, which is independent of activation of SGK1 or Akt (27).
mpkCCD cells appear to behave more like adipocytes than L6 myotubes (17) in that β inhibition does not potentiate that of α. In L6 cells, the effect of p110-α inhibition on Akt phosphorylation is potentiated by p110-β/δ inhibitors, while in adipocytes it is not. The mechanistic basis for differential isoform function in the control of either glucose metabolism or Na+ transport is not immediately apparent; however, it is interesting to speculate that these functional differences play a role in the salt-sensitive hypertension associated with the insulin-resistance syndromes. Notably, it is well established that resistance to insulin-induced glucose uptake in muscle contributes to hyperinsulinemia in prediabetic patients (28). Since the sensitivity of renal tubular Na+ reabsorption to insulin is maintained (30), Na+ retention occurs and contributes to salt-sensitive hypertension (30, 31). Interestingly, a decrease in p110-β expression in muscle has been implicated as a contributing factor in insulin-resistant states (23).
In conclusion, our data strongly support the idea that p110-α is the principal PI3K catalytic subunit isoform required for ENaC-dependent transepithelial Na+ transport, as well as for phosphorylation of the PI3K-dependent kinases SGK1 and Akt in CD cells. These findings underscore the close relationship between pathways involved in the control of glucose metabolism and Na+ transport and are consistent with a role for SGK1 and Akt in hormone-regulated Na+ transport in cortical CD cells. Furthermore, it is of potential pathophysiological and clinical significance that p110-β appears to play a role in insulin action in myotubes but not in CD cells or adipocytes.
GRANTS
Morri Feldman is gratefully acknowledged for careful reading and helpful comments on the manuscript.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-56695 (D. Pearce) and is based upon work supported under a National Science Foundation Graduate Research Fellowship to O. Williams.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Al-Baldawi NF, Stockand JD, Al-Khalili OK, Yue G, Eaton DC. Aldosterone induces ras methylation in A6 epithelia. Am J Physiol Cell Physiol 279: C429–C439, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551, 1996. [PMC free article] [PubMed] [Google Scholar]
- 3.Arteaga MF, Canessa CM. Functional specificity of Sgk1 and Akt1 on ENaC activity. Am J Physiol Renal Physiol 289: F90–F96, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Bhargava A, Fullerton MJ, Myles K, Purdy TM, Funder JW, Pearce D, Cole TJ. The serum- and glucocorticoid-induced kinase is a physiological mediator of aldosterone action. Endocrinology 142: 1587–1594, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Blazer-Yost BL, Punescu TG, Helman SI, Lee KD, Vlahos CJ. Phosphoinositide 3-kinase is required for aldosterone-regulated sodium reabsorption. Am J Physiol Cell Physiol 277: C531–C536, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21: 952–965, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J 15: 5256–5267, 1996. [PMC free article] [PubMed] [Google Scholar]
- 9.Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel (ENaC) by membrane trafficking. Am J Physiol Renal Physiol (First published May 28, 2008). doi: 10.1152/ajprenal.90248.2008. [DOI] [PMC free article] [PubMed]
- 10.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA 96: 2514–2519, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czech MP PIP2 and PIP3: complex roles at the cell surface. Cell 100: 603–606, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20: 7052–7059, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieter M, Palmada M, Rajamanickam J, Aydin A, Busjahn A, Boehmer C, Luft FC, Lang F. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes Res 12: 862–870, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch E, Costa C, Ciraolo E. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J Endocrinol 194: 243–256, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol 17: 615–675, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Knight ZA, Chiang GG, Alaimo PJ, Kenski DM, Ho CB, Coan K, Abraham RT, Shokat KM. Isoform-specific phosphoinositide 3-kinase inhibitors from an arylmorpholine scaffold. Bioorg Med Chem 12: 4749–4759, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125: 733–747, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight ZA, Shokat KM. Features of selective kinase inhibitors. Chem Biol 12: 621–637, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114: 2903–2910, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Lee IH, Dinudom A, Sanchez-Perez A, Kumar S, Cook DI. Akt mediates the effect of insulin on epithelial sodium channels by inhibiting Nedd4-2. J Biol Chem 282: 29866–29873, 2007. [DOI] [PubMed] [Google Scholar]
- 22.McCormick JA, Bhalla V, Pao AC, Pearce D. SGK1: a rapid aldosterone-induced regulator of renal sodium reabsorption. Physiology (Bethesda) 20: 134–139, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia 48: 547–552, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3- kinase-stimulated signaling pathway. EMBO J 18: 3024–3033, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearce D SGK1 regulation of epithelial sodium transport. Cell Physiol Biochem 13: 13–20, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Pearce D The role of SGK1 in hormone-regulated sodium transport. Trends Endocrinol Metab 12: 341–347, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Pochynyuk O, Staruschenko A, Tong Q, Medina J, Stockand JD. Identification of a functional phosphatidylinositol 3,4,5-trisphosphate binding site in the epithelial Na+ channel. J Biol Chem 280: 37565–37571, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Reaven GM The kidney: an unwilling accomplice in syndrome X. Am J Kidney Dis 30: 928–931, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Record RD, Froelich LL, Vlahos CJ, Blazer YB. Phosphatidylinositol 3-kinase activation is required for insulin-stimulated sodium transport in A6 cells. Am J Physiol Endocrinol Metab 274: E611–E617, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Rocchini AP Obesity hypertension, salt sensitivity and insulin resistance. Nutr Metab Cardiovasc Dis 10: 287–294, 2000. [PubMed] [Google Scholar]
- 31.Rocchini AP The relationship of sodium sensitivity to insulin resistance. Am J Med Sci 307, Suppl 1: S75–S80, 1994. [PubMed] [Google Scholar]
- 32.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 287: 1040–1046, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem 280: 39970–39981, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Tong Q, Gamper N, Medina JL, Shapiro MS, Stockand JD. Direct activation of the epithelial Na+ channel by phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate produced by phosphoinositide 3-OH kinase. J Biol Chem 279: 22654–22663, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem 70: 535–602, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Volinia S, Dhand R, Vanhaesebroeck B, MacDougall LK, Stein R, Zvelebil MJ, Domin J, Panaretou C, Waterfield MD. A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p-Vps15p protein sorting system. EMBO J 14: 3339–3348, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Barbry P, Maiyar AC, Rozansky DJ, Bhargava A, Leong M, Firestone GL, Pearce D. SGK integrates insulin and mineralocorticoid regulation of epithelial sodium transport. Am J Physiol Renal Physiol 280: F303–F313, 2001. [DOI] [PubMed] [Google Scholar]