Abstract
We hypothesized that heat shock protein 27 (HSP27), a small heat shock protein with actin-remodeling properties, is involved in the pathogenesis of kidney tubulointerstitial fibrosis. We first examined its expression in the rat unilateral ureteral obstruction (UUO) model of kidney fibrosis and epithelial-to-mesenchymal transition (EMT). Immunoblot analyses showed that UUO resulted in significant upregulation of TGF-β1, α-smooth muscle actin (α-SMA), total and phosphorylated HSP27, and phosphorylated p38MAPK. Immunofluorescence studies showed that HSP27 costained with TGF-β1, α-SMA, and E-cadherin in areas of tubulointerstitial injury. We next attempted to translate these studies in an in vitro model of EMT using rat proximal tubular epithelial cells (NRK52E). TGF-β1 (20 ng/ml) treatment resulted in EMT (upregulation of α-SMA and downregulation of E-cadherin) and significant upregulation of total and phosphorylated HSP27 and p38MAPK after 3 days. Real-time PCR analyses showed that HSP27, vimentin, and fibronectin increased whereas E-cadherin mRNA levels decreased. Double-staining immunofluorescence studies showed intracytoplasmic colocalization of HSP27 with both F-actin and E-cadherin in cells undergoing EMT. HSP27 overexpression by transient transfection significantly increased E-cadherin while decreasing E-cadherin repressor Snail levels. In aggregate, these studies show that HSP27 is involved in the pathogenesis of TGF-β1-induced EMT and chronic tubulointerstitial fibrosis. HSP27 overexpression may delay injury by upregulating E-cadherin through downregulation of Snail.
Keywords: epithelial-to-mesenchymal transition, E-cadherin, NRK52E cells, ureteral obstruction
chronic tubulointerstitial fibrosis is a major contributor to native and transplant kidney failure (16, 26, 42). It is a chronic, progressive, nonspecific, and irreversible histopathological entity that is associated with significant patient morbidity and mortality (30, 40, 42). A major challenge to improving long-term outcomes in patients with kidney disease is to dissect out identifiable causes of chronic tubulointerstitial fibrosis and to develop cause-specific treatment strategies (17).
Interstitial myofibroblasts are the principal source of kidney fibrosis (25, 28). Under stress, resident fibroblasts expand by cell division and generate profibrotic molecules. Up to a third of all disease-related fibroblasts, however, can originate from tubular epithelia at the site of injury through epithelial-to-mesenchymal transition (EMT) (28). EMT is an important profibrotic event in native (28, 29, 37, 46, 49) and transplant kidney injury (8, 21, 27). During EMT, tubular epithelial cells are transformed into myofibroblasts in a stepwise process characterized by downregulation of epithelial markers including E-cadherin and de novo expression of mesenchymal markers such as α-smooth muscle actin (α-SMA). EMT is also associated with actin reorganization, tubular basement membrane disruption, cell migration, and production of profibrotic molecules (28, 29, 37, 46, 49). Transforming growth factor β1 (TGF-β1), oxidative stress (OS), hypoxia, interleukin-1, and tissue-invasive mononuclear cells can all result in EMT (8, 21, 48). A better understanding of the pathogenesis of EMT may result in treatment strategies that delay or inhibit chronic tubulointerstitial fibrosis.
Heat shock proteins (HSPs) are a protein superfamily that respond to heat or any physiological stress (18, 41). HSP27 (in humans and rats) or HSP25 (in mice) belong to the small HSP (sHSP) subfamily and are characterized by a low molecular mass and conserved COOH-terminal domains (the α-crystallin domain). They also contain a WDPF domain in their NH2-terminal part and a nonconserved flexible domain which constitutes the COOH-terminal part of the proteins (4). HSP27 exerts its cytoprotective effects through modulation of the actin cytoskeleton and inhibition of OS and apoptosis (3, 5, 32–34, 39). It also plays a role in inflammation, cell signaling, differentiation, and proliferation (2, 4). It is therefore logical to hypothesize that HSP27 is involved in the pathogenesis of EMT and chronic tubulointerstitial fibrosis at several levels, including its activation by growth factors and OS and its role as an actin-remodeling and antioxidant molecule. To address this hypothesis, we first evaluated the involvement of HSP27 in an established animal model of kidney fibrosis and EMT, and next we examined its role in TGF-β1-induced EMT of rat proximal tubular epithelial cells (NRK52E).
MATERIALS AND METHODS
Cell Culture Experiments
Normal rat kidney proximal epithelial cells (NRK52E) were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and maintained at 37°C in a humidified atmosphere containing 5% CO2. Cells were seeded at 2.5 × 105 cells/well into six-well culture plates in DMEM (high glucose) containing 5% heat-inactivated FBS, 44 mM NaHCO3, 5,000 IU penicillin, and 5,000 μg/ml streptomycin (Cellgro). At 80% confluency, media was changed to serum-free DMEM supplemented with 0.1% BSA for 12 h to arrest growth and synchronize cell activity. TGF-β1 (at indicated concentrations, Sigma-Aldrich, St. Louis, MO) was then added for EMT experiments.
