Abstract
Regulation of the VEGF-VEGF receptor system was examined in the urinary bladder after acute (2–48 h) and chronic (10 days) cyclophosphamide (CYP)-induced cystitis. ELISAs demonstrated significant (P ≤ 0.01) upregulation of VEGF in whole urinary bladder with acute and chronic CYP-induced cystitis; however, the magnitude of increase was greater after acute (2–4 h) cystitis. Immunohistochemistry for VEGF immunoreactivity revealed a significant (P ≤ 0.05) increase in VEGF immunoreactivity in the urothelium, suburothelial vasculature, and detrusor smooth muscle with acute (4 and 48 h) CYP treatment. RT-PCR identified the isoform VEGF-164, the VEGF receptor VEGFR-2, and the VEGF co-receptors neuropilin (Npn)-1 and Npn-2 in the urinary bladder. Quantitative PCR demonstrated upregulation of VEGF-164 transcript with acute and chronic CYP-induced cystitis, but VEGFR-2, Npn-1, and Npn-2 transcripts were upregulated (P ≤ 0.01) in whole bladder only with chronic CYP-induced cystitis. Additional studies demonstrated regulation of VEGF transcript expression in the urinary bladder by nerve growth factor (NGF) in a novel line of NGF-overexpressing mice. These studies demonstrated that urinary bladder inflammation and NGF regulate the VEGF-VEGF receptor system in the urinary bladder. Functional role(s) for the VEGF-VEGF receptor system in urinary bladder inflammation remain to be determined.
Keywords: urothelium, quantitative polymerase chain reaction, immunohistochemistry, detrusor smooth muscle, vasculature, nerve growth factor
vascular endothelial growth factor (VEGF) is a pleiotropic cytokine known for its angiogenic and potent vascular permeability-enhancing activity, but additional roles have been described (18, 37, 47). Trophic effects on neurons and vascular cells have resulted in the demonstration of VEGF-induced neuroprotection in animal models of peripheral or central injury or neurodegenerative diseases (30, 37, 47, 60). Cross talk between the cardiovascular and nervous system has been demonstrated, with VEGF demonstrating neurotrophic effects and nerve growth factor (NGF) demonstrating angiogenic effects (37, 47). In addition, proinflammatory effects of VEGF in inflammatory bowel disease (IBD) have been suggested (15, 23, 33). In humans, multiple isoforms of VEGF-A (VEGF-121, VEGF-145, VEGF-165, VEGF-189, and VEGF-206) have resulted from differential alternative splicing; the corresponding murine forms are shorter by one amino acid (18). VEGF-165 is the most predominant isoform in a number of tissues and is the most potent proinflammatory isoform (6, 18, 53). VEGF signals mainly through two receptor tyrosine kinases: VEGFR-1 [or Fms-like tyrosine kinase-1 (Flt1)] and VEGFR-2 [or fetal liver kinase (Flk-1)/kinase-insert domain receptor] (18). Receptor binding by VEGF results in receptor autophosphorylation and phosphorylation of downstream effectors, including phospholipase Cγ, small GTP-binding proteins, and recruitment of adaptor proteins to the receptor (44). More recently, the neuropilins (Npn-1 and Npn-2) have been identified as co-receptors for specific VEGF isoforms (10, 20).
Patients with interstitial cystitis (IC) or painful bladder syndrome, a painful, chronic urinary bladder inflammation syndrome, exhibit urinary frequency, urgency, and suprapubic and pelvic pain at low-to-moderate bladder pressure (46). Although the etiology and pathogenesis of IC/painful bladder syndrome are unknown, numerous theories, including infection, autoimmune disorder, toxic urinary agents, deficiency in bladder wall lining, and neurogenic causes, have been proposed (17, 24, 28, 46, 51). Numerous studies involving a chemically [cyclophosphamide (CYP)] induced bladder inflammation model (14, 36, 40, 55, 57, 58) have demonstrated alterations in neurochemical (54, 55, 58, 66), electrophysiological (27, 64), organizational (55, 59), and functional properties of bladder afferent neurons in dorsal root ganglia and central reflex micturition pathways, as well as changes in urinary bladder function (13, 25, 26). Neurotrophins, including NGF, have been implicated in mediation of some of these changes (13, 25, 26, 35, 62, 63).
Recent studies have suggested that the VEGF-VEGF receptor system may participate in the pathogenesis of IC/painful bladder syndrome (7, 49, 52) and other inflammatory conditions, including IBD (11, 15, 23, 33). Intravesical corticotropin- releasing hormone and acute restraint stress induce mast cell-dependent VEGF release from bladder explants (7). VEGF can bind and stimulate inflammatory cells (43), and proinflammatory cytokines (e.g., IL-1β and TNFα) can induce VEGF protein expression (2, 8, 43). In addition, bladder biopsies from IC patients with glomerulations following hydrodistension show increased expression of VEGF protein (52). Previous studies have demonstrated upregulation of VEGFR-1 in urinary bladder after LPS-induced bladder inflammation in the mouse (50). More recent studies have demonstrated the presence, accessibility, and functionality of VEGFR-1, VEGFR-2, and Npns in urothelial cells from control and inflamed mouse bladder (49). Although VEGF has been implicated in the pathogenesis of IBD (15, 23, 33), less is known about the potential contribution of VEGF to IC/painful bladder syndrome. The present study used a well-characterized rat model of urinary bladder inflammation to examine the distribution of the VEGF-VEGF receptor system in the urinary bladder and its regulation with CYP-induced cystitis of varying duration. These studies lay the groundwork for future studies to determine the role(s) of the VEGF-VEGF receptor system in the pathogenesis of bladder inflammation and plasticity of the micturition reflex.
In the present study, we determined 1) VEGF protein expression in rat urinary bladder by ELISAs at various durations of CYP treatment, 2) urothelial, suburothelial vasculature, and detrusor smooth muscle expression and regulation of VEGF immunoreactivity (IR) after various durations of CYP-induced cystitis in the rat, 3) VEGF isoforms and VEGF receptor (VEGFR-1, VEGFR-2, Npn-1, and Npn-2) transcript expression and regulation in inflamed bladder by RT-PCR and quantitative PCR, and 4) VEGF transcript expression in urinary bladder isolated from control and CYP-treated mice with NGF overexpression under the control of the uroplakin II promoter.
MATERIALS AND METHODS
Rats.
Adult female Wistar rats (160–180 g; Charles River, St. Constant, PQ, Canada) were housed two per cage and maintained in standard laboratory conditions with free access to food and water. The University of Vermont Institutional Animal Care and Use Committee approved all animal use procedures (Protocol 06-014).
NGF-overexpressing mice.
NGF-overexpressing (OE) mice were generated at Roche Palo Alto (material transfer agreement with Roche Palo Alto and Dr. Debra Cockayne) in collaboration with Dr. Henry Sun (New York University Medical School). Briefly, the BamH I-BglI I NGF cDNA fragment encoding the short NGF transcript was inserted in a pBS SKII+ vector between the uroplakin II promoter (UPII) and a β-globin polyA sequence. The entire UPII-NGF-β-globin polyA transgene was excised en bloc from the plasmid as a Not I fragment, purified, and microinjected into the pronuclei of fertilized C57BL/6J embryos. Animal genotype was confirmed by Southern and/or PCR analyses; all mice have the inbred genetic C57BL/6J background and were derived from F2–F4 generations maintained through a hemizygous backcross strategy with C57BL/6J wild-type (WT) mice. Female mice were bred locally at the University of Vermont. The litters were of normal size, and weight and behaviors (feeding, drinking, and activity patterns) appeared normal. Use of NGF-OE and littermate WT mice was limited to the determination of VEGF transcript regulation in the urinary bladder under basal conditions and with acute (4 h) CYP-induced cystitis.
