Abstract
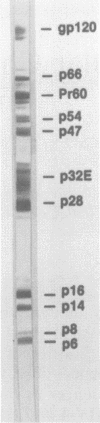
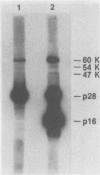
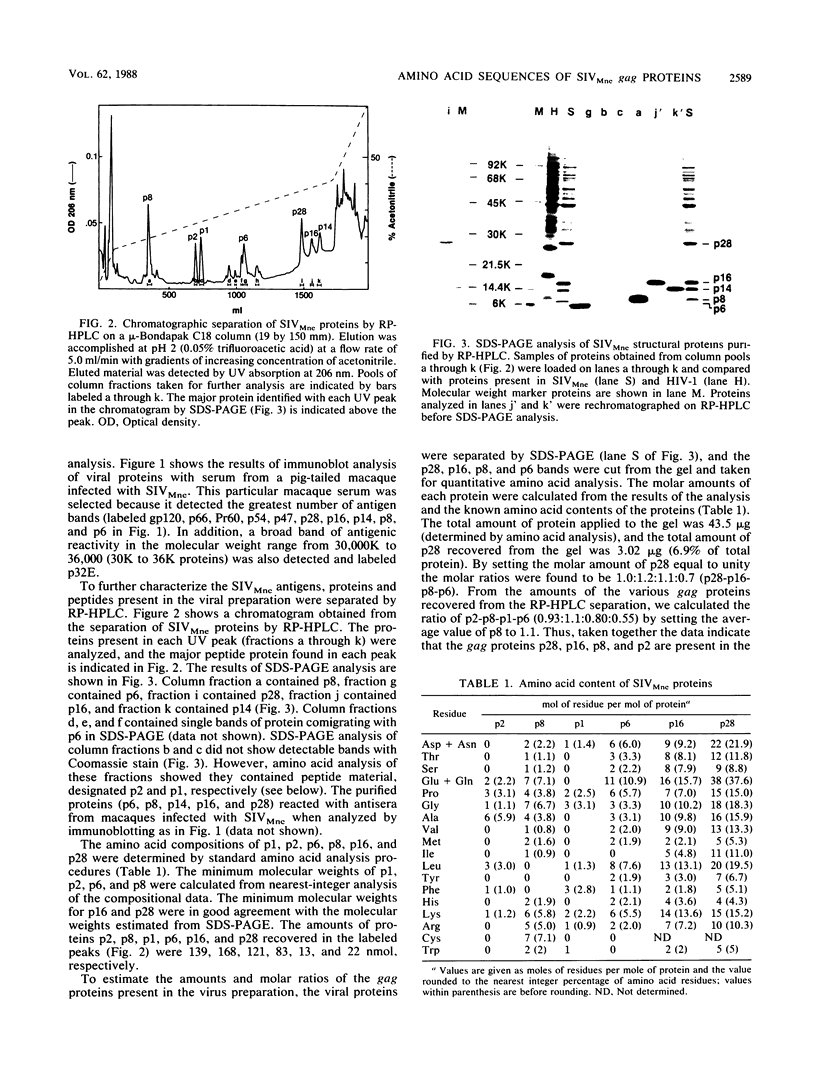
A simian immunodeficiency virus (SIV) designated SIVMne was isolated from a pig-tailed macaque with lymphoma housed at the University of Washington Regional Primate Research Center, Seattle. To better establish the relationship of SIVMne to other immunodeficiency viruses, we purified and determined the partial amino acid sequences of six structural proteins (p1, p2, p6, p8, p16, and p28) from SIVMne and compared these amino acid sequences to the translated nucleotide sequences of SIVMac and human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2). A total of 125 residues of SIVMne amino acid sequence were compared to the predicted amino acid sequences of the gag precursors of SIV and HIVs. In the compared regions 92% of the SIVMne amino acids were identical to predicted residues of SIVMac, 83% were identical to predicted residues of HIV-2, and 41% were identical to predicted residues of HIV-1. These data reveal that the six SIVMne proteins are proteolytic cleavage products of the gag precursor (Pr60gag) and that their order in the structure of Pr60gag is p16-p28-p2-p8-p1-p6. Rabbit antisera prepared against purified p28 and p16 were shown to cross-react with proteins of 60, 54, and 47 kilodaltons present in the viral preparation and believed to be SIVMne Pr60gag and intermediate cleavage products, respectively. SIVMne p16 was shown to contain covalently bound myristic acid, and p8 was identified as a nucleic acid-binding protein. The high degree of amino acid sequence homology between SIVs and HIV-2 around proven proteolytic cleavage sites in SIV Pr60gag suggests that proteolytic processing of the HIV-2 gag precursor is probably very similar to processing of the SIV gag precursor. Peptide bonds cleaved during proteolytic processing of the SIV gag precursor were similar to bonds cleaved during processing of HIV-1 gag precursors, suggesting that the SIV and HIV viral proteases have similar cleavage site specificities.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barin F., M'Boup S., Denis F., Kanki P., Allan J. S., Lee T. H., Essex M. Serological evidence for virus related to simian T-lymphotropic retrovirus III in residents of west Africa. Lancet. 1985 Dec 21;2(8469-70):1387–1389. doi: 10.1016/s0140-6736(85)92556-5. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Arthur L. O., Tsai C. C., Sowder R., Copeland T. D., Henderson L. E., Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986 Nov;60(2):483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Morton W. R., Clark E. A., Tsai C. C., Ochs H. D., Ward J. M., Kuller L., Knott W. B., Hill R. W., Gale M. J. Inoculation of baboons and macaques with simian immunodeficiency virus/Mne, a primate lentivirus closely related to human immunodeficiency virus type 2. J Virol. 1988 Jun;62(6):2091–2101. doi: 10.1128/jvi.62.6.2091-2101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Copeland T. D., Morgan M. A., Oroszlan S. Complete amino acid sequence of the basic nucleic acid binding protein of feline leukemia virus. Virology. 1984 Feb;133(1):137–145. doi: 10.1016/0042-6822(84)90432-x. [DOI] [PubMed] [Google Scholar]
- Copeland T. D., Morgan M. A., Oroszlan S. Complete amino acid sequence of the nucleic acid-binding protein of bovine leukemia virus. FEBS Lett. 1983 May 30;156(1):37–40. doi: 10.1016/0014-5793(83)80243-9. [DOI] [PubMed] [Google Scholar]
