Abstract
The aims of the present study were to determine whether superoxide (O2−) production is enhanced in medullary thick ascending limb (mTAL) of Dahl salt-sensitive (SS) rats compared with a salt-resistant consomic control strain (SS.13BN) and to elucidate the cellular pathways responsible for augmented O2− production. Studies were carried out in 7- to 10-wk-old male SS and SS.13BN rats fed either a 0.4% NaCl diet or a 4.0% NaCl diet for 3 days before tissue harvest. Tissue strips containing mTAL were isolated from the left kidney, loaded with the O2−-sensitive fluorescent dye dihydroethidium, superfused with modified Hanks’ solution, and imaged at ×60 magnification on a heated microscope stage. O2− production was stimulated in mTAL by incrementing superfusate NaCl concentration from 154 to 254 to 500 mM. O2− production was enhanced in mTAL of SS rats compared with SS.13BN rats in response to incrementing bath NaCl. Addition of N-methyl-amiloride (100 μM) or inhibition of NAD(P)H oxidase reduced O2− production in SS mTAL to levels observed in SS.13BN rats. Both amiloride- and ouabain-sensitive pathways of O2− production were elevated following 3 days of high (4.0%) NaCl feeding in mTAL of SS and SS.13BN rats. We conclude that mTAL from SS rats exhibit enhanced amiloride-sensitive O2− production. The amiloride-sensitive O2− response in mTAL is independent of active Na+ transport and appears to be mediated by NAD(P)H oxidase. Amiloride-sensitive O2− production is likely to contribute to augmented outer medullary O2− production observed in SS rats during both normal and high NaCl diets.
Keywords: blood pressure, free radicals, hypertension, kidney, reduced nicotinamide adenine dinucleotide phosphatase oxidase
dahl salt-sensitive (ss) rats exhibit increased blood pressure salt sensitivity and renal medullary oxidative stress compared with consomic, salt-resistant SS.BN13 rats, despite highly similar genetic backgrounds (7). Our laboratory has previously demonstrated that in vivo superoxide (O2−) levels in the renal medulla of SS rats are greater than those of salt-resistant rats (27) and that renal outer medullary oxidative stress contributes to the development of hypertension in SS rats. As the thick ascending limb produces more O2− than any other nephron segment (17), we hypothesized that enhanced O2− production in medullary thick ascending limb (mTAL) mediates the oxidative stress observed in the outer medulla of SS rats (27).
Our laboratory has previously demonstrated that both the Na+-K+-ATPase inhibitor ouabain and the Na+/H+ exchanger (NHE) inhibitor amiloride reduce O2− production in mTAL in response to increased extracellular NaCl (20). Furthermore, Li et al. (18) have recently provided evidence that amiloride-sensitive H+ efflux stimulates O2− production in mTAL and that amiloride-sensitive pathways account for more than 50% of outer medullary oxidative stress in anesthetized Sprague-Dawley rats. Given these data, the first aim of the present study was to determine whether O2− production is enhanced in mTAL isolated from SS rats, providing evidence either for or against a role of mTAL epithelial cells in the development of oxidative stress in the outer medulla of these animals. Our second aim was to determine whether N-methyl-amiloride (NMA)-sensitive O2− production was elevated in SS rats and whether these pathways may be enhanced during high-sodium feeding and the development of hypertension in these animals.
METHODS
Experimental animals.
Studies used 7- to 10-wk-old male SS and SS.13BN rats [MCW inbred strains (7, 26)], weighing 250–350 g, maintained ad libitium on water and a standard pellet diet containing 0.4% NaCl since weaning in the animal resource center of the Medical College of Wisconsin. Where described, animals were switched to a standard pellet diet containing 4.0% NaCl for 3 days’ duration before tissues were harvested. All protocols were approved by the Institutional Animal Care Committee.
Solutions.
Hanks’ balanced salt solution (HBSS) was purchased from Invitrogen (Grand Island, NY) and prepared by adding HEPES (Sigma) to a final concentration of 20 mM. The pH of the resulting solution was adjusted to 7.40 by addition of NaOH. Apocynin, ouabain, NMA, choline chloride (ChCl), and NaCl were purchased from Sigma (St. Louis, MO). Apocynin was dissolved in methanol before being added to the experimental buffer. Dihydroethidium (DHE) and sodium-binding benzofuran isophthalate were purchased from Molecular Probes (Eugene, OR). DHE was prepared in DMSO, as instructed, before being diluted to appropriate concentration in HBSS.
Determination of NaCl concentration in renal medullary interstitial fluid.
Interstitial fluid samples from the outer medulla of SS and SS.13BN rats fed a 0.4% NaCl diet were obtained using the method described by Lee et al. (14, 15).
The Na+ concentration of interstitial fluid samples was determined using the Na+-sensitive fluorescent dye sodium-binding benzofuran isophthalate (Molecular Probes), using a fluorescent plate reader (Spectrafluor Plus; TECAN U.S.). The final sodium concentration of interstitial fluid samples was then determined by comparing the excitation ratio ×340/×405 to samples of known sodium concentration on a standard curve.
