Abstract
The objectives of this study were to determine the effects of chronic angiotensin II (ANG II) infusions on ANG II content and angiotensinogen expression in the mouse kidney and the role of the angiotensin II type 1 receptor (AT1R) in mediating these changes. C57BL/6J male mice were subjected to ANG II infusions at doses of 400 or 1,000 ng·kg−1·min−1 either alone or with an AT1R blocker (olmesartan; 3 mg·kg−1·day−1) for 12 days. Systolic and mean arterial pressures were determined by tail-cuff plethysmography and radiotelemetry. On day 13, blood and kidneys were collected for ANG II determinations by radioimmunoanalysis and intrarenal angiotensinogen expression studies by quantitative RT-PCR, Western blotting, and immunohistochemistry. ANG II infusions at the low dose elicited progressive increases in systolic blood pressure (135 ± 2.5 mmHg). In contrast, the high dose induced a rapid increase (152 ± 2.5, P < 0.05 vs. controls, 109 ± 2.8). Renal ANG II content was increased by ANG II infusions at the low dose (1,203 ± 253 fmol/g) and the high dose (1,258 ± 173) vs. controls (499 ± 40, P < 0.05). Kidney angiotensinogen mRNA and protein were increased only by the low dose to 1.13 ± 0.02 and 1.26 ± 0.10, respectively, over controls (1.00, P < 0.05). These effects were not observed in mice infused at the high dose and those receiving olmesartan. The results indicate that chronic ANG II infusions augment mouse intrarenal ANG II content with AT1R-dependent uptake occurring at both doses, but only the low dose of infusion, which elicited a slow progressive response, causes an AT1R-dependent increase in intrarenal angiotensinogen expression.
Keywords: telemetry, mouse kidney, hypertension
chronic infusions of angiotensin II (ANG II) lead to elevations in intrarenal ANG II content associated with reductions in renal function and sodium excretion and hypertension (26, 29, 31, 35). An increase in intrarenal ANG II content is also associated with enhanced oxidative stress and chronic proinflammatory and proliferative responses that result in progressive tissue injury (7, 18). Indeed, every model of experimental ANG II-dependent hypertension is characterized by augmentation of intrarenal ANG II content to levels much greater than can be explained on the basis of equilibration with the systemic circulation (8, 23, 39).
Augmentation of intrarenal ANG II occurs by several processes. ANG II is actively accumulated in the kidney via internalization mainly by the ANG II type 1 receptor (AT1R) (20, 38, 39). However, megalin, an abundant protein in proximal tubule cells, can also bind and internalize ANG II (6). In addition, the kidneys express all components of the renin-angiotensin system (RAS) and can therefore generate angiotensin peptides from locally formed angiotensinogen (9, 11, 17, 25). Increased ANG II exerts a positive effect on the expression of angiotensinogen mRNA and protein by proximal tubule cells in ANG II-infused rats that translates into an elevation of angiotensinogen excretion in the urine (13, 14, 27). The intrarenal activity of angiotensin-converting enzyme, abundantly expressed in the tubules, is also elevated during ANG II-dependent hypertension (9). Furthermore, principal cells in connecting tubules and collecting ducts express renin, which is also augmented in ANG II-infused rats (30). AT1R abundance in the tubules is also increased during ANG II-dependent hypertension (9). These observations provide the foundation for the hypothesis that intrarenal ANG II synthesis is augmented during ANG II-dependent hypertension despite suppressed renin production by juxtaglomerular cells and elevated arterial pressure.
An important issue that remains unresolved is the quantitative contribution of local generation of ANG II vs. receptor-mediated accumulation from systemic ANG II to the overall intrarenal levels of this hormone (38, 39). These two processes have been difficult to separate in vivo because they are fundamentally linked, as both involve AT1R activation by ANG II. The availability of genetically modified mice opens the possibility for delineation of this matter; however, there is little information presently available regarding the basal activity of the intrarenal RAS in mice. Furthermore, while it is known that mice subjected to chronic infusions of ANG II at subpressor doses develop a blood pressure profile similar to ANG II-infused rats (12, 36), the effects of chronic ANG II infusions on the intrarenal RAS and, in particular, proximal tubule angiotensinogen message and protein are unknown. Accordingly, studies were performed to determine the changes in intrarenal ANG II levels and angiotensinogen expression during ANG II-induced hypertension in mice and to determine the role of the AT1R in mediating these responses.
