Abstract
Apical large-conductance Ca2+-activated K+ (BK) channels in the cortical collecting duct (CCD) mediate flow-stimulated K+ secretion. Dietary K+ loading for 10–14 days leads to an increase in BK channel mRNA abundance, enhanced flow-stimulated K+ secretion in microperfused CCDs, and a redistribution of immunodetectable channels from an intracellular pool to the apical membrane (Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Am J Physiol Renal Physiol 289: F922–F932, 2005). To test whether this adaptation was mediated by a K+-induced increase in aldosterone, New Zealand White rabbits were fed a low-Na+ (LS) or high-Na+ (HS) diet for 7–10 days to alter circulating levels of aldosterone but not serum K+ concentration. Single CCDs were isolated for quantitation of BK channel subunit (total, α-splice variants, β-isoforms) mRNA abundance by real-time PCR and measurement of net transepithelial Na+ (JNa) and K+ (JK) transport by microperfusion; kidneys were processed for immunolocalization of BK α-subunit by immunofluorescence microscopy. At the time of death, LS rabbits excreted no urinary Na+ and had higher circulating levels of aldosterone than HS animals. The relative abundance of BK α-, β2-, and β4-subunit mRNA and localization of immunodetectable α-subunit were similar in CCDs from LS and HS animals. In response to an increase in tubular flow rate from ∼1 to 5 nl·min−1·mm−1, the increase in JNa was greater in LS vs. HS rabbits, yet the flow-stimulated increase in JK was similar in both groups. These data suggest that aldosterone does not contribute to the regulation of BK channel expression/activity in response to dietary K+ loading.
Keywords: ROMK, epithelial sodium channel, mechanoregulation, laminar shear stress, epithelial cell
the late distal convoluted and connecting tubules (CNT) and cortical collecting duct (CCD) of the distal nephron mediate, in large part, the final regulation of urinary K+ excretion (19). The traditional model by which K+ secretion is accomplished in these segments can be summarized as follows. Na+ enters the CNT and principal cell from the urinary fluid through the apical amiloride-sensitive epithelial Na+ channel (ENaC) and is then transported out of the cell at the basolateral membrane in exchange for uptake of K+ via the basolateral Na+-K+-ATPase. The high K+ concentration within the cell and lumen-negative voltage, established by electrogenic Na+ reabsorption, create a favorable electrochemical gradient for K+ to diffuse into the urinary space through apical K+-selective channels. Thus vectorial K+ secretion in these segments requires a favorable electrochemical gradient and an apical permeability to K+.
Two conducting K+-selective channels have been identified by patch-clamp analysis in the apical membrane of the CNT and CCD: the low-conductance inwardly rectifying secretory K+ (SK/ROMK) channel and the large-conductance Ca2+-activated K+ (BK) channel. The relatively abundant SK/ROMK channel, located in CNT and principal cells but not intercalated cells, has a high open probability (Po) at the resting membrane potential and is considered to mediate baseline K+ secretion (15, 16, 69). In contrast, the BK channel, more prevalent in intercalated than principal cells (15, 28, 40), is rarely open at physiological membrane potentials but can be activated by cell depolarization, membrane stretch, and increases in intracellular Ca2+ concentration ([Ca2+]i) (28, 40, 43, 52). Recent studies by ourselves (74, 75) and others (5, 40, 45, 48) suggest that the BK channel plays a major role in flow-stimulated K+ secretion.
The BK channel consists of a pore-forming α-subunit, encoded by the Drosophila slo gene, and a regulatory β-subunit (3, 22). Alternative splicing of the single gene that encodes the α-subunit generates variants that differ in their responses to changes in Ca2+ and voltage, regulation by protein phosphorylation and other signaling cascades (9, 50, 56, 64, 66, 78, 81, 82), as well as cell localization (23, 68, 80). Five distinct variants of the mouse BK α-subunit COOH terminus have been identified, three of which are expressed at significant levels in kidney (% of total renal BK channel mRNA levels) (9): ZERO, resulting from splicing of exon 19 to exon 23 (75%); e21 resulting in insertion of a 59-amino acid, cysteine-rich stress-axis-regulated exon (STREX) between exons 19 and 23 (10%); and Δe23, resulting from the skipping of exon 23, thereby splicing exon 19 to 24, which leads to a frameshift that introduces a premature stop codon within exon 24 (5%). The STREX variant demonstrates a left shift in the Ca2+ sensitivity of the channel compared with the ZERO variant and slower rates of deactivation (9, 50). Δe23 is not functionally expressed at the cell surface and acts as a dominant negative of cell surface expression by trapping other BK channel splice variant α-subunits in the endoplasmic reticulum and perinuclear compartments (9, 23). In rabbit, medullary thick ascending limb cells express two alternatively spliced transcripts of the α-subunit: rbslo1, with a novel insert of 59 novel amino acids and thus likely representing the STREX variant, and rbslo2, in which a COOH terminus deletion creates a frameshift and a truncation of the COOH terminus (34). rbslo1 is expressed at the apical cell membrane, whereas rbslo2 is localized intracellularly (23).
The renal response to dietary K+ loading includes an increase in urinary K+ excretion, due in large part to enhanced K+ secretion in the distal nephron (60, 73, 76). This K+ adaptation is associated with increases in the density of conducting SK channels and the electrochemical driving force favoring K+ secretion across the apical membrane of the CCD (41, 69). Recent data from our group (36) have also demonstrated a role for the BK channel in renal K+ adaptation. Specifically, dietary K+ loading for 10–14 days led to an increase in abundance of message encoding BK α- and β2–4-subunits in single CCDs with a redistribution of immunodetectable channel proteins from an intracellular pool to the apical membrane (36). Additionally, CCDs isolated from K+-loaded animals and microperfused in vitro demonstrated enhanced flow-stimulated net K+ secretion compared with tubules studied from control-fed animals (36). This adaptation could be mediated directly by a transient increase in plasma K+ concentration but could also be initiated by a dietary K+-induced increase in circulating levels of aldosterone (41, 60). Increases in extracellular K+ concentration directly stimulate aldosterone production in zona glomerulosa cells of adrenal glands (6, 59). Although serum aldosterone levels were not measured in the study by Najjar et al. (36), it is safe to assume, on the basis of past studies by others (55, 72), that high-K+-fed rabbits had higher circulating serum aldosterone levels than did their low-K+-fed counterparts.
The goal of the present study was to test the hypothesis that the adaptation in BK channel expression and activity observed in response to an increase in dietary K+ is mediated by increases in endogenous circulating levels of aldosterone. We acknowledge that independent variation of aldosterone and plasma K+ concentration in adrenalectomized rabbits would have allowed us to optimally define the isolated effects of aldosterone on BK-mediated K+ secretion. However, our goal was to study the regulation of the BK channel by dietary manipulation leading to physiologically relevant changes in circulating levels of endogenous mineralocorticoids, not by exogenous administration of supraphysiological doses of steroids.
