Abstract
Reactive oxygen species have emerged as important molecules in cardiovascular dysfunction such as diabetes and hypertension. Recent work has shown that oxidative stress and angiotensin II signaling mutually regulate each other by multiple mechanisms and contribute to the development of hypertension. Most of the known biological actions of angiotensin II can be attributed to AT1 receptors. The present study was carried out to investigate the role of renal AT1 receptor signaling in oxidative stress-mediated hypertension. Male Sprague-Dawley rats received tap water (control) or 30 mM l-buthionine sulfoximine (BSO), an oxidant, with and without 1 mM tempol (an antioxidant) for 2 wk. Compared with control rats, BSO-treated rats exhibited increased oxidative stress and reduced antioxidant levels and developed hypertension. BSO treatment also caused increased renal proximal tubular AT1 receptor protein abundance, message levels, and ligand binding. In these rats, angiotensin II caused significantly higher accumulation of inositol trisphosphate (IP3) and phospholipase C (PLC) activation which was sensitive to blockade by AT1 but not to AT2 antagonist. Also, angiotensin II-mediated, AT1-dependent MAP kinase, Na-K-ATPase, and Na/H exchanger 3 activation was higher in BSO-treated rats than in control rats. Tempol supplementation of BSO-treated rats restored redox status, normalized AT1 receptor expression, and decreased blood pressure. Tempol also normalized the angiotensin II-mediated, AT1-dependent IP3 accumulation and PLC, MAP kinase, Na-K-ATPase, and Na/H exchanger 3 stimulation. These data suggest that oxidative stress leads to AT1 receptor upregulation, which in turn causes overstimulation of sodium transporters and subsequently contributes to sodium retention and hypertension. Tempol, while reducing oxidative stress, normalizes AT1 receptor signaling and decreases blood pressure.
Keywords: l-buthionine sulfoximine, MAP kinase, Na-K-ATPase, Na/H exchanger, tempol
maintenance of renal sodium homeostasis is critical to the regulation of extracellular fluid volume and blood pressure. The renin-angiotensin-aldosterone system (RAS), which affects blood pressure and contributes to the development of hypertension, plays a critical role in the regulation of renal sodium and water metabolism through a variety of physiological pathways (14, 18, 24–26). In particular, ANG II, an important component of the RAS, has known effects on renal hemodynamics, glomerular filtration rate, aldosterone secretion, as well as more direct effects on renal tubular solute transport in the proximal tubule (14, 18, 24–27). In the proximal tubules, the main site for renal water, sodium, and HCO3 reabsorption, it is well established that ANG II increases sodium reabsorption (14, 18, 24–27). Moreover, ANG II stimulates the rate of Na+ uptake in the isolated proximal tubule cells which is inhibited by the AT1 receptor antagonist (30).
ANG II acts through two receptor subtypes, the AT1 and the AT2 receptors, which mediate cardiovascular and other actions of ANG II (18, 22). All classic physiological effects of ANG II, such as vasoconstriction, cell proliferation, aldosterone and vasopressin release, sodium and water retention, and sympathetic facilitation, are mediated by the AT1 receptor (17). The AT2 receptor, in contrast, is upregulated in pathological conditions, and it counteracts several of the growth responses initiated by the AT1 and growth factor receptors (17). AT1 receptors are predominantly coupled to G proteins and signal through phospholipases, inositol phosphates, calcium channels, and a variety of serine/threonine and tyrosine kinases (17). In this regard, ANG II can activate an important serine/threonine protein kinase p42/44 MAP kinase (p42/44-ERK1/2, MAP kinase) pathway (35). ANG II, via AT1 receptor, stimulates the activities of the Na-K-ATPase and Na/H exchanger 3 in renal proximal tubules (11, 18, 30). The Na-K-ATPase is a plasma membrane enzyme that plays a critical role in sodium homeostasis, and it maintains Na+ and K+ gradients between the intra- and extracellular milieu that are important for the maintenance of cell volume and blood pressure regulation (11). Despite the physiological and pathophysiological importance of ANG II, possible signal transduction pathways involved in its regulation of the Na-K-ATPase are poorly understood.
Recently, it has become clear that many of these effects of ANG II are accentuated by reactive oxygen species such as superoxide and hydrogen peroxide and AT1 receptors are upregulated in hypertensive states (8, 29, 33). In spontaneously hypertensive rats (SHR), a commonly used animal model of human essential hypertension, and obese Zucker rats, the hypertension is associated with oxidative stress and AT1 receptor upregulation (6, 8, 29, 31–33, 38). Antioxidants reduce oxidative stress and blood pressure in SHR (31, 32, 38). Our own studies in obese Zucker rats and l-buthionine sulfoximine (BSO)-supplemented hypertensive rats show that antioxidants mitigated oxidative stress and reduced blood pressure (3, 4, 6). Therefore, these data provide a strong link between oxidative stress, AT1 receptor upregulation, and hypertension. However, it is not known whether the upregulated AT1 receptors cause increased stimulation of renal sodium transporters. Thus, the aim of this investigation was to study the ANG II-mediated Na-K-ATPase activation during oxidative stress-associated renal AT1 receptor upregulation and hypertension. We also studied the signaling mechanisms of ANG II-mediated Na-K-ATPase stimulation with particular emphasis on MAP kinase signaling pathway.
METHODS
Animals.
Male Sprague-Dawley (SD) rats (Harlan, Indianapolis, IN) were fed a normal rat diet and divided into following four groups: C (control), animals that were maintained on tap water; BSO, animals that were provided with 30 mM BSO (Sigma, St. Louis, MO); T (tempol), animals that were provided with 1 mM tempol (Sigma); and BSO+T, animals that were provided with BSO plus tempol. BSO, a glutamate cysteine ligase inhibitor, and tempol, a superoxide dismutase (SOD) mimetic compound, were provided in drinking water for 2 wk. All experiments were performed in compliance with University of Houston guidelines and protocols for care and use of laboratory animals. The animal protocol is approved by the IACUC. Blood pressure and glomerular filtration rate (GFR) were measured as detailed earlier (21). Briefly, rats were anesthetized with Inactin (100 mg/kg ip) and tracheotomy was performed to facilitate breathing. To measure blood pressure and heart rate, the left carotid artery was catheterized with PE-50 tubing, connected to a Statham P23AC pressure transducer, and blood pressure and heart rate were recorded on a Grass polygraph (model 7D, Grass Instrument, Quincy, MA). Plasma and urinary creatinine levels were measured by creatinine analyzer (model 2, Beckman, CA) and GFR (ml/min) was calculated based on the creatinine clearance.