Since unstimulated mTAL in our in vitro preparation produce only low levels of O2−, it was necessary to stimulate mTAL O2− production to identify drug treatment or strain effects on mTAL O2− production. As in vivo mTAL are exposed to relatively large fluctuations in both osmolality and extracellular NaCl levels in response to variations in Na+ and water intake (24), we chose to stimulate mTAL O2− production by incrementing superfusate NaCl concentration from 154 (physiologically low) to 254 (physiologically normal) to 500 mM (physiological high). The mean interstitial Na+ concentration determined from fluid obtained from SS and SS.13BN rats was used to estimate physiologically low, normal (×1), and high (×2), the average NaCl levels to which mTAL are exposed in vivo.
Preparation of mTAL for in vitro studies.
Isolation of mTAL was performed as described previously (9). Briefly, rats were anesthetized with pentobarbital sodium (50 mg/kg ip), and the left kidney was perfused with 6 ml of chilled HBSS at 3 ml/min via the abdominal aorta. The left kidney was then excised and sliced sagitally using a razor blade, and a segment of the renal outer medulla was dissected using fine scissors. The segment of outer medulla was then placed in chilled HBSS and microdissected under a Lecia M3Z stereomicroscope to remove a thin strip of tissue from the inner stripe of the outer medulla containing mTAL. The thin tissue strip containing mTAL was placed on a glass coverslip coated with the tissue adhesive Cell-Tak (BD Biosciences, Bedford, MA) for fluorescence imaging.
Fluorescence detection of dyes.
Fluorescence measurements were made using a Nikon TE2000 inverted microscope with a ×60 magnification water immersion (numerical aperture 1.2) objective lens. The signal was detected using a high-resolution digital camera (Photometrics Cascade 512B, Roper Scientific, Tucson, AZ). Excitation was provided by a Sutter DG-4 175 W xenon arc lamp (Sutter Instruments, Novato, CA) that allowed high-speed excitation wavelength switching. DHE and ethidium (Eth) fluorescent signals were stimulated by dual-wavelength excitation at 380 and 480 nm. A 445/40-nm and a 605/55-nm band-pass emission filter were used to collect DHE (excitation 380 nm/emission 445 nm) and Eth (excitation 480 nm/emission 605 nm) signals, respectively. The excitation intensity was adjusted on the DG-4 to minimize bleaching and to balance excitation intensities.
Determination of mTAL O2− production.
Tissue strips containing mTAL were loaded with DHE (50 mmol/l) in HBSS for 1 h at room temperature. Loading buffer was then replaced with HBSS, and tissues rested for a further 15 min before being imaged. Coverslips were placed on a heated imaging chamber maintained at 37°C (Warner Instruments, Hamden, CT) that allowed the rapid exchange of superfusion buffer and were mounted on the stage of an inverted microscope. Five to 10 mTAL epithelial cells were selected within each tissue strip to quantify changes in fluorescent intensity of the DHE dye using Metafluor imaging software (Universal Imaging, Downingtown, PA). The rate of O2− production was calculated as the change in the ratio of Eth fluorescent signal to DHE fluorescent signal. We have found that using the ratio Eth/DHE to determine O2− production in mTAL reduces measurement artifacts associated with cell volume changes and dye bleaching (19).
O2− production in mTAL was stimulated by increasing bath NaCl concentration (20) through 154, 254, and 500 mM, for 200 s at each increment, over a 600-s period, representing approximately physiological isotonic and one and two times normal in vivo NaCl levels in the renal outer medulla. mTAL O2− production was determined in tissue strips obtained from the inner stripe of the outer medulla of SS and SS.13BN rats fed a 0.4% NaCl diet in response to incrementing bath NaCl concentration, and SS and SS.13BN rats fed a 4.0% NaCl diet for 3 days before tissue harvest. Responses to incrementing bath NaCl concentration in mTAL of SS and SS.13BN rats were also determined in the presence of ouabain (4 mM; to inhibit Na+-K+-ATPase) and NMA (100 μM; to inhibit NHE activity). Responses to incrementing bath NaCl concentration in mTAL of 0.4% NaCl-fed SS and SS.13BN rats were also determined in the presence of ouabain (4 mM) + NMA (100 μM; to inhibit both Na+-K+-ATPase and NHE) and apocynin [100 μM; to inhibit NAD(P)H oxidase]. In all cases, tissue strips were incubated with inhibitors for 15 min before recording. In some tissue strips, osmolality was increased in increments of 300, 500, and 1,000 mosmol/kgH2O using ChCl in the presence of ouabain (4 mM; to determine the active Na+ transport independent effects of osmolality) and ChCl in the presence of NMA (100 μM) or urea (to determine the effects of changes in cell volume on mTAL O2− production independent of other osmotic effects).
Eth and DHE fluorescent signals were recorded for 200 s at each increment at 3-s intervals. To conserve time and maximize the number of observations that could be obtained from each animal, positive control stimuli were not tested in all tissue strips. In some tissue strips, diethyldithiocarbamic acid (1 mmol/l; Sigma) to inhibit superoxide dismutase and menadione sodium bisulfite (500 mol/l; Sigma) to stimulate mitochondrial O2− production were added to serve as positive control stimuli to test for dye loading and cell viability. Strong positive control responses were observed in all coverslips tested, indicating that none of the protocols utilized exhausted the DHE dye or adversely affected cell viability.
Western blots.
Rats were prepared as described above. The left kidney was flushed with chilled HBSS, and the outer medulla was snap frozen in liquid nitrogen. Tissue was homogenized, and ∼30 mg of protein were extracted from the outer medulla for Western blot. The membrane was immunoblotted with primary antibody (p22phox-polyclonal antibody; Santa Cruz, Biotechnology, sc-20781) in 1:500 dilution and secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit antibody, Abcam, ab6721) in 1:5,000 dilution. The densitometry values were normalized to the band of β-actin reprobed in the same sample.