To this end, New Zealand White (NZW) rabbits were fed for 7–10 days with low-Na+ (LS) or high-Na+ (HS) diets designed to alter circulating levels of aldosterone but not plasma K+ concentration. Thereafter, single CCDs were isolated for relative quantitation of BK channel subunit (total and α-splice variants and β-isoforms) mRNA abundance by real-time PCR, immunolocalization of BK α-subunit by immunofluorescence microscopy, and measurement of flow-stimulated net transepithelial cation transport via in vitro microperfusion. Our data suggest that aldosterone does not contribute to the regulation of BK channel expression/activity in response to dietary K+ loading and that a kaliuretic factor(s)/pathway other than this hormone regulates renal K+ transport in response to changes in dietary K+ intake, as has been proposed by others (42).
METHODS
Animals.
Adult female NZW rabbits were obtained from Covance (Denver, PA) and housed at the Mount Sinai School of Medicine Center for Comparative Medicine and Surgery. Animals were placed on either a LS diet (Na+: 26 meq/kg; K+: 294 meq/kg) or a HS diet (Na+: 140 meq/kg; K+: 275 meq/kg) for 7–10 days and allowed free access to tap water. Other rabbits were randomized to receive a low-K+ (LK; 0.13% or 34 meq K+/kg) or high-K+ (HK; 1.56%, or 398 meq K+/kg) diet, both containing 0.29% Na+, or 7.5 g/kg diet, for 7–10 days (36). Special diets were obtained from Harlan Teklad (Madison, WI). Animal weights and the amount of food and water consumed were monitored daily. All protocols were approved by the Institutional Animal Care and Use Committee of the Mount Sinai School of Medicine. Animals were euthanized in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. At the time of death, samples of ventricular blood and urine were obtained for measurement of Na+ and K+ concentrations and serum aldosterone concentration.
Measurement of serum aldosterone concentration.
Serum aldosterone levels in the rabbits were quantitatively measured with the use of the Aldosterone Coated-Tube Radioimmunoassay Kit (Diagnostic Systems Laboratories). The procedure follows the basic principle of radioimmunoassay in which a radioactive and a nonradioactive antigen compete for a fixed number of antibody binding sites. The kit contains the following reagents: 1) aldosterone standards, 2) aldosterone I-125 reagent, 3) aldosterone-coated tubes, and 4) aldosterone controls. Rabbit sera were collected as described above and stored at −20°C. Reagents were allowed to reach room temperature and mixed thoroughly before use. Two uncoated tubes for “total counts” and coated tubes in duplicate for standards, controls, and unknowns were labeled. One hundred microliters of the standards, controls, and unknowns were pipetted into the appropriate coated tubes. Five hundred microliters of the I-125 reagent were then added to all tubes. Tubes were shaken gently and then incubated for 3 h on a shaker set at 180 rpm at room temperature (25°C). All tubes, except “total count” tubes, were aspirated, drained on absorbent material for at least 2 min, and then counted in a gamma counter for 1 min.
Results were calculated with a log-linear curve. Mean counts per minute (cpm) for each standard, control, and unknown were calculated. The percent bound of the total (B/T) for each standard, control, and unknown was then calculated. A curve of radioactivity counts (cpm) or % B/T for the aldosterone standards (y-axis) was plotted against the aldosterone concentration (x-axis) on log-linear (semilog) graph paper. A standard curve was drawn through the mean of the duplicate points. Finally, the aldosterone concentration was determined from the means of the duplicate counts of each control and unknown from the standard curve.
Relative quantitation of BK channel subunit and variant mRNA in single CCDs.
CCD microdissection was performed in 1× phosphate-buffered saline (PBS) containing 10 mM vanadyl ribonucleoside complexes (Sigma, St. Louis, MO) to inhibit RNA degradation for no longer than 1 h after the death of the animal. Single tubules were rinsed three times in cold 1× PBS and transferred to a 1.5-ml microcentrifuge tube for extraction of RNA. Approximately 10 mm of total length of CCDs was pooled for each sample. RNA was extracted from the single CCDs and cDNA synthesized with random primers as previously described (36).
Channel transcript expression was analyzed by real-time semiquantitative PCR with the Taqman technique and an ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA). Primers and probes for BK α-, β1-, β2-, and β4-subunits (Table 1), synthesized with Primer Express Software (Applied Biosystems) according to the recommended guidelines based on sequences obtained from GenBank, were identical to those reported previously (36). Quantitation of the β3-subunit was not performed in this study because we have had to use the SYBR Green detection system in the past to quantitate this transcript (36); it should also be noted that in a recent study RT-PCR performed on whole mouse kidney RNA revealed expression of BK β1-, β2-, and β4- but not β3-subunits (18). Additional primers and probes designed to target total and unique splice variants of the α-subunit were also synthesized as above based on sequences reported by Chen et al. (9). The primers for BK α, used for analysis of the expression of α-subunit mRNA in single CCDs isolated from rabbits fed a HS or LS diet (Fig. 1), and those for total BK, used to examine expression of total BK channel α-subunit and splice variant expression in single CCDs in response to changes in dietary Na+ and K+ intake (Fig. 2), were designed to amplify a highly conserved region common to all splice variants (9, 36). ENaC (α-subunit)-specific primers and probe were synthesized based on sequences reported by Audige et al. (4). The 5′ end of each probe was labeled with 6-FAM dye, and the 3′ end was labeled with TAMRA. Primers and probes specific for 18S ribosomal RNA (Applied Biosystems) were selected for the internal positive reference control.
Table 1.
Primers and probes used for real-time PCR
| Forward Primer | Reverse Primer | Probe | |
|---|---|---|---|
| BK | |||
| α | CATCTTTATCAGCACGTGGCTAAC | CCATGGGTCCCCTGAATTCT | CGGCCGGGTTCATCCACTTGG |
| Total BK | CTCAAATGAAATGTACACAGAATATCTG | CTATCATAAGGAGCTTGAGCTTCACA | CCTTCGTGGGTCTGTCCTTCCCTACTGTT |
| α STREX | TTTGGTTGCGGACGTTCTGA | AGGGTGTCCACGTGAC | CTGCTCGTGCATGTCAGGCCGT |
| α ZERO | GCCAAAGAAGTTAAAAGGGCATT | CGGCTGCTCATCTTCAAGC | TGACATCACAGATCCCAAAAGAATAAAAAAATG |
| β1 | ACATCCTGGGAACGACTATGCT | CAGCCGACACAAGGATTCCT | CCCCTCTACCAGAAAAGCGTGTGGACC |
| β2 | TCTTGGATAAAAGGAAAACAGTCACA | GGCCAGTCCCAGGAGGAT | ACTGAAAGCAGGAGAGGACCGGGC |
| β4 | CGTCCAGGTGTACGTGAACAAC | TGGTGCTGGTCGCTGTGTAG | CCGAGTCCAACTCCAGGGCGCT |
| ENaC | |||
| α | CTAGGGTGATGGTGCATGGC | GATGGAGGTCTCCACACCGG | GCCGCAAGTTAAAGCCGCCATCA |
BK, large-conductance Ca2+-activated K+ channel; STREX, stress-axis-regulated exon; ENaC, epithelial Na+ channel.
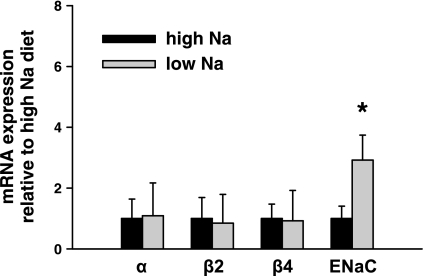
Fig. 1.