Induction of CYP-induced cystitis.
Rats and mice were anesthetized with isoflurane (2%), and acute cystitis was induced with a single injection of CYP (150 mg/kg ip). Rodents were used in studies at 2, 4, 6, and 48 h after treatment (25, 26). Chronic CYP cystitis was induced by administration of CYP (75 mg/kg ip) once every 3 days for 10 days (25, 26). Control rodents were injected with saline or received no treatment.
Preparation of tissue samples for ELISAs.
Tissue processing and ELISAs were performed as described previously (34, 65). Briefly, rats from all experimental groups (n = 6 each) were deeply anesthetized (4% isoflurane), and a thoracotomy was performed. Individual rat bladders were dissected, weighed, solubilized in Tissue Protein Extraction Reagent (1 g tissue/20 ml; Pierce Biotechnology, Woburn, MA) with Complete protease inhibitor cocktail tablets (Roche Applied Science, Mannheim, Germany), and stored at −80°C. On the day of assay, individual bladders were disrupted with a Polytron homogenizer until homogeneous and centrifuged (10,000 rpm for 10 min), and the supernatant was used for total protein estimation and VEGF quantification. Total protein was determined by the Coomassie Plus Protein Assay Reagent Kit (Pierce), and VEGF was quantified using standard 96-well ELISA plates (DuoSet, catalog no. DY564, R & D Systems, Minneapolis, MN) according to the manufacturer's recommendations.
ELISAs for VEGF or NGF in urinary bladder.
The microtiter plates (R & D Systems) were coated with mouse anti-rat VEGF antibody. Sample and standard solutions were run in duplicate. Horseradish peroxidase-streptavidin conjugate was used to detect the antibody complex. Tetramethylbenzidine was the substrate, and the enzyme activity was measured by the change in optical density. The VEGF standard provided with this protocol generated a linear standard curve from 15 to 1,000 pg/ml (R2 = 0.998, P ≤ 0.0001) for bladder samples. The absorbance values of standards and samples were corrected by subtraction of the background value (absorbance due to nonspecific binding). Samples were diluted to bring the absorbance values onto the linear portion of the standard curve. No samples fell below the minimum detection limits of the assay. Curve fitting of standards and evaluation of VEGF content of samples were performed using a least-squares fit. ELISAs for NGF expression in mouse urinary bladder were performed as previously described (57).
Immunohistochemical localization of VEGF in urothelium.
The bladders from control and acute (4 h), intermediate (48 h), and chronic CYP-treated rats (n = 6 in each group) were rapidly dissected, weighed, postfixed in 4% paraformaldehyde, and placed in ascending concentrations (10–30%) of sucrose in 0.1 M PBS for cryoprotection. Cryostat sections (20 μm) of urinary bladder were mounted on gelled (0.5%) microscope slides for on-slide processing, as previously described (13, 34, 65). Briefly, sections were incubated with 400 μl of rabbit anti-VEGF-A (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) in 1% goat serum and 0.1 M phosphate buffer overnight at room temperature. After three 10-min wash cycles with 0.1 M PBS (pH 7.4), the tissues were incubated with Cy3-conjugated donkey anti-goat secondary antibody (1:500 dilution) for 2 h at room temperature. The slides were subjected to three 10-min wash cycles with PBS, and coverslips were applied with Citifluor (Citifluor, London, UK). Control sections incubated in the absence of primary or secondary antibody were also processed and evaluated for specificity or background staining levels. In the absence of primary antibody, no positive immunostaining was observed. The same antiserum used for immunohistochemistry was used in Western blots to verify antibody specificity. With use of bladder tissue homogenates, the VEGF antibody only recognizes the VEGF monomer and dimer forms (p21 and p42, respectively), both of which can be completely absorbed with the appropriate blocking peptide (data not shown).
Visualization and semiquantitative analysis of VEGF IR in urothelium.
VEGF-IR staining in bladder sections was visualized and captured using an Olympus fluorescence photomicroscope, as previously described (13, 34, 65). The filter was set with an excitation range of 560–596 nm and an emission range of 610–655 nm for visualization of Cy3. MetaMorph image analysis software (version 4.5r4, Universal Imaging, Downingtown, PA) was used (13, 34, 65) for semiquantitative analysis of VEGF IR in the urothelium (Fig. 1). Briefly, a predetermined file was used to calibrate each image for specific pixel size. With the help of a free drawing tool, urothelium VEGF-IR-stained area was chosen and measured in total-pixels area (Fig. 2, A, D, G, and J). A threshold encompassing an intensity range of 100–250 gray-scale values was applied to the region of interest (Fig. 2) in the least brightly stained condition first. The threshold was adjusted for each experimental series, with concomitantly processed negative controls used as the guide for setting background fluorescence. The same threshold was subsequently used for all images. VEGF IR was considered to be positive only when it exceeded the established threshold. Percent VEGF expression above threshold in the total area selected was then calculated.
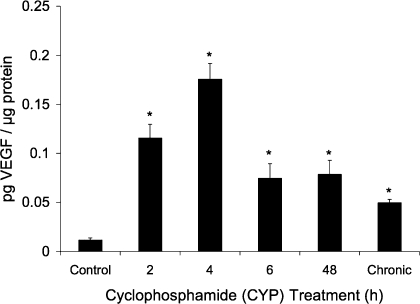
Fig. 1.
VEGF protein expression in the whole bladder with cyclophosphamide (CYP)-induced cystitis measured by ELISAs. Increases in VEGF protein expression were observed at all time points [2–48 h and 10 days (chronic)], but acute (2–4 h) CYP-induced cystitis resulted in a greater-magnitude (9.7–14.7-fold) increase of VEGF protein. *P ≤ 0.01.
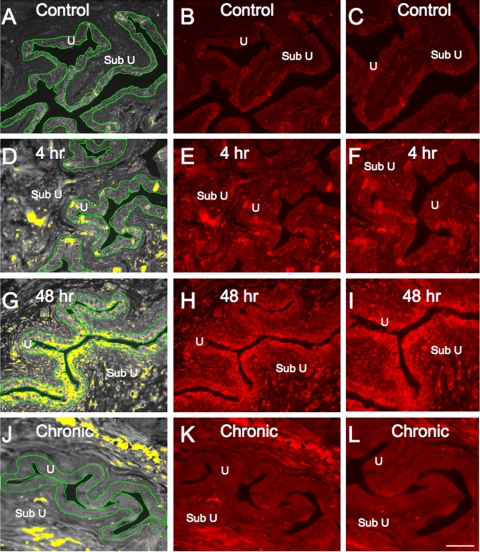
Fig. 2.