Data and statistical analysis.
Background fluorescent signals were subtracted, and the DHE and Eth signals were normalized so that the ratio Eth/DHE at time = 0 was equal to 1. Data are expressed as means ± SE. The responses of intracellular O2− production to incrementing bath NaCl concentration were evaluated with a two-way ANOVA using a Bonferroni post hoc test to compare responses between SS and SS.13BN rat strains (PStrain*Sodium). To determine the effects of treatments, where appropriate, responses were also compared with control responses of mTAL to incrementing sodium from low-sodium (0.4% NaCl)-fed SS.13BN and SS rat in the absence of inhibitors (PTreatment). Where indicated, slopes represent rate of change in ratio of Eth/DHE over the final 100 s of each stimulus period. For all other data, significance was evaluated using an unpaired t-test. The level required to reach significance was P < 0.05.
RESULTS
Interstitial fluid Na+ concentration.
As no significant difference could be detected between the Na+ concentration of interstitial fluid samples obtained from the renal outer medulla of SS and SS.13BN rats fed 0.4% NaCl and maintained ad libitium on water, these data were pooled. The mean Na+ concentration of interstitial fluid extracted from the renal outer medulla of SS and SS.13BN rats was 287 ± 52 mM (n = 10). Given these data, we chose bath NaCl levels of 154, 254, and 500 mM to stimulate O2− production in mTAL in our ex vivo studies, representing physiological isotonic and one and two times normal interstitial NaCl levels in vivo.
O2− responses in mTAL to incrementing NaCl concentration.
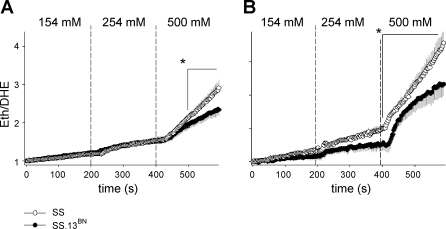
Incrementing bath NaCl concentrations (154 to 254 to 500 mM) stimulated O2− production in mTAL of both SS and SS.13BN rats (Fig. 1A). The rate of O2− production, however, was significantly greater in mTAL of SS rats (n = 13 mTAL, 8 rats) compared with mTAL of SS.13BN rats (n = 16 mTAL, 10 rats) (Fig. 1A: PStrain*Sodium < 0.001), with SS rats producing ∼20% more O2− during the 600-s protocol. Differences in the rate of O2− production between mTAL obtained from SS and SS.13BN rats became significant only during the final 200 s of the protocol in which bath NaCl concentration was raised to 500 mM. During the final 100 s of this period, the rate of O2− production was nearly twice as great in mTAL from SS rats (slope = 0.009 ± 0.002 U/s) compared with mTAL from SS.13BN rats (slope = 0.004 ± 0.001 U/s).
Fig. 1.
Effect of incrementing bath NaCl concentration on superoxide production in medullary thick ascending limb (mTAL) obtained from salt-sensitive (SS) and salt-resistant control SS.13BN rats. A: comparison of superoxide (O2−) response in SS (n = 13) and SS.13BN (n = 16) mTAL of rats fed a 0.4% NaCl diet. B: comparison of O2− response in SS (n = 9) and SS.13BN (n = 10) mTAL of rats fed a 4.0% NaCl diet for 3 days before tissue harvest. Values are means ± SE. y-Axis, rate of mTAL O2− production expressed as normalized ratio ethidium (Eth)/dihyydroethidium (DHE) fluorescent dyes; x-axis, time (seconds). Vertical dashed lines represent time points at which bath NaCl was incremented; ○, data obtained from SS rats; •, data obtained from SS.13BN rats. *Area identified under time point = P < 0.05 for differences between strains, as determined by Bonferroni post hoc test.
In rats fed a 4.0% NaCl diet for 3 days before tissue harvest, O2− responses to incrementing bath NaCl in mTAL from SS (n = 9 mTAL, 5 rats) and SS.13BN rats (n = 10 mTAL, 6 rats) were greater than those observed in mTAL obtained from 0.4% NaCl-fed rats of the same strain (PTreatment*Sodium < 0.001 and < 0.001 for mTAL obtained from SS and SS.13BN rats, respectively). This demonstrates an upregulation of O2−-producing pathways in mTAL during 4.0% NaCl feeding. Importantly, O2− responses were greater in mTAL obtained from SS rats fed a 4.0% NaCl diet compared with mTAL obtained from SS.13BN rats fed a 4.0% NaCl diet (Fig. 1B; PStrain*Sodium < 0.001). Again, differences between strains were most apparent following stimulation with 500 mM NaCl. The rate of O2− production following stimulation with 500 mM bath NaCl was 0.014 ± 0.002 U/s in mTAL of high-NaCl-fed SS rats compared with 0.006 ± 0.002 U/s in mTAL of high-NaCl-fed SS.13BN rats.
Effect of Na+ transport inhibitors on O2− responses to incrementing NaCl concentration.