Preparation of renal proximal tubular suspension.
Renal proximal tubular suspension was prepared as described earlier (21). Briefly, rats were anesthetized with pentobarbital sodium (50 mg/kg ip) and after a midline incision, the aorta was cannulated below the kidneys and an in situ digestion was accomplished by perfusing an enzyme solution of collagenase and hyaluronidase. Enrichment of proximal tubules was carried out using 20% ficoll gradient in Krebs buffer. Cell lysate, cytosol, and membrane fractions were isolated from proximal tubules by centrifugation (21). Protein was determined by bicinchoninic acid method (Pierce Chemical, Rockford, IL) using bovine serum albumin as a standard.
Indexes of oxidative stress.
Renal glutathione levels were assayed by colorimetric assay kit (21023; OXIS, Foster City, CA), 8-isoprostane was measured by RIA kit (516351; Cayman, Ann Arbor, MI), and nitrotyrosine was determined by immunoblotting kit (Upstate, Charlottesville, VA). Malondialdehyde was determined by the method of Mihara and Uchiyama (23). SOD activity was determined as described previously (5).
Assay of phospholipase C activity using exogenously added [3H]phosphatidylinositol 4,5-bisphosphate as a substrate.
Phospholipase C (PLC) activity was determined by using an exogenous source of [3H]phosphatidylinositol 4,5-bisphosphate ([3H]PIP2; PerkinElmer, Shelton, CT) as the primary substrate for PLC and monitoring the release of [3H]inositol trisphosphate ([3H]IP3) as described (5). Briefly, membranes were preincubated with ANG II and the reaction was started by adding [3H]PIP2 (0.005 μCi/assay tube) and phosphatidylserine. After 10 min, the reaction was terminated by addition of lipid extraction medium and radioactive decay was counted in a liquid scintillation spectrophotometer. Inositol trisphosphate (IP3) accumulation was measured by IP3 Biotrak Assay System (TRK1000, GE Healthcare, Piscataway, NJ).
Na-K-ATPase and Na/H exchanger 3 assay.
Na-K-ATPase activity was determined as reported previously (6). Briefly, ANG II-induced Na-K-ATPase stimulation was determined in renal proximal tubular suspensions (1 mg protein/ml) incubated with or without 1 mmol/l ouabain at 37°C for 15 min. Na/H exchanger 3 activity was determined by measurement of 5-(N-methyl-N-isobutyl)-amiloride-sensitive 22Na+ uptake (1).
Immunoblotting and MAP kinase activity.
Proteins were solubilized in Laemmli buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membrane. The membranes were blocked, incubated with antisera directed against AT1 receptor (Santa Cruz Biotechnology, Santa Cruz, CA), GAPDH and ERK1/2 (Calbiochem, La Jolla, CA), in 0.1% Tris-buffered saline followed by incubation with horseradish peroxidase-conjugated secondary antibodies. For ERK1/2 (p42/44) activation, the blots were incubated for 12 h with antiphosphospecific ERK1/2 or antibodies that recognize ERK1/2. GAPDH was used as loading control for whole cell lysate/homogenate and cytosol.
RT-PCR.
To quantify mRNA for AT1 receptor, RT-PCR and real-time detection monitoring the PCR products was employed. Total cellular RNA was extracted from proximal tubules and reverse transcription of total RNA to cDNA was carried out as described earlier (7). The above synthesized cDNA was used as a template in a 25-μl reaction volume, and a no template (a reagent control without added cDNA) was included in all experiments. Specific primers for the rat AT1 receptor and GAPDH are AT1 receptor forward: 5′-GGATGGTTCTCAGAGAGAGTACAT-3′; GAPDH forward: 5′-GGCCTTCCGTGTTCCTACC-3′. The PCR reaction was carried out as described (2).
125I-sar-ANG II binding.
Membranes binding of 125I-sar-ANG II (PerkinElmer) was performed essentially as described earlier (7). The membranes (50 μg protein) were incubated with 30 pM 125I-sar-ANG II at 30°C for 60 min in a shaking water bath. The assay was terminated by rapid filtration on GF/C filters under vacuum. The radioactivity on the filters was counted in gamma counter. Nonspecific binding was determined by performing the binding assay in the presence of 1 μM unlabeled ANG II.
Statistical analyses.
Differences between means were evaluated using the unpaired Student's t-test or ANOVA with Newman-Keuls multiple test, as appropriate. P < 0.05 was considered statistically significant.
RESULTS
The 15-day treatment of SD rats with BSO, tempol, and BSO plus tempol had no effect on body weight and these rats had similar water (data not shown) and food intake as rats that were kept on tap water (control) alone (Table 1). Also, there was no significant difference in GFR among the four experimental groups (Table 1). Compared with control, rats treated with BSO exhibited significant increase in blood pressure (Table 1). Supplementation of BSO treated with tempol normalized the increase in blood pressure, whereas in rats provided with tempol alone the blood pressure was similar to control rats (Table 1).
Table 1.
