Abstract
Both short-term and long-term nitric oxide (NO) blockade were shown to cause an increase in O2− activity. To assess the contribution of such enhanced O2− activity in the kidney, responses to administration of the NO synthase inhibitor nitro-l-arginine methyl ester (l-NAME; 200 μg·min−1·kg body wt−1) were assessed in knockout mice the lacking NAD(P)H oxidase subunit gp91phox (KO; n = 10) and in wild-type (WT; n = 10) mice. Renal blood flow (RBF) and glomerular filtration rate (GFR) were determined by PAH and inulin clearances, respectively. Baseline RBF was higher in KO compared with WT mice (5.8 ± 0.5 vs. 4.5 ± 0.3 ml·min−1·g−1; P < 0.04) without significant differences in GFR (0.62 ± 0.04 vs. 0.73 ± 0.05 ml·min−1·g−1) and in mean arterial pressure (MAP; 91 ± 6 vs. 88 ± 4 mmHg). l-NAME infusion for 60 min caused similar increases in MAP (114 ± 6 vs. 113 ± 3 mmHg) in both groups but resulted in a lesser degree of reduction in RBF in KO compared with WT mice (−7 ± 3 vs. −17 ± 3%; P < 0.02), although GFR remained unchanged in both groups. The natriuretic response to systemic l-NAME infusion was attenuated in KO compared with WT mice (Δ: 3.1 ± 0.7 vs. 5.2 ± 0.6 μmol·min−1·g−1). l-NAME increased urinary 8-isoprostane excretion rate in WT (5.9 ± 1 to 7.7 ± 1 pg·min−1·g−1; P < 0.02) but not in KO mice (5.6 ± 1 to 4.9 ± 0.3 pg·min−1·g−1). In contrast, responses to another vasoconstrictor, norepinephrine, were similar in both strains of mice. These data indicate that activation of NAD(P)H oxidase results in the enhancement of O2− activity that influences renal hemodynamics and excretory function in the condition of NO deficiency.
Keywords: superoxide, oxidative stress, renal blood flow, sodium excretion, 8-isoprostane excretion
current literature reveals an increasing recognition that the interaction between nitric oxide (NO) and superoxide (O2−) plays an important role in the physiology as well as in the pathophysiology of kidney function and in hypertension (8, 19, 23–25, 42). Most of the evidence suggests that there is a balance between the production of NO and O2− that regulates the body function by opposing each other's action. It was demonstrated in vitro that O2− production was increased during NO inhibition in an isolated aortic strip (11). Enhancement of O2− activity by inhibiting SOD enzymes has been shown to decrease renal blood flow (RBF) and sodium excretion (UNaV; Refs. 8, 19, 20, 24, 26), and these responses to SOD inhibition were markedly enhanced during inhibition of the NO synthase (NOS) enzyme (20, 24, 25). It was also observed that the urinary excretion rate of 8-isoprostane (a marker for endogenous O2− activity) increased during acute (8, 25) or chronic (19) NOS inhibition, a response that was ameliorated by administration of tempol (a O2− scavenger), indicating that NOS inhibition caused enhancement of endogenous O2− activity. Tempol administration has also been shown to attenuate the renal excretory responses to NOS inhibition (8, 25). It was demonstrated that O2−, which is locally produced in the juxtaglomerular apparatus, limits NO signaling from the macula densa and enhances tubulo-glomerular feedback responses (41, 42). Collectively, these studies support the notion that NO plays a protective role against the actions of O2− in the kidney. Although NOS inhibition increases O2− activity in the vascular tissue (11, 35) and in the kidney (20, 24, 25), the responsive enzymatic source of such enhancement of O2− production is yet to be identified.
Among the various enzymes, NAD(P)H oxidase is one of the major sources of O2− production in the vascular tissue (1, 10, 32, 33, 37) as well as in the kidney (5, 9, 17). NAD(P)H oxidase is composed of several subunits, where gp91phox acts as a catalytic subunit for the production of O2− (1, 9, 31). Recently, we reported (13) that at the basal condition RBF is higher in knockout (KO) mice lacking the gene for gp91phox of NAD(P)H oxidase compared with the wild-type (WT, C57BL/6) mice. We (13) also reported that urinary excretion of NO metabolites in these KO mice was higher than in the WT mice. Similarly, higher NO bioavailability was also reported in the vascular tissue of KO mice by Gorlach et al. (10). Thus higher NO bioavailability in KO mice appears to be due to lower O2− generation in the absence of the gp91phox subunit of NAD(P)H oxidase.
We hypothesize that the NAD(P)H oxidase enzyme is the source of enhanced O2− activity that results due to a deficiency in NO production and such enhanced O2− activity influences renal hemodynamic and excretory responses to NOS inhibition. Thus, in the present study, we evaluated the functional responses to acute NOS inhibition in mice lacking the gene for the gp91phox subunit of NAD(P)H oxidase to assess the source and contribution of enhanced O2− activity in the kidney during NO deficiency.
MATERIALS AND METHODS
The studies were performed in accordance with the guidelines and practices established by the Tulane University Animal Care and Use Committee. Male gp91phox KO mice and WT C57BL/6 mice (both supplied by Jackson Laboratories, Bar-Harbor, ME) were housed in a temperature- and light-controlled room and allowed free access to a standard diet (Ralston-Purina, St. Louis, MO) and tap water. The gp91phox-deficient mice were developed and maintained in the C57BL/6J strain that was purchased from the Jackson Laboratory (30). Because the C57BL/6 mice (WT) are commonly used as the genetic background for gene-targeted mutations, this strain was used as a control group for the KO mice (31).