Effect of dietary Na+ intake on large-conductance Ca2+-activated K+ (BK) channel and epithelial Na+ channel (ENaC) subunit transcript expression in single cortical collecting ducts (CCDs). Real-time PCR was used to examine the expression of α-, β1-, β2-, and β4-subunits and α-ENaC mRNA in single CCDs isolated from rabbits fed a high (HS)-or low (LS)-Na+ diet. Channel subunit-specific mRNA abundance in CCDs harvested from LS-fed animals, normalized to that of 18S measured in the same sample, is presented as the fold difference relative to the ratio detected in tubules isolated from HS-fed rabbits. BK α (n = 10), β2 (n = 8), and β4 (n = 10) mRNA expression in CCDs isolated from HS-fed animals did not significantly differ from that detected in LS-fed animals (n = 12, 11, 13, respectively; P = nonsignificant). β1-Subunit transcripts were not detected in any CCD sample. α-ENaC mRNA expression in CCDs isolated from LS-fed animals (n = 6) significantly exceeded that detected in HS-fed animals (n = 10). Values are means ± SE. *P < 0.05 compared with HS.
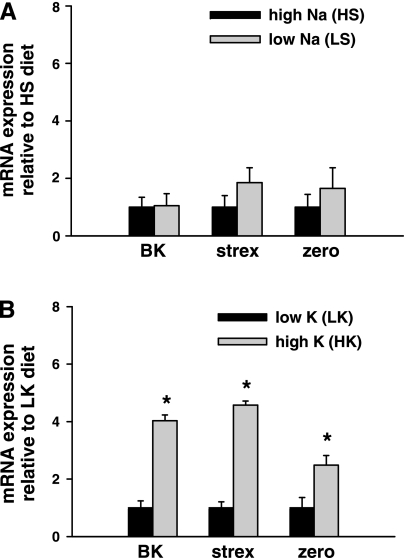
Fig. 2.
Effect of dietary Na+ and K+ intake on BK channel α-subunit splice variant expression in single CCDs. Real-time PCR was used to examine expression of total BK α, stress-axis-regulated exon (STREX), and zero variant mRNA in single CCDs isolated from animals fed a HS or LS (A) or high-K+ (HK) or low-K+ (LK) (B) diet. Channel variant-specific mRNA abundance was normalized to that of 18S measured in the same sample and then normalized to the HS (A) or LK (B) ratio for that variant. A: expression levels of total BK α (n = 7), STREX (n = 7), and zero (n = 7) variant mRNA in CCDs isolated from HS-fed animals did not differ from those detected in LS-fed animals (n = 7, 7, 8, respectively). B: in contrast, CCDs isolated from animals fed a HK diet exhibited significantly greater mRNA abundance for total BK α (n = 9), STREX (n = 10), and zero (n = 9) variants than detected in LK-fed rabbits (n = 9, 9, 9, respectively). Values are means ± SE. *P < 0.03 compared with LK.
Real-time PCR was performed by adding 8 μl of PCR Master Mix containing 0.05 μl of Platinum Taq DNA Polymerase (Invitrogen, Carlsbad, CA), AmpErase UNG (Applied Biosystems), dNTPs with dUTP (Applied Biosystems), passive reference ROX (Invitrogen), optimized buffer component, 0.2 μl (20 pM) of forward and reverse primers, 0.2 μl of Taqman probe, and nuclease-free water to 2 μl of cDNA. For the ENaC studies, 2% DMSO was added to the Master Mix. Each 384-well plate was covered with optical adhesive film, and after the initial steps of 50°C for 2 min for optimal AmpErase UNG enzyme activity and 95°C for 10 min for activation of DNA polymerase, 40 cycles of 95°C for 15 s (melt) and 60°C for 1 min (anneal/extend) were performed.
After PCR amplification, SDS 2.1 software (Applied Biosystems) was utilized to calculate the threshold values (threshold cycle, Ct) for BK and ENaC subunit transcripts. Relative gene expression of channel subunits was calculated with the 2 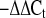 method (30).
method (30).
Immunofluorescence localization of BK channels in kidney.
Coronal sections of rabbit kidneys were fixed in 4% paraformaldehyde and sucrose and embedded in optimal cutting temperature (OCT) compound. Serial 4-μm-thick paraffin sections were cut on a cryostat (Leica CM1900) and collected on Superfrost microscopic slides (Fisher Scientific). Sections were hydrated in PBS for 30 min and then blocked with 1% BSA in PBS-0.02% sodium azide for 30 min.
Tissue sections were incubated with a previously characterized (36) anti-BK channel α-subunit antibody (15 μg/ml) for 75 min at room temperature. For peptide competition experiments, peptide was added to the primary antibody at a 1:100 dilution (stock concentration of 10.5 mg/ml), as previously described (36). After incubation with primary antibody the sections were washed twice for 5 min with high-salt PBS (2.7% NaCl) solution, followed by one 5-min wash in PBS. The secondary anti-chicken IgY antibody, a FITC-conjugated Affinipure F(ab′)2 fragment from donkey, was applied at a 1:50 dilution (stock concentration of 1.5 mg/ml), and sections were colabeled with CY3-conjugated Dolichos biflorus agglutinin (DBA, 5 μg/ml), a marker of principal cells in the rabbit distal nephron (75), prepared in PBS solution, for 60 min at room temperature. Each section was washed twice for 5 min with high-salt PBS solution, followed by one 5-min wash in PBS.
All sections were mounted on coverslips with Vectashield (Vector Labs, Burlingame, CA). Confocal microscopy was performed with a ×40 plan-Apochromat objective (numerical aperture 1.4), with the zoom 2 setting, mounted on a Leica DMRXE equipped with appropriate lasers. The images of the slides incubated with the anti-BK antibody alone and with the anti-BK antibody adsorbed to the immunizing peptide were obtained with identical objective, zoom, and laser settings; these slides were incubated on the same day with identical immunofluorescence staining protocols. The images (1,024 × 1,024 pixels) were saved in a tag-information-file-format (TIFF), and the contrast levels of the images were adjusted in the Photoshop program (Adobe, Mountain View, CA) on a Power PC G-4 Macintosh (Apple, Cupertino, CA). The number of cells showing linear apical BK α-subunit immunofluorescence was counted in individual tubular profiles, as previously described (36).
Microperfusion of single tubules.
Kidneys were removed via a midline incision, and single tubules were dissected in cold (4°C) Ringer solution and microperfused in vitro as previously described (74). Briefly, each isolated tubule was immediately transferred to a temperature and O2/CO2-controlled specimen chamber, mounted on concentric glass pipettes, and perfused and bathed at 37°C with Burg's perfusate containing (in mM) 120 NaCl, 25 NaHCO3, 2.5 K2HPO4, 2.0 CaCl2, 1.2 MgSO4, 4.0 Na lactate, 1.0 Na3 citrate, 6.0 l-alanine, and 5.5 d-glucose, pH 7.4, 290 ± 2 mosmol/kgH2O (74). During the 45-min equilibration period and thereafter, the perfusion chamber was continuously suffused with a gas mixture of 95% O2-5% CO2 to maintain the pH of the Burg's solution at 7.4 at 37°C. The bathing solution was continuously exchanged at a rate of 10 ml/h with a syringe pump (Razel, Stamford, CT).