Food and water intake, oxidative markers, and blood pressure in BSO- and T-treated rats
| C | BSO | T | BSO+T | |
|---|---|---|---|---|
| Body wt, g | 240.0±23.0 | 249.0±18.0 | 255.0±21.0 | 245.0±17.0* |
| Food intake, g/day | 18.8±4.2 | 19.6±3.3 | 19.2±3.9 | 17.6±4.5 |
| 8-Isoprostane urine, pg/mg creatinine | 0.91±0.02 | 1.31±0.02* | 0.89±0.06† | 1.0±0.04† |
| 8-Isoprostane serum, fg/mg creatinine | 39.3±3.1 | 54.0±4.2* | 36.9±3.2† | 42.3±5.3† |
| Malondialdehyde, pmol/mg protein | 88.9±6.3 | 124.9±9.3* | 83.9±7.6† | 93.9±8.3† |
| Nitrotyrosine, arbitrary units | 116.6±11.3 | 183.2±15.3* | 110.9±8.9† | 130.3±10.6† |
| Glutathione, nmol/mg protein | 2.1±0.3 | 0.8±0.1* | 2.4±0.3† | 1.51±0.2*†‡ |
| Superoxide dismutase, EU/mg protein | 2.4±0.2 | 1.3±0.1* | 2.5±0.3† | 2.2±0.2† |
| Glomerular filtration rate, ml/min | 1.49±0.14 | 1.44±0.2 | 1.55±0.17 | 1.52±0.22 |
| Mean blood pressure, mmHg | 102.6±4.3 | 127.7±6.2* | 99.6±5.5† | 109.3±6.6† |
Data are means ± SE from 6–8 rats and were analyzed by ANOVA followed by post hoc Newman-Keuls multiple comparison test. C, control; BSO, l-buthionine-sulfoximine; T, tempol.
P < 0.05 vs. C.
P < 0.05 vs. BSO.
P < 0.05 vs. T.
Oxidative stress.
Treatment of rats with BSO caused a significant increase in oxidative stress. Compared with control, BSO-treated animals exhibited significant increases in plasma and urinary 8-isoprostane levels, renal malondialdehyde and nitrotyrosine content (Table 1). These rats also had decreased levels of renal glutathione and SOD activity (Table 1). Tempol supplementation restored the redox status in BSO-treated rats. There were no significant differences in oxidative or antioxidative markers between control and rats treated with tempol alone (Table 1).
AT1 receptor upregulation in BSO-treated rats.
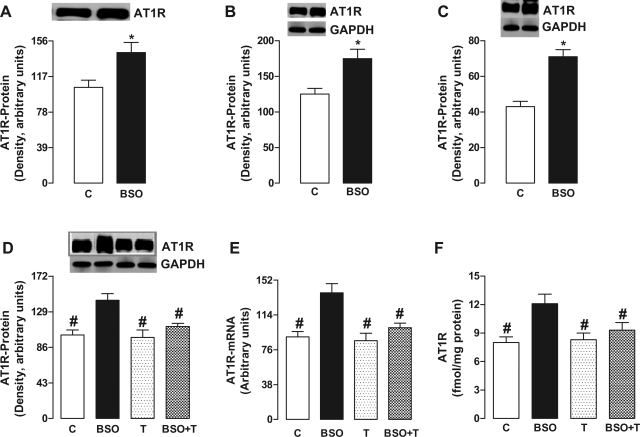
In BSO-treated SD rats, the renal proximal tubular AT1 receptor protein content was increased in cell membranes (Fig. 1A), cytosol (Fig. 1B), and whole cell lysate (homogenate; Fig. 1, C and D), suggesting an increased protein synthesis. In addition, there was an increased AT1 receptor mRNA level in BSO-treated rats compared with control (Fig. 1E). BSO treatment also increased the membrane ligand (125I-sar-Ang) binding compared with control (Fig. 1F). In these animals, tempol decreased the AT1 receptor protein content, ligand binding, and mRNA levels (Fig. 1, D–F). Tempol per se had no effect on these parameters (Fig. 1, D–F).
Fig. 1.
Effect of oxidative stress on AT1 receptor (ATIR) expression in renal proximal tubules from control (C), l-buthionine sulfoximine (BSO)-, tempol (T)-, and BSO plus tempol (BSO+T)-treated rats. AT1R protein content in membrane (A), cytosol (B), and whole cell lysate/homogenate (C and D). AT1R mRNA levels (E) and specific 125I-sar-ANG II membrane binding (F). Bands are representative Western blots. Bars represent means ± SE from 6–8 animals performed in triplicate. *P < 0.05 vs. control using Student's t-test. #P < 0.05 vs. BSO using 1-way ANOVA followed by post hoc Newman-Keuls multiple test.
Oxidative stress accentuated the ANG II-induced IP3 accumulation.
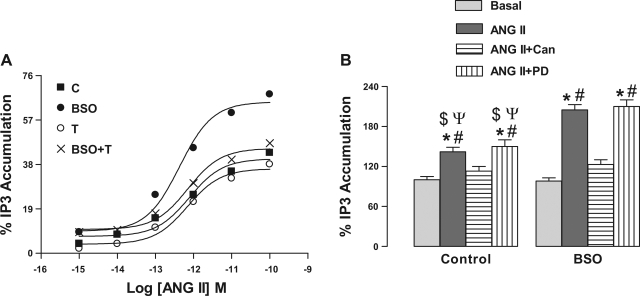
To ascertain that upregulated AT1 receptors are functional, we determined ANG II mediated IP3 production, a second messenger for AT1 receptors. As shown in Fig. 2A, incubation of renal proximal tubules with ANG II caused concentration-dependent accumulation of IP3 in control rats. In proximal tubules from BSO-treated rats, the ANG II-induced IP3 accumulation was significantly higher than control. ANG II-induced IP3 production in tempol- and BSO plus tempol-treated rats was comparable to control (Fig. 2A) and basal IP3 levels were similar in all experimental groups (data not shown). ANG II (10 pM)-induced IP3 production was sensitive to AT1 receptor antagonist candesartan (1 μM), but it was not affected by 1 μM PD-123319, an AT2 receptor antagonist (Fig. 2B).
Fig. 2.
