Abstract
We hypothesized that elevated macula densa intracellular pH (pHi) during tubuloglomerular feedback enhances O2− production from NAD(P)H oxidase. Microdissected thick ascending limbs from rabbits with intact macula densa were cannulated and perfused with physiological saline. When luminal NaCl was switched from 10 to 80 mM, O2− production increased from 0.53 ± 0.09 to 2.62 ± 0.54 U/min (P < 0.01). To determine whether inhibiting the Na/H exchanger blocks O2− production, we used dimethyl amiloride (DMA) to block Na/H exchange. In the presence of DMA, O2− production induced by NaCl was blunted by 40%. To study the effect of pHi on O2− in intact macula densa cells, we measured O2− while pHi was changed by adjusting luminal pH. When the macula densa was perfused with 80 mM NaCl and the pH of the perfusate was switched to 6.8, 7.4, and 8.0, O2− production was significantly enhanced, but not at 10 mM NaCl. To ascertain the source of O2−, we used the NAD(P)H oxidase inhibitor apocynin. In the presence of apocynin (10−5 M), O2− production induced by elevating pHi was blocked. Finally, we measured the optimum pH for O2− production by the macula densa and found optimum extracellular pH is at 7.7 and optimum pHi is ∼8 for O2− production. We found that elevated pHi enhances O2− production from NAD(P)H oxidase induced by increasing luminal NaCl when the lumen is perfused with 80 mM NaCl, not 10 mM, and O2− production is pH sensitive, with an optimum pHi of 8.
Keywords: tubuloglomerular feedback, pH optimum, Na/H exchange, reactive oxygen species
tubuloglomerular feedback (TGF) is a process in which increases in luminal NaCl at the macula densa cause constriction of the afferent arteriole (Af-Art). TGF limits urinary volume and Na+ excretion and decreases the glomerular filtration rate by increasing Af-Art tone in response to increased NaCl at the macula densa (25, 33, 40). TGF is inhibited by a number of factors, including atrial natriuretic factor and nitric oxide (10, 15). In contrast, angiotensin and superoxide (O2−) stimulate TGF (30, 35). O2− production in the macula densa stimulates TGF primarily by scavenging nitric oxide (18, 35). The source of O2− in the macula densa is primarily NAD(P)H oxidase (16).
O2− production by the macula densa is stimulated by increasing luminal NaCl, the same stimulus that initiates TGF. When luminal NaCl is elevated, the activity of luminal membrane Na-K-2Cl cotransporters is enhanced, leading to depolarization of the macula densa. This appears to be the signal that activates enhances O2− production (16). In addition to causing depolarization, elevating luminal NaCl at the macula densa also causes intracellular pH (pHi) to increase due to activation of luminal Na/H exchanger (NHE) (15, 27). However, the effect of this change in pHi and the role of luminal NHE on O2− production has not been studied.
NAD(P)H oxidase produces protons in addition to O2−. NADPH oxidase has also been reported to be a proton pump (1, 22). The activity of the enzyme is enhanced by alkaline pHs (34, 37). It is unclear whether this is due to removal of end-product inhibition or a direct effect of pH on enzyme structure and function. We hypothesized that the increase in pHi caused by elevating luminal NaCl, and consequent activation of NHE, enhances O2− production by NAD(P)H oxidase in the macula densa.
MATERIALS AND METHODS
Isolation and microperfusion of rabbit macula densa.
We used methods similar to those described previously to isolate and microperfuse the thick ascending limb (TAL) of the loop of Henle and attached macula densa (15). This protocol was approved by the Institutional Animal Care and Use Committee at Henry Ford Hospital. Briefly, young male New Zealand White rabbits (1.5–2.0 kg) were anesthetized with ketamine (50 mg/kg im) and given an injection of heparin (500 U iv). The kidneys were removed and sliced along the corticomedullary axis. Single superficial intact glomeruli were microdissected together with adherent tubular segments consisting of portions of the TAL, macula densa, and early distal tubule. Using a micropipette, samples were transferred to a temperature-regulated chamber mounted on an inverted microscope (TE2000-S, Nikon, Yuko, Japan). The glomeruli were positioned so the macula densa could be visualized using a holding pipette. TALs were cannulated with an array of glass pipettes (14, 15). Macula densas were perfused with physiological saline containing (in mM) 10 HEPES, 1.0 CaCO3, 0.5 K2HPO4, 4.0 KHCO3, 1.2 MgSO4, 5.5 glucose, 0.5 Na acetate, 0.5 Na lactate, and 10 or 80 NaCl, pH 7.4. The luminal perfusion rate was 40 nl/min and controlled with a pump (11 plus, Harvard Apparatus, Holliston, MS). The bath consisted of MEM containing 0.15% bovine serum albumin and was exchanged continuously at a rate of 1 ml/min. Microdissection and cannulation were completed within 60 min at 8°C, after which the bath was gradually warmed to 37°C for the rest of the experiment. Once the temperature was stable, a 30-min equilibration period was allowed before any measurements were taken. The imaging system consisted of a microscope (TE2000-S, Nikon), digital CCD camera (IEEE 1394, Hamatsu Photonics, Hamatsu, Japan), and optical filter changer (Lambda 10–2, Sutter Instruments, Novato, CA). Images were displayed and analyzed with SimplePCI imaging software (Compix, Tualatin, OR).