- Copeland T. D., Oroszlan S., Kalyanaraman V. S., Sarngadharan M. G., Gallo R. C. Complete amino acid sequence of human T-cell leukemia virus structural protein p15. FEBS Lett. 1983 Oct 17;162(2):390–395. doi: 10.1016/0014-5793(83)80793-5. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Franchini G., Gurgo C., Guo H. G., Gallo R. C., Collalti E., Fargnoli K. A., Hall L. F., Wong-Staal F., Reitz M. S., Jr Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature. 1987 Aug 6;328(6130):539–543. doi: 10.1038/328539a0. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Swenson R. B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc Natl Acad Sci U S A. 1986 Jul;83(14):5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Sowder R. C., Smythers G. W., Oroszlan S. Primary structure of the low molecular weight nucleic acid-binding proteins of murine leukemia viruses. J Biol Chem. 1981 Aug 25;256(16):8400–8406. [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R. C., Smythers G. W., Oroszlan S. Chemical and immunological characterizations of equine infectious anemia virus gag-encoded proteins. J Virol. 1987 Apr;61(4):1116–1124. doi: 10.1128/jvi.61.4.1116-1124.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R., Copeland T. D., Smythers G., Oroszlan S. Quantitative separation of murine leukemia virus proteins by reversed-phase high-pressure liquid chromatography reveals newly described gag and env cleavage products. J Virol. 1984 Nov;52(2):492–500. doi: 10.1128/jvi.52.2.492-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R., Smythers G., Benveniste R. E., Oroszlan S. Purification and N-terminal amino acid sequence comparisons of structural proteins from retrovirus-D/Washington and Mason-Pfizer monkey virus. J Virol. 1985 Sep;55(3):778–787. doi: 10.1128/jvi.55.3.778-787.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Mullins J. I. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell. 1987 May 8;49(3):307–319. doi: 10.1016/0092-8674(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Hizi A., Henderson L. E., Copeland T. D., Sowder R. C., Hixson C. V., Oroszlan S. Characterization of mouse mammary tumor virus gag-pro gene products and the ribosomal frameshift site by protein sequencing. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7041–7045. doi: 10.1073/pnas.84.20.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Townsley K., Varmus H. E., Majors J. Two efficient ribosomal frameshifting events are required for synthesis of mouse mammary tumor virus gag-related polyproteins. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4298–4302. doi: 10.1073/pnas.84.12.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki P. J., Kurth R., Becker W., Dreesman G., McLane M. F., Essex M. Antibodies to simian T-lymphotropic retrovirus type III in African green monkeys and recognition of STLV-III viral proteins by AIDS and related sera. Lancet. 1985 Jun 8;1(8441):1330–1332. doi: 10.1016/s0140-6736(85)92818-1. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., M'Boup S., Ricard D., Barin F., Denis F., Boye C., Sangare L., Travers K., Albaum M., Marlink R. Human T-lymphotropic virus type 4 and the human immunodeficiency virus in West Africa. Science. 1987 May 15;236(4803):827–831. doi: 10.1126/science.3033826. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., McLane M. F., King N. W., Jr, Letvin N. L., Hunt R. D., Sehgal P., Daniel M. D., Desrosiers R. C., Essex M. Serologic identification and characterization of a macaque T-lymphotropic retrovirus closely related to HTLV-III. Science. 1985 Jun 7;228(4704):1199–1201. doi: 10.1126/science.3873705. [DOI] [PubMed] [Google Scholar]