Effect of oxidative stress on ANG II-induced inositol trisphosphate (IP3) accumulation in renal proximal tubules from C, BSO-, T-, and BSO+T-treated rats. A: renal proximal tubular membranes were challenged with indicated doses of ANG II for 10 min. The data are expressed as a percentage of stimulation produced by the indicated concentration of ANG II. A single experiment is shown, representative of 6–8 individual experiments performed in triplicate. B: membranes were incubated with 10 pM ANG II for 10 min in the presence and absence of 1 μM candesartan (Can) or PD-123319 (PD). Bars represent means ± SE of 6–8 different experiments performed in triplicate. *P < 0.05 vs. basal. #P < 0.05 vs. ANG II + Can. $P < 0.05 vs. ANG II from BSO group. ΨP < 0.05 vs. ANG II + PD from BSO group using 1-way ANOVA followed by post hoc Newman-Keuls multiple test.
Oxidative stress enhanced the ANG II-induced PLC activation.
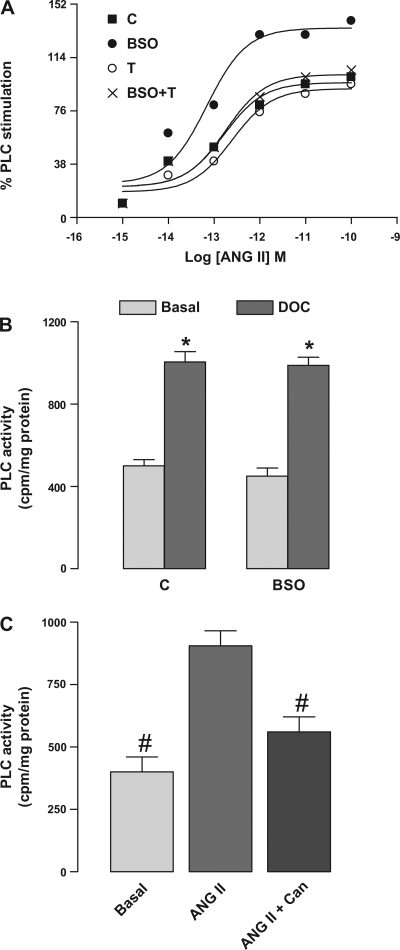
To determine whether higher ANG II-mediated IP3 accumulation in BSO-treated rats was due to decreased turn over or increased production of IP3, we also determined the PLC activity, an enzyme responsible for IP3 synthesis, in renal proximal tubular membranes. As shown in Fig. 3A, incubation of renal proximal tubules with ANG II caused concentration-dependent activation of PLC in all experimental groups. However, as observed with IP3 accumulation, the hormonal response was significantly higher in BSO-treated rats compared with other groups (Fig. 3A). The basal PLC activity was similar in all the groups (data not shown). Also, 3 mM deoxycholate (DOC), a direct activator of PLC, caused similar stimulation of PLC in control and BSO-treated rats (Fig. 3B). Furthermore, preincubation of proximal tubular membranes from control rats with 1 μM candesartan blocked the ANG II (10 pM)-mediated PLC activation (Fig. 3C), while 1 μM PD-123319 had no effect (data not shown).
Fig. 3.
Effect of oxidative stress on ANG II-induced phospholipase C (PLC) stimulation in renal proximal tubules from C, BSO-, T-, and BSO+T-treated rats. A: renal proximal tubular membranes were incubated with [3H]phosphatidylinositol-4,5-bisphosphate ([3H]PIP2) and challenged with indicated doses of ANG II for 10 min. The data are expressed as a percentage of stimulation produced by the indicated concentration of ANG II. A single experiment is shown, representative of 6–8 individual experiments performed in triplicate. B: membranes from control and BSO-treated rats were incubated with 3 mM deoxycholate (DOC). C: membranes from control rats were incubated with 10 pM ANG II for 10 min in the presence and absence of 1 μM Can. Bars represent means ± SE of 6–8 different experiments performed in triplicate. *P < 0.05 vs. basal using Student's t-test. #P < 0.05 vs. ANG II using 1-way ANOVA followed by post hoc Newman-Keuls multiple test.
Oxidative stress exaggerated the ANG II-induced Na-K-ATPase and Na/H exchanger 3 stimulation.
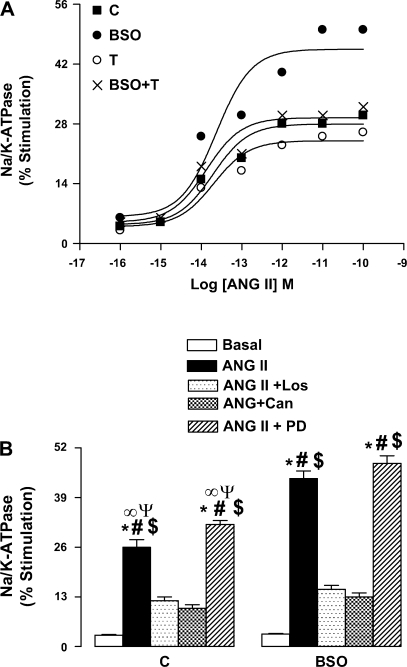
As shown in Fig. 4A, ANG II caused concentration-dependent stimulation of Na-K-ATPase in proximal tubules from control animals. However, the stimulatory effect of ANG II was more robust in proximal tubules from BSO-treated rats (Fig. 4A). Tempol supplementation of BSO-treated rats normalized the hormonal stimulation of Na-K-ATPase (Fig. 4A). The stimulatory effect of ANG II was similar in control and tempol-treated rats (Fig. 4A). The basal activity of Na-K-ATPase (nmol Pi·mg protein−1·min−1) was similar in all the four groups, C 232.3 ± 16.1, BSO 248.4 ± 19.3, T 225.0 ± 15.6, and BSO+T 238.9 ± 21.6. The stimulatory effect of ANG II was AT1 receptor specific because preincubation of proximal tubules with either 1 μM losartan or 1 μM candesartan abolished the ANG II (10 pM)-induced Na-K-ATPase stimulation, whereas PD-123319 at similar concentration had no effect (Fig. 4B).
Fig. 4.