METHODS
Animal Preparation and Sample Collection
All protocols were approved by the Tulane University Health Sciences Center Animal Care and Use Committee. Mice (C57BL/6J, 8- to 10-wk-old males) were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained in a temperature-controlled room on a 12:12-h light-dark cycle with free access to food (Na+ content 0.4%) and water.
First series.
In this series, mice were trained for systolic blood pressure (SBP) determinations by tail-cuff plethysmography before recording of initial pretreatment measurements. Following this period, mice were anesthetized with 1–2% isoflurane inhalation in 100% O2 to allow subcutaneous implantation of osmotic minipumps (Alzet 1002, Durect, Cupertino, CA). ANG II was infused at doses of 400 or 1,000 ng·kg−1·min−1 with a separate group of each series treated with olmesartan (an AT1R blocker, 3 mg·kg−1·day−1) for 12 days before sample collection. Sham-operated animals were used as controls. SBP was recorded every 3 days using the Visitech BP2000 system (Visitech Systems, Apex, NC) as the average of 30 readings/animal taken between 1:00 and 5:00 P.M.. On day 13, the mice were killed by conscious decapitation to collect blood and tissue samples. The right kidney was utilized for ANG II determinations. The left kidney cortex was utilized for mRNA and protein expression studies. The kidneys were processed as described previously (14, 30).
Second series.
In this series, mice were subjected to isoflurane anesthesia and a catheter connected to a radiotelemetry device was inserted in the left carotid artery to monitor heart rate and BP by telemetry in conscious, unrestrained conditions (3, 21) (model PA-C10, Data Sciences International, St. Paul, MN). After a recovery phase (7–14 days), basal mean arterial pressure (MAP) levels were established and animals were again subjected to general anesthesia for minipump implantation. Data were collected, stored, and analyzed using Dataquest A.R.T 4.0 software (Data Sciences International). ANG II was infused only at the lower dose (400 ng·kg−1·min−1) for 12 days before sample collection. On day 13, animals were given an isoflurane overdose and the kidneys were collected and fixed in zinc-saturated formalin and further processed for immunohistochemistry.
Determinations of ANG II, Plasma Renin Concentration, and Kidney Renin Content by RIA
For analysis of plasma ANG II, trunk blood from mice in the first series was collected in chilled tubes containing 5.0 mmol/l EDTA and a protease inhibitor cocktail. For intrarenal ANG II, the right kidneys were homogenized in methanol immediately after extraction. Later, plasma and kidney samples were processed as previously described (10, 16, 30). A rabbit anti-ANG II antisera (Peninsula Laboratories, San Carlos, CA) was used for ANG II determinations by RIA (10, 16, 30). Plasma renin concentration (PRC) and kidney renin content (KRC) were also determined by RIA (GammaCoat, DiaSorin, Stillwater, MN) as the generation of ANG I after addition of excess renin substrate in samples collected without inhibitors.
Angiotensinogen Expression Studies
mRNA expression as determined by quantitative real-time RT-PCR.
RNA ISOLATION.
Five to eight milligrams of renal cortices were used to isolate total RNA using a commercial kit following the manufacturer's recommendations (RNeasy Mini kit, Qiagen, Valencia, CA) that include DNase treatment. Total RNA was then tested for quality and quantity.
QUANTITATIVE RT-PCR.