On the day of experiments, mice weighing ∼25 g were anesthetized with inactin (thiobutabarbital sodium, 110 mg/kg body wt ip; dissolved in saline; stock solutions were kept in the refrigerator at 4°C). Supplemental doses of inactin (10 mg/kg ip) were administered as required. This dose helped to maintain a stable level of systemic blood pressure during the long experimental period. The mice were placed on a servo-controlled surgical table that maintained the their body temperature at 37°C, and a tracheostomy was performed. The mice were allowed to breathe air enriched with O2 by placement of the exterior end of the tracheal cannula inside a small plastic chamber into which humidified 95% O2-5% CO2 was continuously passed. (4, 13). The right carotid artery was cannulated with PE-10 tubing connected to PE-50 tubing for continuous measurement of arterial pressure and blood sampling. Arterial pressure was measured with a pressure transducer connected to the carotid cannula and was monitored with the AcKnowledge data acquisition system (Biopac). The right jugular vein was catheterized with a PE-10 tube for fluid infusion at the rate of 4 μl/min via a CMA microdyalysis pump. During surgery, an isotonic saline solution containing 6% albumin (bovine serum; Calbiochem, La Jolla, CA) was infused. After surgery, the infusion fluid was changed to isotonic saline containing 2% albumin, 7.5% Inulin (Inutest; Laevosan, Linz/Donau, Austria), and 1.5% PAH (Merck Sharpe & Dohme, West Point, PA). The bladder was catheterized with a PE-90 tube via a suprapubic incision for urine collection.
After a 60-min equilibration period after the completion of surgery, the experimental protocol was started with urine collection for a 30-min clearance period to determine the baseline level. Then an infusion of nitro-l-arginine methyl ester (l-NAME; 200 μg·min−1·kg−1 iv; Refs. 6, 35) was initiated. After a 10-min stabilization, urine was collected for two consecutive 30-min clearance periods. After the second collection period during l-NAME infusion, an arterial blood sample (500 μl) was taken for measurements of hematocrit and plasma PAH, inulin, and sodium/potassium concentrations. To maintain a stable preparation during the experimental period, only one blood collection was made at the end and the values obtained from this sample were used to calculate inulin and PAH clearances for all the collection periods as reported in our earlier studies (4, 13). Since a period of at least 30 min period is required to observe the full effects of l-NAME infusion (21, 23), the values obtained from the second collection period were considered for calculating the responses to l-NAME. At the end of the experimental protocol, the animals were killed with a high dose of inactin anesthesia and the kidneys were removed and weighed.
Separate groups of age-matched KO and WT mice were used to evaluate and compare the renal responses to another vasoconstrictor agent, norepinephrine (NE) with those observed with l-NAME treatment. NE was infused at a rate of 5 μg·min−1·kg−1 iv, as this dose showed a comparable renal vasoconstrictor response similar to l-NAME infusion. The protocol followed in these experiments was exactly the same as that with l-NAME. Similar to l-NAME treated groups, the values of renal parameters obtained during the 2nd collection period were considered as the responses to NE.
Analytical procedures.
Blood and urine samples collected during systemic experiments were analyzed for inulin, PAH, and sodium/potassium concentrations. Inulin and PAH concentrations were determined by spectrophotometry and sodium/potassium concentrations were determined by flame photometry. The value for inulin clearance was considered as the glomerular filtration rate (GFR), and the value for PAH clearance was considered as renal plasma flow (RPF) as reported previously in mice (13). The formulas for measurements of GFR and RPF are as follows: GFR (inulin clearance) = (urinary conc. of inulin × urine volume)/plasma inulin conc.; RPF (PAH clearance) = [(urinary conc. of PAH × urine volume)/plasma PAH conc.]. RBF was calculated from RPF and hematocrit (Hct) value [RBF = RPF/(1-Hct)]. Renal vascular resistance (RVR) was calculated by dividing the value of mean arterial pressure with the value of RBF. Urinary concentration of 8-isoprostane was measured by enzyme immunoassay (Cayman Chemical). All values were normalized per gram of kidney weight. Results are means ± SE. Statistical comparison among the groups was conducted by ANOVA. Statistical analyses were performed using a Student's t-test. Significance level was deemed as P < 0.05.
RESULTS
Renal hemodynamic responses to l-NAME in KO and WT mice.
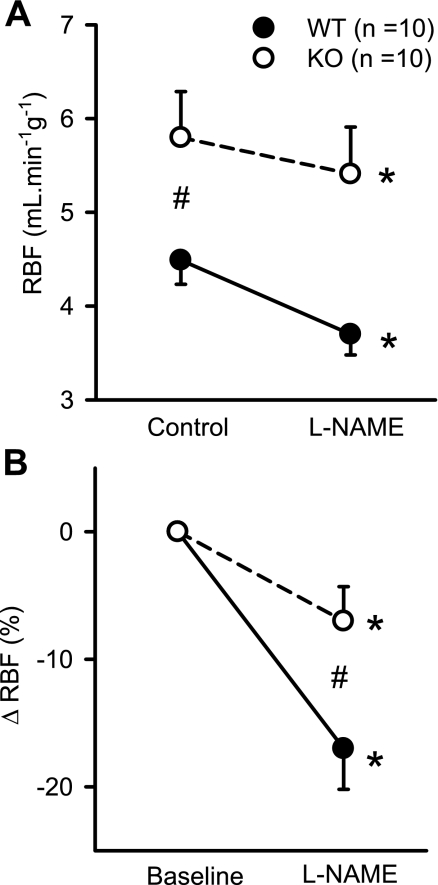
Figure 1A shows the RBF responses to systemic administration of l-NAME. Baseline RBF was higher in KO compared with WT mice (5.8 ± 0.5 vs. 4.5 ± 0.2 ml·min−1·g−1; P < 0.04), as reported previously (13). However, it was noted that the baseline value of RBF in mice in the present study was higher than what was observed in our earlier study (13). Although we have no clear explanation of this difference in baseline RBF between these two studies, it may be due to the use of a single vs. multiple anesthetic agents. In our earlier study (13), a combination of inactin and ketalar (a mixture of ketamine and xylazine) was used as anesthetic agent. However, only inactin was used in the present study.