VEGF immunoreactivity (IR) in cryostat sections of urothelium after CYP treatment. Urothelium (U) was outlined (green) and measured in total-pixels area (A, D, G, and J). A threshold encompassing an intensity range of 100–250 gray-scale values was applied to the region of interest (A, D, G, and J). The same threshold was subsequently used for all images. Percentage of VEGF expression above threshold in the total area selected was then calculated. CYP (48 h; G–I) significantly (P ≤ 0.05) upregulated percentage of VEGF IR in urothelium (G, yellow stain within urothelium) compared with all other groups. Fluorescence images of VEGF expression in urinary bladder sections of control rats (B), rats treated with CYP for 4 h (E) and 48 h (H), and rats chronically treated with CYP (K) are shown. C, F, I, and L: higher-power fluorescence images of B, E, H, and K, respectively. For all images, exposure times were held constant and all tissues were processed simultaneously. In rats treated with CYP for 4 and 48 h, VEGF expression was visible in urothelium (D–F and G–I), whereas control rats (A–C) and rats chronically treated with CYP (J–L) showed little or no VEGF IR. Some VEGF IR was observed in suburothelial (Sub U) region at each time point of CYP-induced cystitis (D–L). Scale bars, 50 μm (A, B, D, E, G, H, J, and K) and 25 μm (C, F, I, and L).
Assessment of immunohistochemical staining in urinary bladder regions.
Immunohistochemistry and subsequent semiquantification of VEGF IR in bladder sections or whole-mount preparations were performed on control and experimental tissues simultaneously to reduce the incidence of staining variation that can occur between tissues processed on different days. Staining in experimental tissue was compared with that in experiment-matched negative controls. Urinary bladder sections or whole mounts exhibiting IR that was greater than the background level in experiment-matched negative controls were considered positively stained.
Immunohistochemical localization of VEGF in detrusor smooth muscle and suburothelial vasculature in urinary bladder whole mounts.
The urinary bladder from control (n = 5) and experimental treatments (n = 5 each) was dissected and placed in Krebs solution. The bladder was cut open along the midline and pinned to a Sylgard-coated dish. While the bladder was pinned, the bladder neck on one side was notched for orientation purposes and regional analyses of IR. The bladder was incubated for 1.5 h at room temperature in cold fixative (2% paraformaldehyde + 0.2% picric acid). Fine-tip forceps and iris scissors were used to dissect the urothelium from the underlying detrusor smooth muscle with the aid of a dissecting microscope (13, 34, 65, 67). Urothelium and bladder musculature were processed for VEGF IR (see above). Whole mounts stained for VEGF were also treated with YOYO-1 (1:10,000 dilution; Molecular Probes, Eugene, OR) to stain RNA and visualize cell nuclei. In some whole mounts processed for VEGF IR, nerve fibers in the suburothelial nerve plexus were also stained with the pan-neuronal marker protein gene product (1:15 dilution; PGP9.5, Abcam, Cambridge, MA). No VEGF-IR nerve fibers (PGP-positive) were observed in any preparation from control or CYP-treated rats (data not shown). After they were washed, the whole mounts were placed on microscope slides, and coverslips were applied (see above).
Visualization and semiquantitative analysis of VEGF IR in suburothelial vasculature and detrusor smooth muscle.
Whole mounts from control (n = 5) and experimental groups (n = 5 each) were examined under an Olympus fluorescence photomicroscope (see above). Cy3 was visualized with a filter with an excitation range of 560–596 nm and an emission range of 610–655 nm. YOYO-1 was visualized with a filter with an excitation range of 470–490 nm and an emission range of 510–530 nm. Semiquantification of VEGF expression in the suburothelial vasculature and detrusor smooth muscle was performed as previously described (31) and modified from Brady et al. (5). Gray-scale images acquired in tiff format were imported into ImageJ (1), and images were thresholded. Images were acquired from the bladder neck of control and treated rats for suburothelial vasculature analyses and from the bladder dome, body, and neck of control and treated rats for detrusor analyses. A rectangle of fixed dimension (500 × 500 pixels) was placed on the section according to a random selection of x and y coordinates. This process was repeated seven times for each image of detrusor or suburothelial vasculature. The average optical density of VEGF IR of detrusor smooth muscle or suburothelial vasculature was then calculated.
RNA extraction, RT, and PCR.
Total RNA was extracted from the bladder of control and CYP-treated (2, 4, 6, and 48 h and chronic) rats (n = 5 each) and NGF-OE and littermate WT mice (n = 5 each) with RNA/mRNA STAT-60 isolation reagent (Tel-Test, Friendswood, TX) and reverse transcribed as described previously (22, 32, 45). Briefly, from 2 μg of total RNA, first-strand cDNA was synthesized using SuperScript reverse transcriptase and oligo(dT) primers with the SuperScript II Preamplification Kit (Invitrogen, Carlsbad, CA). Amplification of cDNA was performed with AmpliTaq DNA polymerase (Applied Biosystems, Norwalk, CT) using oligonucleotide primers specific for rat VEGF, VEGFR-1, VEGFR-2, Npn-1, Npn-2, and β-actin (45). Oligonucleotide primer sequences for mouse NGF were as follows: AGTGAGGTGCATAGCGTAAT (upper, mNGF 279U20) and AGTGGAGTCTCCGTTTCTTA (lower, mNGF 527L20). Amplification of cDNA was performed according to the following parameters: initial denaturation and enzyme activation at 94°C for 10 min, denaturation at 94°C for 30 s, annealing at primer-specific annealing temperature for 30 s, extension at 72°C for 45 s with 30–35 cycles, and final extension at 72°C for 5 min. PCR products were resolved by 1.6% agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV light. Routine controls included cDNA synthesis in the absence of RNA or reverse transcriptase and amplification with omission of template or primers.
Real-time quantitative RT-PCR.
Total RNA was extracted using the STAT-60 total RNA/mRNA isolation reagent (Tel-Test), as previously described (22, 32). cDNA was synthesized from 1–2 μg of RNA per sample using SuperScript II reverse transcriptase and random hexamer primers with the SuperScript II Preamplification System (Invitrogen) in a 20-μl final reaction volume.
The quantitative PCR standards for all transcripts were prepared with the amplified rat VEGF, VEGFR-1, VEGFR-2, Npn-1, Npn-2, L32, 18S, and L32 cDNA products ligated directly into pCR2.1 TOPO vector using the TOPO TA cloning kit (Invitrogen). The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility). For estimation of the relative expression of the receptor transcripts, 10-fold serial dilutions of stock plasmids were prepared as quantitative standards. The range of standard concentrations was determined empirically.
Real-time quantitative PCR was performed using SYBR Green I detection (22, 32). cDNA templates, diluted fivefold to minimize the inhibitory effects of the RT reaction components, were assayed using SYBR Green I JumpStart Taq ReadyMix (Sigma-Aldrich, St. Louis, MO) containing 5 mM MgCl2; dATP, dGTP, dCTP, and dTTP (200 mM each); 0.64 U of Taq DNA polymerase; and primer (300 nM each) in a final 25-μl reaction volume. The real-time quantitative PCR was performed on an real-time PCR system (7500 Fast, Applied Biosystems, Foster City, CA) (22, 32) as follows: 1) 94°C for 2 min and 2) amplification over 40 cycles at 94°C for 15 s and at 58–62°C (depending on the primer set) for 30 s.
SYBR Green I melting analysis of the amplified product from these amplification parameters was carried out by ramping the temperature of the reaction samples from 60°C to 95°C. A single DNA melting profile demonstrating amplification of a single unique product free of primer dimers or other anomalous products was observed under these dissociation assay conditions.