Effect of oxidative stress on ANG II-induced Na-K-ATPase stimulation in renal proximal tubules from C, BSO-, T-, and BSO+T-treated rats. A: renal proximal tubules were challenged with the indicated doses of ANG II for 10 min. The data are expressed as a percentage of stimulation produced by the indicated concentration of ANG II. A single experiment is shown, representative of 6 individual experiments performed in triplicate. B: renal proximal tubules were incubated with 1 μM losartan (Los) or Can or PD followed by challenge with 10 pM ANG II for 10 min. Bars represent means ± SE of 6–8 different experiments performed in triplicate. *P < 0.05 vs. basal. #P < 0.05 vs. ANG II + Los. $P < 0.05 vs. ANG II + Can. ∞P < 0.05 vs. ANG II from BSO group. ΨP < 0.05 vs. ANG II + PD from BSO group using 1-way ANOVA followed by post hoc Newman-Keuls multiple test.
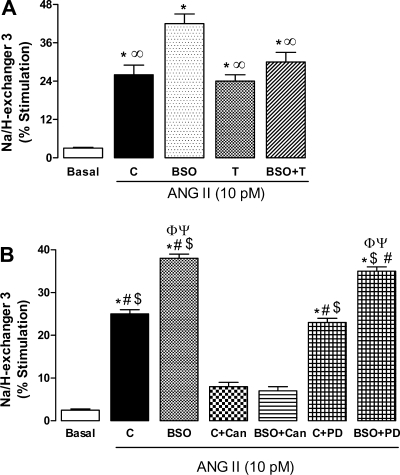
Incubation of proximal tubules with ANG II (10 pM) also caused significantly higher activation of Na/H exchanger 3 in BSO-treated rats compared with control (Fig. 5A). Tempol mitigated the exaggerated stimulation of Na/H exchanger 3 in BSO-treated rats, while having no effect when given alone (Fig. 5A). The basal activity of Na/H exchanger 3 (nmol 22Na+·mg protein−1·min−1) was similar in all animal groups, C 3.3 ± 0.4, BSO 2.9 ± 0.3, T 3.5 ± 0.6, and BSO+T 2.8 ± 0.3. Similar to Na-K-ATPase, the ANG II-mediated Na/H exchanger 3 stimulation was sensitive to AT1 receptor blocker, while AT2 blocker had no effect (Fig. 5B).
Fig. 5.
Effect of oxidative stress on ANG II-induced Na/H exchanger 3 stimulation in renal proximal tubules from C, BSO-, T-, and BSO+T-treated rats. A: renal proximal tubules were challenged with 10 pM ANG II for 10 min. B: renal proximal tubules were incubated with 1 μM Can or PD followed by challenge with 10 pM ANG II for 10 min. Bars represent means ± SE of 6–8 different experiments performed in triplicate. *P < 0.05 vs. basal. ∞P < 0.05 vs. BSO. #P < 0.05 vs. C + Can. $P < 0.05 vs. BSO + Can. ΦP < 0.05 vs. C. ΨP < 0.05 vs. C + PD using 1-way ANOVA followed by post hoc Newman-Keuls multiple test.
Oxidative stress increased ANG II-mediated mitogen-activated protein kinase activation.
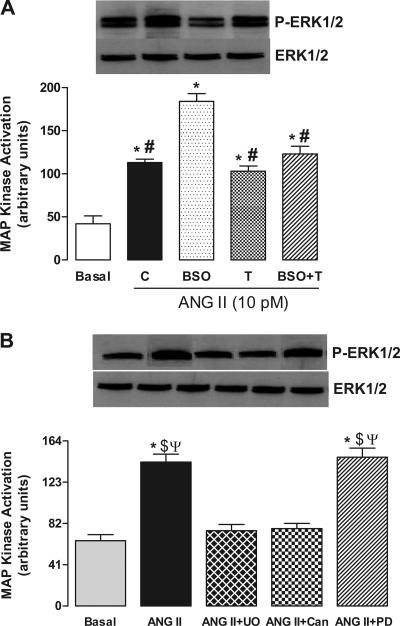
To determine the ANG II-mediated mitogen-activated protein (MAP) kinase activation, we analyzed the phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) in renal proximal tubules. The immunobloting experiments showed that incubation of proximal tubules with 10 pM ANG II caused a significant increase in ERK1/2 phosphorylation (Fig. 6A), without affecting the ERK1/2 protein expression, in both control and BSO-treated rats (Fig. 6A). However, the ANG II-mediated phosphorylation of ERK1/2 was markedly higher in BSO-treated rats compared with control (Fig. 6A). Tempol abolished the increased phosphorylation of ERK1/2 in BSO-treated rats, while showing no effect when provided alone (Fig. 6A). The ANG II (10 pM)-mediated ERK1/2 activation was blocked by candesartan (1 μM) and UO126 (10 μM), a MAP kinase kinase (MEK) inhibitor which prevents MAP kinase stimulation, while PD-123319 (1 μM) had no effect (Fig. 5B).
Fig. 6.
Effect of oxidative stress on ANG II-induced MAP kinase activation in renal proximal tubules from C, BSO-, T-, and BSO+T-treated rats. A: renal proximal tubules were challenged with 10 pM ANG II for 10 min. B: renal proximal tubules from control rats were incubated with 1 μM Can or 10 μM UO126 (UO) followed by challenge with 10 pM ANG II for 10 min. Bands are representative Western blots and bars are means ± SE of 6–8 different experiments performed in triplicate. *P < 0.05 vs. basal. #P < 0.05 vs. BSO. $P < 0.05 vs. ANG II + UO. ΨP < 0.05 vs. ANG II + Can using 1-way ANOVA followed by post hoc Newman-Keuls multiple test.
Role of MAP kinase in ANG II-mediated Na-K-ATPase stimulation.