Fig. 1.
Responses to systemic administration of nitro-l-arginine methyl ester (l-NAME; 200 ng·min−1·g−1) on absolute (A) and percent changes (B) in renal blood flow (RBF) in anesthetized knockout (KO) mice lacking the gene for gp91phox subunit of NAD(P)H oxidase and their genetic control (C57BL6) wild-type (WT) mice. *P < 0.05 vs. control/baseline; #P < 0.01 between groups.
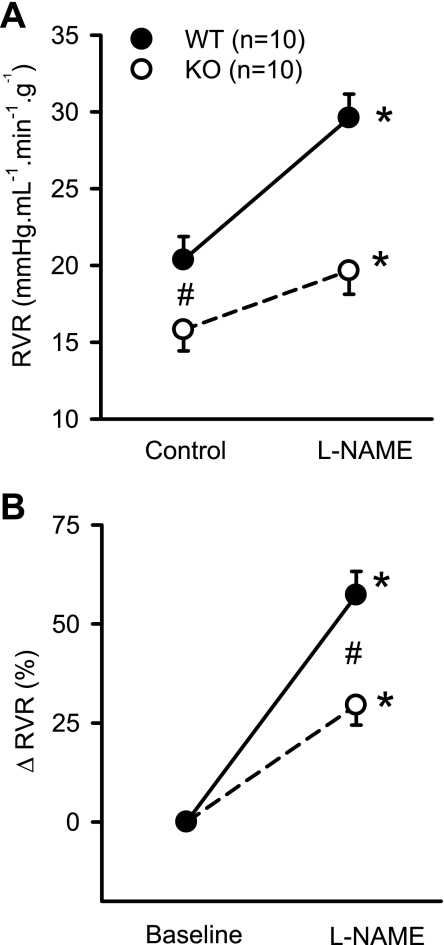
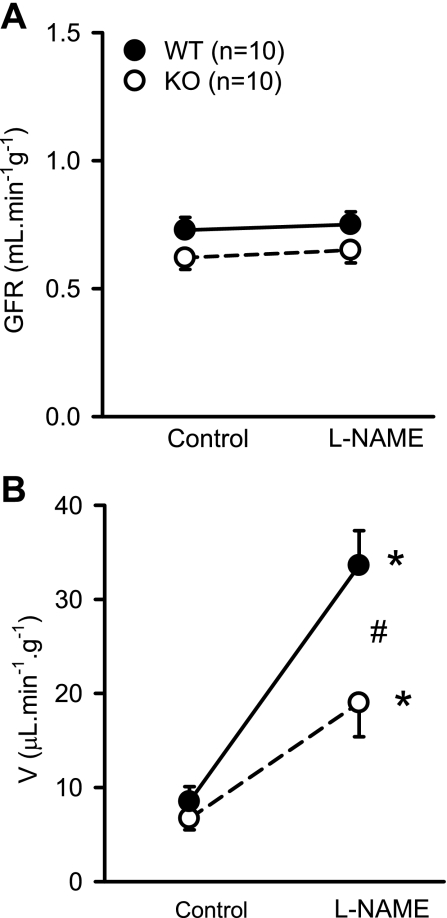
Figure 1 illustrates the RBF responses to NOS inhibition in WT and KO mice. In response to l-NAME, RBF decreased from 4.5 ± 0.2 to 3.7 ± 0.2 ml·min−1·g−1 (P < 0.002) in WT mice and from 5.8 ± 0.5 to 5.4 ± 0.5 ml·min−1·g−1 (P < 0.04) in KO mice (Fig. 1A). The decrease in RBF (−7 ± 2.7%) in response to l-NAME in KO mice was significantly (P < 0.03) lower than in WT mice (−17 ± 3.2%; Fig. 1B). Intravenous administration of l-NAME caused a similar increase in MAP in both groups of mice. MAP increased from 91 ± 6 to 114 ± 5 mmHg (25 ± 4%) in KO mice and from 88 ± 4 to 113 ± 3 mmHg (31 ± 6%) in WT mice. Figure 2 illustrates the RVR response to l-NAME. Baseline RVR in KO (15.8 ± 1.4 mmHg·ml−1·min·g) was lower than in WT (20.4 ± 1.5 mmHg·ml−1·min·g; P < 0.04). In response to l-NAME, RVR increased from 15.8 ± 1.4 to 21.6 ± 2.3 mmHg·ml−1·min·g (P < 0.007) in KO mice and from 20.4 ± 1.5 to 31.3 ± 1.6 mmHg. ml·min−1·g−1 (P < 0.001) in WT mice (Fig. 2A). The increase in RVR in response to l-NAME in KO mice (29.5 ± 5%) was significantly (P < 0.005) lower than in WT mice (57.3 ± 6%, Fig. 2B). l-NAME administration did not cause a significant change in GFR (from 0.62 ± 0.05 to 0.60 ± 0.06 ml·min−1·g−1) in KO or in WT mice (from 0.73 ± 0.05 to 0.74 ± 0.06 ml·min−1·g−1; Fig. 3A).