For data analyses, a standard curve was constructed by amplification of serially diluted plasmids containing the target sequence. Data were analyzed at the termination of each assay using Sequence Detection Software version 1.3.1 (Applied Biosystems). In standard assays, default baseline settings were selected. The increase in SYBR Green I fluorescence intensity was plotted as a function of cycle number, and the threshold cycle was determined by the software as the amplification cycle at which the change in SYBR Green I fluorescence intensity first intersects the established baseline. All data are expressed as the relative quantity of the gene of interest normalized to the relative quantity of the housekeeping gene L32 for rat samples or 18S for mouse samples. Control samples are set equal to 100%.
Split bladder preparation and assessment of potential contamination of bladder layers.
VEGF and NGF mRNA and NGF protein expression were determined in the urothelium/suburothelium and detrusor smooth muscle layers of the urinary bladder of NGF-OE and WT mice. The urothelium/suburothelium was dissected from the detrusor smooth muscle with use of fine-tip forceps under a dissecting microscope, as previously described (13, 31). To confirm the specificity of our split bladder preparations, we examined urothelium/suburothelium and detrusor samples for the presence of α-smooth muscle actin (1:1,000 dilution; Abcam) and uroplakin II (1:25 dilution; American Research Products, Belmont, MA) by Western blotting or RT-PCR (31). In urothelium/suburothelium layers, only uroplakin II was present. Conversely, in detrusor samples, only α-smooth muscle actin was present. In the urothelium/suburothelium layer, the suburothelial vasculature exhibits vascular reactivity in response to agonist application, and spontaneous and field stimulation induced Ca2+ flashes and Ca2+ waves (J. Layne and M. T. Nelson, personal communication). Thus the suburothelial vasculature retains function after dissection from the detrusor smooth muscle.
Materials.
All standard chemicals (analytic or laboratory grade) were obtained from Sigma-Aldrich or Fisher.
Figure preparation.
Digital images were obtained using a charge-coupled device camera (MagnaFire SP, Optronics, Optical Analysis, Nashua, NH) and LG-3 frame grabber attached to an Olympus microscope (Optical Analysis). Exposure times were held constant during acquisition of images from control and experimental animals processed and analyzed on the same day. Images were imported into Photoshop 7.0 (Adobe Systems, San Jose, CA), where groups of images were assembled and labeled.
Statistics.
Values are means ± SE. Data were compared using ANOVA. Percentages from image analysis were arcsin transformed to meet the requirements of this statistical test. Animals, processed and analyzed on the same day, were tested as a block in the ANOVA. When F ratios exceeded the critical value (P ≤ 0.05), Dunnett's post hoc test was used to compare the control means with each experimental mean.
RESULTS
VEGF protein expression in urinary bladder with CYP-induced cystitis.
VEGF protein expression in whole urinary bladders as determined by ELISAs significantly (P ≤ 0.01) increased at all time points of CYP treatment (Fig. 1). Acute (2–4 h) CYP treatment exhibited the greatest (9.7- to 14.7-fold) increase in VEGF protein expression in the urinary bladder. CYP treatment from 6 h (acute) to 10 days (chronic) resulted in comparable VEGF expression in the urinary bladder (4.2- to 6.6-fold increase; Fig. 1).
VEGF IR in urothelium with CYP-induced cystitis.
The expression of VEGF IR was very weak in the urothelium of the urinary bladder from control (Figs. 2 and 3) rats. With acute (4 h) CYP treatment, some diffuse VEGF IR was observed in the urothelium and lamina propria (Fig. 2). Significant (P ≤ 0.05) increases in VEGF IR in the urothelium were observed 48 h after CYP treatment, and more obvious VEGF IR was also observed in the lamina propria (Figs. 2 and 3). The increased expression of VEGF IR in the urothelium was not maintained with chronic CYP treatment, where expression of VEGF IR returned to the level observed in control (noninflamed) rats (Figs. 2 and 3). No regional differences in VEGF IR in the urothelium of the dome, body, or neck of the urinary bladder were observed in control or CYP-treated rats. VEGF IR was observed in all cell layers (apical, intermediate, and basal) of the urothelium.
Fig. 3.
Semiquantitative analysis of VEGF IR in urothelium with CYP-induced cystitis. Values are means ± SE (n = 6 for each group). *P ≤ 0.05.
VEGF IR in suburothelial vascular bed with CYP-induced cystitis.
In whole-mount preparations, VEGF IR was observed in a suburothelial vascular bed throughout the urinary bladder. The density of the VEGF-IR vascular bed was greatest in the neck region, and our analysis of CYP-induced effects was restricted to this region. A significant (P ≤ 0.01) increase in the density of the VEGF-IR vasculature in the bladder neck region was observed with 4 h (4.8-fold increase) and 48 h (6.1-fold increase) of CYP treatment (Fig. 4). No changes in the VEGF-IR vasculature were observed with chronic CYP treatment (Fig. 4). The pattern of VEGF IR in the vascular bed was distinct, and no PGP9.5 staining of the vascular bed was observed (data not shown). No VEGF IR was observed in the suburothelial nerve plexus (PGP9.5-positive) in any control or CYP-treated group.
Fig. 4.
Fluorescence images of VEGF IR in suburothelial vasculature in the bladder neck region in whole-mount preparations of urinary bladder in control rats (A and B) and rats treated with CYP for 4 h (C and D) and 48 h (E and F). VEGF IR in the vasculature in the neck region increased in density with CYP-induced cystitis (4 and 48 h). Insets in A, C, E are shown in B, D, and F, respectively. Scale bar, 80 μm. G: increase in density of VEGF IR in suburothelial vasculature with CYP-induced cystitis. OD, optical density. Values are means ± SE. *P ≤ 0.01.
VEGF IR in detrusor smooth muscle with CYP-induced cystitis.
In whole-mount preparations of the urinary bladder from control rats, detrusor smooth muscle expression of VEGF IR was weak in all bladder regions (dome, body, and neck; Fig. 5). A significant (P ≤ 0.01) increase in VEGF IR in detrusor smooth muscle was observed in all urinary bladder regions examined at 4 h (8-fold increase) and 48 h (9.2-fold increase) of CYP-induced cystitis (Fig. 5). No changes in VEGF IR in detrusor smooth muscle were observed with chronic CYP-induced cystitis (Fig. 5).
Fig. 5.
Fluorescence images of VEGF IR in detrusor smooth muscle in the bladder neck region in whole-mount preparations of urinary bladder in control rats (A and B) and rats treated with CYP for 4 h (C and D) and 48 h (E and F). VEGF IR in the detrusor smooth muscle increased in density with CYP-induced cystitis (4 and 48 h). Insets in A, C, and E are shown in B, D, and F, respectively. Detrusor smooth muscle nuclei were stained with YOYO-1 (yellow-green, B and F). Scale bar, 80 μm. G: increase in density of VEGF IR in detrusor smooth muscle with CYP-induced cystitis. Values are means ± SE. *P ≤ 0.01.
VEGF-164, VEGFR-1, VEGFR-2, Npn-1, and Npn-2 mRNA expression in whole bladder of rats with and without CYP-induced cystitis.