Measurement of O2−.
As we described (16), an O2−-sensitive fluorescent dye, dihydroethidium, was used to detect O2− production by the macula densa. The cells were loaded with 10 μM dihydroethidium in 0.1% DMSO plus 0.1% pluronic acid from the lumen for 30 min and then washed for 20 min. Dihydroethidium is irreversibly converted to oxyethidium in the presence of O2− (38, 39, 42). Therefore, the rate of oxyethidium accumulation reflects O2− production. An increase or decrease in this rate indicates enhanced or blunted O2− production. O2− production is expressed as the rate of increase in the ratio of oxyethidium to dihydroethidium fluorescence intensity (units/min). Macula densa cells were exposed to 380- and 490-nm light to excite dihydroethidium and oxyethidium, respectively. Emitted fluorescence from dihydroethidium was recorded using a 420-nm dichroic mirror with a 460/50-nm band-pass filter; for oxyethidium, we used a 565-nm dichroic mirror with a 605/55-nm band-pass filter (Chroma). Square-shaped regions of interest were set inside the cytoplasm of macula densa cells, and mean intensity within them was recorded every 5 s. Changes in O2− concentration were expressed as the increase in oxyethidium/dihydroethidium ratio (units/s).
All studies were performed in the presence of the nitric oxide synthase (NOS) inhibitor N-nitro-l-arginine methyl ester (l-NAME; 10−4 M) while O2− was measured to eliminate its reaction with nitric oxide.
Calibration and measurement of pHi.
Once TALs were perfused, macula densa cells were loaded with BCECF-acetoxymethyl ester (AM) from the lumen at 37 ± 1°C for 15 min and then washed for 10 min. Intracellular dye was excited alternately at 440 and 490 nm, and emitted fluorescence was recorded at 540 nm. Ratiometric measurements of the images were analyzed with the imaging system. The ratio was calibrated as described previously (7, 15). The pH calibration solutions contained (in mM) 95 KCl, 5 NaCl, 30 N-methyl-d-glucamine, 2.5 NaH2PO4, 1.5 MgSO4, 5 glucose, 2 CaCl2, 25 HEPES, and 0.01 nigericin and were titrated to selected pH values between 6.4 and 8.0. Nigericin was prepared as a 10 mM stock in methanol and diluted in calibration solution just before use. All solutions had an osmolality of 290 mosmol/kgH2O adjusted with mannitol.
Chemicals.
Dihydroethidium, BCECF-AM, DMSO, and pluronic acid were obtained from Molecular Probes (Eugene, OR). All other chemicals were purchased from Sigma.
Statistics.
Data were collected as repeated measures over time under different conditions. We tested only the effects of interest, using a paired t-test or ANOVA for repeated measures. When ANOVA was used, the three pairwise comparisons were then evaluated using three paired t-tests. Significance was judged as P < 0.05 or an adjusted value using Hochberg's method for multiple testing.
RESULTS
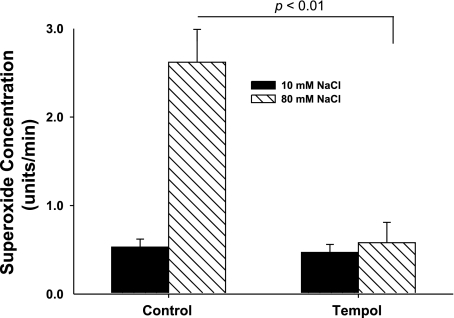
First, we demonstrated that increasing luminal NaCl increases O2− production, as we have reported (16). When luminal NaCl was 10 mM, the rate of change in the OE/DHE ratio was 0.53 ± 0.09 U/min. After a switch to 80 mM NaCl, it was 2.62 ± 0.54 U/min (P < 0.01 vs. 10 mM NaCl; n = 5). To show that changes in the OE/DHE ratio are due to changes in O2−, we used tempol (10−4 M), a stable membrane-permeant superoxide dismutase (SOD) mimetic. In the presence of tempol, when the lumen was perfused with 10 mM NaCl, the rate of increase in the OE/DHE ratio was 0.47 ± 0.09 U/min. When NaCl was switched to 80 mM NaCl, it was 0.58 ± 0.23 U/min (n = 5; Fig. 1). Thus increases in the OE/DHE ratio are due to increases in O2− concentration, and increasing luminal NaCl stimulates O2− production by the macula densa.