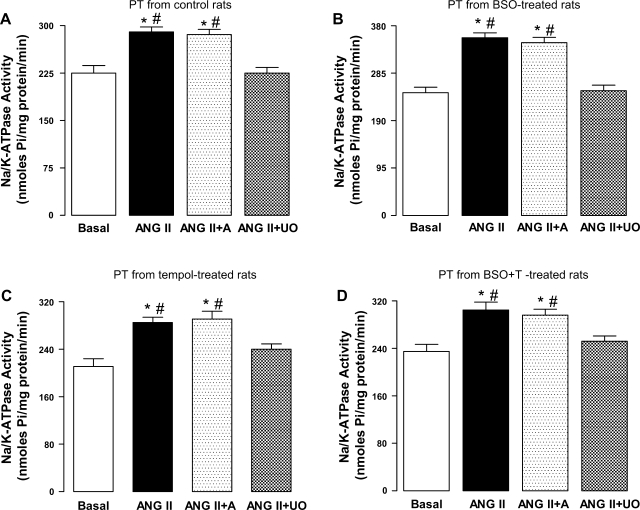
As shown in Fig. 7, A–D, the incubation of proximal tubules, from all the experimental groups, with 10 pM ANG II caused a significant increase in Na-K-ATPase activity. This ANG II-mediated Na-K-ATPase was abolished when proximal tubules were preincubated with MEK inhibitor UO126 (10 μM), which also blocked ANG II-induced MAP kinase stimulation (Fig. 6B), while amiloride (10 μM), a Na/H exchanger 3 inhibitor, showed no effect on ANG II response (Fig. 7, A–D).
Fig. 7.
Effect of oxidative stress on ANG II-induced Na-K-ATPase activation in renal proximal tubules from C (A), BSO (B)-, T (C)-, and BSO+T (D)-treated rats. Renal proximal tubules (PT) were incubated with 10 μM amiloride (A) or 10 μM UO followed by challenge with 10 pM ANG II for 10 min. Bars are means ± SE of 6–8 different experiments performed in triplicate. *P < 0.05 vs. basal. #P < 0.05 vs. ANG II + UO using 1-way ANOVA followed by post hoc Newman-Keuls multiple test.
DISCUSSION
The link between oxidative stress and high blood pressure has been demonstrated in both hypertensive animal models and humans, but understating of the intermediate molecules involved is incomplete. Our studies show that SD rats treated with BSO, a prooxidant compound, exhibited significant oxidative stress and increase in blood pressure. In these animals, oxidative stress caused renal AT1 receptor upregulation which led to exaggerated ANG II response/signaling leading to an increase in blood pressure. ANG II via AT1 receptors caused significantly higher IP3 accumulation and PLC activation. Also, the stimulation of sodium transporters Na-K-ATPase and Na/H exchanger 3 in response to ANG II was higher in BSO-treated hypertensive rats compared with normotensive control animals. Our data also show that rapid ANG II stimulation of Na-K-ATPase was mediated by MAP kinase. Tempol supplementation of BSO-treated rats decreased oxidative stress and normalized AT1 receptor expression and signaling and blood pressure.
Our observation that BSO-treated rats with oxidative stress, as evidenced by increased levels of malondialdehyde, 8-isoprostane, and nitrotyrosine and decreased antioxidant markers like glutathione and SOD activity, exhibited high blood pressure is consistent with previous observations that hypertensive humans and animal models have unbalanced oxidative status (13, 19, 20, 28, 36). In humans with essential hypertension, administration of vitamin C has been shown to lower plasma isoprostane and attenuate abnormal endothelium-dependent vasodilation and decrease blood pressure (34). Studies in SHR showed that chronic administration of antioxidants decreased oxidative stress and blood pressure (31, 32, 38). Our data also showed that tempol supplementation of BSO-treated rats restored the redox homeostasis and decreased blood pressure (3, 4). Tempol has been shown to reduce oxidative stress, prevent oxidative remodeling, improve renovascular function, and lower the blood pressure (12, 31, 32, 34, 38).
Various studies showed that hypertension in different animal models, including SHR, is associated with oxidative stress and AT1 receptor upregulation (8, 29, 32, 33). Recent evidences also indicate that AT1 receptors are upregulated during hypertensive states and oxidants can accentuate the ANG II signaling (8, 29, 32, 33). We found that in BSO-treated rats oxidative stress increased AT1 receptor expression, while antioxidant tempol normalized the AT1 receptor upregulation and blood pressure, suggesting that AT1 receptor upregulation may serve as a link between oxidative stress and hypertension. While the mechanisms by which reactive oxygen species modulate the function of AT1 receptor in different organs are unclear, recent findings indicate that NF-κB, a redox-sensitive transcription factor, upregulates AT1 receptor involving two binding sites within the 5′-flanking region of AT1 receptor gene (9, 10). Also, NF-κB is necessary for cytokine-induced upregulation of both AT1 receptor mRNA and protein expression (9, 10). Our cell fractionation experiments showed an increased AT1 receptor protein expression in whole cell lysate, membrane, and cytosol in BSO-treated rats compared with control, indicating an increased protein synthesis. Also, there was an increase in AT1 receptor message level in these rats. Taken together, these data show that oxidative stresses can transcriptionally upregulate AT1 receptors which may involve NF-κB activation. This is in agreement with our earlier studies in obese Zucker rats and BSO-treated hypertensive rats showing oxidative stress activated NF-κB and antioxidants while mitigating oxidative stress, attenuated NF-κB, and reduced blood pressure (3, 6).