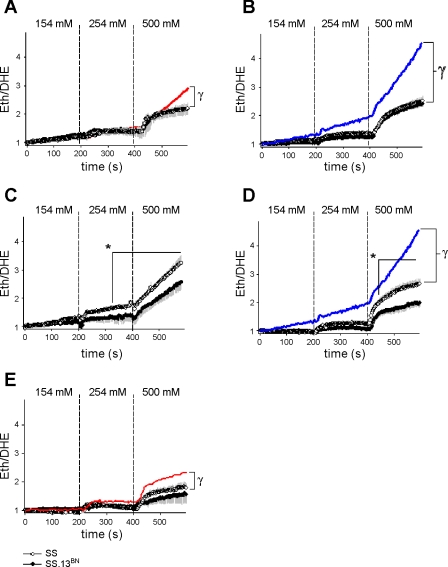
As shown in Fig. 2A, addition of NMA (100 μM) to the bath attenuated O2− production in response to incrementing bath NaCl levels in mTAL obtained from 0.4% NaCl-fed SS rats (n = 8 mTAL, 5 rats; PTreatment*Sodium < 0.001) but not 0.4% NaCl-fed SS.13BN rats (n = 7 mTAL, 5 rats; note: SS and SS.13BN traces superimposed in Fig. 2A). NMA also inhibited O2− production in both 4.0% NaCl-fed SS rats (n = 7 mTAL, 7 rats; PTreatment*Sodium < 0.001) and 4.0% NaCl-fed SS.13BN rats (n = 7 mTAL, 7 rats; PTreatment*Sodium < 0.001; Fig. 2B). Importantly, in both 0.4% and 4.0% NaCl-fed rats, NMA abolished differences in the rate of O2− production between strains (note: SS and SS.13BN traces superimposed in Fig. 2B).
Fig. 2.
Effect of incrementing bath NaCl concentration on superoxide production in mTAL obtained from SS and SS.13BN rats in the presence of amiloride and/or ouabain. A: comparison of O2− response in SS (○; n = 8) and SS.13BN (•; n = 7) mTAL of rats fed a 0.4% NaCl diet in the presence of 100 μM N-methyl-amiloride (SS and SS.13BN data appear superimposed). Red line shows path of 0.4% NaCl-fed SS rat depicted in Fig. 1A for comparison. B: comparison of O2− response in SS (n = 9) and SS.13BN (n = 7) mTAL of rats fed a 4.0% NaCl diet in the presence of 100 μM N-methyl-amiloride (SS and SS.13BN data appear superimposed). C: comparison of O2− response in SS (n = 7) and SS.13BN (n = 6) mTAL of rats fed a 0.4% NaCl diet in the presence of 4 mM ouabain. D: comparison of O2− response in SS (n = 7) and SS.13BN (n = 7) mTAL of rats fed a 4.0% NaCl diet in the presence of 4 mM ouabain. Blue lines in B and D show path of 4.0% NaCl-fed SS rat depicted in Fig. 1B for comparison. E: comparison of O2− response in SS (n = 7) and SS.13BN (n = 7) mTAL of rats fed a 0.4% NaCl diet in the presence of 100 μM N-methyl-amiloride and 4 mM ouabain. Red line shows path of 0.4% NaCl-fed SS rat with N-methyl-amiloride depicted in Fig. 2A for comparison. *Area identified under time point = P < 0.05 for differences between strains, as determined by Bonferroni post hoc test; γsignificant difference between treatment groups.
Ouabain alone did not ameliorate differences in O2− production in response to incrementing bath NaCl in either 0.4% NaCl-fed or 4.0% NaCl-fed rats (Fig. 2, C and D). Interestingly, in the presence of ouabain, strain differences between mTAL of 0.4% NaCl-fed SS (n = 7 mTAL, 5 rats) and SS.13BN (n = 6 mTAL, 5 rats) rats were more pronounced, with differences being observed even during conditions of normal (254 mM) bath NaCl. These differences, however, could be completely abolished by addition of NMA in combination with ouabain (Fig. 2E). While in mTAL of 0.4% NaCl-fed SS and SS.13BN rats O2− responses in ouabain-treated mTAL were not significantly different from control responses, in both mTAL of 0.4% NaCl-fed rats treated with NMA + ouabain and 4.0% NaCl-fed rats treated with ouabain alone, O2− responses were reduced, indicating ouabain had an inhibitory action on mTAL O2− production.
mTAL O2− responses to incrementing bath osmolality and osmotic cell shrinkage.