Fig. 2.
Responses to systemic administration of l-NAME (200 ng·min−1·g−1) on absolute (A) and percent changes (B) in renal vascular resistance (RVR) in anesthetized KO mice lacking the gene for gp91phox subunit of NAD(P)H oxidase and their genetic control (C57BL6) WT mice. *P < 0.05 vs. control/baseline; #P < 0.05 between groups.
Fig. 3.
Responses to systemic administration of l-NAME (200 ng·min−1·g−1) on glomerular filtration rate (GFR; A) and urine flow (V; B) in anesthetized KO mice lacking the gene for gp91phox subunit of NAD(P)H oxidase and their genetic control (C57BL6) wild type (WT) mice. *P < 0.05 vs. control; #P < 0.05 between groups.
Renal excretory responses to l-NAME in KO and WT mice.
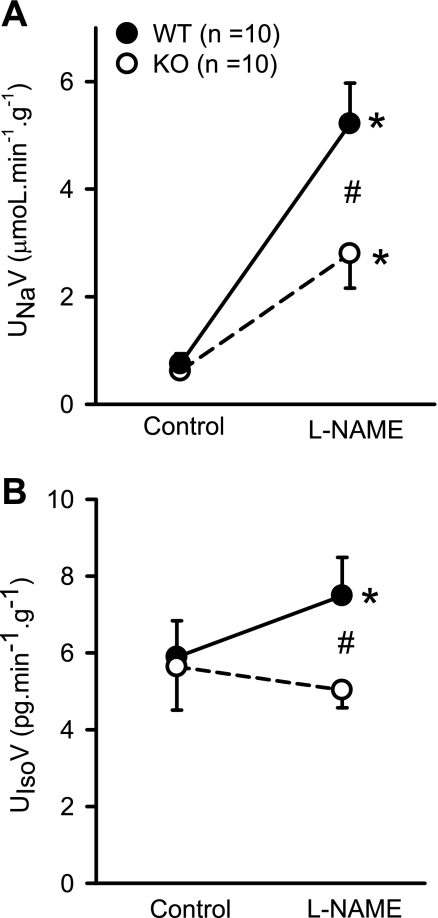
Systemic administration of l-NAME increased urine flow (V) from 6.7 ± 1.2 to 19 ± 3.6 μl·min−1·g−1 (202 ± 57%) in KO and from 8.5 ± 1.6 to 33.6 ± 3.7 μl·min−1·g−1 (444 ± 81%) in WT mice (Fig. 3B). Figure 4A shows the UNaV responses to systemic administration of l-NAME. In response to l-NAME, UNaV increased from 0.62 ± 0.1 to 3.7 ± 0.8 μmol·min−1·g−1 (550 ± 170% ) in KO and 0.75 ± 0.2 to 5.9 ± 0.76 μmol·min−1·g−1 (1,116 ± 195%) in WT mice. Compared with KO mice, the increases of UNaV were higher in WT mice (550 ± 170 vs. 1,116 ± 195%; P < 0.05). In response to l-NAME, fractional excretion of sodium (FENa) increased from 0.69 ± 0.16 to 4.09 ± 1.0% in KO mice and from 0.55 ± 0.08 to 5.2 ± 0.6% in WT mice, which was not statistically different between the groups. In conscious animals in our earlier study (13), a significant difference was observed in 24-h urinary excretion of sodium between KO and WT mice. However, the UNaV noted in the anesthetized animals was not significantly different between the strains in the present as well in our earlier study (13). There was no difference in potassium excretion (UKV) between the KO and WT mice in the basal condition (0.91 ± 0.1 vs. 1.03 ± 0.2 μmol·min−1·g−1) or in response to l-NAME (0.96 ± 0.16 vs. 0.99 ± 0.07 μmol·min−1·g−1). Interestingly, l-NAME infusion increased urinary 8-isoprostane excretion (UIsoV; marker for endogenous O2− activity) in WT but not in KO mice (Fig. 4B). In WT mice, UIsoV increased (5.9 ± 1 to 7.7 ± 1 pg·min−1·g−1; P < 0.02), while it did not increase in KO mice (5.6 ± 1 to 4.9 ± 0.3 pg·min−1·g−1).
Fig. 4.
Responses to systemic administration of l-NAME (200 ng·min−1·g−1) on urinary excretion rate of sodium (UNaV; A) and 8-isoprostane (UIsoV; B) in anesthetized KO mice lacking the gene for gp91phox subunit of NAD(P)H oxidase and their genetic control (C57BL6) WT mice. *P < 0.05 vs. control; #P < 0.01 between groups.
NE induced renal responses in KO and WT mice.
