Abstract
The cholinergic anti-inflammatory pathway is a mechanism whereby local inflammation is modulated by the brain via the vagus nerve and nicotinic acetylcholine receptors (nAChRs). The nAChR family are ligand-gated ion channels that consist of many different subtypes formed by the specific assembly of five polypeptide subunits including α1–10, β1–4, γ, δ, and ɛ. The α7 receptor (α7nAChR) mediates the anti-inflammatory effects of cholinergic stimulation. We recently demonstrated that cholinergic agonists attenuate renal ischemia-reperfusion (I/R) injury in rats. We also showed that tubular epithelial cells express functional nAChRs in vitro. The current studies report the expression, localization, and regulation of the α7nAChR in the rat kidney after I/R injury. We also examined, in this model, potential interactions between cholinergic stimulation and the STAT3 pathway, a key signaling cascade that has been linked to α7nAChR activation. RT-PCR and immunohistochemistry showed constitutive expression of many nAChR subunits. Immunohistochemistry localized basal α7nAChR expression to the endothelium of cortical peritubular capillaries, and its distribution was upregulated after I/R injury. Western blotting also showed an increase in α7nAChR subunit protein after renal I/R injury. Interestingly, pretreatment with nicotine, which improves the outcome after renal I/R injury, reduced the α7nAChR protein after I/R injury. Finally, we found that I/R injury stimulated the STAT3 pathway, whereas pretreatment with nicotine downregulated its activation. These results suggest that the α7nAChR plays an important role in the pathophysiology of renal I/R injury.
Keywords: renal inflammation, cholinergic anti-inflammatory pathway, renal injury, STAT3, proteasome activity
the cholinergic anti-inflammatory pathway (CAP) is a physiological mechanism by which local inflammation activates the afferent (sensory) fibers of the vagus nerve to signal the brain to trigger an anti-inflammatory response through firing of the efferent (motor) vagus nerve. Acetylcholine released by stimulation of the vagus nerve binds to nicotinic acetylcholine receptors (nAChRs) expressed by macrophages and other immunocompetent cells that modulate or participate in the inflammatory response to suppress proinflammatory cytokine production. Cholinergic agonists (including nicotine and GTS-21) produce effects similar to the electrical stimulation of the vagus nerve (5, 23, 32–34).
The nAChR family are ligand-gated ion channels that mediate diverse physiological functions and were originally identified in the nervous system. They consist of many different subtypes formed by the specific assembly of five polypeptide subunits including α1–10, β1–4, γ, δ, and ɛ (10, 12, 16). The subunits fall into two broad groups: neuronal nicotinic receptors (consisting of α2–10 and β2–4) and muscle nicotinic receptors (consisting of α1, β1, γ, δ, and ɛ). Functional neuronal nAChR subtypes are either homomeric (consisting of 5 identical α-subunits, as in α7- or α9nAChR) or heteromeric (consisting of combinations of the α- and β-subunits, such as α3β2nAChR).
We and others have recently shown the expression of these receptors in nonneuronal tissue (2, 17–19, 28, 34, 38). The α7 receptor (α7nAChR) has been well-characterized and shown to mediate the anti-inflammatory effects of cholinergic stimulation (29, 33, 34, 38). Both electrical and pharmacological stimulation of the CAP significantly reduce proinflammatory cytokine production, including tumor necrosis factor (TNF), IL-1β, IL-6, IL-8, and high-mobility group box 1. This reduction in cytokine synthesis is associated with improved outcomes in experimental endotoxemia, hemorrhagic shock, pancreatitis, peritonitis, and other diseases associated with excessive cytokine release (3, 5, 11, 23, 33).
The intracellular mechanisms by which cholinergic stimulation blunts cytokine production are not completely understood, but current knowledge suggests that ligand-receptor interaction on cytokine-expressing cells decreases nuclear translocation of NF-κB and may involve the JAK2/STAT3 and suppressor of cytokine signaling-3 cascade (8, 30). Therefore, the CAP represents a physiological anti-inflammatory mechanism that can be exploited to treat clinical conditions associated with significant inflammation, such as renal ischemia-reperfusion (I/R) injury. Renal I/R injury is a common clinical condition that affects both native and transplanted kidneys and is associated with high morbidity and mortality. The pivotal role of inflammation as a key mechanism in renal I/R injury is well-established, but despite the advances in our understanding of the pathophysiological mechanisms involved, the prognosis for this type of renal injury has not improved much (4, 36). Thus there is a critical need to develop novel therapeutic agents to prevent complications of renal I/R injury.