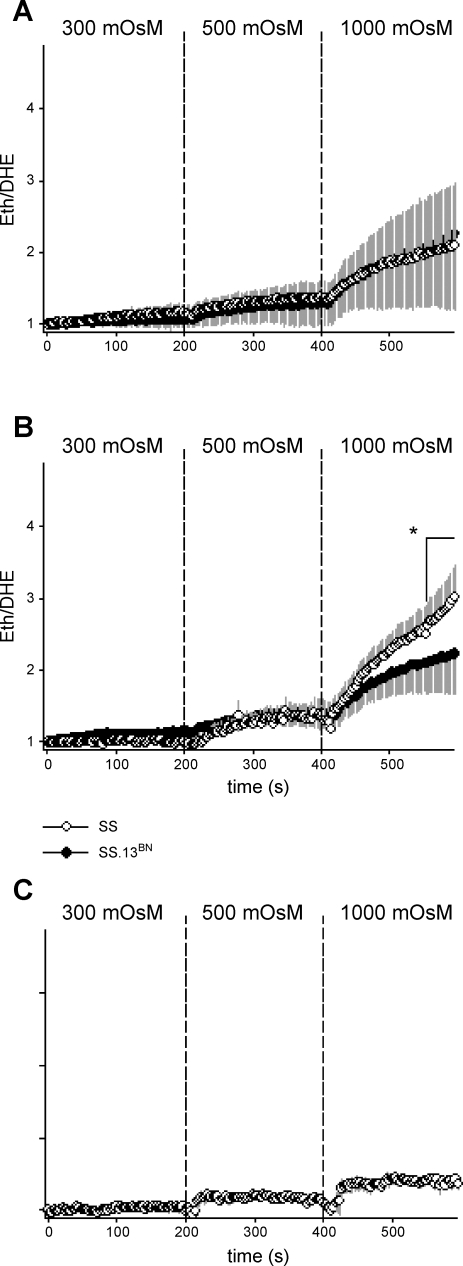
As shown in Fig. 3B, in the presence of ouabain (4 mM) to inhibit active Na+ transport, incrementing bath osmolality using ChCl, in equimolar amounts to that using NaCl in other protocols, stimulated O2− production in mTAL from both SS (n = 6 mTAL, 5 rats) and SS.13BN rats (n = 6 mTAL, 4 rats). Increases in O2− production induced by ChCl were not significantly different from those observed in response to stimulation with NaCl. Consistent with our results using NaCl, O2− production was significantly greater in mTAL obtained from SS rats compared with that from SS.13BN rats in response to increasing bath osmolality in increments of 300, 500, and 1,000 mosmol/kgH2O using ChCl (PStrain+ChCl < 0.001). Importantly, differences in O2− production in response to incrementing ChCl between SS and SS.13BN rats were abolished by addition of NMA, suggesting ChCl was stimulating O2− production through a similar pathway to that stimulated by NaCl (Fig. 3A; note: SS and SS.13BN traces superimposed). Increasing bath osmolality to the same degree using the cell-permeable urea as that achieved using NaCl or ChCl did not stimulate significant O2− production in mTAL (studies performed in mTAL obtained from SS rats only; n = 6 mTAL, 4 rats), indicating that cell shrinkage was required to stimulate O2− production.
Fig. 3.
Effect of incrementing bath osmolality on superoxide production in mTAL obtained from SS and SS.13BN rats. A: comparison of O2− response in SS (○; n = 7) and SS.13BN (•; n = 7) mTAL of rats fed a 0.4% NaCl diet in response to incrementing bath osmolality using choline-chloride (ChCl) in the presence of 100 μM N-methyl-amiloride (SS and SS.13BN data appear superimposed). B: comparison of O2− response in SS (n = 6) and SS.13BN (n = 6) mTAL of rats fed a 0.4% NaCl diet in response to incrementing bath osmolality using ChCl in the presence of 4 mM ouabain. C: O2− response in SS (n = 6) mTAL of rats fed 0.4% NaCl in response to incrementing bath osmolality using urea. *Area identified under time point = P < 0.05 for differences between strains, as determined by Bonferroni post hoc test.
Effect of inhibition of NAD(P)H oxidase on O2− responses to incrementing bath NaCl.
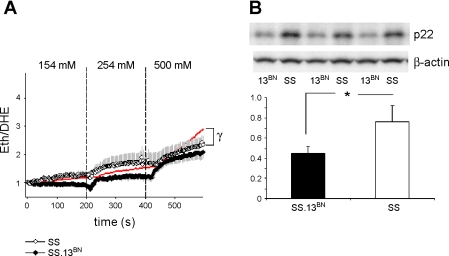
Figure 4A shows that preincubation of tissue strips with the NAD(P)H oxidase inhibitor apocynin (100 μM) significantly ameliorated O2− production in mTAL obtained from SS (n = 5 mTAL, 5 rats; PTreatment*Sodium < 0.001) but not SS.13BN rats (n = 5 mTAL, 5 rats). There was no significant difference in the rate of O2− production between mTAL from SS and SS.13BN rats treated with apocynin, indicating that NAD(P)H oxidase is the source of enhanced O2− production observed in mTAL obtained from SS rats. Western blot analysis of outer medullary tissue homogenates from SS and SS.13BN rats indicated protein expression of p22phox, a subunit of the enzyme NAD(P)H oxidase, was more highly expressed in the outer medulla of SS rats (n = 6) compared with that of SS.13BN rats (n = 6; Fig. 4B; P = 0.001).
Fig. 4.
Role of NAD(P)H oxidase on superoxide production in mTAL obtained from SS and SS.13BN rats in response to incrementing bath NaCl. A: comparison of O2− response in SS (○; n = 5) and SS.13BN (•; n = 5) mTAL of rats fed a 0.4% NaCl diet in the presence of 100 μM apocynin. Red line shows path of 0.4% NaCl-fed SS rat depicted in Fig. 1A for comparison. B, bottom: Western blot analysis of p22phox protein expression in renal outer medullary homogenates from SS (n = 6) and SS.13BN (n = 6) rats. Top: representative example of exposed film demonstrating difference in protein expression of p22phox in outer medullary samples from SS and SS.13BN rats but not in control protein β-actin. *P < 0.05 for differences between strains, as determined by Bonferroni post hoc test; γsignificant difference between treatment groups.