Fig. 1.
Tempol blocks the increase in superoxide induced by NaCl. When NaCl was switched from 10 to 80 mM, superoxide concentration increased significantly. In the presence of tempol, a stable membrane-permeant SOD mimetic, the NaCl-induced increase in superoxide was blocked (n = 5).
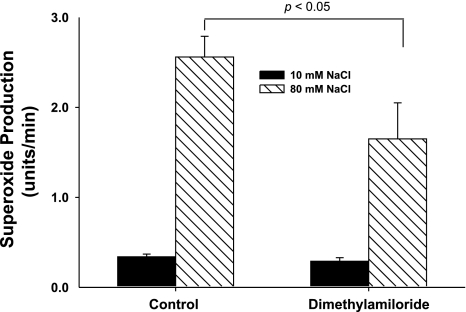
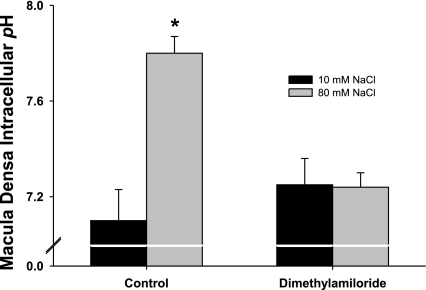
Increasing luminal NaCl at the macula densa elevates pHi due to activation of NHE. To begin to study whether macula densa pHi regulates O2− production and the role of NHE, we measured the effect of dimethyl amiloride (DMA), a NHE inhibitor, on O2− production and pHi. During the control period, when luminal NaCl was switched from 10 to 80 mM, O2− production increased from 0.34 ± 0.15 to 2.56 ± 0.31 U/min (P < 0.01; n = 7; Fig. 2). When we repeated the switch in the presence of DMA (10−4 M), O2− production only increased from 0.29 ± 0.14 to 1.65 ± 0.36 U/min, 40% less (P < 0.05 vs. without DMA; n = 7; Fig. 2). Time controls showed no significant changes (O2− production increased from 0.42 ± 0.17 to 2.83 ± 0.22 U/min and from 0.47 ± 0.21 to 2.74 ± 0.35 U/min in the first and second luminal NaCl switches, respectively). When we repeated these experiments but measured pHi instead of O2−, we found that in the absence of DMA increasing luminal NaCl elevated pHi from 7.1 ± 0.1 to 7.7 ± 0.2 (P < 0.01; n = 4; Fig. 3) and in the presence of DMA this increase was completely blocked (Fig. 3). Control experiments showed no significant effects of time on NaCl-induced changes in pHi (pHi increased from 7.0 ± 0.1 to 7.7 ± 0.1 and 7.1 ± 0.2 to 7.8 ± 0.2 in the first and second luminal NaCl switches, respectively). Taken together, these data suggest that the changes in macula densa pHi induced by activating NHE enhance O2− production.
Fig. 2.
Blocking the Na/H exchanger blunts the superoxide production induced by increasing luminal NaCl. In a control experiment, increasing luminal NaCl stimulated superoxide production. When the Na/H exchanger was blocked with dimethylamiloride, NaCl-induced reduction in superoxide production was inhibited by 40% (n = 7).
Fig. 3.
Blocking the Na/H exchanger inhibits the increase in intracellular pH (pHi) induced by increasing luminal NaCl. When macula densa NaCl was switched from 10 to 80 mM, pHi increased from 7.1 ± 0.1 to 7.7 ± 0.2 (P < 0.01; n = 4). In the presence of dimethylamiloride, a Na/H exchanger inhibitor, NaCl-induced changes in pH were blocked.