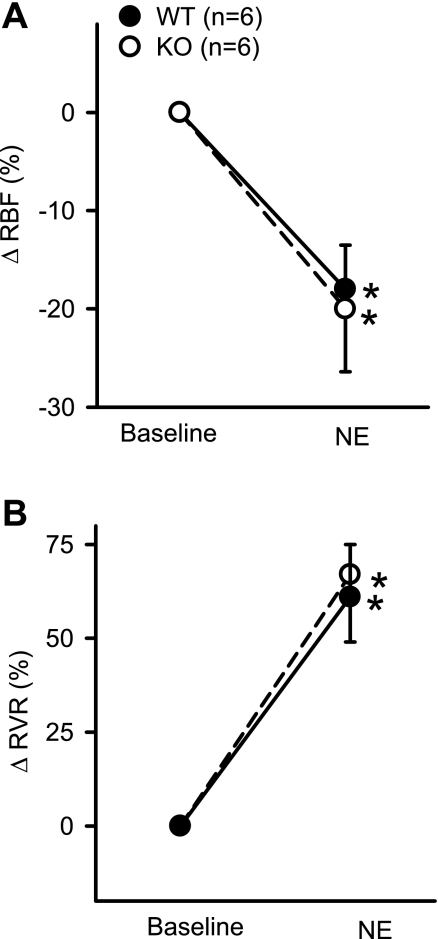
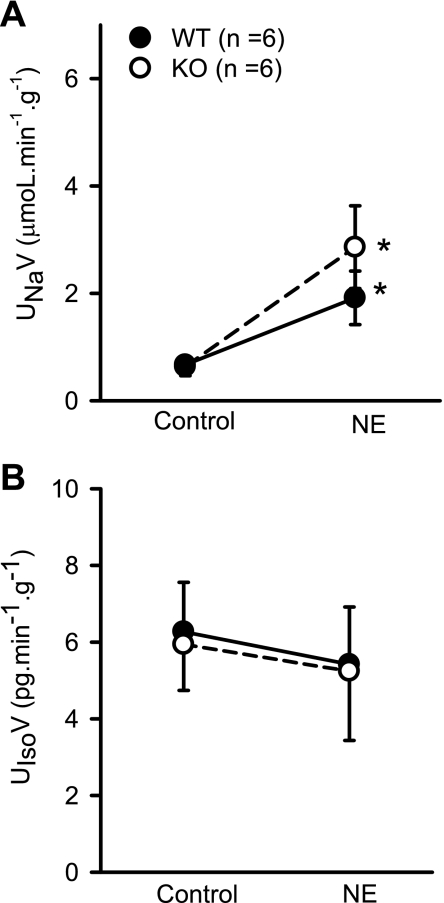
In response to NE administration, RBF decreased from 7.1 ± 0.2 to 5.6 ± 0.4 ml·min−1·g−1 (P < 0.04) in KO mice and from 5.9 ± 0.35 to 4.83 ± 0.3.5 ml·min−1·g−1 (P < 0.02) in WT mice. The decrease in RBF in response to NE was similar (20 ± 6.4 vs. 18 ± 4.5%) in the respective KO and WT mice (Fig. 5A). There was also no difference in the degree of increase in RVR between KO and WT mice in responses to NE. RVR increased from 13.2 ± 1.9 to 21.3 ± 2.7 mmHg·ml−1·min·g (67 ± 18%) in KO and from 16.2 ± 2.5 to 25.4 ± 3.7 mmHg·ml−1·min·g (61 ± 14%) in WT (Fig. 5B). MAP increased from 92 ± 11 to 120 ± 13 mmHg (30 ± 7%) in KO and from 91 ± 8 to 114 ± 13 mmHg (24 ± 7%) in WT mice. NE did not cause significant change in GFR in KO (from 0.80 ± 0.06 to 0.90 ± 0.13 ml·min−1·g−1) or in WT mice (from 0.73 ± 0.06 to 0.76 ± 0.09 ml·min−1·g−1). There were increases in V, UNaV, and FENa in both the KO and WT mice in response to NE. UNaV increased from 0.64 ± 0.2 to 2.4 ± 0.7 μmol·min−1·g−1 (P < 0.02; 309 ± 114%) in KO and 0.75 ± 0.2 to 5.9 ± 0.76 μmol·min−1·g−1 (P < 0.03; 216 ± 93%) in WT mice (Fig. 6A). However, the magnitude of these responses was not different between the KO and WT mice in response to NE (Fig. 6A). UKV did not change in either the KO or the WT mice in response to NE. There were also no significant changes in UIsoV responses to NE in either group of mice (Fig. 6B). In KO mice, UIsoV altered from 5.9 ± 1.2 to 5.1 ± 1.6 pg·min−1·g−1and in WT mice from 6.3 ± 0.1 to 5.5 ± 1.6 pg·min−1·g−1.
Fig. 5.
Percent changes in the responses to systemic administration of norepinephrine (NE; 25 ng·min−1·g−1) on RBF (A) and RVR (B) in anesthetized KO mice lacking the gene for gp91phox subunit of NAD(P)H oxidase and their genetic control (C57BL6) WT mice. *P < 0.05 vs. baseline; #P < 0.01 between groups.
Fig. 6.
Responses to systemic administration of NE (25 ng·min−1·g−1) on urinary excretion rate of UNaV (A) and UIsoV (B) in anesthetized KO mice lacking the gene for gp91phox subunit of NAD(P)H oxidase and their genetic control (C57BL6) WT mice. *P < 0.05 vs. control.