In screening studies, RT-PCR analyses demonstrated that, among the major isoforms of VEGF, only VEGF-164 mRNA was detected in the whole urinary bladder of control and CYP-treated rats at all time points (Fig. 6). VEGFR-1, VEGFR-2, Npn-1, and Npn-2 transcripts were present in whole urinary bladder (Figs. 7–9). The regulation of VEGF-164, VEGFR-2, Npn-1, and Npn-2 transcripts was subsequently examined by quantitative PCR (Figs. 7–9). VEGFR-1 was not examined by quantitative PCR, inasmuch as screening studies suggested the absence of regulation in the urinary bladder with CYP-induced cystitis. Quantitative PCR demonstrated a significant (P ≤ 0.01) increase in VEGF mRNA in whole urinary bladder with 6 h of CYP treatment and chronic CYP-induced cystitis (Fig. 6). No changes in VEGF mRNA in whole urinary bladder with 2 and 4 h of CYP treatment were demonstrated (Fig. 6). VEGFR-2, Npn-1, and Npn-2 mRNA expression in whole urinary bladder were regulated similarly with CYP-induced cystitis and demonstrated a significant (P ≤ 0.01) increase in transcript expression only with chronic CYP-induced cystitis (Figs. 7–9). Shorter durations of CYP treatment did not regulate VEGFR-2, Npn-1, or Npn-2 mRNA expression in the urinary bladder (Figs. 7–9).
Fig. 6.
Regulation of urinary bladder VEGF-164 transcript level in control rats, after 2–6 h of CYP treatment, and after chronic (10 day) CYP-induced bladder inflammation. Top: semiquantitative RT-PCR showing urinary bladder expression of VEGF-164 transcript from control and CYP-treated rats. Bottom: VEGF transcript expression as a percentage of control and normalized to expression of the housekeeping gene L32 obtained from quantitative PCR. Values are means ± SE (n = 5 for each group). *P ≤ 0.01 vs. control.
Fig. 7.
Regulation of urinary bladder VEGF receptor (VEGFR-2) transcript level in control rats, after 2–6 h of CYP treatment, and after chronic (10 day) CYP-induced bladder inflammation. Top: semiquantitative RT-PCR showing urinary bladder expression of VEGFR-2 transcript from control and CYP-treated rats. Bottom: VEGFR-2 transcript expression as a percentage of control and normalized to expression of the housekeeping gene L32 obtained from quantitative PCR. Values are means ± SE (n = 5 for each group). *P ≤ 0.01 vs. control.
Fig. 8.
Regulation of urinary bladder VEGF co-receptor neuropilin-1 (Npn-1) transcript level in control rats, after 2–6 h of CYP treatment, and after chronic (10 day) CYP-induced bladder inflammation. Top: semiquantitative RT-PCR showing urinary bladder expression of Npn-1 transcript from control and CYP-treated rats. Bottom: Npn-1 transcript expression as a percentage of control and normalized to expression of the housekeeping gene L32 obtained from quantitative PCR. Values are means ± SE (n = 5 for each group). *P ≤ 0.01 vs. control.
Fig. 9.
Regulation of urinary bladder VEGF co-receptor neuropilin-2 (Npn-2) transcript level in control rats, after 2–6 h of CYP treatment, and after chronic (10 day) CYP-induced bladder inflammation. Top: semiquantitative RT-PCR showing urinary bladder expression of Npn-2 transcript from control and CYP-treated rats. Bottom: Npn-2 transcript expression as a percentage of control and normalized to expression of the housekeeping gene L32 obtained from quantitative PCR. Values are means ± SE (n = 5 for each group). *P ≤ 0.01 vs. control.
Characterization of NGF expression in urinary bladder of NGF-OE mice.
Quantitative PCR and ELISAs were used to determine NGF mRNA and protein expression in the urothelium/suburothelium and detrusor layers of the urinary bladder in NGF-OE and WT littermate mice (Fig. 10). Quantitative PCR revealed a 230-fold increase in NGF mRNA expression in urothelium/suburothelium of NGF-OE mice compared with WT mice (Fig. 10A). NGF mRNA expression was similar in detrusor of NGF-OE and WT mice (Fig. 10A). NGF protein expression was significantly (P ≤ 0.001) increased in whole urinary bladder from NGF-OE mice compared with WT mice (Fig. 10B). NGF protein expression was significantly (P ≤ 0.001) greater in urothelium/suburothelium from NGF-OE mice than WT mice (Fig. 10B). NGF protein expression was similar in detrusor layers of NGF-OE and WT mice (Fig. 10B).
Fig. 10.
Characterization of nerve growth factor (NGF) mRNA and protein expression in urinary bladder from NGF-overexpressing (OE) mice by quantitative PCR and ELISAs. A: NGF transcript expression in urothelium/suburothelium and detrusor layers in NGF-OE and wild-type (WT) mice normalized to expression of the housekeeping gene 18S. Values are means ± SE (n = 5 for each group). *P ≤ 0.001: NGF-OE urothelium/suburothelium vs. WT urothelium/suburothelium and detrusor (with horizontal bars) and within NGF-OE bladder layers (without horizontal bars). B: NGF protein expression in whole urinary bladder, urothelium/suburothelium, and detrusor layers in NGF-OE and WT mice. Values are means ± SE (n = 5–10 for each group). *P ≤ 0.001: urothelium/suburothelium vs. detrusor from NGF-OE mice (with horizontal bars) and NGF-OE vs. WT (without horizontal bars).
VEGF mRNA expression in urothelium/suburothelium and detrusor smooth muscle in NGF-OE and WT mice and regulation with acute (4 h) CYP-induced cystitis.
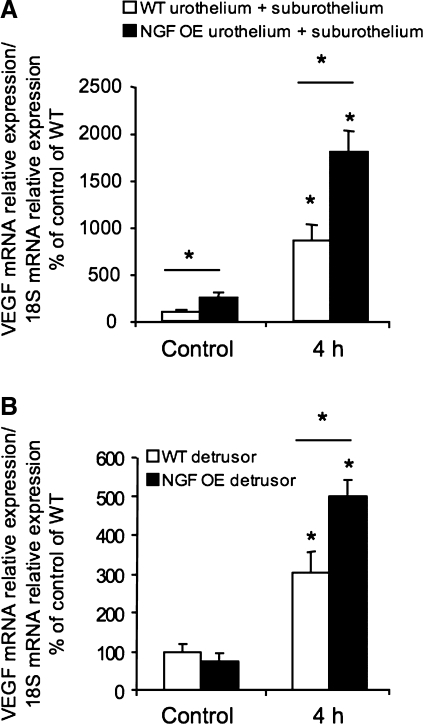
VEGF mRNA expression was determined by quantitative PCR in the urothelium/suburothelium and detrusor smooth muscle of NGF-OE and WT mice without inflammation (control) or after acute (4 h) CYP treatment. Basal expression of VEGF mRNA was significantly (P ≤ 0.05) greater in the urothelium/suburothelium of control NGF-OE mice than littermate WT mice (Fig. 11A). In detrusor smooth muscle, no differences in basal VEGF mRNA expression were observed between NGF-OE and WT mice (Fig. 11B). With acute (4 h) CYP-induced cystitis, a significant (P ≤ 0.05) increase in VEGF mRNA was observed in the urothelium/suburothelium in NGF-OE and WT mice (Fig. 11A). However, with 4 h of CYP treatment, VEGF mRNA expression in the urothelium/suburothelium was significantly (P ≤ 0.05) greater in NGF-OE mice than in WT mice (Fig. 11A). With 4 h of CYP treatment, there was also a significant (P ≤ 0.05) increase in VEGF mRNA in detrusor smooth muscle of NGF-OE and WT mice (Fig. 11B). However, the VEGF mRNA expression was significantly (P ≤ 0.05) greater in the detrusor of NGF-OE mice than WT mice (Fig. 11B).