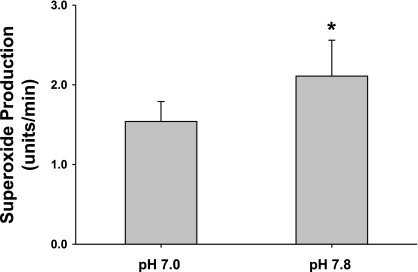
The inhibition of NaCl-induced O2− production caused by DMA could be either due to blocking changes in pHi or intracellular Na, because NHE transports both protons and Na (19). To directly study whether the effect of DMA is due to changes in macula densa pHi, we clamped pHi with a K+/H+ ionophore, nigericin, to equilibrate luminal and pHi without switching luminal NaCl. The macula densa was perfused with 80 mM NaCl, and nigericin (10−5 M) was added to the perfusate for 20 min. When pHi was adjusted to 7.0, O2− production was 1.54 ± 0.24 U/min. When we raised pHi to 7.8, O2− production by the macula densa increased to 2.11 ± 0.28 U/min (P < 0.01; n = 5; Fig. 4). These data indicate that elevated macula densa pHi enhances O2− production in 80 mM NaCl rather than changes in intracellular Na.
Fig. 4.
Effect of pHi on superoxide production by the macular densa. pHi was clamped with a K+/H+ ionophore, nigericin, to equilibrate luminal pH and pHi. When pHi was raised from 7.0 to 7.8 without a change in NaCl concentration, superoxide production by the macula densa was increased significantly (P < 0.01; n = 5).
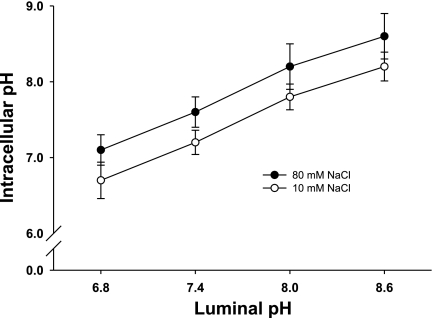
Clamping pHi with nigericin has limitations and potential unintended consequences. Thus we next tested whether we could effectively set pHi by changing luminal pH in intact macula densa cells in both 10 and 80 mM NaCl. When the macula densa was perfused with 10 mM NaCl and luminal pH was raised from 6.8 to 7.4, 8.0, and 8.6, pHi was 6.69 ± 0.24, 7.20 ± 0.16, 7.82 ± 0.17, and 8.23 ± 0.19, respectively. When the experiment was repeated with 80 mM NaCl, pHi increased from 7.13 ± 0.26 to 7.57 ± 0.22, 8.16 ± 0.26, and 8.65 ± 0.28, respectively (Fig. 5; n = 6). These data indicate that changing luminal pH can be used as a mechanism to alter pHi and to further study the role of pH in regulating O2− production.
Fig. 5.
Effect of changes in luminal pH on macula densa pHi. When luminal pH was increased from 6.8 to 8.6, there was a linear correlation between changes in pHi and extracellular pH of the macula densa. At any given extracellular pH, pHi of the macula densa at 80 mM NaCl was 0.5, higher than at 10 mM (n = 6).
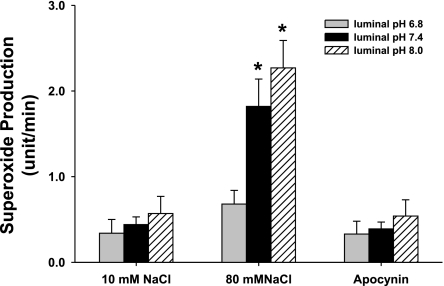
Our data with DMA indicate that pH may have less of an effect on O2− production when the macula densa is perfused with 10 mM than 80 mM NaCl. To examine this issue in more detail, we measured O2− production while the luminal perfusate, and thus pHi, was adjusted to different pHs, in the presence of either 10 or 80 mM NaCl. When the macula densa was perfused with 10 mM NaCl and the pH of the perfusate was switched from 6.8 to 7.4, and 8.0, there was no significant increase in O2− (0.37 ± 0.16, 0.44 ± 0.09, and 0.57 ± 0.20 U/min, respectively) (Fig. 6; n = 5). In contrast, when the macula densa was perfused with 80 mM NaCl and the pH of the perfusate was switched from 6.8 to 7.4 and 8.0, O2− production was increased as pH increased (0.69 ± 0.16, 1.81 ± 0.32, and 2.27 ± 0.32 U/min, respectively) (P < 0.01 vs. pH 6.8; Fig. 6; n = 5). These data suggest that increasing macula densa pHi enhances O2− generation only when the lumen is perfused with 80 mM NaCl, not 10 mM. To study whether the effect of pH was on NAD(P)H oxidase, we used the NAD(P)H oxidase inhibitor apocynin. In the presence of apocynin when the pH of the perfusate was switched from 6.8 to 7.4 and 8.0 with 80 mM NaCl in the lumen, O2− production it was 0.33 ± 0.15, 0.39 ± 0.08, and 0.54 ± 0.19 U/min, respectively (Fig. 6: n = 5). These data suggest that NAD(P)H oxidase is the primary source of O2−.