Recently, we demonstrated that the cholinergic agonists nicotine and GTS-21 attenuate renal I/R injury in rats. We also showed that rat tubular epithelial cells express functional nAChRs in vitro (38). GTS-21 is a selective α7nAChR agonist, suggesting a specific role of the α7nAChR in mediating the renoprotective effects. This conclusion is supported by a report by Sadis et al. (29) showing that nicotine treatment protects wild-type mice but not α7nAChR-deficient mice from renal I/R injury. However, to effectively harness the CAP to treat renal I/R injury, a detailed knowledge and understanding of the identity, cellular localization, and regulation of the nAChRs in the kidney is essential. By using a rat model of renal I/R injury, the present study was designed to test the hypothesis that the kidney constitutively expresses nicotinic acetylcholine receptors that are modulated during renal I/R injury and mediate the anti-inflammatory effects observed following the administration of cholinergic agonists.
MATERIALS AND METHODS
Reagents.
The α7nAChR nAChR primary (monoclonal) antibody used for Western blotting was obtained from Abcam (Cambridge, MA). The α2nAChR and α3nAChR primary (polyclonal) antibodies and the primary antibody for total STAT3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Primary antibody for pSTAT3Tyr705 was obtained from Cell Signaling Technology (Danvers, MA). All other reagents were commercial products of the highest quality.
Animals.
Male Sprague-Dawley rats (250–300 g) purchased from Taconic Farms (Germantown, NY) were housed in a light-controlled room with a 12:12-h light-dark cycle and allowed free access to water and standard rat chow. Rats were acclimatized for 1 wk before experimentation. All animals received humane care in compliance with the National Research Council's Guide for the Care and Use of Laboratory Animals. The animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the North Shore-Long Island Jewish Health System.
Experimental groups and renal ischemia model.
An established model of renal I/R injury in rats was used (27). In brief, male Sprague-Dawley rats were anesthetized by intramuscular injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). A midline abdominal incision was made to expose the left kidney. Blood supply to the left kidney was interrupted by the application of nontraumatic microvascular clamps (Fine Science Tools, Foster City, CA) around the left renal artery. Ischemia was confirmed by blanching of the kidneys. After 45 min, the clamp was removed and reperfusion was confirmed visually. The wound was then closed in two layers with a 4-0 silk suture, and the animals were allowed to recover with free access to food and water. During the experiment, the animals were kept hydrated with normal saline instilled intraperitoneally and were kept on warm heating pads to maintain body temperature. The rats were euthanized by CO2 inhalation, and the left kidneys were harvested after 2, 4, 6, or 24 h of reperfusion. There were three rats in each group. In addition, frozen kidney samples and formalin-fixed paraffin sections from rats treated with nicotine (1 mg/kg) were available from our previous experiments.
RT-PCR.
Total RNA was extracted from the kidney and RT-PCR was performed using primers as described previously (38). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control to verify the efficiency of RNA isolation and cDNA synthesis. PCR products were run on a 1.5% agarose gel containing ethidium bromide and visualized under ultraviolet illumination.
Quantitative real-time (TaqMan) RT-PCR.
Gene-specific oligonucleotide primers and probes were designed using Primer Express software (Applied Biosystems, Foster City, CA). The primers and probes were used for the α7nAChR gene (forward, 5-ATCTGATTCTGTGCCCTTGATAGC-3; reverse, 5-CACAATCACTGTCACCACTACAGAGA-3; probe, 5-FAM-AATACTTCGCCAGCACCATGATCATCGT-3) and for the GAPDH gene (forward, 5-GGCCTACATGGCCTCCAA-3; reverse, 5-GGCCTCTCTCTTGCTCTCAGTATC-3; probe, 5-FAM-AGTAAGAAACCC CTGGACCACCCAGC-3). One-step real-time PCR was performed on an ABI Prism 7700 sequence detection system (Applied Biosystems) using the Eurogentec RT-QPCR master mix. The thermal cycler conditions included an initial RT step of 30 min at 48°C and then a denaturing step at 95°C for 10 min, followed by 45 cycles at 95°C for 15 s and 60°C for 1 min. Amplification products were verified by melting point curves. All reactions were run in duplicate and included a negative control. The relative gene expression was determined using the ΔΔCt method. The results were normalized with data from the housekeeping gene GAPDH and were expressed relative to the mean of the control (normal healthy kidney) group.
Western blotting.