HSP27 transfection studies in cells were performed using an empty pCI Mammalian Expression Vector (Promega, Madison, WI) or pCI vector containing a 780-bp human Hsp27 gene insert (HuHSP27, GenBank no. X54079) under the control of the human cytomegalovirus early enhancer/promoter. The vector was constructed by cloning the EcoRI insert of pGAD10-HuHSP27 (14), a generous gift from Dr. Jacques Landry. Cells were transfected for 5 h before the addition of TGF-β1, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the recommended protocol. Cells were harvested before the addition of TGF-β1 (time 0), on days 1, 2, and 3 and used for immunoblotting, real-time PCR, and immunofluorescence studies.
Animal Studies
Adult (9- to 11-wk-old) male Lewis rats were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Animals were housed in the animal care facility at the William Middleton Veterans Affairs (VA) Hospital in Madison, WI, and the procedures were performed in accordance with the Animal Care Policies at the VA Hospital and the University of Wisconsin. A unilateral ureteral obstruction (UUO) procedure was performed under general anesthesia with isoflurane. Briefly, the left ureter was ligated with 6-0 silk at two points and then severed between the ligatures to prevent retrograde urinary tract infection. Animals were killed after 2 wk by exsanguination through cardiac puncture under general anesthesia. Both kidneys were harvested and sectioned longitudinally in half. One-half was snap frozen immediately and used for immunoblot and real-time PCR analyses, whereas the other half was formalin-fixed and paraffin-embedded for immunohistochemical analyses. The right kidney served as the control to the left obstructed kidney.
RNA Extraction, Purification, and Real-Time PCR Analyses
Total RNA extraction was performed as previously described (22, 23). Briefly, RNA was extracted from cell lysates using the TRIzol protocol (GIBCO BRL, Life Technologies, Rockville, MD). Following centrifugation, the clear supernatant containing RNA was transferred into a fresh tube and chloroform (200 μl/tube) was added for phase separation. Isopropanol (500 μl/tube) was then added, and following another centrifugation the supernatant was removed and the RNA pellet was washed with 1 ml 75% EtOH in diethylpyrocarbonate (DEPC)-treated water. The sample was centrifuged and the supernatant was removed, allowing the pellet to dry at room air. Pellets were then resuspended in DEPC-treated water, and an RNA Easy kit (catalog no. 74104, Qiagen) was used for purification. RNA samples were eluted with sterile DEPC-treated water, and spectrophotometric readings were done at 260/280 nm to determine the final concentration. We utilized rat HSP27 (Unigene ID: Rn.3841, exon boundary 2–3), vimentin (Unigene ID: Rn.2710, exon boundary 2–3), fibronectin (Unigene ID: Rn.1604, exon boundary 23–24), S100A4 (Unigene ID: Rn.504, exon boundary 1–2), E-cadherin (Unigene ID:Rn.1303, exon boundary 3–4), and S26 internal control (Refseq: XM 001066146.1 exon boundary 1–1) primers. cDNA synthesis was performed using a First Strand cDNA synthesis kit (Roche catalog no. 1483188) as described previously (22, 23). Briefly, RNA (2.5 μg), 10× reaction buffer (4.0 μl), 25 mM MgCl (8.0 μl), dNTPs (4.0 μl), RNase inhibitor (2.0 μl), AMV RT polymerase (1.6 μl), and oligo dT (4.0 μl) were mixed in a 500-μl Eppendorf tube. Samples were mixed and incubated at 25°C for 10 min, 42°C for 60 min, and 99°C for 5 min, followed by a 4°C hold in a thermocycler. PCR reactions were then performed using the primers described above and the GeneAmp 5700 Sequence detection system (Applied Biosystems). Mean Ct, SD, and ΔCt (compared with S26 internal control) for HSP27 and matrix remodeling genes (vimentin, fibronectin, and E-cadherin) at baseline (time 0), and days 2 and 3 of treatment with TGF-β1 were evaluated and presented. Experiments were performed in triplicate, and representative samples are presented.