Transport measurements were performed in the absence of transepithelial osmotic gradients, and thus water transport was assumed to be zero. Three or four samples of tubular fluid were collected under water-saturated light mineral oil by timed filling of a calibrated 30-nl volumetric constriction pipette at slow (∼1 nl·min−1·mm−1) and fast (∼ 5 nl·min−1·mm−1) flow rates. The K+ and Na+ concentrations of perfusate (C0) and collected tubular fluid (CL) were determined by helium glow photometry. The rates of K+ and Na+ transport (Jx, in pmol·min−1·mm−1) were calculated as follows: J = (C0 − CL) × VL/L, where VL is the rate of collection of tubular fluid determined from the time (in min) required to fill the precalibrated volumetric pipette and L is the tubule length in millimeters. To determine the concentration of Na+ and K+ delivered to the tubular lumen, ouabain (100 μM) was added to the bath at the conclusion of each experiment to inhibit all active transport, and an additional three or four samples of tubular fluid were obtained for analysis. The rates of net transport (in pmol·min−1·mm tubular length−1) were calculated with standard flux equations, and the calculated ion fluxes were averaged to obtain a mean rate of ion transport for the CCD at each flow rate, as previously described (51). The flow rate was varied by adjusting the height of the perfusate reservoir. The sequence of flow rates was randomized within each group of tubules to minimize any bias induced by time-dependent changes in ion transport.
Statistics.
All results are expressed as means ± SE; n is the number of animal or tubule samples used for in vitro microperfusion studies and real-time PCR. Comparisons were made by paired and unpaired t-tests as appropriate, with commercially available statistical software (SPSS, Chicago, IL). Significance was asserted if P < 0.05.
Real-time PCR data are represented as the fold change in mRNA expression, normalized to that of 18S, relative to the values observed under the condition that did not lead to an increase in plasma aldosterone concentration. The standard deviation of the fold change was calculated from the standard deviations (S) of each subunit or splice variant value under the different dietary conditions with the formula s = √S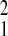 + S
+ S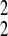 , as we described previously (70).
, as we described previously (70).
RESULTS
Effect of diet on serum and urine electrolytes and serum aldosterone concentration.
Table 2 summarizes the weight gain, serum and urine Na+ and K+ concentrations, and serum aldosterone levels in animals on each diet. Weight gain was identical in the HS- and LS-fed animals during the period of dietary Na+ manipulation. As expected, LS rabbits excreted virtually no urinary Na+ and had higher circulating levels of aldosterone than HS animals; there were no significant differences between serum Na+ and K+ and urinary K+ concentrations between the two experimental groups.
Table 2.
Biochemical profiles of high- and low-Na+-fed rabbits
| Diet | Weight Gain, kg | Serum Na+, meq/l | Serum K+, meq/l | Urine Na+, meq/l | Urine K+, meq/l | Serum Aldosterone, ng/dl |
|---|---|---|---|---|---|---|
| HS | 0.27±0.02 (n=12) | 139.3±1.2 (n=24) | 4.5±0.1 (n=24) | 72.3±7.6 (n=19) | 278.4±23.2 (n=19) | 31.0±4.2 (n=7) |
| LS | 0.26±0.02 (n=12) | 137.4±0.9 (n=22) | 4.5±0.1 (n=22) | 0.1±0.04*(n=23) | 248.5±17.4 (n=23) | 113.8±13.9*(n=14) |
Values are means ± SE for n animals studied. HS, high Na+; LS, low Na+.
P < 0.05 vs. HS diet.
Effect of dietary Na+ and K+ intake on expression of BK channel subunit mRNA.
The relative abundance of BK channel α-, β1-, β2-, and β4-subunit and α-ENaC transcripts was examined by real-time PCR of single CCDs isolated from LS and HS rabbits. Single CCDs expressed BK α-, β2-, and β4-subunit mRNAs (Fig. 1); the β1-subunit was not detected in CCDs from either group, as previously described (36). BK α (n = 10), β2 (n = 8), and β4 (n = 10) mRNA expression in CCDs isolated from HS animals did not significantly differ from that detected in LS animals [n = 12, 11, 13, respectively: P = not significant (NS)] (Fig. 1). In contrast, α-ENaC mRNA expression in CCDs isolated from LS animals (n = 6) significantly exceeded that detected in HS animals (n = 10, P < 0.05) (Fig. 1).
Alternative splicing of the pore-forming α-subunits of BK channels modifies the functional properties of BK channels, as summarized above. Alternative splicing of STREX is controlled by circulating stress and sex hormones, including corticosterone and dexamethasone, as well as cellular excitability (24, 25, 77, 78). BK α (n = 7), STREX (n = 7), and zero (n = 7) mRNA expression in CCDs isolated from HS animals did not differ from that detected in LS animals (n = 7, 7, 8, respectively; P = NS) (Fig. 2A). In contrast, CCDs isolated from animals fed a HK diet exhibited significantly greater mRNA abundance for BK α (n = 9), STREX (n = 10), and zero (n = 9) variants than tubules from LK fed rabbits (n = 9, 9, 9, respectively; P < 0.03) (Fig. 2B).
Effect of dietary Na+ intake on immunolocalization of BK channel α-subunit.
We previously reported (36) that K+ adaptation in the rabbit is associated with an increase in steady-state abundance of BK channel subunit-specific mRNAs, immunodetectable apical α-subunit (consistent with redistribution from an intracellular pool to the plasma membrane), and flow-stimulated net K+ secretion. However, in that study, Western blotting of whole kidney failed to identify any differences in BK channel α-subunit abundance among control, high-K+-fed, and low-K+-fed rabbits. We speculated that our inability to detect differences in protein expression among the three groups was due to diet-induced alterations in BK channel expression in only a subset of nephron segments representing a minor fraction of the total renal mass. Given this concern, and the fact that immunoblotting whole kidney would not provide information about the cellular localization of channels, we sought to utilize an immunolocalization approach to determine whether changes in dietary Na+ intake altered the subcellular localization of BK channel in the CCD, as did changes in dietary K+ intake (36).
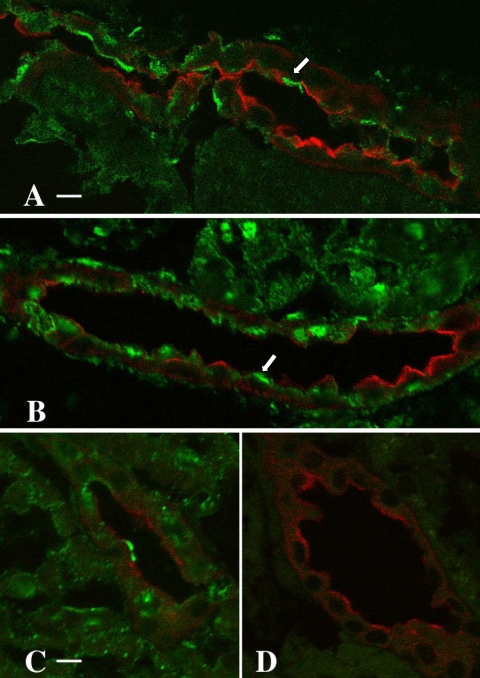
Cryosections of kidneys from HS- and LS-fed rabbits (n = 4 per group) were colabeled with CY3-conjugated DBA (red) to identify principal cells in the distal nephron and a previously characterized anti-BK channel α-subunit antibody (36), the latter visualized with a FITC-labeled (green) secondary antibody. Indirect immunofluorescence microscopy revealed immunodetectable BK α-subunit in a predominantly linear pattern along the apical membranes of DBA-negative cells, i.e., intercalated cells, in both LS (Fig. 3A, arrow)- and HS (Fig. 3B)-fed animals. Analysis of the number of cells showing linear apical BK α-subunit immunofluorescence in individual tubular profiles revealed apical BK immunolabeling in 11% of 542 cells (in 41 tubules) in LS-fed rabbits and 12% of 747 cells (in 51 tubules) in HS-fed rabbits. These results suggest that differences in dietary Na+ intake, and thus presumably circulating concentrations of aldosterone, do not alter cellular expression or localization of immunodetectable BK channel α-subunit in the CCD.