Pieces of frozen kidney were homogenized in RIPA buffer containing a protease and phosphatase inhibitor cocktail (Sigma, St. Louis, MO). Protein concentration was quantified using the bicinchoninic acid (BCA) protein assay (Pierce Biotechnology, Rockford, IL). Proteins (60 μg/lane) were separated by SDS-polyacrylamide gel electrophoresis (Invitrogen, Carlsbad, CA) and then transferred to a polyvinylidene difluoride membrane (Amersham Biosciences, Piscataway, NJ). Nonspecific binding to the membrane was blocked with Odyssey blocking buffer for 1 h at room temperature. The membrane was then incubated with the primary antibody (α7nAChR, 1:200; pSTAT3Tyr705, 1:1,000; total STAT3, 1:200) for 1 h at room temperature (in the case of the pSTAT3 antibodies, the incubation was carried out overnight at 4°C). The blot was then incubated with the appropriate near-infrared-fluorescently labeled secondary antibody (1:15,000) for 1 h at room temperature. After washing, protein bands were revealed using the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE).
Immunohistochemistry.
Formalin-fixed paraffin sections were used. After routine deparaffinization, heat-induced epitope retrieval was done using a citrate-based buffer. Endogenous peroxidase was quenched by incubation with 3% H2O2 in phosphate-buffered saline (PBS; pH 7.4) for 5 min. The sections were permeabilized using 0.1% (wt/vol) Triton X-100 in PBS for 15 min. Nonspecific binding was minimized by incubating sections with 5% normal serum from the species in which the secondary antibody was raised (diluted in 5% bovine serum albumin/PBS) for 60 min at room temperature. Endogenous biotin and avidin binding sites were blocked by sequential incubation with avidin and biotin for 15 min (Vector Laboratories, Burlingame, CA). The slides were then rinsed and incubated with the primary antibodies or non-immune serum (diluted 1:50) overnight at 4°C. The slides were rinsed with PBS (3 times) and incubated with the biotinylated secondary antibody for 30 min at room temperature. Binding was revealed using an ABC peroxidase kit and the substrate 3,3′-diaminobenzidine (Santa Cruz Biotechnology). Slides were counterstained with hematoxylin, dehydrated in alcohol, cleared in xylene, and mounted. The slides were examined by a pathologist (M. Susin) who was blinded to the experimental conditions.
Analysis of proteasome activity.
The chymotrypsin-like activity of the proteasome was measured using the fluorogenic substrate succinyl-LLVY-7-amino-4-methylcoumarin (Biomol Research Laboratory, Plymouth Meeting, PA). Proteasome activity was determined in tissue homogenates as described by Powell et al. (26). In brief, kidney samples were homogenized in HEPES buffer (50 mmol/l) containing KCl (20 mmol/l), MgCl2 (5 mmol/l), DTT (1 mmol/l), pH 7.5, and then centrifuged at 10,000 g for 20 min at 4°C. The supernatant was collected and used immediately for determining the proteasome activity. Supernatant (30 μg of protein) was added to assay buffer and incubated for 1 h at 37°C with the proteasome substrate at a final concentration of 20 μmol/l. The samples were monitored at 360-nm excitation and 460-nm emission wavelengths using a fluorescence multiwell plate reader (Perspective Biosystems, Framingham, MA). The results are expressed as relative fluorescence per 30 μg of tissue lysate.
Statistical analysis.
All data are means ± SE. Multiple group comparisons were performed using analysis of variance followed by Dunnett's post hoc testing or Student's t-test for data with only two subgroups. Statistical significance was set at P < 0.05.
RESULTS
The kidney constitutively expresses nAChRs.
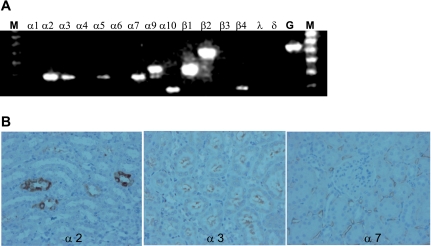
To gain insight into basal renal expression of nAChRs, we examined mRNA expression by RT-PCR using total mRNA samples from healthy rat kidneys. We observed constitutive expression of the α2, α3, α5, α7, α9, α10, β1, β2, and β4 subunit mRNA (Fig. 1A). The primers for each subunit yielded products of expected size. Based on the mRNA expression profile, we determined that most of the functional nAChRs on the cell membrane would contain either one or more of the α2, α3, or α7 receptor subunits. Using immunohistochemistry, we confirmed basal expression of α2, α3, and α7 receptor subunit proteins in the kidney (Fig. 1B).
Fig. 1.
The rat kidney constitutively expresses many nicotinic acetylcholine receptor (nAChR) subunits. A: constitutive renal nAChR mRNA expression was determined by RT-PCR. GAPDH was used as an internal control. The α1, α4, α6, β3, δ, and γ transcripts were not detected. M, molecular mass marker; G, GAPDH. B: constitutive renal α2, α3, and α7 nAChR subunit protein expression was determined using immunohistochemistry.
Renal I/R injury downregulates α7nAChR subunit mRNA.