Fig. 6.
Effect of luminal pH on superoxide production. When the macula densa was perfused with 10 mM NaCl and luminal pH was switched to 6.8, 7.4, and 8.0, there was no significant increase in superoxide production. When the macula densa was perfused with 80 mM NaCl and luminal pH was switched to 6.8, 7.4, and 8.0, superoxide production was significantly enhanced. In the presence of apocynin (10−5 M), NaCl-induced superoxide production was blocked (n = 5; *P < 0.01 vs. pH 6.8).
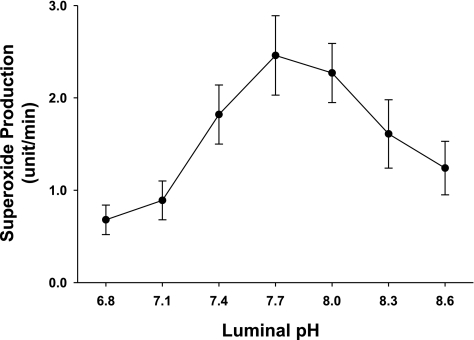
Finally, we measured the optimum pH for O2− production by the macula densa. When the pH of the perfusate was switched from 6.8 to 7.1, 7.4, 7.7, 8.0, 8.3, and 8.6 with 80 mM NaCl in the lumen, O2− was 0.69 ± 0.16, 0.89 ± 0.21, 1.81 ± 0.32; 2.46 ± 0.43, 2.26 ± 0.31, 1.61 ± 0.37, and 1.24 ± 0.29 U/min, respectively (Fig. 7; n = 5). These data suggest that the optimum extracellular pH is at 7.7, corresponding to a pHi of ∼8, for O2− production.
Fig. 7.
Optimum pH for superoxide production at the macula densa. The macula densa was perfused with 80 mM NaCl, and luminal pH was switched from 6.8 to 8.6. Superoxide production was inhibited significantly at both low and high pH values, with a optimum luminal pH at 7.7 (pHi ≈ 8.); n = 5.
DISCUSSION
When the luminal NaCl concentration increases at the macula densa, the cells depolarize (2) and pHi increases due to activation of NHE (15, 27). We reported that depolarization activates NAD(P)H oxidase and induces O2− production (16). However, the role of pHi and NHE in O2− production is unknown. We hypothesized that the elevation in macula densa pHi caused by increasing luminal NaCl, and consequent activation of NHE, enhances O2− production from NAD(P)H oxidase.
To begin to test our hypothesis, we first studied the effect of inhibiting luminal NHE on the increase in O2− production caused by raising luminal NaCl at the macula densa. Luminal NHEs are important regulators of pHi in the macula densa (5, 15, 26, 27), and an increase in luminal NaCl stimulates their activity (5). We found that the NHE-selective inhibitor DMA reduced the increase in O2− production caused by raising luminal NaCl from 10 to 80 mM by 40%. These data directly indicate that activation of luminal NHE that occurs when luminal NaCl is elevated stimulates O2− production. However, they do not indicate whether the increase in pHi or the increase in intracellular Na resulting from NHE activation stimulates O2− production.
Our results showing the inhibition of NHE in the macula densa are similar to results found in other nephron segments. In the thick ascending limb, blocking NHE with methylisobutyl-amiloride or dimethylamiloride reduced O2− production (13, 21). However, unlike the macula densa, inhibiting NHE in thick limbs completely prevented the stimulation of O2− production caused by pH, high glucose, or NaCl (13, 21).
The Na/H exchanger not only regulates pHi but also contributes to intracellular Na+. When the Na/H exchanger is inhibited by DMA, intracellular Na+ concentration is decreased and pHi elevation is blocked (12, 15). To test whether the effect of DMA on O2− production is due to pHi or Na+ inhibition, we used a K+/H+ ionophore, nigericin, to equilibrate luminal and pHi and measured O2− production. We found that when we increased pHi from 7.0 to 7.8 with nigericin while keeping luminal NaCl at 80 mM, there was a significant increase in O2− production. These data suggest that 1) the effects of DMA are due to its ability to prevent changes in pHi rather than a reduction in intracellular Na; and 2) elevated macula densa pHi enhances O2− production.