Fig. 3.
Immunolocalization of BK α-subunit in the kidney of adult rabbits maintained on LS (A, C, D) and HS (B) diets. Cryosections of kidneys from LS- and HS-fed animals were colabeled with CY3-conjugated Dolichos biflorus agglutinin (DBA; red), a principal cell marker, and an anti-BK channel α-subunit antibody, visualized with an FITC-labeled (green) secondary antibody. The pattern of apical expression, localized almost exclusively to DBA-negative cells (arrows), was similar in both experimental groups (compare A and B, same magnification; scale bar in A = 10 μm). Two sections of LS kidney cortex (C and D, same magnification) were labeled on the same day, one with the anti-BK channel antibody (C; scale bar = 5 μm) and the other (D) with antibody in the presence of excess immunizing peptide. Preincubation of the BK channel antibody with the immunizing peptide completely abolished anti-BK channel antibody labeling (D).
The specificity of the anti-BK channel antibody was confirmed by labeling cryosections obtained from a single LS-fed rabbit kidney with DBA and either the α-subunit antibody alone (Fig. 3C) or the channel antibody adsorbed to its immunizing peptide (Fig. 3D). Labeling and image acquisition were performed under identical conditions on the same day. Preincubation of the BK channel antibody with the immunizing peptide completely abolished anti-BK channel antibody labeling (Fig. 3D), thus confirming the specificity of the anti-BK channel staining in these studies.
Effects of diet on flow-stimulated net cation transport in microperfused CCDs.
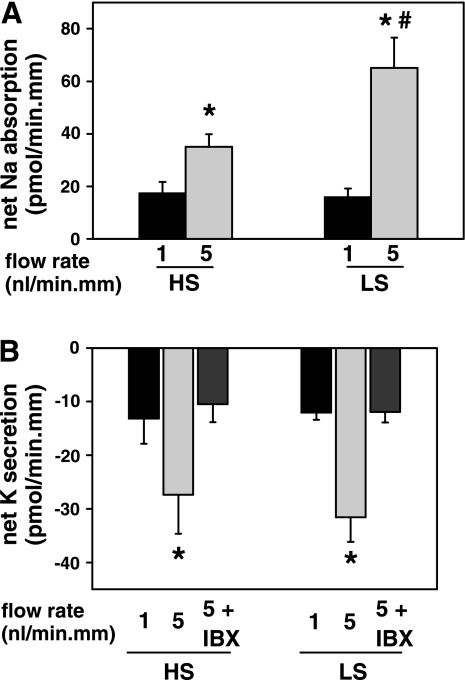
At a slow flow rate of 1 nl·min−1·mm−1, the rate of net K+ secretion (in pmol·min−1·mm−1) in CCDs isolated from LS animals (−12.0 ± 1.4, n = 7) did not differ from that measured in HS animals (−13.2 ± 4.7; n = 6; P = NS) (Fig. 4B). An increase in tubular fluid flow rate to 5 nl·min−1·mm−1 elicited equivalent approximately twofold increases in the rate of net K+ secretion in CCDs isolated from animals in the two experimental groups (−31.6 ± 4.6 and −27.4 ± 7.3 pmol·min−1·mm−1, respectively). Luminal addition of 50 nM iberiotoxin (IBX) inhibited flow-stimulated net K+ secretion in CCDs from both LS-fed (to −11.9 ± 2.0 pmol·min−1·mm−1; n = 3) and HS-fed (to −10.5 ± 3.4 pmol·min−1·mm−1; n = 3) animals. These results are consistent with the notion that flow-stimulated K+ secretion is mediated by an IBX-sensitive BK channel and that dietary Na+ manipulation, in contrast to dietary K+ alterations (36), does not modify BK channel function.
Fig. 4.
Effect of dietary Na+ intake on flow-stimulated net cation transport in isolated perfused CCDs. A: net Na+ absorption increased as tubular flow rate was increased from ∼1 to 5 nl·min−1·mm−1 in CCDs harvested from HS (n = 6)- and LS (n = 7 )-fed rabbits. However, the flow-stimulated increase in net Na+ absorption in LS-fed animals significantly exceeded that measured in animals fed a HS diet. B: a 5-fold increase in luminal flow rate increased net K+ secretion to an equivalent degree in the same set of tubules described in A. The flow-stimulated increment in net K+ secretion in both experimental groups was sensitive to iberiotoxin (IBX), an inhibitor of BK but not ROMK channels. Values are means ± SE. *P < 0.05 compared with transport rate at 1 nl·min−1·mm−1 in same tubules; #P < 0.05 compared with transport rate in HS-fed animals studied at same transport rate.
Net Na+ absorption was measured in the same tubules as above. At a flow rate of ∼1 nl·min−1·mm−1, the rate of net Na+ absorption (in pmol·min−1·mm−1) was identical in CCDs isolated from the LS-fed (15.8 ± 3.4; n = 7) and HS-fed (17.4 ± 4.3; n = 6; P = NS) rabbits (Fig. 4A). After an increase in tubular flow rate, net Na+ absorption increased significantly in both groups, although the increase in Na+ absorption (in pmol·min−1·mm−1) in the LS-fed group (to 65.1 ± 11.5) was greater than that detected in the HS-fed group (to 35.0 ± 4.8; P = 0.044).
DISCUSSION
We previously reported (36) that dietary K+ loading of rabbits for 7–10 days leads to an increase in BK channel function (assayed as IBX-sensitive flow-stimulated net K+ secretion in in vitro microperfused tubules), steady-state abundance of subunit (α, β2, β4) mRNA, and apical immunodetectable channel proteins in the CCD. The mechanisms underlying the upregulation of BK channel expression and function associated with this K+ adaptation are not known. Dietary K+ loading is associated with transient increases in plasma K+ concentration and circulating levels of mineralocorticoids (41, 44, 60), either of which could stimulate net K+ secretion. In support of a role for mineralocorticoids in the regulation of BK channel activity was the finding by Engbretson and Stoner (13) that microperfused CCDs from rabbits fed a low-Na+ diet for 4–10 days showed a greater flow dependence of net K+ secretion in the range of 0.5–3 nl/min than segments isolated from control-fed animals. It should be noted that in a similar study by Schwartz and Burg (55), CCDs isolated from rabbits subject to dietary Na+ restriction for 6–21 days failed to show a significant increase in net K+ secretion compared with control-fed animals. However, in the latter study, CCDs were microperfused at 5–10 nl/min (55), a range within and beyond which flow stimulation of net K+ secretion in the rabbit CCD saturates (13, 51, 61).