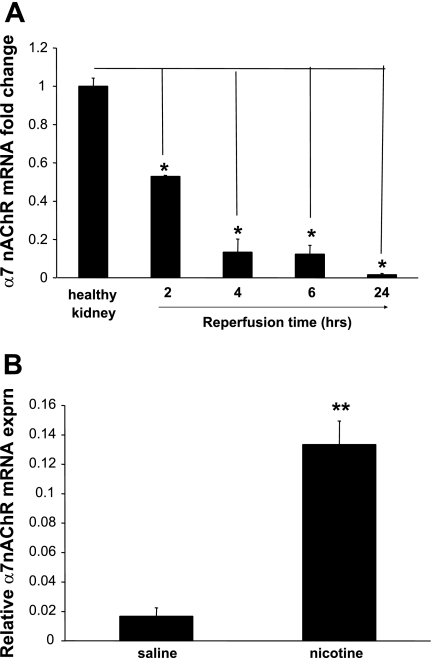
Animals underwent unilateral renal artery occlusion for 45 min, followed by varying periods of reperfusion (2–24 h). This insult leads to acute kidney injury and a robust inflammatory response mediated by cytokines, chemokines, and inflammatory cells, including neutrophils and macrophages. The α7nAChR is important in mediating the anti-inflammatory effects of cholinergic receptor activation. Using real-time PCR, we found a time-dependent decrease in the α7nAChR mRNA (Fig. 2A). Preischemic treatment with nicotine, a cholinergic agonist, significantly increased the mRNA expression relative to vehicle treatment (Fig. 2B).
Fig. 2.
The α7nAChR subunit mRNA is downregulated by renal ischemia-reperfusion (I/R) injury. A: after 45 min of ischemia, rat kidneys were harvested at 2, 4, 6, or 24 h postreperfusion. Quantitative PCR was performed using total mRNA. The α7nAChR expression relative to healthy control is shown. *P < 0.05 vs. healthy control group. B: pretreatment with nicotine (1 mg/kg) significantly increased the mRNA expression relative to vehicle treatment. Values are means ± SE (n = 3 rats/group). **P < 0.005 vs. vehicle-treated group.
Renal I/R injury increases α7nAChR subunit protein expression, whereas pretreatment with nicotine decreases expression.
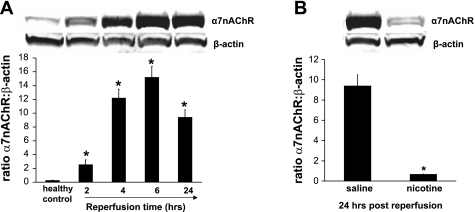
We studied the expression and the changes in the α7nAChR subunit protein following renal I/R injury using Western blotting (Fig. 3A). The α7nAChR subunit protein was expressed constitutively in healthy (control) kidneys, but contrary to the mRNA expression, the subunit protein was increased by I/R injury. The protein was identified as a band with an apparent molecular mass of ∼56 kDa. Protein expression was similar in sham-operated animals and healthy animals (data not shown). After their synthesis, α7nAChR subunits assemble into (homomeric) pentameric channels and are transported from the endoplasmic reticulum through the Golgi to the cell membrane. Previous studies showed that pretreatment with cholinergic agonists (nicotine and GTS-21) attenuates renal damage following I/R injury. Therefore, we assessed the effect of nicotine treatment on α7nAChR expression following renal I/R injury using Western blotting methods. Compared with vehicle treatment, nicotine treatment clearly reduced the α7nAChR expression (Fig. 3B).
Fig. 3.
The α7nAChR subunit protein is upregulated by renal I/R injury. A: representative Western blotting showing levels of α7nAChR and β-actin from kidneys of sham-operated rats and those that underwent 45 min of ischemia followed by 2, 4, 6, or 24 h of reperfusion (top) and quantitative analysis of the relative abundance of α7nAChR after normalization with β-actin (bottom). Values are means ± SE (n = 3 rats/group). *P < 0.01 vs. healthy control group. B: pretreatment with nicotine (1 mg/kg) decreased the protein expression compared with vehicle treatment at 24 h postreperfusion. Values are means ± SE (n = 3 rats/group). *P < 0.0001 vs. vehicle-treated group.
Renal I/R injury decreases proteasome activity.
The ubiquitin-proteasome system is a major cellular protein degradation pathway that regulates cellular responses to various stimuli (14) and has been shown to regulate nicotinic receptor expression (6). To explore the basis for the observed differential expression of α7nAChR mRNA and protein after renal I/R injury, we hypothesized that the increased protein was due to I/R-induced impairment in proteasome activity. Using a fluorogenic substrate, we measured the 20S proteasome activity in kidney homogenates. Our results show reduction in proteasome activity that was most significant at 2 h postreperfusion and persisted at 24 h postreperfusion (Table 1).