DISCUSSION
The results of the present investigation demonstrated that the renal vasoconstrictor response to administration of a NOS inhibitor, l-NAME, was significantly attenuated in KO mice lacking the gene for gp91phox subunits of NADPH oxidase compared with the response in WT mice. These KO mice have higher basal RBF and lower vascular resistances than in WT mice. Although these KO mice had higher NO bioavailability than WT mice (13), NO blockade in these mice did not cause greater response on RBF or vascular resistance than in WT as might be expected but rather showed an attenuated response. On the other hand, the renal vasoconstrictor response to NE was not different between these KO and WT mice. It was observed that there was an increase in UIsoV (a marker for endogenous O2− activity) in response to l-NAME in WT mice but not in KO mice. NE administration did not cause any change in UIsoV. Previous studies (8, 12, 17, 18, 19, 25) from many laboratories have validated that a change in UIsoV is linearly correlated with endogenous O2− activity. The increase in UIsoV in response to l-NAME treatment in WT mice indicates that NO blockade caused enhancement of O2− activity. Enhancement of O2− activity in response to NOS inhibition was also reported in the aortic vasculature (11) and in the kidney (16, 19, 25). The findings that UIsoV remained unaltered in KO mice during l-NAME infusion suggest that an activation of NAD(P)H oxidase is required for the enhancement of O2− activity in response to NOS inhibition. As such increase in O2− activity was absent in KO mice, this resulted in attenuation of the vasoconstrictor response to l-NAME in KO mice. In an earlier study (13), we also demonstrated that ANG II induced renal vasoconstriction was attenuated in KO compared with WT mice, which was attributed to the lack of ANG II induced increase in O2− activity. Collectively, these results demonstrate that the mechanism involved in mediating renal vasoconstrictor response to NOS inhibition is not limited to only a decrease in NO activity but also to a concomitant increase in O2− activity. The findings of the present study are in agreement with the results from a recent study by Just et al. (16) in rats that showed an increase in RBF in response to apocynin (NADPH oxidase inhibitor) or tempol (O2− scavenger) administration in l-NAME pretreated rats, indicating that enhanced O2− activity partially contributes to the renal vasoconstrictor response to NOS inhibition.
Although Just et al. (16) also showed an attenuation of the vasoconstrictor response to NE in apocynin/tempol-treated rats, such attenuation of NE response was not seen in KO mice in the present study. The apparent discrepancy in the implications of these results could be related to the differences in the choice of experimental protocols and the routes of NE administration in both the studies. In the present study, assessment of the renal responses to a systemic infusion of NE was made for more than an hour, while the study by Just et al. (16) assessed the RBF response to a bolus administration of NE infused directly in the renal artery for only 2 min. Thus such direct short-term effects of NE in the renal vasculature could be different from the effects of comparatively longer period administration of NE systemically. However, other previous studies also reported that NE administration in vitro did not affect vascular O2− production in aortic vascular segments (32) or NO production in carotid vessels (34). The vasoconstrictor response to NE was shown to remain unaffected by l-NAME pretreatment in isolated aortic vessels (35).
The dose of l-NAME used in the present study was shown to prevent the vasodepressor action of acetylcholine or bradykinin in earlier studies (6, 23, 36) conducted in our laboratory. Functional evidence from those initial studies (6, 23, 36) indicated that this dose of l-NAME exerted a maximal renal vasoconstrictor response, indicating a near complete blockade of NO formation in the kidney. Thus it was reasonable to assume that this dose achieved a maximal inhibition of NOS in these mice. However, it could be argued that the effective inhibitory dose of l-NAME could be different in KO mice than in WT mice as the former had higher NO bioavailability compared with the later. This seems unlikely, as this dose of l-NAME resulted in similar increases in arterial pressure in both WT and KO mice. Although a direct measure of NO or O2− production in the renal vasculature or an assessment of the renal regional differences in their levels was not performed in the present study, it was observed earlier (13) that KO mice have a higher urinary excretion rate of NO metabolites indicating increased NO bioavailability compared with WT mice. As O2− reacts with NO and limits its bioavailability, it is expected that a reduction in O2− production would lead to higher NO bioavailability in these KO mice. Although a direct measure of O2− production before and during NOS inhibition was not made in the present study, previous studies have clearly demonstrated that NOS inhibition enhances vascular O2− production in rats (39), in mice (7), and also in humans (11). The exact mechanism that resulted in an increased endogenous level of O2− production during NOS inhibition is not yet clear. Both NO and O2− are constant products of cellular metabolism, and both of these molecules are constantly interacting with each other in biological tissues (14, 20). Normally, the O2− level in the tissue is kept to a minimal level by the anti-oxidative function of NO as well as SOD. However, when NO production is diminished in the tissue, it is expected that this balance is altered, allowing accumulation of O2− in the tissue because of its inadequate removal by NO. Although no direct evidence is currently available, it may be possible that the intact NO activity may regulate the activity of the NAD(P)H enzyme. However, as reported previously (13), the basal UIsoV was not different between KO and WT mice in the present study. The reason could be the involvement of other enzymatic (xanthine oxidase, cyclooxygenase enzymes) or nonenzymatic (mitochondria) mechanisms in the compensatory regulation of the basal level of O2− activity in KO mice. However, further in vivo as well as in vitro experiments are required to delineate these interactions between NO and O2− at the enzymatic level in the kidney. It should be emphasized here that the elucidation of the exact molecular mechanism involved in this process was not the primary question that was addressed in the present study. Such assessment of molecular mechanisms is beyond the scope of these present in vivo experiments and will require separate in vitro studies with appropriate protocols to address more focal questions.
It could be argued how the interaction of NO and O2− may provides a reno-protective function (18–20, 24–26) when it is known that the reaction between NO and O2− results in the formation of peroxynitrite (ONOO−), a powerful cytotoxic agent. Although the exact mechanism of this protective effect of ONOO− is not yet clearly understood, we have recently reported (27) that while an intra-arterial infusion of a high dose of ONOO− in the kidney caused decreases in RBF and GFR, a lower dose resulted in vasodilator and hyperfiltration responses that were abolished in the condition of NO blockade. These findings support the hypothesis that OONO− acts as a NO-dependant vasodilator and provides reno-protective function at low physiologic concentrations but acts as an oxidant radical and contributes to the renal pathophysiology at higher concentrations. However, more comprehensive studies are required to examine this issue further.