Immunoblotting
Western blotting was performed on protein lysates obtained from whole kidney tissue or cell lysates as described earlier (22, 23). After separation by SDS-PAGE (10–20% gradient PAGE, Bio-Rad, Hercules, CA), proteins were transferred electrophoretically (100 V, 30 min) to nitrocellulose membranes (Bio-Rad) that were then blocked with a solution containing 5% Carnation nonfat milk, 50 mM Tris·HCl, pH 7.4, 150 mM NaCl, and 0.05% Tween 20 (TBS-Tween) overnight at 4°C. Membranes were incubated the next day with antibodies against total human Hsp27 (clone G3.1, SPA-800, 1:1,000, Stressgen), total rat Hsp27 (385877, 1:2,000, Calbiochem, La Jolla, CA), and phosphorylated (S85) Hsp27 (Ab5594, 0.25:1,000, Abcam). The immunogen is a synthetic peptide, RQLSpSGVSEIR, corresponding to amino acids 82–92 of rat Hsp27. The antibody does not detect the unphosphorylated form of the protein but cross reacts with both human and rat phosphoHSP27. Other antibodies included E-cadherin (clone 34, mouse IgG2b, immunogen human E-cadherin, AA 735–883, C37020, 150 μg, 1:100, 4°C overnight, BD Transduction Labs); α-SMA (clone 1A4, A2547, 2 ml, 1:2,000, Sigma); Snail (Ab17732, 1:1,000, Abcam Transduction Labs); total p38 (Ab19329, 1:20,000, Abcam); TGF-β1 (AHG0051, 1:1,000, Invitrogen); and β-actin (A0760–40, 1:7,500, US Biologicals). All primary antibodies were diluted in the solution containing 5% Carnation nonfat milk, 50 mM Tris·HCl, pH 7.4, 150 mM NaCl, and 0.05% Tween 20 (TBS-Tween). Binding of primary antibodies was followed by incubation for 1 h at room temperature with a secondary horseradish peroxidase-conjugated IgG in 1% nonfat milk. Signals were visualized by enhanced chemiluminescence signals captured on X-ray film. Data were normalized to β-actin. Densitometry was done using National Institutes of Health Image J software, downloaded from http://rsb.info.nih.gov/ij. Results were displayed as representative assays of three sets of independent experiments.
Immunostaining
Cell culture studies.
NRK cells were cultured on 0.1% gelatin-coated coverslips in a 24-well plate. Cells were fixed with 4% paraformaldehyde for 10 min at room temperature, washed, and permeabilized with 1% Triton X-100 for 3 min. Anti-HSP27 (1:100, Calbiochem) staining was done at room temperature for 1 h followed by an anti-rabbit Alexa 488 secondary antibody (1:1,000, Invitrogen, Carlsbad, CA). Double staining was done with phalloidin-Alexa 568 (1:40, Invitrogen). Cells were stained with anti E-cadherin-FITC (1:2.5 BD Bioscience, San Jose, CA) overnight at 4°C and double stained with anti-HSP27 (1:100) followed by anti-rabbit Alexa 594 secondary antibody (1:1,000). ProLong Gold Antifade reagent with 4,6-diamidino-2-phenylindole (Invitrogen) was used to mount the coverslips to slides.
Tissue sections.
Formalin-fixed, paraffin-embedded sections were cut into 4- to 5-μm sections. Slides were deparaffinized and rehydrated from xylene through a graded ethanol series to dH2O. Antigen retrieval was performed in a 1 mM EDTA (pH = 8.0) solution at 25 psi for 2 min. Nonspecific background staining was blocked using Sniper (Biocare Medical, Concord, CA). TGF-β1 (1:400) primary staining was done at room temperature for 1 h followed by an anti-mouse Alexa 594 secondary antibody (1:1,000). Double staining with HSP27 (1:200) was completed using anti-rabbit Alexa 488 (1:1,000). For the immunoperoxidase assays, anti-TGF-β1 and -HSP27 antibody concentrations were 1:500 and 1:200, respectively. The anti-TGF-β1 antibody [rabbit polyclonal antibody LC1–30-1 (s)] used for staining experiments was a generous gift from Dr. Randy Wolff at the University of Wisconsin. α-SMA (1:50,000, Sigma-Aldrich) primary staining was done at room temperature for 1 h followed by an anti-mouse Alexa 488 secondary antibody (1:1,000). Double staining with HSP27 (1:100) was completed using anti-rabbit Alexa 594 (1:1,000). E-cadherin primary staining of tissue was done using an unconjugated antibody (1:50, BD Bioscience) overnight at 4°C followed by anti-mouse Alexa 488 (1:1,000). Double staining with HSP27 was completed as previously mentioned. Coverslips were secured using the ProLong Gold Antifade reagent with 4,6-diamidino-2-phenylindole. Slides were viewed on a Nikon Eclipse E600 microscope with an Olympus DP70 camera. Images were analyzed using DP70 imaging software.
RESULTS
HSP27 Was Upregulated in UUO and Colocalized with TGF-β1, α-SMA, and E-cadherin in Areas of Injury
We first sought to evaluate the expression of HSP27 and its signaling pathway in an established animal model of kidney fibrosis and EMT (15, 31). Lewis rats underwent UUO of the left kidney, and we examined HSP27, its signaling pathway, and matrix-remodeling molecules by immunoblot and immunofluorescence studies. Immunoblot analyses showed that UUO resulted in a significant upregulation of TGF-β1, α-SMA, total and phosphorylated HSP27, and phosphorylated p38MAPK while E-cadherin levels remained grossly unchanged (Fig. 1).
Fig. 1.