Table 1.
Proteasome activity is reduced after renal I/R injury
| Duration | 20S Proteasome Activity |
|---|---|
| No ischemia | 1,225±99 |
| 2 h Postreperfusion | 609±55* |
| 4 h Postreperfusion | 880±148 |
| 6 h Postreperfusion | 882±66 |
| 24 h Postreperfusion | 854±43 |
Rats underwent 45 min of renal ischemia followed by 2, 4, 6, or 24 h of reperfusion (I/R). Kidneys were harvested at the appropriate time and analyzed for proteasome activity. Results are expressed as the relative fluorescence per 30 μg of tissue lysate. Values are means ± SE (3 separate experiments representing 3 rats/group).
P < 0.05.
Localization of α7nAChR by immunohistochemistry.
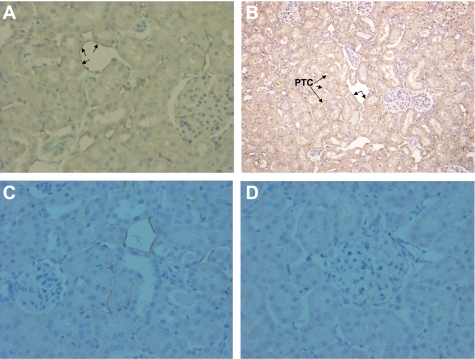
The cellular distribution of the α7nAChR was examined with immunohistochemical staining using formalin-fixed paraffin kidney sections and polyclonal α7nAChR antibody (Santa Cruz Biotechnology). In healthy kidneys, α7nAChR immunostaining was localized predominantly in the endothelium of cortical peritubular capillaries (Fig. 4A). The extent of α7nAChR-positive staining increased within the cortex after renal I/R injury (Fig. 4B). No medullary staining was present in healthy tissue; however, peritubular capillary staining was seen in the medulla after renal I/R injury (not shown). Also, staining of the endothelium of venules was seen in both healthy kidneys and after renal I/R injury. Pretreatment with nicotine resulted in less staining compared with vehicle treatment (Fig. 4C). No significant signal was apparent in the other parts of the kidney, especially glomeruli or tubules. Infiltrating cells stained only rarely, suggesting that the increase in α7nAChR protein detected by Western blotting is not due to infiltrating cells. The specificity of the labeling was confirmed by absence of signal when the primary antibody was omitted and also when the sections were incubated with isotype control antibodies (Fig. 4D).
Fig. 4.
Immunolocalization of basal and I/R injury-induced α7nAChR subunit protein by immunohistochemistry. Representative photomicrographs show (A) staining in cortical peritubular capillaries (PTC). B: widespread expression of α7nAChR subunit protein within the endothelium of the PTC at 24 h postreperfusion. Unlabeled arrows indicate staining of the endothelium of a venule. C: relatively less extensive staining was observed in animals pretreated with nicotine. D: no staining was observed when the primary antibody was omitted or when the sections were incubated with isotype control antibodies. Original magnification, ×200.
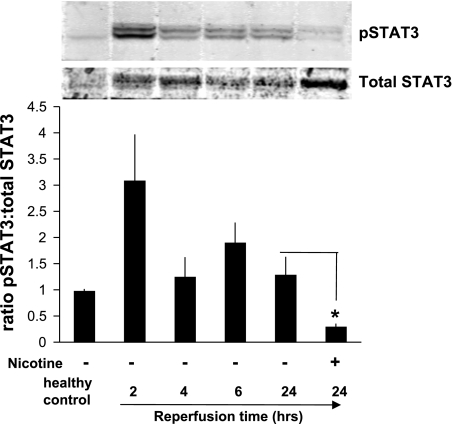
Renal I/R-induced STAT3 activation is inhibited by nicotine.
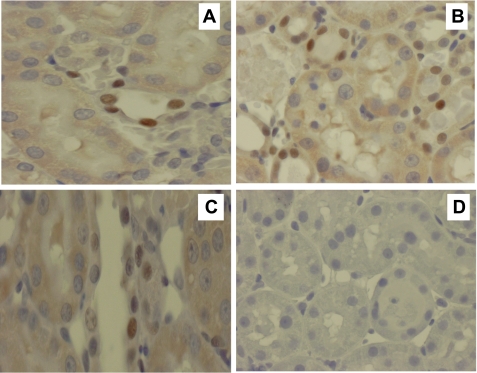
The JAK2/STAT3 signaling pathway is one of the major mechanisms for cytokine signal transduction, and recent studies have shown that activation of this pathway plays a detrimental role following renal I/R injury. We observed an increase in activation of STAT3 (pSTAT3) in the kidney within 2 h of reperfusion. Next, we explored whether nicotine affected the STAT3 activation in this model. We found that nicotine pretreatment reduced the pSTAT3 expression after renal I/R injury (Fig. 5). The Western blot data are supported by immunohistochemical studies showing a significant increase in nuclear staining (for pSTAT3) in vehicle-treated animals compared with nicotine-treated or sham-operated animals (Fig. 6).