Our earlier findings indicated that depolarization of the macula densa activates NAD(P)H oxidase and stimulates O2− production (16). Nigericin may affect membrane potential, which in turn might modulate O2− production by the macula densa. To make sure that pHi influences O2− production in the macula densa, we sought the least invasive method possible to alter pHi. Thus we studied the effect changing pHi by adjusting luminal pH. We found that increasing luminal pH proportionately increased pHi. Similar to our results, Fowler et al. (5) reported that a significant elevation of pHi at the macula densa was induced by increasing luminal NaCl. Similar findings have also been reported for other cell types (8, 37). This effect is likely mediated by altering NHE activity. Because we had already shown that changes in intracellular Na that may accompany changes in NHE activity do not affect O2− production, increasing luminal pH was chosen as the method to study the effect of pHi on O2− production in intact macula densa cells. We found that when the macula densa was perfused with 10 mM NaCl, increasing extracellular pH did not enhance O2− production. However, at 80 mM NaCl, increasing extracellular pH significantly enhanced O2− production. The former data seem to indicate that pHi does not modify O2− production, while the latter data seem to indicate that it does. Our explanation for these results is that pHi only modulates O2− production only when the macula densa is depolarized and NAD(P)H oxidase activated, as occurs when 80 mM NaCl is in the lumen. Under these circumstances, the macula densa is depolarized and depolarization activates O2− production as we have previously reported (16). In contrast, when 10 mM NaCl is in the lumen, the macula densa is hyperpolarized and O2− production is not activated. Thus pHi has no effect. Our data indicate that pHi is an important modulator for O2− production induced by NaCl, accounting for ∼30% of O2−. We believe that depolarization of the macula densa is a major factor in control O2− production as we reported previously (16).
The above data imply that NAD(P)H oxidase is the source of O2− because we have shown that depolarization stimulates O2− production by this enzyme. To study whether this was in fact the case, we tested the ability apocynin to block the effects of pHi on O2− production in the presence of 80 mM NaCl. Similar to our previous report (16), we found that apocynin blocked the stimulation of O2− production caused by increasing pHi. These data suggest that NAD(P)H oxidase is the primary source of O2− induced by NaCl and affected by pHi.
Our findings that O2− production is pH dependent only after NAD(P)H oxidase is activated are in agreement with published reports. After NAD(P)H oxidase is activated by agonists to induce O2− production, elevating pHi pharmacologically with nigericin (11, 24), raising the external sodium concentration (34), or increasing extracellular pH (8, 23, 37) raises O2− concentrations in macrophages, leukocyte, neutrophils, and eosinophils.
To further understand the pH dependence of O2− production in the macula densa, we varied pHi over a greater range. We found that O2− production first increased as pHi was raised, reaching a maximum ∼pH 8.0, and then declined. Thus the correlation of NAD(P)H oxidase with pH was not a linear response. A number of studies have reported similar effects of pH on NAD(P)H oxidase activity and shown profound inhibition by either a low or high pH, with the optimum of ∼6.0–7.5 (20, 36). In phorbol myristate acetate-activated normal human neutrophils, a single enzymatic entity that was dormant in unstimulated cells, optimally active at pH 7.0, above or below the optimal pH significantly inhibits O2− production (36). Morgan et al. (20) studied the pH dependence of NAD(P)H oxidase by patch-clamping human eosinophils. They found that in intact eosinophils, NAD(P)H oxidase is highly sensitive to pH and has an optimum ∼7.5, decreasing drastically at higher or lower values. However, some reports found a linear response between pH and O2− production in zymosan-treated neutrophils and in chemotactic factor-stimulated human neutrophils. In each instance, the amount of O2− release correlated directly with pHi and was enhanced by intracellular alkalinization from 7.0 to 8.1 (6, 34). Two other studies reported that lowering pH to 6.0 had no effect on O2− generation (28, 31). The explanation for these apparent discrepancies is unclear.
Exactly how pHi regulates NAD(P)H oxidase is not fully understood. Since protons are generated together with O2− by NAD(P)H oxidase (1, 22), elevation pH may just relieve product induced inhibition of the enzyme. It has long been found that addition or removal of proton ions changes the charges on the protein structure of the enzyme, disrupting the ability to couple with its substrate (4). Experiments with a cell-free O2−-forming system suggested that this decline in respiratory burst activity at low pH was due to inefficient activation of the O2−-forming enzyme under acidic conditions (6). Bormann et al. (3) reported that activation of PLA2 in rabbit neutrophils is pH sensitive. The optimum pH was 8.0–8.5, with activity rapidly decreasing at pH values below 7.0. PLA2 is important for regulation of NAD(P)H oxidase activity. It controls the arachidonate-sensitive assembly of the complete oxidase complex by modulating the translocation of both p47phox and p67phox (32, 41). It is tempting to speculate that pHi might be acting through this route.