The gp91phox subunit of NAD(P)H oxidase has been reported to be distributed in the kidney with a prominent expression in renal vessels, glomeruli, mesangial cells, as well as tubular epithelial cells, including the macula densa (5, 9). Although this isoform is abundantly expressed in the endothelial cell layer, reports (1) are also available that suggests the presence of this isoform in other layers of vasculature. Although the available reports do not provide any strong indication, it may be possible that a lack of this isoform alters the characters and functional activity of other subunits of NAD(P)H oxidase in an adaptive or compensatory manner in KO mice. However, such characterization of NAD(P)H oxidase subunits needs separate studies using in vitro preparations and specific targeted protocols. However, it should be emphasized here that the results from the present study provide strong support for the notion that gp91phox plays an important role in the regulation of kidney function during NO deficiency.
It was noted that the basal blood pressure was not different in these KO mice compared with that in WT mice in the present as well as in our earlier study (13). Similar results were also reported in studies from other laboratories (33, 38) except one (3) that showed a slightly lower blood pressure in KO compared with WT mice. As multiple factors are involved in controlling blood pressure, there may be a compensatory role of many other factors that maintain the blood pressure in KO mice. It should be noted that our present experiments mainly focused on the kidney functions but not on other organ or vascular beds. Thus the importance of gp91phox in the regulation of total peripheral vascular resistance is yet to be determined.
Although l-NAME-induced reductions in RBF and vascular resistance were attenuated in KO mice, GFR was mostly unaffected by l-NAME in both strains. Baseline values of GFR in KO mice were not significantly different from those in WT mice as reported previously (13). The inhibition of NOS was also reported to have none or minimal effect on GFR in many studies (6, 19, 23, 24, 29, 36). These findings are consistent with the concept that NO exerts a proportionate influence on both preglomerular and postglomerular resistance segments (28). It should also be noted that the magnitude of GFR depends on the filtration forces that depend on the balance between pre- and postglomerular vascular resistances. In conditions in which an equipotent balance is maintained, GFR may remain the same despite changes in RBF. Such a situation is noted in cases of administration of many agents, such as ANG II, ACh, or l-NAME (28).
It was observed that systemic l-NAME infusion in mice in the present study resulted in marked increases in V and UNaV as similar responses were also also reported in rats (2, 6). Although inhibition of renal NO generation leads to antidiuretic and antinatriuretic effects (21, 23), such diuretic and diuretic responses to systemic l-NAME administration were suggested to be linked with marked concomitant increases in systemic arterial pressure (15, 36). However, it is interesting to note that such increases in V and UNaV in response to l-NAME were significantly lower in KO mice compared with WT mice, despite a similar increase in arterial pressure in both the strains. Baseline values of V and UNaV as well as systemic arterial pressure were not significantly different between WT and KO mice as reported previously (13). Although the diuretic and natriuretic responses to l-NAME administration were suggested to be the effects of associated increase in arterial pressure (15), it was also demonstrated that inhibition of renal NO production abolishes arterial pressure induced increases in UNaV (21, 22). Thus the exact reason for such diuretic and natriuretic responses to systemic l-NAME is not yet clearly defined. Moreover, it is interesting that these diuretic and natriuretic responses to systemic l-NAME administration were markedly attenuated in KO mice that are lacking a subunit of NAD(P)H oxidase. It should also be noted that NE administration also caused a similar increase in arterial pressure in both KO and WT mice as with l-NAME administration. Nevertheless, NE caused only a mild diuresis and natriuresis and the responses are not significantly different between the strains. Thus these results seem to indicate that a lack of NAD(P)H enzyme activity contributes to these attenuated diuretic and natriuretic responses to systemic l-NAME administration in KO mice. This notion is somewhat difficult to explain at present as all the available data (24, 25, 30) indicate that the enhancement of O2− activity leads to sodium retention as opposed to observed natriuresis in response to l-NAME in WT mice. However, the reduced natriuretic response to systemic administration of l-NAME in KO mice cannot be explained by the lower NO production or autoinhibition of NOS in KO mice, as this response was observed in the presence of NOS blockade (2, 6). The present findings clearly indicates that the full expression of this natriuretic response to systemic l-NAME administration is linked to the intact NAD(P)H oxidase enzyme activity. It is possible that other natriuretic agents, such as atrial natriuretic peptide and endothelin, may be released in the oxidative stress condition (40) induced by NOS inhibition in WT but not in KO and thus contribute to the difference in the responses in both the strains. Further comprehensive studies will be required to examine this possibility.
In conclusion, the results of the present investigation indicate that the increase in O2− production during NOS inhibition results from the activation of NAD(P)H oxidase and such enhancement of O2− activity partially contributes to the renal vasoconstriction in the condition of NO deficiency.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute Grant HL-66432 and the Louisiana Board of Regents Grant through the Millennium Trust Health Excellent Fund (2001–2006).
Acknowledgments
We thank Alexander Castillo for excellent technical assistance.