Fig. 5.
Renal I/R injury-induced STAT3 activation is suppressed by nicotine. Representative Western blots show levels of phosphorylated STAT3 (pSTAT3) and total STAT3 from kidneys of sham-operated animals and rats that underwent 45 min of ischemia followed by 2, 4, 6, or 24 h of reperfusion (top) and quantitative analysis by the ratio of pSTAT3 to total STAT3. Pretreatment with nicotine (1 mg/kg) decreased pSTAT3/total STAT3 compared with vehicle treatment. Values are means ± SE (n = 3 rats/group). *P < 0.05 vs. vehicle-treated group at 24 h postreperfusion.
Fig. 6.
Immunohistochemical staining of rat kidneys for pSTAT3. Representative photomicrographs of pSTAT3 are shown at 24 h postreperfusion. A: only occasional pSTAT3 nuclear staining was observed in tubular cells in sham-operated rats. B: brown nuclear staining of pSTAT3 in tubular and infiltrating cells increased markedly in vehicle-treated rats following renal I/R injury. C: pretreatment with nicotine (1 mg/kg) reduced the extent and intensity of pSTAT3 nuclear staining to a level similar to that in sham-operated animals. D: no staining was observed when the primary antibody was omitted.
DISCUSSION
The purpose of this study was to examine the expression and localization of nAChR subunits in the normal rat kidney and to elucidate the regulation of the α7nAChR after renal I/R injury. Our results reveal, for the first time, constitutive expression of many nAChR subunits in the rat kidney in vivo. Real-time PCR showed downregulation of the α7nAChR mRNA, Western blot analysis showed an increase in the α7nAChR protein after renal I/R injury, and immunohistochemical staining localized this increase mainly to the endothelium of cortical peritubular capillaries. Interestingly, pretreatment with nicotine increased the α7nAChR mRNA and downregulated the α7nAChR protein expression after I/R injury, which might be a mechanism associated with the renoprotection observed with cholinergic stimulation. Furthermore, we found that STAT3 signaling was increased by renal I/R injury, whereas it was inhibited by nicotine pretreatment. The results presented support a key role for the α7nAChR in modulating the renal response to I/R injury and may potentially be applicable to other inflammatory conditions of the kidney.
The nAChRs have been extensively studied in the nervous system, where they are known to mediate many physiological functions, including modulation of neuronal signaling at both the pre- and postsynaptic levels (12). The recent identification of these receptors in nonneuronal tissues/cells has coincided with the discovery of the anti-inflammatory effects of cholinergic stimulation, termed the “cholinergic anti-inflammatory pathway.” In particular, the dominant role of the α7nAChR in mediating this anti-inflammatory effect is now well accepted.
The functional importance of nAChRs in I/R injury is supported by recent data. Fujiki et al. (9) demonstrated the neuroprotective effects of donepezil, a nicotinic acetylcholine receptor activator, after cerebral infarction in rats, an effect that was prevented by coinjection with mecamylamine, a nAChR antagonist, indicating that protection was mediated by nAChR activation (9). Crockett et al. (7) showed the protection of early phase hepatic I/R injury by cholinergic agonists. Finally, our laboratory (38) and Sadis et al. (29) have shown that nAChR stimulation with cholinergic agonists protects the rat and mouse, respectively, from renal I/R injury. Together, our reports convincingly show the specific importance of the α7nAChR in mediating renoprotection. These findings come at a time when there is a critical need to develop novel therapeutic/preventive agents that will markedly improve the morbidity and mortality associated with I/R injury in humans. Our current studies explore the role of the α7nAChR in the pathophysiology of acute renal injury.
The precise physiological function of these receptors in the kidney is not known. Acetylcholine, the neurotransmitter at the vagal nerve ending, is the endogenous ligand for the nAChRs. The constitutive expression of these receptors in the kidney provides an intrarenal target for cholinergic stimulation and is consistent with acetylcholine being an autocrine/paracrine hormone in the kidney (24, 25). Acetylcholine has long been known to influence renal vascular tone under both physiological and pathological conditions (13). However, the use of acetylcholine for clinical purposes is very limited on account of its very short half-life. Therefore, nicotine and other less toxic cholinergic agonists such as GTS-21, which are exogenous ligands to these receptors, provide an opportunity to exploit the CAP to treat human disease.