We are aware of the recent publication (9) in which the authors conclude that apocynin may act as a scavenger of reactive oxygen species rather than a specific inhibitor of NAD(P)H oxidase in some cells. However, this conclusion is primarily based on Fig. 3 of the paper in which apocynin at a concentration of 1,000 μM reduces the amount of superoxide detected by either oxyethidium or lucigenin when produced by cell-free systems. Importantly, at concentrations of 100 and 10 μM, apocynin showed no scavenging activity of superoxide in these two assays. In addition, 1 mM apocynin did not have any effect on superoxide levels in HEK293 cells transfected with Nox 2 and p47 measured with lucigenin. These data clearly show that apocynin is not simply a superoxide scavenger. In our preparation we used apocynin at a concentration of 10 μM. Given that this concentration is 100 times less than that needed to scavenge superoxide, it is unlikely that apocynin is acting via this mechanism in our preparation. In conclusion, we found that elevated pHi enhances the O2− production induced by increasing luminal NaCl. O2− production is very sensitive to pH, and optimum pHi is ∼8. We have recently reported that increasing luminal NaCl at the macula densa activates nNOS by elevating pHi (15, 17). In physiological situations, both nNOS and NAD(P)H oxidase activate during TGF. NO blunts tubuloglomerular feedback via the cGMP pathway (29). Superoxide at the macula densa augments tubuloglomerular feedback by scavenging nitric oxide (18), since after blocking nNOS with 7-NI, superoxide has no effect on tubuloglomerular feedback. The interaction and resulting balance between O2− and NO in the macula densa will modify the TGF response. Enhanced O2− generation and/or impaired production of NO or its metabolites (such as peroxynitrate) by the macula densa might contribute to pathophysiological changes in hypertension.
GRANTS
This work was supported by Grants SDG-0630288N from the American Heart Association (R. Liu) and RO1-HL-86767 from the National Heart, Lung, and Blood Institute (R. Liu).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Banfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, Krause KH. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science 287: 138–142, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Bell PD, Lapointe JY, Cardinal J. Direct measurement of basolateral membrane potentials from cells of the macula densa. Am J Physiol Renal Fluid Electrolyte Physiol 257: F463–F468, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Bormann BJ, Huang CK, Mackin WN, Becker EL. Receptor-mediated activation of a phospholipase A2 in rabbit neutrophil plasma membrane. Proc Natl Acad Sci USA 81: 767–770, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon M The effect of pH on the affinities of enzymes for substrates and inhibitors. Biochem J 55: 161–170, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler BC, Chang YS, Laamarti A, Higdon M, Lapointe JY, Bell PD. Evidence for apical sodium proton exchange in macula densa cells. Kidney Int 47: 746–751, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Gabig TG, Bearman SI, Babior BM. Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood 53: 1133–1139, 1979. [PubMed] [Google Scholar]
- 7.Garvin JL, Hong NJ. Nitric oxide inhibits sodium/hydrogen exchange activity in the thick ascending limb. Am J Physiol Renal Physiol 277: F377–F382, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Henderson LM, Chappell JB, Jones OT. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem J 255: 285–290, 1988. [PMC free article] [PubMed] [Google Scholar]
- 9.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Huang CL, Cogan MG. Atrial natriuretic factor inhibits maximal tubuloglomerular feedback response. Am J Physiol Renal Fluid Electrolyte Physiol 252: F825–F828, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Jensen MS, Bainton DF. Temporal changes in pH within the phagocytic vacuole of the polymorphonuclear neutrophilic leukocyte. J Cell Biol 56: 379–388, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juncos R, Hong NJ, Garvin JL. Differential effects of superoxide on luminal and basolateral Na+/H+ exchange in the thick ascending limb. Am J Physiol Regul Integr Comp Physiol 290: R79–R83, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Zhang G, Yi FX, Zou AP, Li PL. Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle. Am J Physiol Renal Physiol 289: F1048–F1056, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Liu R, Bell PD, Peti-Peterdi J, Kovacs G, Johansson A, Persson AEG. Purinergic receptor signaling at the basolateral membrane of macula densa cells. J Am Soc Nephrol 13: 1145–1151, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Liu R, Carretero OA, Ren Y, Garvin JL. Increased intracellular pH at the macula densa activates nNOS during tubuloglomerular feedback. Kidney Int 67: 1837–1843, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Liu R, Garvin JL, Ren Y, Pagano PJ, Carretero OA. Depolarization of the macula densa induces superoxide production via NAD(P)H oxidase. Am J Physiol Renal Physiol 292: F1867–F1872, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Liu