Heat shock protein 27 (HSP27) was upregulated after ureteral obstruction. Three-month-old Lewis rats (n = 6) underwent unilateral ureteral obstruction (UUO) of the left kidney. A: representative immunoblot analyses of right (control) and left (UUO) kidneys. All studies were repeated 3 times. B: bar graph of average protein levels normalized to GAPDH. UUO resulted in a significant upregulation of transforming growth factor (TGF)-β1, phosphorylated p38MAPK, total and phosphorylated HSP27, and α-smooth muscle actin (α-SMA).
Immunohistochemical and immunofluorescence studies showed that HSP27 was primarily present in blood vessels, glomeruli, and a few distal tubules in control kidneys (see Figs. 2–4). UUO resulted in greater HSP27 and TGF-β1 staining in areas of tubulointerstitial injury compared with control kidneys (Fig. 2, A–E). Furthermore, a significant number of tubular epithelial cells showed colocalization of both molecules (Fig. 2, C and E, yellow arrows). Similarly, α-SMA stained the vessels (Fig. 3) and E-cadherin the basolateral membranes of distal tubules (Fig. 4). However, in obstructed kidneys, HSP27 and α-SMA costained areas of tubulointerstitial stress and injury (Fig. 3F) and E-cadherin lost its membrane-bond expression while costaining with HSP27 in the cytoplasm of cells under stress (yellow arrow, Fig. 4F). Together, these studies suggest that HSP27 is involved in the pathogenesis of kidney tubulointerstitial fibrosis.
Fig. 2.
HSP27 and TGF-β1 colocalized in areas of tubulointerstitial fibrosis. Immunohistochemical and immunofluorescence studies were performed for HSP27 and TGF-β1 in normal control (D) and UUO kidneys (A, B, C, and E). C: merged image from A and B. UUO resulted in greater HSP27 and TGF-β1 staining in areas of tubulointerstitial injury compared with the control kidney (A–E). Furthermore, a significant number of tubular epithelial cells showed colocalization of both molecules (C and E, yellow arrows).
Fig. 4.
HSP27 and cytoplasmic E-cadherin colocalized in areas of tubulointerstitial fibrosis. Immunofluorescence studies were performed for HSP27 (Alexa 594, red) and E-cadherin (Alexa 488, green). A–C: control right kidney. B–E: left kidney undergoing UUO. C and F: merged image analyses. In the control kidney, HSP27 had a sporadic tubular expression (A) while E-cadherin was membrane-bound (B). No colocalization was observed. UUO resulted in downregulation of membrane-bound E-cadherin and cytoplasmic colocalization of HSP27 and E-cadherin (F, yellow arrow).
Fig. 3.
HSP27 and α-SMA colocalized in areas of tubulointerstitial fibrosis. Immunofluorescence studies were performed for HSP27 (Alexa 594, red) and α-SMA (Alexa 488, green). A–C: control right kidney. B–E: left kidney undergoing UUO. C and F: merged image analyses, including 4,6-diamidino-2-phenylindole (DAPI) staining for the nuclei. In UUO kidneys, HSP27 and α-SMA costained areas of tubulointerstitial stress and injury (*). V, vessel, G, glomerulus.
HSP27 Protein Levels Increased During TGF-β1-Induced EMT
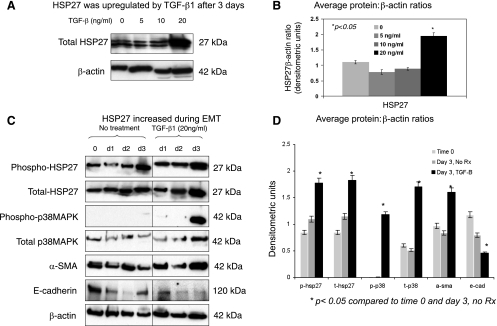
TGF-β1 is a key inducer of EMT and fibrosis in kidneys (12, 48). We also showed that TGF-β1 and HSP27 levels were both increased after UUO (Figs. 1 and 2). However, there is no information on whether TGF-β1 regulates HSP27 expression in renal tubular epithelial cells. To address this question, NRK52E cells were treated with increasing concentrations of TGF-β1 (0, 5, 10, or 20 ng/ml) for 3 days as described in materials and methods. Western blot analyses showed that there was a significant increase in HSP27 levels when cells were treated with 20 ng/ml of TGF-β1 (Fig. 5, A and B). This dose range was based on previous studies examining TGF-β1-induced EMT in NRK52E cells (13, 43). Double bands on immunoblots (Fig. 5A) are consistent with previously reported in vitro and animal studies and are probably related to the phosphorylation state of HSP27 (1, 6, 19). We next used this dose of TGF-β1 and examined the expression of HSP27 and its signaling pathway during TGF-β1-induced EMT. TGF-β1 treatment resulted in EMT (upregulation of α-SMA and downregulation of E-cadherin) and significant upregulation of total and phosphorylated HSP27 and p38MAPK after 3 days, suggesting that HSP27 is involved in the pathogenesis of TGF-β1-induced EMT via the activation of the p38MAPK pathway.
Fig. 5.