Fig. 11.
Regulation of urinary bladder VEGF-164 transcript level in urothelium/suburothelium (A) and detrusor (B) in control mice and 4 h after CYP-induced bladder inflammation in NGF OE and WT mice. A: VEGF transcript expression in urothelium/suburothelium of WT and NGF-OE mice as a percentage of control and normalized to expression of the housekeeping gene 18S obtained from quantitative PCR. Values are means ± SE (n = 5 for each group). *P ≤ 0.05: control vs. 4 h (without horizontal bars) and NGF OE vs. WT (with horizontal bars). B: VEGF transcript expression in detrusor of WT and NGF-OE mice as a percentage of control and normalized to expression of the housekeeping gene 18S obtained from quantitative PCR. Values are means ± SE (n = 5 for each group). *P ≤ 0.05: control vs. 4 h (without horizontal bars) and NGF-OE vs. WT (with horizontal bars).
DISCUSSION
These studies demonstrate several novel findings with respect to the VEGF-VEGF receptor system in the urinary bladder and its modification with CYP-induced cystitis of varying duration. VEGF protein expression in the urothelium, suburothelial vasculature, and detrusor smooth muscle increases most dramatically with acute (2–48 h) CYP-induced cystitis. VEGF protein and transcript expression in the whole urinary bladder is significantly increased with acute (2–48 h) and chronic CYP-induced cystitis, with a greater magnitude of protein increase with acute cystitis. In contrast, VEGFR-2, Npn-1, and Npn-2 transcript expression in the whole urinary bladder is significantly increased with chronic CYP-induced cystitis. NGF overexpression in the urothelium under the control of the uroplakin II promoter results in a significant increase in basal and acute CYP-induced expression of VEGF mRNA. These studies have identified some key elements of the VEGF-VEGF receptor system in the urinary bladder and regulation with CYP-induced cystitis. VEGF has been demonstrated to have a proinflammatory role and to confer neuroprotection in certain systems with neural injury or disease (18, 30, 37, 47, 60). Future studies are needed to address the functional contribution of the VEGF-VEGF receptor system to anatomic and functional plasticity of the micturition reflex after urinary bladder inflammation.
IC/painful bladder syndrome is a chronic inflammatory bladder disease syndrome characterized by urinary frequency, urgency, and suprapubic and pelvic pain (17, 46). Altered visceral sensations from the urinary bladder (i.e., pain at low or moderate bladder filling) that accompany IC/painful bladder syndrome (17, 24, 28, 46, 51) may be mediated by many factors, including changes in the properties of peripheral bladder afferent pathways, such that bladder afferent neurons respond in an exaggerated manner to normally innocuous stimuli (allodynia). These changes may be mediated, in part, by inflammatory changes in the urinary bladder. Among potential mediators of inflammation, neurotrophins (e.g., NGF) (3, 16, 61), as well as proinflammatory cytokines, have been implicated in this sensitization process.
VEGF expression is regulated in various tissues by numerous factors, including hypoxia, inflammatory mediators, hormonal milieu, cyclooxygenase-2 (COX-2), and mechanical forces, and in tumors by oncogenes and tumor suppressor genes (18, 37, 47, 48). Previous studies have demonstrated upregulation of COX-2 and numerous inflammatory mediators in the urinary bladder with CYP-induced cystitis in the rat (21, 31, 41). Robust changes in a number of urinary bladder cytokines, including IL-1β, IL-2, IL-4, and IL-6, and more modest changes in TNFα or TNFβ with CYP-induced cystitis have also been demonstrated (41). Most recently, we demonstrated upregulation of the chemokine, fractalkine, and fractalkine receptor in the urinary bladder and, specifically, in the urothelium with CYP-induced cystitis (65). Recent studies also demonstrated the regulation of numerous urinary bladder cytokines, chemokines, and associated receptors in CYP-induced cystitis in mice (21). In addition, the present study also demonstrates increased expression of VEGF mRNA in the urothelium/suburothelium in mice with NGF overexpression under the control of the uroplakin II promoter under basal conditions and a greater increase in VEGF mRNA in the urothelium/suburothelium after acute (4 h) CYP-induced cystitis. The NGF-OE mouse exhibits a 230-fold increase in NGF mRNA in the urothelium/suburothelium compared with detrusor smooth muscle, together with a significant increase in NGF protein expression in the urothelium/suburothelium compared with the detrusor layer. Recent studies demonstrated a number of roles for VEGF in the nervous system, in addition to angiogenesis, including neurotrophic, neurotropic, and neuroprotection roles (37, 47). In addition, NGF is also an angiogenic factor (37, 47). Thus cross talk between VEGF and NGF has been suggested through interactions with TrkA and VEGFR-2 (37, 47). However, the present understanding of this cross talk is limited, and additional investigation is necessary. Increased expression of VEGF transcript in the detrusor smooth muscle with acute CYP-induced cystitis in the NGF-OE mouse compared with the WT mouse may reflect interactions between the urothelium with NGF overexpression and inflammatory infiltrates, interstitial cells, myofibroblasts, and/or detrusor smooth muscle. Thus NGF, proinflammatory cytokines, chemokines, and COX-2 activation in the inflamed urinary bladder may also contribute to urinary bladder VEGF expression. The NGF-OE mouse model will enable us to continue to explore NGF and VEGF interactions at the level of the urinary bladder, including determining whether NGF-expressing cells in the urinary bladder also express VEGF. In addition, increases in VEGF mRNA in the urinary bladder were demonstrated at an earlier (4 h) time point after CYP treatment in the mouse studies (NGF-OE and WT) than in the rat studies, suggesting species differences in response to CYP-induced cystitis.
The contribution of the VEGF-VEGF receptor system to the pathogenesis of IC/painful bladder syndrome is not known, but an involvement in IBD has been suggested (15, 23, 33). Changes in VEGF expression have been reported in bladder biopsies from areas of glomerulations in IC/painful bladder syndrome patients (52). Previous studies demonstrated that VEGF signaling is a key mechanism downstream of protease-activated receptor activation in response to proinflammatory stimulation of the urinary bladder (50). More recently, functional VEGF receptors (VEGFR-1 and VEGFR-2) and co-receptors (Npn-1 and Npn-2) in mouse urothelial cells and upregulation with protease-activated receptor activation have been demonstrated (49). We have extended these studies by demonstrating regulation of VEGF receptor with varying duration of CYP-induced cystitis, as well as by demonstrating expression of VEGF protein in the urothelium, suburothelial vasculature, and detrusor smooth muscle with CYP-induced cystitis. Although the tissue and cellular distribution of VEGF receptors and co-receptors was not addressed in the present study, recent studies demonstrated VEGF receptor and VEGF co-receptor immunolocalization to urothelial cells in the mouse (49). In addition, a role for VEGF in IBD has been suggested (11, 15, 23, 33). The potential contribution of VEGF to IBD is not limited to VEGF's well-established role in angiogenesis but also involves roles in proliferation, migration, and recruitment of inflammatory cells leading to the perpetuation of chronic inflammation (15, 23, 33). Future studies are needed to determine whether the VEGF-VEGF receptor system may also perpetuate bladder inflammation in IC/painful bladder syndrome.