DISCUSSION
The results of the present study indicate that O2− production is enhanced in mTAL of SS rats compared with a salt-resistant control strain (SS.13BN). Incrementing extracellular osmolality with NaCl increased mTAL O2− production in both SS and SS.13BN rats, confirming our laboratory's previous observations that extracellular NaCl stimulates mTAL O2− production (20). In the absence of pharmacological inhibition, differences in O2− production between the strains were observed when mTAL were exposed to biologically hypertonic media. In the presence of ouabain, however, the rate of O2− production was greater in mTAL of SS, even during incubation in physiologically normal NaCl concentration (254 mM; Fig. 2C). As these differences could be completely abolished by treatment with amiloride (Fig. 2E), our data indicate amiloride-sensitive pathways of O2− production are augmented in SS mTAL under normal conditions, and that these differences are exacerbated during hypertonic NaCl stress.
Since in vivo high-sodium feeding results in increased outer medullary oxidative stress and the development of hypertension in SS rats (27), in addition to prehypertensive 0.4% NaCl-fed rats, we also examined responses in mTAL from SS and SS.13BN rats fed a high (4.0%) NaCl diet for 3 days before tissue harvest. A relatively early time point (3 days) after initiation of high-sodium feeding was chosen, since hypertension is only beginning to develop at this time, limiting the confounding effects of severe chronic hypertension and associated renal tubular damage (21). Our results demonstrate that this 4.0% NaCl diet increased the rate of O2− production in mTAL obtained from both rat strains in response to acute increments of bath NaCl. Importantly, however, O2− production in mTAL of SS rats remained elevated above that of SS.13BN rats.
Both amiloride- and ouabain-sensitive pathways of O2− production were enhanced following 3 days of high (4.0%) NaCl feeding in mTAL of SS and SS.13BN rats compared with mTAL of 0.4% NaCl-fed rats. Importantly, while amiloride-sensitive O2− production was greater in both mTAL of SS or SS.13BN rats by high NaCl, strain differences in O2− production between mTAL of SS and SS.13BN rats remained. The factors that result in upregulation of mTAL O2− production in the early stages of a high-sodium diet remain incompletely understood. Enhanced O2− production could, however, be mediated by a variety of factors, including increased active transport of Na+, enhanced mitochondrial-derived O2− production, upregulation of NAD(P)H oxidase, or reduced levels of free radical scavengers, such as superoxide dismutase, as our laboratory has previously demonstrated that these processes are altered in the outer medulla of high-NaCl-fed SS rats (27, 28). Our data are consistent with the published data of Taylor et al., who, using in vivo microdialysis techniques, demonstrated differences in outer medullary O2− levels in both 0.4% NaCl-fed SS and SS.13BN rats (27), and SS and SS.13BN rats fed 4.0% NaCl for 6 wk (28).
Evidence that the activity of NHE linked to O2− production in rat mTAL.
The amiloride-sensitive O2− production observed in our studies appears to be mediated primarily by cell shrinkage. A direct effect of increased extracellular Na+ concentration or changes in active Na+ transport can be excluded for two reasons. First, when ChCl was used to increase bath osmolality, extracellular NaCl concentration remained unchanged, yet O2− responses were the same as during stimulation with NaCl, and these responses were also sensitive to amiloride. Second, amiloride-sensitive O2− responses to increases in bath NaCl were present when Na+ transport was inhibited by ouabain. Unlike NaCl and ChCl, mTAL are highly permeable to urea, so incrementing bath urea concentration does not largely affect cell volume (13). Since increasing bath osmolality using urea to the magnitude of that reached with NaCl and ChCl did not stimulate significant mTAL O2− production, we conclude that the amiloride-sensitive component of the O2− response was mediated by cell shrinkage rather than other factors associated with increasing bath osmolality.
Our data are consistent with the results of Li et al. (18), who demonstrated H+ efflux through amiloride-sensitive, NHE-stimulated, NAD(P)H oxidase-dependent O2− production in mTAL obtained from Sprague-Dawley rats. The hypothesis that cellular extrusion of H+ through NHE mediates O2− responses to incrementing bath NaCl levels is attractive, since NHE-1 is known to be present on the basolateral membrane of mTAL (2) and the activity of NHE-1 is known to be increased in response to cell shrinkage (8, 10). It should also be noted here that, in protocols in which we incremented bath osmolality using ChCl, NaCl (154 mM) was present, and thus NHE would not have been inhibited (23). While the analog of amiloride used in our study, NMA, is a relatively specific inhibitor of the basolateral NHE, this amiloride analog does not allow us to confirm which isoform of NHE may be involved in the observed O2− responses, or if nonspecific inhibition of other transporters may have occurred. Further studies utilizing novel analogs of amiloride capable of specific inhibition of NHE isoforms will be required to resolve these issues.