After renal I/R injury, the α7nAChR, which mediates the anti-inflammatory effects of cholinergic stimulation, was markedly increased in the endothelium of the peritubular capillaries. The peritubular capillaries directly supply the tubules and are essential for maintaining the structure and function of the renal tubules (1, 21). Many studies have shown the involvement of the vascular endothelium in the pathophysiology of renal dysfunction following I/R injury. For example, it has been shown that the endothelium of the peritubular capillaries overexpresses intracellular adhesion molecule-1 and P-selectin, which are important in mediating leukocyte trafficking during renal I/R injury (15, 22). The vascular expression of the α7nAChR is consistent with the known vasodilatory effect of acetylcholine in the kidney and supports the dramatic attenuation of tubular damage after renal I/R injury observed in rats pretreated with cholinergic agonists (38).
In this model, the α7nAChR mRNA expression was already decreased by ∼50% at 2 h and was further decreased at 24 h postreperfusion. The reasons for the reduction in the mRNA expression and its effect on I/R injury outcome are not known. One possible reason for the reduction in the α7nAChR mRNA may be renal tubular damage, from either apoptosis or necrosis. In this case, the observed relative increase in the α7nAChR mRNA following nicotine pretreatment may be a direct reflection of the renoprotection, as evidenced by the reduced acute tubular necrosis and apoptosis noted in previous reports (29, 38). In contrast to the mRNA regulation by renal I/R injury, Western blotting and immunohistochemical staining showed an increase in the α7nAChR subunit protein after renal I/R injury. The differential regulation of the mRNA and the protein expression indicates that the receptor expression is posttranscriptionally regulated (20). Our results showing a reduction in proteasome activity after renal I/R injury are consistent with this. The significance of the increased α7nAChR protein level after renal I/R injury is not known. I/R injury causes an increase in calcium influx into cells that may be through both voltage- and ligand-gated calcium channels. The role of excess intracellular calcium as a major mechanism of cell injury and death during I/R injury is now well accepted (31, 35). The α7nAChR is a calcium channel that may augment the calcium influx during renal I/R injury. The observed reduction in receptor protein expression following nicotine treatment may represent an important survival mechanism. Similarly, this reduction in receptor expression may be directly or indirectly related to the reduction in TNF production (38).
The JAK2/STAT3 signaling cascade has been linked to α7nAChR activation (8). Our data suggest that the activation of STAT3 is associated with a worse outcome after renal I/R injury. This observation is supported by a recent report showing that blockade of JAK2/STAT3 signaling with a selective JAK2 inhibitor attenuates renal I/R injury in rats (37).
In summary, we have demonstrated constitutive expression of nAChRs in the rat kidney and that renal I/R injury regulates expression of the α7nAChR, a ligand-gated ion channel that mediates the anti-inflammatory effects of cholinergic stimulation. We also have shown evidence that administration of nicotine, a cholinergic agonist, protects against I/R injury, probably by modulating membrane expression of the α7nAChR. Our data suggest that the observed increase in the α7nAChR subunit protein without an increase in the mRNA expression following renal I/R injury is, at least in part, due to injury-induced proteasome dysfunction. The anti-inflammatory effects of cholinergic stimulation are increasingly being appreciated, and our findings represent a significant advancement in the understanding of the role of the nAChRs in renal I/R injury. Further elucidation of the downstream signaling mechanisms of α7nAChR-mediated renoprotection may lead to development of novel therapeutic strategies against renal I/R injury and other inflammatory diseases.
GRANTS
M. M. Yeboah is supported by the Elmezzi Graduate School of Molecular Medicine. C. N. Metz is funded by National Institute of General Medical Sciences Grant R01 GM070727.