- Kannagi M., Kiyotaki M., Desrosiers R. C., Reimann K. A., King N. W., Waldron L. M., Letvin N. L. Humoral immune responses to T cell tropic retrovirus simian T lymphotropic virus type III in monkeys with experimentally induced acquired immune deficiency-like syndrome. J Clin Invest. 1986 Nov;78(5):1229–1236. doi: 10.1172/JCI112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Rein A., Shibuya M., Odaka T., Oroszlan S. Murine leukemia virus maturation: protease region required for conversion from "immature" to "mature" core form and for virus infectivity. Virology. 1985 Sep;145(2):280–292. doi: 10.1016/0042-6822(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Li Y., Naidu Y. M., Butler C. V., Ochs M. F., Jaenel G., King N. W., Daniel M. D., Desrosiers R. C. Comparison of simian immunodeficiency virus isolates. Nature. 1988 Feb 18;331(6157):619–622. doi: 10.1038/331619a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Lowenstine L. J., Pedersen N. C., Higgins J., Pallis K. C., Uyeda A., Marx P., Lerche N. W., Munn R. J., Gardner M. B. Seroepidemiologic survey of captive Old-World primates for antibodies to human and simian retroviruses, and isolation of a lentivirus from sooty mangabeys (Cercocebus atys). Int J Cancer. 1986 Oct 15;38(4):563–574. doi: 10.1002/ijc.2910380417. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Rangan S. R., Baskin G. B., Gormus B. J., Wolf R. H., Andes W. A., West M., Montelaro R. C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986 May 22;321(6068):435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Copeland T. D. Primary structure and processing of gag and env gene products of human T-cell leukemia viruses HTLV-ICR and HTLV-IATK. Curr Top Microbiol Immunol. 1985;115:221–233. doi: 10.1007/978-3-642-70113-9_14. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Henderson L. E., Stephenson J. R., Copeland T. D., Long C. W., Ihle J. N., Gilden R. V. Amino- and carboxyl-terminal amino acid sequences of proteins coded by gag gene of murine leukemia virus. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1404–1408. doi: 10.1073/pnas.75.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Oroszlan S. In vivo modification of retroviral gag gene-encoded polyproteins by myristic acid. J Virol. 1983 May;46(2):355–361. doi: 10.1128/jvi.46.2.355-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Tsai S. C., Kung H. F., Oroszlan S., Moss J., Vaughan M. Hydroxylamine-stable covalent linkage of myristic acid in G0 alpha, a guanine nucleotide-binding protein of bovine brain. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1234–1239. doi: 10.1016/0006-291x(87)90780-7. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Translational readthrough of an amber termination codon during synthesis of feline leukemia virus protease. J Virol. 1985 Sep;55(3):870–873. doi: 10.1128/jvi.55.3.870-873.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Smythers G. W., Oroszlan S. Bovine leukemia virus protease: purification, chemical analysis, and in vitro processing of gag precursor polyproteins. J Virol. 1986 Mar;57(3):826–832. doi: 10.1128/jvi.57.3.826-832.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]