Addition of apocynin (100 μM) ameliorated the O2− response to incrementing bath NaCl in mTAL from SS but not SS.13BN rats, and no differences in the rate of O2− production between rat strains were detected in the presence of apocynin. We conclude from these data that the enzyme NAD(P)H oxidase is linked to the enhanced rate of O2− production observed in mTAL from SS rats. These data are consistent with our laboratory's previous data, indicating that, in vivo, O2− production in the intact renal outer medulla of SS rats can be reduced by interstitial infusion of apocynin (27). Furthermore, we have previously demonstrated that NAD(P)H oxidase activity is enhanced in outer medullary tissue homogenates of SS rats (27). Many of the subunits of NAD(P)H oxidase have been found to be more highly expressed in the outer medulla of SS rats compared with SS.13BN rats, including gp47phox and p22phox (27), as confirmed by Western blot analysis of protein expression of p22phox in the present study. While our data indicate that NAD(P)H oxidase is an important mediator of the enhanced O2− production observed in mTAL from SS rats, it has recently been demonstrated that apocynin acts as a nonspecific antioxidant in vascular smooth muscle cells and other nonphagocytic cells lacking the enzyme myeloperoxidase, which converts apocyinin to its active form, diapocynin (11). Whether other peroxidases capable of converting apocynin to its active form are present in mTAL is currently unknown. The renal medulla does, however, exhibit endogenous peroxidase activity (3–5, 29). Our data are also consistent with previous studies demonstrating that NAD(P)H oxidase is a major producer of O2− in mTAL using various pharmacological approaches (6, 18). At present, the cellular pathways by which NHE may stimulate NAD(P)H oxidase remain unclear. Further studies will be required to elucidate such pathways and to determine whether, in addition to NAD(P)H oxidase, NHE expression or other constituents of such a pathway may be upregulated in mTAL of SS rats.
Physiological significance.
In vivo mTAL are exposed to large fluctuations in extracellular osmolality and NaCl concentration in response to variations in Na+ and water intake (24). These osmotic fluctuations lead changes in cell volume, which, to maintain cellular function, must be offset by activating regulatory transporters, such as NHE-1, which return cell volume to normal (8, 10). While in vivo relatively small changes in cell volume and the production of reactive oxygen species in the renal medulla may have powerful consequences on renal function and tubular Na+ reabsorption, experimentally these alterations may be short lived and are difficult to quantify. To identify differences between strains of animals in pathways of O2− production within the tubular segment responsible for the greatest amount of O2− production (mTAL), in vitro studies are required. Given that we were more likely to identify differences between strains under conditions of high O2− production, large concentrations of NaCl were used, recognizing that such concentrations occur in vivo only in conditions of severe dehydration (15, 16). While recognizing this caveat, we have found good consistency between in vitro data, using similar approaches in freshly isolated mTAL and results from whole animal in vivo studies in which physiologically relevant stimuli are utilized (9, 22, 25, 31).
There is evidence indicating that the pathways identified under maximal NaCl stimulation in the present study are of consequence under normal physiological conditions. Consistent with the data of Zhou et al. (30), indicating that TAL produce the majority of O2− in the renal medulla, our present data indicate that mTAL are likely to be responsible in large part for differences in outer medullary oxidative stress observed in SS and SS.13BN rats (27, 28). Our data are also consistent with the data of Li et al. (18), who provided evidence that amiloride-sensitive pathways account for >50% of outer medullary oxidative stress in Sprague-Dawley rats in vivo under normal physiological conditions. We confirm that much of the O2− generated by mTAL is amiloride sensitive and have found that these amiloride-sensitive pathways are upregulated in SS rats. The observation that amiloride-sensitive pathways of O2− production may be upregulated in SS rats has immediate physiological relevance, given that our laboratory has previously demonstrated that enhanced outer medullary oxidative stress in SS rats leads to the development of hypertension and renal injury in these animals (21, 27). We believe that the present findings provide a strong impetus for future experimental studies aimed at ameliorating renal damage and hypertension in SS animals with amiloride analogs directed at inhibition of NHEs such as NMA.
Our finding that amiloride-sensitive pathways account for much of the excess O2− produced by mTAL of SS rats does not exclude the possibility that other pathways of reactive oxygen species production contribute to the outer medullary oxidative stress observed in SS hypertension. Our laboratory has previously demonstrated that both increased luminal Na+ concentration and increased luminal flow increased the rate of O2− production in isolated, perfused mTAL of Sprague-Dawley rats (1). Furthermore, Hong and Garvin (12) have demonstrated that, in response to increased luminal NaCl delivery, O2− production is increased in mTAL via a furosemide-inhibitable pathway, indicating that active transport of Na+ stimulates mTAL O2− production. This group has also recently demonstrated that tubular stretch increases mTAL O2− production. Importantly, Hong and Garvin (12) were unable to inhibit O2− production in NaCl-perfused mTAL with amiloride, indicating that the pathways of O2− production that they observed are different from the amiloride-sensitive pathways observed in the present study. As both mTAL luminal NaCl delivery and flow are likely to be increased during high-NaCl feeding, it is probable that many of these alternative mechanisms of mTAL O2− production are activated and contribute to elevated outer medullary oxidative stress in high-sodium-fed SS rats. While it is clear from the present study that amiloride-sensitive pathways of O2− production are differentially regulated in SS and SS.13BN and likely contribute to the elevated hypertension and renal damage observed in SS rats compared with salt-resistant SS.13BN rats, whether alternative pathways are also elevated in SS mTAL remain unclear (27, 28).
Summary and conclusions.