HSP27 protein levels increased during TGF-β1-induced epithelial-to-mesenchymal transition (EMT). NRK52E cells were seeded at 2.5 × 105 cells/well into 6-well culture plates in DMEM (high glucose) containing 5% heat-inactivated FBS. At 80% confluency, media was changed to serum-free DMEM supplemented with 0.1% BSA for 12 h to arrest growth and synchronize cell activity. TGF-β1 (at indicated concentrations) was added for EMT experiments. A: representative Western blot evaluating the dose-response. B: bar graph representing average HSP27:β-actin ratios. C: representative Western blot evaluating EMT and HSP27 signaling at baseline and days 1, 2, and 3 with or without TGF-β1 treatment (20 ng/ml). D: bar graph representing average protein:β-actin ratios. All experiments were repeated at least 3 times. TGF-β1 treatment resulted in EMT (upregulation of α-SMA and downregulation of E-cadherin) and greater total and phosphorylated HSP27 and p38MAPK levels.
HSP27 mRNA Increased in TGF-β1-Induced-EMT: Real-Time PCR Analyses
We next evaluated HSP27, vimentin, fibronectin, and E-cadherin mRNA levels by real-time PCR at baseline (time 0) and days 2 and 3 of treatment with TGF-β1 (20 ng/ml) (Fig. 6). HSP27, vimentin, and fibronectin mRNA levels increased with time and TGF-β1 while E-cadherin levels decreased. The differences were statistically significant for HSP27, fibronectin, and E-cadherin between TGF-β1 and no treatment groups on day 3. Although the magnitude of the mRNA increase was not as significant as protein changes observed in Fig. 5, these findings suggest that HSP27 transcription may contribute to greater protein levels during TGF-β1-induced EMT.
Fig. 6.
HSP27 mRNA increased during TGF-β1-induced-EMT. A: table representing mean Ct, SD, ΔCt (compared to S26 internal control) for HSP27 and matrix-remodeling genes (vimentin, fibronectin, and E-cadherin) at baseline (time 0) and days 2 and 3 of treatment with TGF-β1. All experiments were done in triplicate and repeated at least 3 times. B and C: bar graphs representing average fold-changes for genes of interest compared with baseline. These studies showed that HSP27, vimentin, and fibronectin increased with time and TGF-β1 treatment (20 ng/ml) whereas E-cadherin mRNA levels decreased. Differences were statistically significant for HSP27, fibronectin, and E-cadherin between TGF-β1 and no treatment groups on day 3.
HSP27 Colocalized with F-Actin and E-Cadherin in TGF-β1-Induced EMT
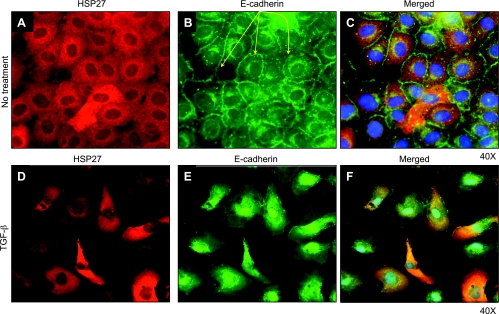
To examine the cellular localization of HSP27 in TGF-β1-induced EMT, we performed immunofluorescence studies for HSP27 and EMT biomarkers F-actin and E-cadherin. Cells were treated or not with TGF-β1 (20 ng/ml) for 3 days. In untreated cells, HSP27 expression was homogenous and intracytoplasmic (Fig. 7A), while F-actin localized under the cell membrane (Fig. 7B). Merged images did not show significant colocalization (Fig. 7C). In TGF-β1-treated cells, we observed features of EMT with the loss of the cobblestone pattern, spindle-shaped cells, and the presence of F-actin stress filaments (Fig. 7, D–F). HSP27 and F-actin colocalized in cells undergoing the most significant phenotypic changes (marked with a yellow *, Fig. 7F), suggesting that HSP27 interacts with F-actin during EMT. Similar studies examining HSP27 and E-cadherin showed that in untreated cells, E-cadherin was primarily localized on the cell membrane (yellow arrows, Fig. 8B). TGF-β1-treated cells displayed features of EMT including downregulation of membrane-bound E-cadherin (Fig. 8E). Merged images showed significant cytoplasmic colocalization of HSP27 and E-cadherin in most cells, especially those with pronounced features of EMT.
Fig. 7.
HSP27 and F-actin colocalized in TGF-β1-induced EMT. Immunofluorescence studies are shown evaluating HSP27 (Alexa 488) and F-actin (phalloidin, Alexa 568 antibody) staining in untreated (A–C) or TGF-β1-treated (20 ng/ml) cells (D–F) for 3 days. C and F: merged images that include DAPI (blue) staining for nuclei. All experiments were performed at least 3 times. In untreated cells, HSP27 expression was intracytoplasmic (A), while F-actin localized under the cell membrane (B). Merged images did not show significant colocalization (C). TGF-β1-treated cells showed features of EMT with the loss of the cobblestone pattern, spindle-shaped cells, and the presence of F-actin stress filaments (D–F). HSP27 and F-actin colocalized in cells undergoing the most significant phenotypic changes (F, yellow asterisks).