Upon binding of ANG II to its receptor, AT1 receptor interacts and activates several heterotrimeric G proteins including Gq/11 (14, 17). Activation of Gq/11 leads to stimulation of PLC, which hydrolyses phosphatidylinositol 4,5-bisphosphate to IP3 and diacylglycerol (14, 17). These two major second messengers produced by AT1 receptor activation lead to intracellular Ca2+ elevation, protein kinase activation, and other cellular effects (14, 17). To ascertain that the upregulated AT1 receptors in BSO-treated rats are functional, we determined the ANG II-mediated IP3 accumulation in proximal tubules from control and BSO-treated rats. We found that ANG II caused a significant accumulation of IP3 in both control and BSO-treated rats; however, the accumulation was markedly higher in BSO-treated rats compared with control. To rule out that increased IP3 accumulation in BSO-treated rats is not due to decreased turnover of the second messenger, we also measured the ANG II-mediated PLC activation. Similar to IP3 accumulation, ANG II-mediated PLC activation was significantly higher in BSO-treated rats compared with control. It should be noted that both ANG II-mediated IP3 accumulation and PLC activation were sensitive to AT1 receptor antagonist candesartan but not to AT2 antagonist PD-123319, suggesting that upregulated AT1 receptors are functional and exaggerated response to ANG II was AT1 receptor specific. In addition, supplementation of BSO-treated rats with tempol normalized the ANG II-induced PLC activation and IP3 accumulation, indicating that oxidative stress contributes to AT1 receptor upregulation and increased hormonal response to this peptide during hypertension.
It is well established that ANG II not only regulates blood pressure via its vascular effects but also as a principal determinant of fluid volume homeostasis (18). One of the mechanisms by which ANG II maintains salt and water balance and blood pressure is via the regulation of sodium transports, Na-K-ATPase and Na/H exchanger 3, in renal proximal tubules where 70% of filtered sodium is reabsorbed (16, 18). Herein, we found that the incubation of proximal tubules with ANG II caused a concentration-dependent activation of Na-K-ATPase in control rats. At similar ANG II concentration the stimulation of Na-K-ATPase was markedly higher in BSO-treated rats. The stimulation of Na-K-ATPase was sensitive to candesartan but not to PD-123319, indicating that the exaggerated hormonal response of Na-K-ATPase is due to upregulation of AT1 receptors. In proximal tubules from tempol- or tempol plus BSO-treated rats the response to ANG II was similar to control animals, suggesting the role of oxidative stress in AT1 receptor-dependent sodium transport regulation. Similar to Na-K-ATPase regulation, ANG II-mediated Na/H exchanger 3 stimulation in renal proximal tubules was higher in BSO-treated rats than in control rats and tempol supplementation of BSO-treated rats normalized this ANG II response. The basal activities of both the transporters were similar in all experimental groups, which also indicate that enhanced activation of these transporters is due to AT1 receptor upregulation associated with oxidative stress.
The ability of ANG II to significantly increase Na-K-ATPase activity and expression has been demonstrated in several systems, but understanding of the intracellular signal transduction pathways involved is incomplete. Our observation of rapid ANG II (10 min) stimulation of Na-K-ATPase activity is consistent with previous observations that ANG II, acting via AT1 receptors, can acutely regulate sodium transporters in various tissues including kidney. The data presented in this paper are novel because they indicate that the stimulation of the proximal tubular Na-K-ATPase by ANG II involves ERK1/2 pathways as the activation of Na-K-ATPase was blocked with pharmacological inhibition of MAP kinase. With regard to the MAP kinase pathway, the role of ERK1/2 MAP kinase activation in ANG II-stimulated Na-K-ATPase activity was assessed, using ERK1/2 (P42/44) inhibitor UO126. This inhibitor suppressed phosphorylation of ERK1/2 in proximal tubules treated with ANG II. Furthermore, pretreatment of tubules with UO126 also abolished ANG II-stimulated Na-K-ATPase activity. Interestingly, the ANG II-mediated ERK1/2 phosphorylation was higher in BSO-treated rats and was sensitive to AT1 antagonist while AT2 antagonist had no effect. Also, tempol normalized the ANG II-mediated ERK1/2 phosphorylation in BSO-treated rats. Thus, these data demonstrate that MAP kinase signaling is necessary for the both normal and exaggerated ANG II stimulation of the Na-K-ATPase in proximal tubules.
The effect of the Na/H exchanger 3 on ANG II action was also investigated because it has been previously suggested that an increase in Na+ influx is required for ANG II-stimulated Na-K-ATPase activity (15). A variety of growth factors and vasoconstrictors activate Na/H exchanger 3, leading to increases in intracellular Na+ and intracellular alkalinization, with secondary activation of the Na-K-ATPase to restore homeostasis (37). In the current study, however, ANG II-mediated stimulation of Na-K-ATPase was not inhibited by amiloride, a relatively specific inhibitor of Na/H exchanger 3, suggesting that this acute stimulation was not dependent on Na/H exchanger 3 activation.
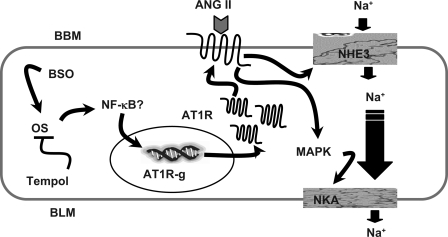
In conclusion, the data indicate that oxidative stress causes renal AT1 receptor upregulation and hypertension. The upregulated renal AT1 receptors led to enhanced ANG II signaling causing overstimulation of effector molecules like PLC and MAP kinase and increased accumulation of second messenger IP3. This in turn caused higher stimulation of Na-K-ATPase and Na/H exchanger 3 in renal proximal tubules. Therefore, the enhanced sodium transport activity in proximal tubules could contribute to sodium retention and subsequent hypertension (Fig. 8). Antioxidant tempol ameliorated the oxidative stress, which in turn normalized AT1 receptor signaling and blood pressure.
Fig. 8.
Proposed mechanism of oxidative stress-mediated angiotensin AT1R upregulation and overstimulation of sodium transporters. AT1R-g, angiotensin AT1R gene; BBM, brush-border membrane; BLM, basolateral membrane; NKA, Na-K-ATPase; NHE3, Na/H exchanger 3; MAPK, MAP kinase. Proposed signaling (→), inhibition (—l), unknown mechanism (?).