The results of the present study demonstrate that a physiologically relevant approximately fourfold increase in endogenous circulating levels of aldosterone induced by dietary Na+ restriction for 7–10 days, in the absence of significant changes in serum K+ concentration, fails to alter the abundance of message encoding the BK channel, localization of immunodetectable protein, and channel function (IBX-sensitive flow-stimulated net K+ secretion). These results differ from those reported by Engbretson and Stoner (13), summarized above, and may reflect our selection of a diet designed not to alter serum K+ concentration. We thus propose that the increases in flow-stimulated net K+ secretion and BK channel expression that we have reported in rabbits fed a high-K+ diet (36) are not mediated, either directly or indirectly, by aldosterone.
In general, chronic elevation of the ambient aldosterone concentration in vivo, by either dietary Na+ restriction (13, 76) or exogenous administration of mineralocorticoid (55, 61, 65), stimulates net K+ secretion in the CCD. This response appears to be due primarily to a steroid-induced increase in the apical expression of conducting Na+ channels (31, 41, 69) and net Na+ absorption (55, 61), generating a more favorable electrochemical driving force for K+ secretion into the urinary fluid. A key player in the response of the aldosterone-sensitive distal nephron to mineralocorticoids is serum- and glucocorticoid-inducible kinase (sgk)1, which is rapidly (within 15 min) induced by aldosterone (10, 37) but must be phosphorylated via signaling cascades involving phosphatidylinositol 3-kinase (PI3-kinase)- and 3-phosphoinositide-dependent kinases for its activation (reviewed in Ref. 26); sgk1 inactivates the ubiquitin protein ligase Nedd4-2, which in turn reduces endocytotic retrieval of ENaC at the cell surface (11, 57, 58). While dietary Na+ restriction or administration of exogenous mineralocorticoids does not affect the density of SK/ROMK channels in the rat CCD (16, 41), the SK channel has been reported to be present in far more patches of primary cultures of rabbit CCD cells grown in medium containing aldosterone than when cells were grown in the absence of hormone (29). The data from the present study, demonstrating that baseline net K+ secretion in CCDs harvested from rabbits fed a LS diet is not different from that detected in tubules harvested from HS-fed animals (Fig. 4), provide additional support for the notion that physiological increases in circulating levels of mineralocorticoids do not stimulate SK/ROMK channel expression/activity in native epithelia (Fig. 4).
The effect of mineralocorticoids on renal epithelial cell BK channel expression and activity is uncertain. The prevalence of BK channels in cell-attached patches of CCDs harvested from rats subject to chronic (10 day) DOCA treatment (54) or low-Na+ diet (40) was the same or less, respectively, as that detected in rats on a normal diet. Similarly, the presence of aldosterone in the culture medium was reported not to alter the frequency of BK channels in cell-attached patches of rabbit CCD principal cells grown in culture (29). However, it is likely that physiological BK channel activation requires membrane stretch and/or an increase in [Ca2+]i, and thus changes in activity may not be readily detected in the standard cell-attached patch configuration.
Chronic dietary K+ supplementation enhances renal K+ secretion (60, 76), due predominantly to an aldosterone-induced increase in driving force favoring K+ secretion in the distal nephron, described above, as well as an aldosterone-independent increase in density of SK channels, demonstrated in the adult rat (41, 69). Indeed, an increase in dietary K+ intake for as little as 6 h increases SK channel density in rat CCD, an adaptation well described after 10–14 days of high K+ intake (41, 69). While this effect appears to require an increase in plasma K+, the observation that adrenalectomized rats fail to exhibit an increase in SK channel density in response to high K+ intake suggests that circulating steroid levels play a permissive role in this process, at least in this species (41). In contrast, microperfused CCDs isolated from K+-adapted rabbits exhibit enhanced K+ secretion even after adrenalectomy (73). Furthermore, the apical K+ conductance of the CCD is increased in both control and adrenalectomized rabbits fed a high-K+ diet (35). These data suggest that mineralocorticoids may be necessary for K+ adaptation in rat, but not necessarily in rabbit.
The effect of dietary K+ loading on BK channel activity is inconsistent. K+ loading fails to stimulate BK channel activity in the rat CCD assayed by patch-clamp analysis in most (7, 20, 43), but not all (28), studies, although it does increase the density of these channels in rat distal colon (7), mouse distal nephron (5), and amphibian collecting tubule (62). These somewhat disparate results suggest that regulation of the BK channel during K+ adaptation may be species- and/or tissue specific.
BK channels are composed of pore-forming α-subunits and accessory β-subunits. Alternative splicing of the COOH terminus of the α-subunit results in channels that exhibit phenotypic diversity that can be further modified by association with distinct β-subunits and protein phosphorylation (50, 56, 66, 78). Whereas endogenous (corticosterone) and exogenous (dexamethasone) glucocorticoids regulate Slo splicing, and specifically STREX inclusion, in pituitary and chromaffin cells (78), the present study reveals that a physiologically relevant increase in circulating levels of mineralocorticoids does not affect STREX expression in rabbit CCD (Fig. 2A). While changes in dietary Na+ intake did not alter the expression of the STREX BK channel variant in CCDs, we observed an increase in expression of the STREX variant in rabbits maintained on a HK diet. The significance of the latter observation remains to be clarified.
Rabbits maintained on the LS diet for at least 1 wk demonstrated an elevation in plasma aldosterone levels as expected (Table 2). Dietary Na+ restriction and/or exogenous aldosterone increases α-ENaC mRNA (Fig. 1) and protein in the mammalian kidney (2, 33), enhances the translocation of preexisting channels from intracellular sites to the plasma membrane in the distal nephron (14, 31–33), and leads to proteolytic cleavage of α- and γ-subunits of the channel protein (14, 33). The net effect is an increase in Na+ channel activity (41) and net transepithelial Na+ absorption (13, 55). Our finding that the rate of net Na+ absorption at a slow tubular fluid flow rate was similar in LS- and HS-fed rabbits suggests that a minimal “threshold” tubular fluid flow rate, and thus shear stress, may be essential to detect a mineralocorticoid-induced stimulation of Na+ absorption. While shear directly activates ENaC (1, 8, 53), other shear-regulated factors may indirectly affect ENaC activity. For example, a shear-induced activation of PI3-kinase, as has been demonstrated to occur within 15 s of a shear of 0.5 dyn/cm2 in vascular endothelial cells (17), would be expected to activate sgk and ENaC.
In general, increases in net Na+ absorption in the CCD are accompanied by increases in net K+ secretion (51), as would be predicted for two transport processes tightly coupled to the basolateral Na+-K+-ATPase. However, we observed that flow-stimulation of net K+ secretion in CCDs isolated from HS- and LS-fed rabbits did not differ, despite a significantly greater flow stimulation of net Na+ absorption in the latter (Fig. 4). Cumulative evidence now indicates that uncoupling of NaCl reabsorption and K+ secretion in the distal nephron may be mediated by the prevailing functional balance of WNK (with no lysine) kinase isoforms.