Fig. 8.
HSP27 and E-cadherin colocalized in TGF-β1-induced EMT. Immunofluorescence studies show HSP27 (Alexa 594) and E-cadherin (FITC) staining in untreated (A–C) or TGF-β1-treated (20 ng/ml) cells (D–F) for 3 days. C and F: merged images that include DAPI (blue) staining for nuclei. All experiments were performed at least 3 times. In untreated cells, E-cadherin was primarily localized on the cell membrane (B, yellow arrows). TGF-β1-treated cells displayed features of EMT including downregulation of membrane-bound E-cadherin (E). Merged images showed cytoplasmic colocalization of HSP27 and E-cadherin in cells undergoing the most dramatic phenotypic changes.
EMT is a dynamic and time-dependent event, and therefore cells may be at different stages of transition depending on the time after treatment. Additionally, although all cells are treated with TGF-β1, some undergo EMT while others may become apoptotic or alternatively resist stress and maintain their phenotype (12, 24, 38, 47). Our observations simply suggest that HSP27 and F-actin interact in cells undergoing significant phenotypic changes after the addition of TGF-β1.
HSP27 Overexpression Increased E-Cadherin While Downregulating Snail
To examine the effects of HSP27 overexpression on EMT, NRK52E cells were transiently transfected with human HSP27 or control vector. Regardless of TGF-β1 treatment, human HSP27 transfection resulted in significantly greater levels of human HSP27, phosphorylated HSP27, and E-cadherin while the levels of E-cadherin repressor Snail decreased (Fig. 9). There were no significant changes in intrinsic rat HSP27 levels. Together, these studies indicated that HSP27 may modulate EMT through upregulation of E-cadherin expression by regulating Snail expression.
Fig. 9.
HSP27 overexpression increased E-cadherin while downregulating Snail. NRK52E cells were transiently transfected with human HSP27 or empty vector, followed by treatment with TGF-β1 for 3 days. A: representative blots. B and C: bar graphs representing average protein levels normalized to the internal control β-actin. All experiments were performed at least 3 times. These studies showed that in TGF-β1-treated cells, human (hu)HSP27 transfection resulted in significantly greater levels of human HSP27, phosphorylated HSP27, and E-cadherin while Snail levels decreased. There were no significant changes in intrinsic rat HSP27 levels.
DISCUSSION
These studies show that HSP27 is involved in the pathogenesis of TGF-β1-induced EMT and kidney tubulointerstitial fibrosis. Furthermore, our findings suggest that HSP27 may modulate EMT by upregulating E-cadherin through the inhibition of E-cadherin repressor Snail. Because TGF-β1 is the principal profibrotic cytokine in the kidney (12, 48), these findings may be relevant to our understanding of the pathobiology of kidney injury. A few other studies support our observations. For example, cortical HSP27 levels were increased in kidney allografts with chronic tubulointerstitial fibrosis (22). HSP27 activation was required for TGF-β1-induced cell motility in prostate epithelial cells (20). HSP27 induction was found as an adaptive response of the human kidney to congenital unilateral ureteropelvic junction obstruction (45). Furthermore, a p38-MAPK-mediated increase in Hsp27 phosphorylation maintained cell adhesion and suppressed apoptosis in nephrotoxicant 1,2-(dichlorovinyl)-l-cysteine-treated LLC-PK1 cells (19). Last, adenosine-induced p38MAPK activation resulted in mediated Hsp27 (total and phosphorylated) upregulation and was renoprotective in mice (35).