In recent years, the functional contribution of the urothelial lining of the urinary bladder has advanced beyond the view that the urothelium is a passive barrier to the notion that it is an active sensor with a potential signaling (i.e., sensory) role, especially in the context of urinary bladder inflammation (4). Although previous studies demonstrated the presence of VEGF receptors in the urinary bladder (49), the present study demonstrates expression and regulation of VEGF in the urothelium, as well as in the suburothelial vasculature and detrusor smooth muscle, with CYP-induced cystitis. VEGF IR was expressed under control (noninflamed) conditions in the suburothelial vasculature, but expression in the urothelium, suburothelial vasculature, and detrusor smooth muscle was significantly increased with acute (4 and 48 h) CYP-induced cystitis. For unknown reasons, VEGF IR in the suburothelial vascular bed was greatest in the neck region of the urinary bladder. Future studies are needed to determine whether this differential distribution is observed only with VEGF immunostaining or with other vascular markers as well. ELISAs demonstrated increased VEGF protein expression in urinary bladder as early as 2 and 4 h after CYP treatment; however, increased VEGF mRNA expression was observed beginning 6 h after CYP treatment. This difference is likely attributed to greater variability at 2 and 4 h after CYP treatment than at 6 h after CYP treatment; however, reasons underlying this variability are unknown. Increased VEGF expression in the inflamed urinary bladder is not surprising, given the numerous reports that document inflammation-induced upregulation of VEGF (9, 11, 19, 38, 39). Serum and tissue concentrations of VEGF appear to follow periods of IBD activity vs. quiescence (23). Serum concentrations of VEGF and VEGFR-2 correlate to parameters of inflammation and to bone destruction in early arthritis (12) and osteoarthritis (42). VEGF has been implicated in the pathogenesis of diabetic retinopathy (29). Given the wide involvement of VEGF in inflammation, it is viewed as a target to inhibit disease processes.
Targeting VEGF to inhibit progression of inflammatory disease processes is driven by the proinflammatory and angiogenic roles of VEGF-165 and VEGF-164, both of which can contribute to and perpetuate chronic inflammation (11, 18, 19, 37, 47). However, neuroprotective roles of VEGF have also been described (30, 60). Protection from delayed degeneration by VEGF has been demonstrated, and clinical use of VEGF in amyotrophic lateral sclerosis and other neurodegenerative diseases has been suggested (30, 60). The exact role(s) of the VEGF-VEGF receptor system in the context of urinary bladder inflammation is not known, but the time course of VEGF upregulation in the urinary bladder, on the basis of ELISAs and immunohistochemistry, demonstrates greater upregulation of VEGF with acute (2–48 h) cystitis. In contrast, VEGF receptor and co-receptor mRNA are significantly increased with chronic CYP-induced cystitis. The present studies have not examined the urinary bladder for expression of other VEGF family members, including VEGF-C or VEGF-D; expression of these family members in urinary bladder is unknown. Previous studies suggested VEGF involvement in the pathogenesis of arthritis during disease onset (12). Thus, whether inhibiting or augmenting VEGF at the level of the urinary bladder in the context and progression of urinary bladder inflammation should be pursued awaits determination of the functional role(s) of VEGF in the inflamed urinary bladder.
Conclusions.
These studies demonstrate increased VEGF expression in the urothelium, suburothelial vasculature, and detrusor smooth muscle with CYP-induced cystitis. Acute (2–48 h) CYP-induced cystitis significantly increased VEGF expression to a greater extent than chronic CYP-induced cystitis. In contrast, the VEGF receptor VEGFR-2 and the VEGF co-receptors Npn-1 and Npn-2 exhibit increased transcript expression in the urinary bladder after chronic CYP-induced cystitis. Whereas VEGFR-1 transcript expression was observed in whole urinary bladder, regulation with CYP-induced cystitis was not suggested, although LPS-induced cystitis is associated with VEGFR-1 transcript expression (50). In addition, the present studies demonstrate increased basal and acute CYP-induced cystitis-induced expression of VEGF mRNA in the urothelium of NGF-OE mice. It is not known whether the VEGF-VEGF receptor system is exerting angiogenic, proinflammatory, neuroprotective, and/or other role(s) in the urinary bladder with CYP-induced cystitis. In summary, urinary bladder inflammation and NGF can regulate the VEGF-VEGF receptor system in the urinary bladder.
GRANTS
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-051369, DK-060481, and DK-065989 and also by National Institutes of Health Centers for Bioresearch Excellence Program for Research Resources Grant P20 RR-16435.
Acknowledgments
The authors gratefully acknowledge the technical expertise and support provided by the Vermont Cancer Center DNA Analysis Facility.
Present address of B. P. Cheppudira: Department of Pharmacology, University of Illinois at Chicago, Chicago, IL 60612.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int 11: 36–42, 2004. [Google Scholar]
- 2.Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res 13: 2825–2830, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Aβ fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci 19: 859–867, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birder LA Urinary bladder urothelium: molecular sensors of chemical/thermal/mechanical stimuli. Vascul Pharmacol 45: 221–226, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Brady CM, Apostolidis AN, Harper M, Yiangou Y, Beckett A, Jacques TS, Freeman A, Scaravilli F, Fowler CJ, Anand P. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int 93: 770–776, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med 9: 777–794, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao J, Boucher W, Kempuraj D, Donelan JM, Theoharides TC. Acute stress and intravesical corticotropin-releasing hormone induces mast cell dependent vascular endothelial growth factor release from mouse bladder explants. J Urol 176: 1208–1213, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho AT, Souza H, Carneiro AJ, Castelo-Branco M, Madi K, Schanaider A, Silv F, Pereira Junior FA, Pereira MG, Tortori C, Dines I, Carvalho J, Rocha E, Elia C. Therapeutic and prophylactic thalidomide in TNBS-induced colitis: synergistic effects on TNF-α, IL-12 and VEGF production. World J Gastroenterol 13: 2166–2173, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med 7: 425–429, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 19: 547–559, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Chidlow JH Jr, Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol 293: G5–G18, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Clavel G, Bessis N, Lemeiter D, Fardellone P, Mejjad O, Menard JF, Pouplin S, Boumier P, Vittecoq O, Le Loet X, Boissier MC. Angiogenesis markers (VEGF, soluble receptor of VEGF and angiopoietin-1) in very early arthritis and their association with inflammation and joint destruction. Clin Immunol 124: 158–164, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R125–R134, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Cox PJ Cyclophosphamide cystitis—identification of acrolein as the causative agent. Biochem Pharmacol 28: 2045–2049, 1979. [DOI] [PubMed] [Google Scholar]
- 15.Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A, Fiocchi C. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology 130: 2060–2073, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Dray A Inflammatory mediators of pain. Br J Anaesth 75: 125–131, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Driscoll A, Teichman JMH. How do patients with interstitial cystitis present? J Urol 166: 2118–2120, 2001. [PubMed] [Google Scholar]
- 18.Ferrara N Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol 237: 1–30, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Frieri M Advances in the understanding of allergic asthma. Allergy Asthma Proc 28: 614–619, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL. Neuropilin-2 is a receptor for semaphoring IV: insight into the structural basis of receptor function and specificity. Neuron 21: 1079–1092, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Girard B, Malley S, Braas K, Waschek J, May V, Vizzard MA. Exaggerated expression of inflammatory mediators in vasoactive intestinal polypeptide knockout (VIP−/−) mice with cyclophosphamide (CYP) induced cystitis. J Mol Neurosci. In press. [DOI] [PMC free article] [PubMed]
- 22.Girard BM, May V, Bora SH, Fina F, Braas KM. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regul Pept 109: 89–101, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Goebel S, Huang M, Davis WC, Jennings M, Siahaan TJ, Alexander JS, Kevil CG. VEGF-A stimulation of leukocyte adhesion to colonic microvascular endothelium: implications for inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 290: G648–G654, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Ho N, Koziol JA, Parsons CL. Epidemiology of interstitial cystitis. In: Interstitial Cystitis, edited by Sant GR. Philadelphia: Lippincott-Raven, 1997, p. 9–16.