The primary findings of our study are that O2− responses in mTAL obtained from SS rats in response to increments in bath NaCl are greater than that of mTAL obtained from salt-resistant SS.13BN rats. Addition of both amiloride and apocynin normalized the rate of O2− production in mTAL from SS to that of SS.13BN rats, indicating that the amiloride-sensitive pathways may mediate enhanced O2− production in mTAL from SS rats via an interaction with NAD(P)H oxidase. Amiloride-sensitive O2− production in SS rats is largely independent of active Na+ transport and is mediated by hyperosmotic cell shrinkage, consistent with activation of NHE-1. Whereas both amilioride-sensitive and amiloride-insensitive pathways of O2− production are elevated in mTAL of SS and SS.13BN rats fed a high-NaCl diet, differences in O2− production between the strains are abolished by amiloride. We conclude that amiloride-sensitive pathways active in mTAL epithelial cells are likely to be responsible for the enhanced levels of O2− observed in the outer medulla of the SS kidney. Future studies aimed at the further characterization of such pathways, as well as studies utilizing amiloride analogs directed toward inhibition of NHE, may be important in understanding and preventing oxidative stress and salt-sensitive hypertension.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant HL-29587. P. M. O'Connor is a recipient of American Heart Association Postdoctoral Fellowship 0625793Z.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe M, O'Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW Jr. Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol Renal Physiol 291: F350–F357, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Borensztein P, Froissart M, Laghmani K, Bichara M, Paillard M. RT-PCR analysis of Na+/H+ exchanger mRNAs in rat medullary thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1224–F1228, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Carmagnol F, Sinet PM, Jerome H. Selenium-dependent and non-selenium-dependent glutathione peroxidases in human tissue extracts. Biochim Biophys Acta 759: 49–57, 1983. [DOI] [PubMed] [Google Scholar]
- 4.Cavallo T Cytochemical localization of endogenous peroxidase activity in renal medullary collecting tubules and papillary mucosa of the rat. Lab Invest 34: 223–228, 1976. [PubMed] [Google Scholar]
- 5.Cavallo T Fine structural localization of endogenous peroxidase activity in inner medullary interstitial cells of the rat kidney. Lab Invest 31: 458–464, 1974. [PubMed] [Google Scholar]
- 6.Chen Y, Gill PS, Welch WJ. Oxygen availability limits renal NADPH-dependent superoxide production. Am J Physiol Renal Physiol 289: F749–F753, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Cowley AW, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Demaurex N, Grinstein S. Na+/H+ antiport: modulation by ATP and role in cell volume regulation. J Exp Biol 196: 389–404, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Dickhout JG, Mori T, Cowley AW Jr. Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced medullary vasoconstriction. Circ Res 91: 487–493, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Gillis D, Shrode LD, Krump E, Howard CM, Rubie EA, Tibbles LA, Woodgett J, Grinstein S. Osmotic stimulation of the Na+/H+ exchanger NHE1: relationship to the activation of three MAPK pathways. J Membr Biol 181: 205–214, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol 292: F993–F998, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Kaji DM, Diaz J, Parker JC. Urea inhibits Na-K-2Cl cotransport in medullary thick ascending limb cells. Am J Physiol Cell Physiol 272: C615–C621, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Lewis AF, Williams PG. A method for investigating renal papillary solute concentrations. J Physiol 214: 9P–11P, 1971. [PubMed] [Google Scholar]
- 15.Lee J, Williams PG. Changes of sodium and urea concentrations in the renal papillary interstitial fluid on dehydration of rats. J Physiol 218: 195–204, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Williams PG. The effect of vasopressin (Pitressin) administration and dehydration on the concentration of solutes in renal fluids of rats with and without hereditary hypothalamic diabetes insipidus. J Physiol 220: 729–743, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol 282: F1111–F1119, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Li N, Zhang G, Yi FX, Zou AP, Li PL. Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle. Am J Physiol Renal Physiol 289: F1048–F1056, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Mori T, Cowley AW Jr. Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension 42: 588–593, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Mori T, Cowley AW Jr. Renal oxidative stress in medullary thick ascending limbs produced by elevated NaCl and glucose. Hypertension 43: 341–346, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW Jr. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol. In press. [DOI] [PMC free article] [PubMed]
- 22.O'Connor PM, Cowley AW Jr. Vasopressin-induced nitric oxide production in rat inner medullary collecting duct is dependent on V2 receptor activation of the phosphoinositide pathway. Am J Physiol Renal Physiol 293: F526–F532, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Orlowski J Heterologous expression and functional properties of amiloride high affinity (NHE-1) and low affinity (NHE-3) isoforms of the rat Na/H exchanger. J Biol Chem 268: 16369–16377, 1993. [PubMed] [Google Scholar]
- 24.Palm F, Carlsson PO. Thick ascending tubular cells in the loop of Henle: regulation of electrolyte homeostasis. Int J Biochem Cell Biol 37: 1554–1559, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Park F, Zou AP, Cowley AW Jr. Arginine vasopressin-mediated stimulation of nitric oxide within the rat renal medulla. Hypertension 32: 896–901, 1998. [DOI] [PubMed] [Google Scholar]
- 26.PGA The Medical College of Wisconsin. PhysGen (Online). http://pga.mcw.edu [2008].
- 27.Taylor NE, Glocka P, Liang M, Cowley AW Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47: 692–698, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Taylor NE, Maier KG, Roman RJ, Cowley AW Jr. NO synthase uncoupling in the kidney of Dahl S rats: role of dihydrobiopterin. Hypertension 48: 1066–1071, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Vejrazka M, Micek R, Stipek S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta 1722: 143–147, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Zou AP, Li N, Cowley AW Jr. Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Zou AP, Wu F, Cowley AW Jr. Protective effect of angiotensin II-induced increase in nitric oxide in the renal medullary circulation. Hypertension 31: 271–276, 1998. [DOI] [PubMed] [Google Scholar]