Present address for M. Z. Haque: Department of Physiology, Wayne State University, 421 East Canfield, 1129 Elliman Building, Detroit, MI 48201.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Babior BM NADPH oxidase: an update. Blood 93: 1464–1476, 1999. [PubMed] [Google Scholar]
- 2.Baylis C, Harton P, Engels K. Endothelial derived relaxing factor controls renal hemodynamics in the normal rat kidney. J Am Soc Nephrol 1: 875–881, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 105: 293–296, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Cervenka L, Mitchell KD, Navar LG. Renal function in mice: effects of volume expansion and angiotensin II. J Am Soc Nephrol 10: 2631–2636, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 39: 269–274, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Chin SY, Wang CT, Majid DS, Navar LG. Renoprotective effects of nitric oxide in angiotensin II-induced hypertension in the rat. Am J Physiol Renal Physiol 274: F876–F882, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Cosentino F, Barker JE, Brand MP, Heales SJ, Werner ER, Tippins JR, West N, Channon KM, Volpe M, Luscher TF. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol 21: 496–502, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Dutta UK, Lane J, Roberts LJ II, Majid DS. Superoxide formation and interaction with nitric oxide modulate systemic arterial pressure and renal function in salt-depleted dogs. Exp Biol Med (Maywood) 231: 269–276, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res 87: 26–32, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Guzik TJ, West NE, Pillai R, Taggart DP, Channon KM. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension 39: 1088–1094, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Haas JA, Krier JD, Bolterman RJ, Juncos LA, Romero JC. Low-dose angiotensin II increases free isoprostane levels in plasma. Hypertension 34: 983–986, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Haque MZ, Majid DS. Assessment of renal functional phenotype in mice lacking gp91PHOX subunit of NAD(P)H oxidase. Hypertension 43: 335–340, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Huie RE, Padmaja S. The reaction of NO with superoxide. Free Radic Res Commun 18: 195–199, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Johnson RA, Freeman RH. Sustained hypertension in the rat induced by chronic blockade of nitric oxide production. Am J Hypertens 5: 919–922, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Just A, Olson AJ, Whitten CL, Arendshorst WJ. Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide. Am J Physiol Heart Circ Physiol 292: H83–H92, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol 14: 2775–2782, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Kopkan L, Huskova Z, Vanourkova Z, Thumova M, Skaroupkova P, Cervenka L, Majid DS. Superoxide and its interaction with nitric oxide modulates renal function in prehypertensive Ren-2 transgenic rats. J Hypertens 25: 2257–2265, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Kopkan L, Majid DS. Enhanced superoxide activity modulates renal function in NO-deficient hypertensive rats. Hypertension 47: 568–572, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Majid DS, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol 34: 946–952, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Majid DS, Navar LG. Nitric oxide in the control of renal hemodynamics and excretory function. Am J Hypertens 14: 74S–82S, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Majid DS, Navar LG. Nitric oxide in the mediation of pressure natriuresis. Clin Exp Pharmacol Physiol 24: 595–599, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Majid DS, Navar LG. Suppression of blood flow autoregulation plateau during nitric oxide blockade in canine kidney. Am J Physiol Renal Fluid Electrolyte Physiol 262: F40–F46, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Majid DS, Nishiyama A. Nitric oxide blockade enhances renal responses to superoxide dismutase inhibition in dogs. Hypertension 39: 293–297, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Majid DS, Nishiyama A, Jackson KE, Castillo A. Inhibition of nitric oxide synthase enhances superoxide activity in canine kidney. Am J Physiol Regul Integr Comp Physiol 287: R27–R32, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Majid DS, Nishiyama A, Jackson KE, Castillo A. Superoxide scavenging attenuates renal responses to ANG II during nitric oxide synthase inhibition in anesthetized dogs. Am J Physiol Renal Physiol 288: F412–F419, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Matavelli LC, Kadowitz PJ, Majid DSA. The effects of peroxynitrite administration on renal hemodynamics and excretory function in rats (Abstract). J Am Soc Nephrol 18: 21A, 2007. [Google Scholar]
- 28.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Obst M, Gross V, Janke J, Wellner M, Schneider W, Luft FC. Pressure natriuresis in AT(2) receptor-deficient mice with l-NAME hypertension. J Am Soc Nephrol 14: 303–310, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol 283: F957–F962, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 9: 202–209, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ Res 89: 408–414, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Somoza B, Gonzalez MC, Gonzalez JM, Abderrahim F, Arribas SM, Fernandez-Alfonso MS. Modulatory role of the adventitia on noradrenaline and angiotensin II responses role of endothelium and AT2 receptors. Cardiovasc Res 65: 478–486, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava P, Hegde LG, Patnaik GK, Dikshit M. Role of endothelial-derived reactive oxygen species and nitric oxide in norepinephrine-induced rat aortic ring contractions. Pharmacol Res 38: 265–274, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Takenaka T, Mitchell KD, Navar LG. Contribution of angiotensin II to renal hemodynamic and excretory responses to nitric oxide synthesis inhibition in the rat. J Am Soc Nephrol 4: 1046–1053, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Touyz RM, Mercure C, He Y, Javeshghani D, Yao G, Callera GE, Yogi A, Lochard N, Reudelhuber TL. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension 45: 530–537, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Usui M, Egashira K, Kitamoto S, Koyanagi M, Katoh M, Kataoka C, Shimokawa H, Takeshita A. Pathogenic role of oxidative stress in vascular angiotensin-converting enzyme activation in long-term blockade of nitric oxide synthesis in rats. Hypertension 34: 546–551, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol 582–593, 2006. [DOI] [PubMed]
- 41.Welch WJ, Tojo A, Wilcox CS. Roles of NO and oxygen radicals in tubuloglomerular feedback in SHR. Am J Physiol Renal Physiol 278: F769–F776, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Wilcox CS, Welch WJ. Interaction between nitric oxide and oxygen radicals in regulation of tubuloglomerular feedback. Acta Physiol Scand 168: 119–124, 2000. [DOI] [PubMed] [Google Scholar]