- 25.Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol 284: R574–R585, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol 173: 1016–1021, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Jennings LJ, Vizzard MA. Cyclophosphamide-induced inflammation of the urinary bladder alters electrical properties of small diameter afferent neurons from dorsal root ganglia (Abstract). FASEB J 13: A57, 1999. [Google Scholar]
- 28.Johansson SL, Ogawa K, Fall M. The pathology of interstitial cystitis. In: Interstitial Cystitis, edited by Sant GR. Philadelphia: Lippincott-Raven, 1997, p. 143–152.
- 29.Kern TS Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res 2007: 95103, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilic U, Kilic E, Jarve A, Guo Z, Spudich A, Bieber K, Barzena U, Bassetti CL, Marti HH, Hermann DM. Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating ERK-1/2 and Akt pathways. J Neurosci 26: 12439–12446, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klinger MB, Dattilio A, Vizzard MA. Expression of cyclooxygenase-2 in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R677–R685, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Klinger MB, Girard B, Vizzard MA. p75(NTR) expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol 507: 1379–1392, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Koutroubakis IE, Tsiolakidou G, Karmiris K, Kouroumalis EA. Role of angiogenesis in inflammatory bowel disease. Inflamm Bowel Dis 12: 515–523, 2006. [DOI] [PubMed] [Google Scholar]
- 34.LaBerge J, Malley SE, Zvarova K, Vizzard MA. Expression of corticotropin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 291: R692–R703, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain 5: 150–156, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Lantéri-Minet M, Bon K, de Pommery J, Michiels JF, Menétrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-Fos and Krox-24 proteins. Exp Brain Res 105: 220–232, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Lazarovici P, Marcinkiewicz C, Lelkes PI. Cross talk between the cardiovascular and nervous systems: neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF)—implications in drug development. Curr Pharm Des 12: 2609–2622, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Lemstrom KB, Krebs R, Nykanen AI, Tikkanen JM, Sihvola RK, Aaltola EM, Hayry PJ, Wood J, Alitalo K, Yla-Herttuala S, Koskinen PK. Vascular endothelial growth factor enhances cardiac allograft arteriosclerosis. Circulation 105: 2524–2530, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Lu J, Kasama T, Kobayashi K, Yoda Y, Shiozawa F, Hanyuda M, Negishi M, Ide H, Adachi M. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J Immunol 164: 5922–5927, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Maggi CA, Lecci A, Santiciolo P, Del Biance E, Giuliani S. Cyclophosphamide cystitis in rats: involvement of capsaicin-sensitive primary afferents. J Auton Nerv Syst 38: 201–208, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics 9: 5–13, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Murata M, Yudoh K, Masuko K. The potential role of vascular endothelial growth factor (VEGF) in cartilage: how the angiogenic factor could be involved in the pathogenesis of osteoarthritis? Osteoarthritis Cartilage 16: 279–286, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Ng YS, Krilleke D, Shima DT. VEGF function in vascular pathogenesis. Exp Cell Res 312: 527–537, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Ortega N, Hutchings H, Plouet J. Signal relays in the VEGF system. Front Biosci 4: D141–D152, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Pavelock K, Braas K, Ouafik L, Osol G, May V. Differential expression and regulation of the vascular endothelial growth factor receptors neuropilin-1 and neuropilin-2 in rat uterus. Endocrinology 142: 613–622, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Petrone RL, Agha AH, Roy JB, Hurst RE. Urodynamic findings in patients with interstitial cystitis (Abstract). J Urol 153: 290A, 1995. [Google Scholar]
- 47.Raab S, Plate KH. Different networks, common growth factors: shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol (Berl) 113: 607–626, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Ruas JL, Lendahl U, Poellinger L. Modulation of vascular gene expression by hypoxia. Curr Opin Lipidol 18: 508–514, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Saban MR, Backer JM, Backer MV, Maier J, Fowler B, Davis CA, Simpson C, Wu XR, Birder L, Freeman MR, Soker S, Hurst RE, Saban R. VEGF receptors and neuropilins are expressed in the urothelial and neuronal cells in normal mouse urinary bladder and are up-regulated in inflammation. Am J Physiol Renal Physiol 295: F60–F72, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saban MR, Hellmich H, Nguyen NB, Winston J, Hammond TG, Saban R. Time course of LPS-induced gene expression in a mouse model of genitourinary inflammation. Physiol Genomics 5: 147–160, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Sant G, Hanno PM. Interstitial cystitis: current issues and controversies in diagnosis. Urology 57: 82–88, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Tamaki M, Saito R, Ogawa O, Yoshimura N, Ueda T. Possible mechanisms inducing glomerulations in interstitial cystitis: relationship between endoscopic findings and expression of angiogenic growth factors. J Urol 172: 945–948, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Usui T, Ishida S, Yamashiro K, Kaji Y, Poulaki V, Moore J, Moore T, Amano S, Horikawa Y, Dartt D, Golding M, Shima DT, Adamis AP. VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest Ophthalmol Vis Sci 45: 368–374, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Vizzard MA Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21: 125–138, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Vizzard MA Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol 278: R1027–R1039, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Vizzard MA Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161: 273–284, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Vizzard MA Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol 420: 335–348, 2000. [PubMed] [Google Scholar]
- 59.Vizzard MA, Boyle MM. Increased expression of growth-associated protein (GAP-43) in lower urinary tract pathways following cyclophosphamide (CYP)-induced cystitis. Brain Res 844: 174–187, 1999. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA, Jin K. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J Neurosci 27: 304–307, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woolf CJ, Doubell TP. The pathophysiology of chronic pain-increased sensitivity to low threshold A-β fiber inputs. Curr Opin Neurobiol 4: 525–534, 1994. [DOI] [PubMed] [Google Scholar]
- 62.Yoshimura N Bladder afferent pathways and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog Neurobiol 57: 583–606, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci 26: 10847–10855, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 19: 4644–4653, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuridullah R, Corrow KA, Malley SE, Vizzard MA. Expression of fractalkine and fractalkine receptor in urinary bladder after cyclophosphamide (CYP)-induced cystitis. Auton Neurosci 126–127: 380–389, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zvarova K, Vizzard MA. Changes in galanin immunoreactivity in rat micturition reflex pathways after cyclophosphamide-induced cystitis. Cell Tissue Res 324: 213–224, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Zvarova K, Vizzard MA. Distribution and fate of cocaine- and amphetamine-regulated transcript peptide (CARTp)-expressing cells in rat urinary bladder: a developmental study. J Comp Neurol 489: 501–517, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]