Acknowledgments
We thank Dr. Xue-Ping Wang for assistance with real-time PCR studies. We also acknowledge statistical support by Dr. Chengke Tang and Nina Kohn.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol 290: F103–F110, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, Tracey KJ. Cholinergic anti-inflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg 36: 1231–1236, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Christianson JC, Green WN. Regulation of nicotinic receptor expression by the ubiquitin-proteasome system. EMBO J 23: 4156–4165, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crockett ET, Galligan JJ, Uhal BD, Harkema J, Roth R, Pandya K. Protection of early phase hepatic ischemia-reperfusion injury by cholinergic agonists. BMC Clin Pathol 6: 3, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-Stat3 signaling pathway. Nat Immunol 6: 844–851, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Fujiki M, Kobayashi H, Uchida S, Inoue R, Ishii K. Neuroprotective effect of donepezil, a nicotinic acetylcholine-receptor activator, on cerebral infarction in rats. Brain Res 1043: 236–241, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 27: 482–491, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini A, Marini R, Adamo EB, Venuti FS, Squadrito F. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation 107: 1189–1194, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Kalamida D, Poulas K, Avramopoulou V, Fostieri E, Lagoumintzis G, Lazaridis K, Sideri A, Zouridakis M, Tzartos SJ. Muscle and neuronal nicotinic acetylcholine receptors. Structure, function and pathogenicity. FEBS J 274: 3799–845, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Kamata K, Hosokawa M, Matsumoto T, Kobayashi T. Acetylcholine-induced vasodilation in the perfused kidney of the streptozotocin-induced diabetic rat: role of prostacyclin. J Smooth Muscle Res 42: 159–170, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17: 1807–1819, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Lien YH, Yong KC, Cho C, Igarashi S, Lai LW. S1P1-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int 69: 1601–1608, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G. Structure and function of neuronal nicotinic acetylcholine receptors. Prog Brain Res 109: 125–137, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Lips KS, Bruggmann D, Pfeil U, Vollerthun R, Grando SA, Kummer W. Nicotinic acetylcholine receptors in rat and human placenta. Placenta 26: 735–746, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Liu Mizuta M RH, Matsukura S. The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes. J Pharmacol Exp Ther 310: 52–58, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther 287: 435–359, 1998. [PubMed] [Google Scholar]
- 20.Mousavi M, Hellstrom-Lindahl E, Guan ZZ, Shan KR, Ravid R, Nordberg A. Protein and mRNA levels of nicotinic receptors in brain of tobacco using controls and patients with Alzheimer's disease. Neuroscience 122: 515–520, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Nangaku M Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Okusa MD, Linden J, Huang L, Rieger JM, Macdonald TL, Huynh LP. A2A adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol Renal Physiol 279: F809–F818, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, Larosa GJ, Miller EJ, Tracey KJ, Al-Abed Y. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med 35: 1139–1144, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Pirola CJ, Alvarez AL, Finkielman S, Nahmod VE. Release of acetylcholine from isolated canine renal tissue. Am J Physiol Renal Fluid Electrolyte Physiol 260: F198–F203, 1991. [DOI] [PubMed] [Google Scholar]
- 25.Pirola CJ, Alvarez AL, Balda MS, Finkielman S, Nahmod VE. Evidence for cholinergic innervation in dog renal tissue. Am J Physiol Renal Fluid Electrolyte Physiol 257: F746–F754, 1989. [DOI] [PubMed] [Google Scholar]
- 26.Powell SR, Davies KJ, Divald A. Optimal determination of heart tissue 26S-proteasome activity requires maximal stimulating ATP concentrations. J Mol Cell Cardiol 42: 265–269, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabb H, Mendiola CC, Saba SR, Dietz JR, Smith CW, Bonventre JV, Ramirez G. Antibodies to ICAM-1 protect kidneys in severe ischemic reperfusion injury. Biochem Biophys Res Commun 211: 67–73, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Razani-Boroujerdi S, Boyd RT, Dávila-García MI, Nandi JS, Mishra NC, Singh SP, Pena-Philippides JC, Langley R, Sopori ML. T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J Immunol 179: 2889–2898, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Sadis C, Teske G, Stokman G, Kubjak C, Claessen N, Moore F, Loi P, Diallo B, Barvais L, Goldman M, Florquin S, Le Moine A. Nicotine protects kidney from renal ischemia/reperfusion injury through the cholinergic anti-inflammatory pathway. PLoS ONE 2: e469, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeed RW, Varma S, Peng-Nemeroff Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med 201: 1113–1123, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka T, Nangaku M, Miyata T, Inagi R, Ohse T, Ingelfinger JR, Fujita T. Blockade of calcium influx through L-type calcium channels attenuates mitochondrial injury and apoptosis in hypoxic renal tubular cells. J Am Soc Nephrol 15: 2320–2333, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Tracey KJ The inflammatory reflex. Nature 420: 853–859, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der Poll T. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 130: 1822–1830, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Won SJ, Kim DY, Gwag BJ. Cellular and molecular pathways of ischemic neuronal death. J Biochem Mol Biol 35: 67–86, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Yang N, Luo M, Li R, Huang Y, Zhang R, Wu Q, Wang F, Li Y, Yu X. Blockage of JAK/STAT signaling attenuates renal ischaemia-reperfusion injury in rat. Nephrol Dial Transplant 23: 91–100, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Yeboah MM, Xue X, Duan B, Ochani M, Tracey KJ, Susin M, Metz CN. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int 74: 62–69, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]