Among the WNK kinases, full-length WNK1 (L-WNK1) and WNK4 are expressed in the aldosterone-sensitive distal nephron (71); mutations in these kinases cause pseudohypoaldosteronism type II (71). L-WNK1 stimulates ENaC via activation of sgk1 (79) and inhibits ROMK by reducing surface expression of the channel (27, 67). Wild-type WNK4 inhibits ROMK and ENaC (21) (49). An alternatively spliced kinase-deficient WNK1 isoform specifically expressed in the kidney (kidney-specific WNK1, or KS-WNK1)(12, 39) acts as an antagonist of L-WNK1 with respect to its effects on ROMK as well as WNK4 (27, 63, 67). Thus a high ratio of L-WNK1 to KS-WNK1 in the CCD is predicted to enhance net Na+ reabsorption via ENaC and limit ROMK-mediated K+ secretion, a dissociation in cation fluxes similar to that observed in the present study. In contrast, a decrease in this ratio (due to an increase in the abundance of KS-WNK1), as is detected in response to acute (67) and chronic (7 day; Ref. 38) dietary K+ loading of the rat and mouse, respectively, presumably releases L-WNK1-mediated inhibition of ROMK, thereby enhancing urinary K+ secretion. It is unknown whether WNK kinases regulate BK channel activity at the apical membrane. In contrast to the clear differences in WNK isoform expression in kidneys harvested from rodents provided variable K+ intakes, mice fed a LS (0.03%) or HS (3%) diet for 6 days revealed only a marginally significant downregulation of KS-WNK1 mRNA abundance; expression of L-WNK1 and WNK4 was similar in both treatment groups (38).
In sum, our data lend further support to the premise that aldosterone is not an essential or primary regulator of renal K+ excretion and that a kaliuretic factor(s)/pathway other than this hormone regulates renal K+ transport in response to changes in dietary K+ intake (42), a notion that has been advanced by others based on the failure to document measurable increases in plasma K+ in response to increased K+ intake (46, 47) and the observation that high dietary K+ intake enhances K+ secretion in the distal nephron in adrenalectomized rabbits (73). The extent to which dietary K+-induced modifications in the functional balance of the unique WNK kinases participate in flow-stimulated K+ secretion remains to be explored.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants DK-038470 (to L. M. Satlin), DK-051391 (to T. R. Kleyman), and K08-HD-045524 (NPS).
Acknowledgments
The authors gratefully acknowledge Beth Zavilowitz (Mount Sinai School of Medicine) and Christy Smolak (University of Pittsburgh) for their technical support, and Dr. Robert Wilson (Mount Sinai School of Medicine) for performing the measurements of serum aldosterone concentration.
Abstracts of this work were presented at the Annual Meetings of the American Society of Nephrology in 2006 and 2007 (San Diego, CA and San Francisco, CA).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Althaus M, Bogdan R, Clauss WG, Fronius M. Mechano-sensitivity of epithelial sodium channels (ENaCs): laminar shear stress increases ion channel open probability. FASEB J 21: 2389–2399, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Asher C, Wald H, Rossier BC, Garty H. Aldosterone-induced increase in the abundance of Na+ channel subunits. Am J Physiol Cell Physiol 271: C605–C611, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science 253: 551–555, 1991. [DOI] [PubMed] [Google Scholar]
- 4.Audige A, Yu ZR, Frey BM, Uehlinger DE, Frey FJ, Vogt B. Epithelial sodium channel (ENaC) subunit mRNA and protein expression in rats with puromycin aminonucleoside-induced nephrotic syndrome. Clin Sci (Lond) 104: 389–395, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int 70: 51–59, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Boyd JE, Palmore WP, Mulrow PJ. Role of potassium in the control of aldosterone secretion in the rat. Endocrinology 88: 556–565, 1971. [DOI] [PubMed] [Google Scholar]
- 7.Butterfield I, Warhurst G, Jones MN, Sandle GI. Characterization of apical potassium channels induced in rat distal colon during potassium adaptation. J Physiol 501: 537–547, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carattino MD, Sheng S, Kleyman TR. Epithelial Na+ channels are activated by laminar shear stress. J Biol Chem 279: 4120–4126, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Tian L, MacDonald SH, McClafferty H, Hammond MS, Huibant JM, Ruth P, Knaus HG, Shipston MJ. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) alpha-subunits generated from a single site of splicing. J Biol Chem 280: 33599–33609, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA 96: 2514–2519, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20: 7052–7059, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaloy C, Lu J, Houot AM, Disse-Nicodeme S, Gasc JM, Corvol P, Jeunemaitre X. Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol Cell Biol 23: 9208–9221, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engbretson BG, Stoner LC. Flow-dependent potassium secretion by rabbit cortical collecting tubule in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 253: F896–F903, 1987. [DOI] [PubMed] [Google Scholar]
- 14.Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol 291: F683–F693, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol 287: F1030–F1037, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Frindt G, Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F143–F151, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Go YM, Park H, Maland MC, Darley-Usmar VM, Stoyanov B, Wetzker R, Jo H. Phosphatidylinositol 3-kinase gamma mediates shear stress-dependent activation of JNK in endothelial cells. Am J Physiol Heart Circ Physiol 275: H1898–H1904, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-beta subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol 293: F350–F359, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch J, Leipziger J, Frobe U, Schlatter E. Regulation and possible physiological role of the Ca2+-dependent K+ channel of cortical collecting ducts of the rat. Pflügers Arch 422: 492–498, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R. Primary sequence and immunological characterization of beta-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J Biol Chem 269: 17274–17278, 1994. [PubMed] [Google Scholar]
- 23.Kwon SH, Guggino WB. Multiple sequences in the C terminus of MaxiK channels are involved in expression, movement to the cell surface, and apical localization. Proc Natl Acad Sci USA 101: 15237–15242, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai GJ, McCobb DP. Opposing actions of adrenal androgens and glucocorticoids on alternative splicing of Slo potassium channels in bovine chromaffin cells. Proc Natl Acad Sci USA 99: 7722–7727, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai GJ, McCobb DP. Regulation of alternative splicing of Slo K+ channels in adrenal and pituitary during the stress-hyporesponsive period of rat development. Endocrinology 147: 3961–3967, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 86: 1151–1178, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103: 1615–1620, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li D, Wang Z, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Inhibition of MAPK stimulates the Ca2+-dependent big-conductance K channels in cortical collecting duct. Proc Natl Acad Sci USA 103: 19569–19574, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling BN, Hinton CF, Eaton DC. Potassium permeable channels in primary cultures of rabbit cortical collecting tubule. Kidney Int 40: 441–452, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, Rossier BC, Kaissling B. Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol 279: F252–F258, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol 280: F675–F682, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–23, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morita T, Hanaoka K, Morales MM, Montrose-Rafizadeh C, Guggino WB. Cloning and characterization of maxi K+ channel alpha-subunit in rabbit kidney. Am J Physiol Renal Physiol 273: F615–F624, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Muto S, Sansom S, Giebisch G. Effects of a high potassium diet on electrical properties of cortical collecting ducts from adrenalectomized rabbits. J Clin Invest 81: 376–380, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289: F922–F932, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Naray-Fejes-Toth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Toth G. sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial Na+ channels. J Biol Chem 274: 16973–16978, 1999. [DOI] [PubMed] [Google Scholar]