E-cadherin expression may be cytoplasmic or membrane bound and was regulated by TGF-β1 and HSP27 in our studies. TGF-β1 decreased E-cadherin expression while HSP27 overexpression resulted in significant upregulation of E-cadherin in the presence or absence of TGF-β1. Although E-cadherin levels were not decreased in vivo compared with in vitro studies, the molecule was consistently “recompartmentalized” from the membrane to the cytoplasm under stress. The difference regarding total E-cadherin levels may result from the lack of serum and most physiological repair mechanisms that characterized our in vitro conditions. In other words, the stress of the cell culture environment was significantly greater than in vivo, resulting in more drastic phenotypic changes. Another question was how E-cadherin could be downregulated in response to TGF-β1, which also increased HSP27 expression, while overexpression of HSP27 itself resulted in increased expression of E-cadherin. HSP27 is a stress-response protein that protects cells when stress is mild to moderate. In a high-stress environment, EMT, apoptosis, or necrosis may ensue (12, 18). It is therefore possible that in vitro, the burden of exogenous TGF-β1 overcomes the repair capacity of endogenous HSP27, resulting in E-cadherin downregulation. If HSP27 levels are increased by transfection, cell repair capacity is enhanced and downregulation of E-cadherin is prevented. Another possibility is the role of signaling pathways (e.g., Smad, Slug, Wnt) that could operate independently from HSP27 in this context and result in downregulation of E-cadherin (36, 37). Our studies suggest that HSP27-mediated upregulation of E-cadherin was dependent, at least in part, on the inhibition of the E-cadherin repressor Snail (7, 10, 11). Immunofluorescence studies were in concert with these findings showing costaining of E-cadherin and HSP27 in TGF-β1-treated cells and the UUO model, indicating that HSP27 may interact with E-cadherin during EMT and tubulointerstitial fibrosis. Indeed, recent data support this hypothesis by demonstrating a dialog between HSP27 and adherens junction/focal adhesion (1, 9, 44). For example, overexpression of HSP27 in human melanoma cell lines upregulated E-cadherin while decreasing matrix metalloproteinases 2 and 9 and tumor markers MUC18/MCAM, suggesting an inhibitory-regulatory role for HSP27 in melanoma progression (1). Conversely, desmosome signaling may also activate HSP27 (9). Berkowitz et al. (9) showed that pemphigus vulgaris pathogenic antibodies activate p38MAPK and HSP27 signaling pathways after binding to desmoglein-3, the desmosomal cadherin (9). The activation of HSP27 resulted in acantholysis, suggesting that the inhibition of p38MAPK and/or HSP27 phosphorylation may provide novel treatments for desmosome-associated blistering diseases (9). Furthermore, HSP27 was recruited to sites of cell-cell and cell-substrate attachment in renal epithelial cells undergoing injury by heat stress or ATP depletion, indicating a potential role for HSP27 in protection or regulation of epithelial cell-cell and cell-substrate attachments (44).
Our study limitations include the in vitro and in vivo models of fibrosis. EMT is a complex and dynamic process difficult to capture in vivo, and the extent of its contribution to chronic kidney injury is unknown. Similarly, UUO includes a significant inflammatory component mediated by monocytes/macrophages that is not universal to all models of kidney fibrosis. HSP27 is a molecule with pleiotropic characteristics. It may play different roles in normal and pathological cells (4). These effects may also be cell or tissue specific. For example, in normal cells, HSP27 contributes to cytoskeleton, redox state, and protein-folding homeostasis and the protection of cells in case of stress. In pathological cells, it may have deleterious effects by protecting cancer cells against the immune system or chemotherapy. It is therefore clear that therapies targeting HSP27 should be tailored to the underlying disease state. In conclusion, our studies demonstrate that HSP27 is involved in the pathogenesis of matrix remodeling and kidney tubulointerstitial fibrosis with a potential protective role. We define in vitro and in vivo models where genetic manipulation of HSP27 may shed further light on potential therapies that inhibit chronic kidney fibrosis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant D-K067981–04 (A. Djamali).
Acknowledgments
We thank Dr. Jacques Landry and Herman Lambert from Centre de Recherche Hôtel-Dieu de Québec (Québec, Canada G1R 2J6) for invaluable contributions to the transfection studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aldrian S, Kindas-Mugge I, Trautinger F, Frohlich I, Gsur A, Herbacek I, Berger W, Micksche M. Overexpression of Hsp27 in a human melanoma cell line: regulation of E-cadherin, MUC18/MCAM, and plasminogen activator (PA) system. Cell Stress Chaperones 8: 249–257, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alford KA, Glennie S, Turrell BR, Rawlinson L, Saklatvala J, Dean JL. Heat shock protein 27 functions in inflammatory gene expression and transforming growth factor-beta-activated kinase-1 (TAK1)-mediated signaling. J Biol Chem 282: 6232–6241, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Arrigo AP, Firdaus WJ, Mellier G, Moulin M, Paul C, Diaz-Latoud C, Kretz-Remy C. Cytotoxic effects induced by oxidative stress in cultured mammalian cells and protection provided by Hsp27 expression. Methods 35: 126–138, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Arrigo AP, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, az-Latoud C, Vicart P. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett 581: 3665–3674, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal 7: 414–422, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Basile DP, Donohoe D, Cao X, Van Why SK. Resistance to ischemic acute renal failure in the Brown Norway rat: a new model to study cytoprotection. Kidney Int 65: 2201–2211, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Becker KF, Rosivatz E, Blechschmidt K, Kremmer E, Sarbia M, Hofler H. Analysis of the E-cadherin repressor Snail in primary human cancers. Cells Tissues Organs 185: 204–212, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Bedi S, Vidyasagar A, Djamali A. Epithelial-to-mesenchymal transition and chronic allograft tubulointerstitial fibrosis. Transplant Rev 22: 1–5, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, Rubenstein DS. Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J Biol Chem 280: 23778–23784, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Blechschmidt K, Kremmer E, Hollweck R, Mylonas I, Hofler H, Kremer M, Becker KF. The E-cadherin repressor snail plays a role in tumor progression of endometrioid adenocarcinomas. Diagn Mol Pathol 16: 222–228, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Blechschmidt K, Sassen S, Schmalfeldt B, Schuster T, Hofler H, Becker KF. The E-cadherin repressor Snail is associated with lower overall survival of ovarian cancer patients. Br J Cancer 98: 489–495, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol 13: 2600–2610, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Burns WC, Twigg SM, Forbes JM, Pete J, Tikellis C, Thallas-Bonke V, Thomas MC, Cooper ME, Kantharidis P. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol 17: 2484–2494, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol 20: 7602–7612, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chevalier RL Obstructive nephropathy: towards biomarker discovery and gene therapy. Nat Clin Pract Nephrol 2: 157–168, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Colvin RB Chronic allograft nephropathy. N Engl J Med 349: 2288–2290, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Cornell LD, Colvin RB. Chronic allograft nephropathy. Curr Opin Nephrol Hypertens 14: 229–234, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Creagh EM, Sheehan D, Cotter TG. Heat shock proteins—modulators of apoptosis in tumour cells. Leukemia 14: 1161–1173, 2000. [DOI] [PubMed] [Google Scholar]
- 19.de Graauw M, Tijdens I, Cramer R, Corless S, Timms JF, van de Water B. Heat shock protein 27 is the major differentially phosphorylated protein involved in renal epithelial cellular stress response and controls focal adhesion organization and apoptosis. J Biol Chem 280: 29885–29898, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Di K, Wong YC, Wang X. Id-1 promotes TGF-beta1-induced cell motility through HSP27 activation and disassembly of adherens junction in prostate epithelial cells. Exp Cell Res 313: 3983–3999, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Djamali A Oxidative stress as a common pathway to chronic tubulointerstitial injury in kidney allografts. Am J Physiol Renal Physiol 293: F445–F455, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Djamali A, Reese S, Oberley T, Hullett D, Becker B. Heat shock protein 27 in chronic allograft nephropathy: a local stress response. Transplantation 79: 1645–1657, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Djamali A, Reese S, Yracheta J, Oberley T, Hullett D, Becker B. Epithelial-to-mesenchymal transition and oxidative stress in chronic allograft nephropathy. Am J Transplant 5: 500–509, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Docherty NG, O'Sullivan OE, Healy DA, Murphy M, O'Neill AJ, Fitzpatrick JM, Watson RW. TGF-β1-induced EMT can occur independently of its proapoptotic effects and is aided by EGF receptor activation. Am J Physiol Renal Physiol 290: F1202–F1212, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Essawy M, Soylemezoglu O, Muchaneta-Kubara EC, Shortland J, Brown CB, el Nahas AM. Myofibroblasts and the progression of diabetic nephropathy. Nephrol Dial Transplant 12: 43–50, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Halloran PF, Melk A, Barth C. Rethinking chronic allograft nephropathy: the concept of accelerated senescence. J Am Soc Nephrol 10: 167–181, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Hertig A, Verine J, Mougenot B, Jouanneau C, Ouali N, Sebe P, Glotz D, Ancel PY, Rondeau E, Xu-Dubois YC. Risk factors for early epithelial to mesenchymal transition in renal grafts. Am J Transplant 6: 2927–2946, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan B, Meier-Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant 2: 970–974, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861–F875, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem 268: 3420–3429, 1993. [PubMed] [Google Scholar]
- 33.Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem 268: 24210–24214, 1993. [PubMed] [Google Scholar]
- 34.Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol 15: 505–516, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HT, Kim M, Jan M, Penn RB, Emala CW. Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int 71: 1249–1261, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172: 973–981, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15: 1–12, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Mao H, Li Z, Zhou Y, Li Z, Zhuang S, An X, Zhang B, Chen W, Nie J, Wang Z, Borkan SC, Wang Y, Yu X. HSP72 attenuates renal tubular cell apoptosis and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 295: F202–F214, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehlen P, Schulze-Osthoff K, Arrigo AP. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem 271: 16510–16514, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation 75: 1291–1295, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones 7: 167–176, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nangaku M Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med 43: 9–17, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, Lee HB. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol 16: 667–675, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Shelden EA, Borrelli MJ, Pollock FM, Bonham R. Heat shock protein 27 associates with basolateral cell boundaries in heat-shocked and ATP-depleted epithelial cells. J Am Soc Nephrol 13: 332–341, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Valles P, Jorro F, Carrizo L, Manucha W, Oliva J, Cuello-Carrion FD, Ciocca DR. Heat shock proteins HSP27 and HSP70 in unilateral obstructed kidneys. Pediatr Nephrol 18: 527–535, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 159: 1465–1475, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoo J, Ghiassi M, Jirmanova L, Balliet AG, Hoffman B, Fornace AJ Jr, Liebermann DA, Bottinger EP, and Roberts AB. Transforming growth factor-beta-induced apoptosis is mediated by Smad-dependent expression of GADD45B through p38 activation. J Biol Chem 278: 43001–43007, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 24: 5764–5774, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med 82: 175–181, 2004. [DOI] [PubMed] [Google Scholar]