GRANTS
This study was supported by National Institutes of Health Grant AG-25056 from the National Institute of Aging.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Albrecht FE, Xu J, Moe OW, Hopfer U, Simonds WF, Orlowski J, Jose PA. Regulation of NHE3 activity by G protein subunits in renal brush-border membranes. Am J Physiol Regul Integr Comp Physiol 278: R1064–R1073, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Ansar S, Vikman P, Nielsen M, Edvinsson L. Cerebrovascular ETB, 5-HT1B, and AT1 receptor upregulation correlates with reduction in regional CBF after subarachnoid hemorrhage. Am J Physiol Heart Circ Physiol 293: H3750–H3758, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Banday AA, Fazili FR, Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and hypertension via mechanisms that involve nuclear factor-kappaB and protein kinase C. J Am Soc Nephrol 18: 1446–1457, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Banday AA, Lau YS, Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and salt-sensitive hypertension in Sprague-Dawley rats. Hypertension 51: 367–375, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Banday AA, Lokhandwala MF. Oxidative stress reduces renal dopamine D1 receptor-Gq/11αG protein-phospholipase C signaling involving G protein-coupled receptor kinase 2. Am J Physiol Renal Physiol 293: F306–F315, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Banday AA, Marwaha A, Tallam LS, Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes 54: 2219–2226, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Banday AA, Siddiqui AH, Menezes MM, Hussain T. Insulin treatment enhances AT1 receptor function in OK cells. Am J Physiol Renal Physiol 288: F1213–F1219, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Becker M, Umrani D, Lokhandwala MF, Hussain T. Increased renal angiotensin II AT1 receptor function in obese Zucker rat. Clin Exp Hypertens 25: 35–47, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Cowling RT, Gurantz D, Peng J, Dillmann WH, Greenberg BH. Transcription factor NF-kappa B is necessary for upregulation of type 1 angiotensin II receptor mRNA in rat cardiac fibroblasts treated with tumor necrosis factor-alpha or interleukin-1 beta. J Biol Chem 277: 5719–5724, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Cowling RT, Zhang X, Reese VC, Iwata M, Gurantz D, Dillmann WH, Greenberg BH. Effects of cytokine treatment on angiotensin II type 1A receptor transcription and splicing in rat cardiac fibroblasts. Am J Physiol Heart Circ Physiol 289: H1176–H1183, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Feraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev 81: 345–418, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Haj-Yehia AI, Nassar T, Assaf P, Nassar H, Anggard EE. Effects of the superoxide dismutase-mimic compound TEMPOL on oxidant stress-mediated endothelial dysfunction. Antioxid Redox Signal 1: 221–232, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med 346: 1954–1962, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 112: 417–428, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Isenovic ER, Jacobs DB, Kedees MH, Sha Q, Milivojevic N, Kawakami K, Gick G, Sowers JR. Angiotensin II regulation of the Na+ pump involves the phosphatidylinositol-3 kinase and p42/44 mitogen-activated protein kinase signaling pathways in vascular smooth muscle cells. Endocrinology 145: 1151–1160, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Jose PA, Eisner GM, Felder RA. Renal dopamine receptors in health and hypertension. Pharmacol Ther 80: 149–182, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press 12: 70–88, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Lip GY, Edmunds E, Nuttall SL, Landray MJ, Blann AD, Beevers DG. Oxidative stress in malignant and non-malignant phase hypertension. J Hum Hypertens 16: 333–336, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Manning RD, Meng S, Tian N. Renal and vascular oxidative stress and salt sensitivity of arterial pressure. Acta Physiol Scand 179: 243–250, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Marwaha A, Banday AA, Lokhandwala MF. Reduced renal dopamine D1 receptor function in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol 286: F451–F457, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86: 271–278, 1978. [DOI] [PubMed] [Google Scholar]
- 24.Navar LG, Nishiyama A. Why are angiotensin concentrations so high in the kidney? Curr Opin Nephrol Hypertens 13: 107–115, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Navar LG, Saccomani G, Mitchell KD. Synergistic intrarenal actions of angiotensin on tubular reabsorption and renal hemodynamics. Am J Hypertens 4: 90–96, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Quan A, Baum M. Endogenous angiotensin II modulates rat proximal tubule transport with acute changes in extracellular volume. Am J Physiol Renal Physiol 275: F74–F78, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redon J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, Saez GT. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 41: 1096–1101, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Reja V, Goodchild AK, Phillips JK, Pilowsky PM. Upregulation of angiotensin AT1 receptor and intracellular kinase gene expression in hypertensive rats. Clin Exp Pharmacol Physiol 33: 690–695, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Saccomani G, Mitchell KD, Navar LG. Angiotensin II stimulation of Na+-H+ exchange in proximal tubule cells. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1188–F1195, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 32: 59–64, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension 33: 424–428, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Shah S, Hussain T. Enhanced angiotensin II-induced activation of Na+, K+-ATPase in the proximal tubules of obese Zucker rats. Clin Exp Hypertens 28: 29–40, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Taddei S, Virdis A, Ghiadoni L, Magagna A, Favilla S, Pompella A, Salvetti A. Restoration of nitric oxide availability after calcium antagonist treatment in essential hypertension. Hypertension 37: 943–948, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Viedt C, Soto U, Krieger-Brauer HI, Fei J, Elsing C, Kubler W, Kreuzer J. Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol 20: 940–948, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Ward NC, Hodgson JM, Puddey IB, Mori TA, Beilin LJ, Croft KD. Oxidative stress in human hypertension: association with antihypertensive treatment, gender, nutrition, and lifestyle. Free Radic Biol Med 36: 226–232, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Weissberg PL, Little PJ, Cragoe EJ Jr, Bobik A. Na-H antiport in cultured rat aortic smooth muscle: its role in cytoplasmic pH regulation. Am J Physiol Cell Physiol 253: C193–C198, 1987. [DOI] [PubMed] [Google Scholar]
- 38.Welch WJ, Mendonca M, Blau J, Karber A, Dennehy K, Patel K, Lao YS, Jose PA, Wilcox CS. Antihypertensive response to prolonged tempol in the spontaneously hypertensive rat. Kidney Int 68: 179–187, 2005. [DOI] [PubMed] [Google Scholar]