R, Pittner J, Persson AE. Changes of cell volume and nitric oxide concentration in macula densa cells caused by changes in luminal NaCl concentration. J Am Soc Nephrol 13: 2688–2696, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Molony DA, Reeves WB, Andreoli TE. Na+:K+:2Cl− cotransport and the thick ascending limb. Kidney Int 36: 418–426, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Morgan D, Cherny VV, Murphy R, Katz BZ, DeCoursey TE. The pH dependence of NADPH oxidase in human eosinophils. J Physiol 569: 419–431, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori T, Cowley AW Jr. Renal oxidative stress in medullary thick ascending limbs produced by elevated NaCl and glucose. Hypertension 43: 341–346, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Murillo I, Henderson LM. Expression of gp91phox/Nox2 in COS-7 cells: cellular localisation of the protein and the detection of outward proton currents. Biochem J 385: 649–657, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy JK, Forman HJ. Effects of sodium and proton pump activity on respiratory burst and pH regulation of rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 264: L523–L532, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Nasmith PE, Grinstein S. Impairment of Na+/H+ exchange underlies inhibitory effects of Na+-free media on leukocyte function. FEBS Lett 202: 79–85, 1986. [DOI] [PubMed] [Google Scholar]
- 25.Navar LG, Bell PD, Thomas CE, Ploth DW. Influence of perfusate osmolality on stop-flow pressure feedback responses in the dog. Am J Physiol Renal Fluid Electrolyte Physiol 235: F352–F358, 1978. [DOI] [PubMed] [Google Scholar]
- 26.Peti-Peterdi J, Bell PD. Regulation of macula densa Na:H exchange by angiotensin II. Kidney Int 54: 2021–2028, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Peti-Peterdi J, Chambrey R, Bebok Z, Biemesderfer D, St. John PL, Abrahamson DR, Warnock DG, Bell PD. Macula densa Na+/H+ exchange activities mediated by apical NHE2 and basolateral NHE4 isoforms. Am J Physiol Renal Physiol 278: F452–F463, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Reeves EP, Lu H, Jacobs HL, Messina C, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416: 291–297, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Ren Y, Garvin JL, Carretero OA. Role of macula densa nitric oxide and cGMP in the regulation of tubuloglomerular feedback. Kidney Int 58: 2053–2060, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension 39: 799–802, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Rotstein OD, Nasmith PE, Grinstein S. The bacteroides by-product succinic acid inhibits neutrophil respiratory burst by reducing intracellular pH. Infect Immun 55: 864–870, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin BB, Downey GP, Koh A, Degousee N, Ghomashchi F, Nallan L, Stefanski E, Harkin DW, Sun CX, Sun CX, Smart BP, Gelb MH. Cytosolic phospholipase A2-alpha is necessary for platelet-activating factor biosynthesis, efficient neutrophil-mediated bacterial killing, and the innate immune response to pulmonary infection. J Biol Chem 280: 7519–7529, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnermann J, Persson AE, Agerup Tubuloglomerular feedback B. Nonlinear relation between glomerular hydrostatic pressure and loop of Henle perfusion rate. J Clin Invest 52: 862–869, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simchowitz L Intracellular pH modulates the generation of superoxide radicals by human neutrophils. J Clin Invest 76: 1079–1089, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki S, Franco-Saenz R, Mulrow PJ, McPartland RP, Sustarsic D, Rapp JP. Effects of rat urinary arginine esterases on rat kidney to release renin. Endocrinology 108: 1639–1642, 1981. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki Y, Lehrer RI. NAD(P)H oxidase activity in human neutrophils stimulated by phorbol myristate acetate. J Clin Invest 66: 1409–1418, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swallow CJ, Grinstein S, Sudsbury RA, Rotstein OD. Relative roles of Na+/H+ exchange and vacuolar-type H+ ATPases in regulating cytoplasmic pH and function in murine peritoneal macrophages. J Cell Physiol 157: 453–460, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Thompson RP, Simson JAV, Currie MG. Atriopeptin distribution in the developing rat heart. Anat Embryol 175: 227–233, 1986. [DOI] [PubMed] [Google Scholar]
- 39.Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol 29: 2571–2583, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Welch WJ, Wilcox CS. Tubuloglomerular feedback and macula densa-derived NO. In: Nitric Oxide and the Kidney: Physiology and Pathophysiology, edited by Goligorsky MS and Gross SS. New York: Chapman and Hall, 1997, p. 216–232.
- 41.Zhao X, Bey EA, Wientjes FB, Cathcart MK. Cytosolic phospholipase A2 (cPLA2) regulation of human monocyte NADPH oxidase activity. cPLA2 affects translocation but not phosphorylation of p67phox and p47phox. J Biol Chem 277: 25385–25392, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Zou AP, Li N, Cowley AW Jr. Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001. [DOI] [PubMed] [Google Scholar]