- 38.O'Reilly M, Marshall E, Macgillivray T, Mittal M, Xue W, Kenyon CJ, Brown RW. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J Am Soc Nephrol 17: 2402–2413, 2006. [DOI] [PubMed] [Google Scholar]
- 39.O'Reilly M, Marshall E, Speirs HJ, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol 14: 2447–2456, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Pacha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 261: F696–F705, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer LG, Frindt G. Aldosterone and potassium secretion by the cortical collecting duct. Kidney Int 57: 1324–1328, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. Am J Physiol Renal Physiol 292: F966–F973, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol Renal Physiol 277: F805–F812, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Pluznick JL, Wei P, Carmines PK, Sansom SC. Renal fluid and electrolyte handling in BKCa-β1−/− mice. Am J Physiol Renal Physiol 284: F1274–F1279, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Rabinowitz L Aldosterone and potassium homeostasis. Kidney Int 49: 1738–1742, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Rabinowitz L, Green DM, Sarason RL, Yamauchi H. Homeostatic potassium excretion in fed and fasted sheep. Am J Physiol Regul Integr Comp Physiol 254: R357–R380, 1988. [DOI] [PubMed] [Google Scholar]
- 48.Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int 72: 566–573, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Ring AM, Cheng SX, Leng Q, Kahle KT, Rinehart J, Lalioti MD, Volkman HM, Wilson FH, Hebert SC, Lifton RP. WNK4 regulates activity of the epithelial Na+ channel in vitro and in vivo. Proc Natl Acad Sci USA 104: 4020–4024, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito M, Nelson C, Salkoff L, Lingle CJ. A cysteine-rich domain defined by a novel exon in a slo variant in rat adrenal chromaffin cells and PC12 cells. J Biol Chem 272: 11710–11717, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Satlin LM Postnatal maturation of potassium transport in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 266: F57–F65, 1994. [DOI] [PubMed] [Google Scholar]
- 52.Satlin LM, Palmer LG. Apical Na+ conductance in maturing rabbit principal cell. Am J Physiol Renal Fluid Electrolyte Physiol 270: F391–F397, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Schlatter E, Bleich M, Hirsch J, Markstahler U, Frobe U, Greger R. Cation specificity and pharmacological properties of the Ca2+-dependent K+ channel of rat cortical collecting ducts. Pflügers Arch 422: 481–491, 1993. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz GJ, Burg MB. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 235: F576–F585, 1978. [DOI] [PubMed] [Google Scholar]
- 56.Shipston MJ Alternative splicing of potassium channels: a dynamic switch of cellular excitability. Trends Cell Biol 11: 353–358, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J Biol Chem 277: 5–8, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Snyder PM, Steines JC, Olson DR. Relative contribution of Nedd4 and Nedd4-2 to ENaC regulation in epithelia determined by RNA interference. J Biol Chem 279: 5042–5046, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev 84: 489–539, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Stanton BA, Giebisch GH. Potassium transport by the renal distal tubule: effects of potassium loading. Am J Physiol Renal Fluid Electrolyte Physiol 243: F487–F493, 1982. [DOI] [PubMed] [Google Scholar]
- 61.Stokes JB Potassium secretion by cortical collecting tubule: relation to sodium absorption, luminal sodium concentration, and transepithelial voltage. Am J Physiol Renal Fluid Electrolyte Physiol 241: F395–F402, 1981. [DOI] [PubMed] [Google Scholar]
- 62.Stoner LC, Viggiano SC. Environmental KCl causes an upregulation of apical membrane maxi K and ENaC channels in everted Ambystoma collecting tubule. J Membr Biol 162: 107–116, 1998. [DOI] [PubMed] [Google Scholar]
- 63.Subramanya AR, Yang CL, Zhu X, Ellison DH. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol 290: F619–F624, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem 276: 7717–7720, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Tomita K, Pisano JJ, Knepper MA. Control of sodium and potassium transport in the cortical collecting duct of the rat. Effects of bradykinin, vasopressin, and deoxycorticosterone. J Clin Invest 76: 132–136, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron 13: 1315–1330, 1994. [DOI] [PubMed] [Google Scholar]
- 67.Wade JB, Fang L, Liu J, Li D, Yang CL, Subramanya AR, Maouyo D, Mason A, Ellison DH, Welling PA. WNK1 kinase isoform switch regulates renal potassium excretion. Proc Natl Acad Sci USA 103: 8558–8563, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang SX, Ikeda M, Guggino WB. The cytoplasmic tail of large conductance, voltage- and Ca2+-activated K+ (MaxiK) channel is necessary for its cell surface expression. J Biol Chem 278: 2713–2722, 2003. [DOI] [PubMed] [Google Scholar]
- 69.Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 259: F494–F502, 1990. [DOI] [PubMed] [Google Scholar]
- 70.Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem 282: 6455–6462, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. [DOI] [PubMed] [Google Scholar]
- 72.Wingo CS Potassium transport by medullary collecting tubule of rabbit: effects of variation in K intake. Am J Physiol Renal Fluid Electrolyte Physiol 253: F1136–F1141, 1987. [DOI] [PubMed] [Google Scholar]
- 73.Wingo CS, Seldin DW, Kokko JP, Jacobson HR. Dietary modulation of active potassium secretion in the cortical collecting tubule of adrenalectomized rabbits. J Clin Invest 70: 579–586, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. [DOI] [PubMed] [Google Scholar]
- 75.Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol 285: F629–F639, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Wright FS, Strieder N, Fowler NB, Giebisch G. Potassium secretion by distal tubule after potassium adaptation. Am J Physiol 221: 437–448, 1971. [DOI] [PubMed] [Google Scholar]
- 77.Xie J, Black DL. A CaMK IV responsive RNA element mediates depolarization-induced alternative splicing of ion channels. Nature 410: 936–939, 2001. [DOI] [PubMed] [Google Scholar]
- 78.Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science 280: 443–446, 1998. [DOI] [PubMed] [Google Scholar]
- 79.Xu BE, Stippec S, Chu PY, Lazrak A, Li XJ, Lee BH, English JM, Ortega B, Huang CL, Cobb MH. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci USA 102: 10315–10320, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zarei MM, Eghbali M, Alioua A, Song M, Knaus HG, Stefani E, Toro L. An endoplasmic reticulum trafficking signal prevents surface expression of a voltage- and Ca2+-activated K+ channel splice variant. Proc Natl Acad Sci USA 101: 10072–10077, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Joiner WJ, Bhattacharjee A, Rassendren F, Magoski NS, Kaczmarek LK. The appearance of a protein kinase A-regulated splice isoform of slo is associated with the maturation of neurons that control reproductive behavior. J Biol Chem 279: 52324–52330, 2004. [DOI] [PubMed] [Google Scholar]
- 82.Zhou XB, Arntz C, Kamm S, Motejlek K, Sausbier U, Wang GX, Ruth P, Korth M. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J Biol Chem 276: 43239–43245, 2001. [DOI] [PubMed] [Google Scholar]