The sequence of oligonucleotide primers and Taqman probes specific for mouse angiotensinogen and mouse β-actin used were as follows: For angiotensinogen, forward 5′-TATCCACTGACCCAGTTCTTT-3′, reverse 5′-AAGTGAACGTAGGTGTTGAAA-3′, and probe 5′-6-AMCTGTGACAGGGTGGAAGATGAACTTGCCA-BHQ1-3′. For β-actin, forward 5′-ATCATGAAGTGTGACGTTGA-3′, reverse 5′-GATCTTCATGGTCGTAGGAGC-3′, and probe 5′-HEX-TCTATGCCAACACAGTGCTGTCTGGT-BHQ2-3′. All samples were amplified in triplicate in a Stratagene MX 3000P multiplex quantitative (q) RT-PCR system using Stratagene PCR master mix II (Stratagene, La Jolla, CA). Twenty nanograms of the sample were used as a template, and angiotensinogen and β-actin were coamplified in the same reaction. The conditions for the reaction were set as one individual cycle of 50°C (30 min) and 95°C (10 min) followed by 45 cycles of 95° (15 s), 56°C (60 s), and 72°C (60 s). Assay quality was determined based on standard curve values of R2 ≥ 0.99, Y ≤ 3.9, and an efficiency of ≥80 and ≤110%. Quantitative values were extrapolated from individual standard curves for angiotensinogen and β-actin whose expression was not affected by the experiments. Results were normalized to β-actin expression.
Angiotensinogen protein expression as determined by Western blotting and immunohistochemistry.
PROTEIN ISOLATION.
Renal cortices were thawed and homogenized in 500 μl of lysis buffer. After that, samples were sonicated and centrifuged to collect the supernatant. Protein concentration was determined using the Bradford method (Bio-Rad protein assay, Bio-Rad, Hercules, CA).
WESTERN BLOTTING.
Five micrograms of protein of each sample were subjected to gel electrophoresis and transferred to nitrocellulose membranes following protocols described elsewhere (16).
The membranes were subjected to successive probing with a polyclonal primary rabbit anti-mouse/rat angiotensinogen antibody in a 1:200 dilution overnight at room temperature (code no. 99666, IBL, Gumma, Japan) and a secondary fluorescent-tagged goat anti-rabbit IgG antibody (IRDye 800 CW, Licor Biosciences). β-Actin detection was performed with a mouse monoclonal antibody (Abcam, 1:5,000) and a secondary fluorescent-tagged anti-mouse IgG antibody (IRDye 680 goat anti-mouse, Licor Biosciences). Fluorescent intensity was collected in two separate channels using an infrared Odyssey system (Licor Biosciences). The intensity of each angiotensinogen band was normalized to β-actin detected in the same membrane.
IMMUNOHISTOCHEMISTRY.
From every paraffin-embedded kidney, 10 consecutive 3-μm tissue slices were obtained, of which one slice per mouse was selected randomly for staining. After antigen activation, a Dako Envision plus system-HRP (Dakocytomation, Carpinteria, CA) was used to detect the binding of rabbit primary antibodies against mouse/rat angiotensinogen (IBL, see above) in the slice. Each tissue slice was probed at a concentration of 0.25 μg/ml for 15 min. Samples were also counterstained with hematoxylin before analysis. The staining protocol was performed to completion in an Autostainer Plus (Dako). Immunoreactivity was semiquantitatively evaluated in a blind manner as described elsewhere (16). Briefly, 20 microscopic fields/slide were selected at random for evaluation. Examination was performed using a microscope with ×200 magnification (Olympus BX51-TRF) and an integrated digital camera system (Magnafire SP). In every field, the sum of intensities of positive areas was calculated and averaged by the field area using Image Pro-plus software (Media Cybernetics, Bethesda, MD). The field area was calculated as the total field area minus the areas corresponding to glomeruli, blood vessels, and empty spots in the field. The average of intensities of the 20 fields represented the total intensity per slide and therefore per mouse.
Statistical Analyses
All data are presented as means ± SE. Two-way ANOVA with Bonferroni's posttest were used when blood pressure changes were analyzed. A paired t-test was applied when changes in body weight were studied. For the rest of the data, one-way ANOVA with Bonferroni's posttest were applied. A value of P < 0.05 was regarded as significant.
RESULTS
Body and Kidney Weights
Control mice gained weight during the period of the study (23.4 ± 0.6 before vs. 24.5 ± 0.3 g at the completion of the study, n = 6). Mice infused at a low dose of ANG II (400 ng·kg−1·min−1) showed a similar gain (24.1 ± 0.3 before vs. 25.1 ± 0.3 g after, n = 8, P < 0.05). Mice subjected to a high dose of ANG II (1,000 ng·kg−1·min−1) failed to show such progression [23.1 ± 0.4 before vs. 22.9 ± 0.5 g after treatment, n = 7, not significant (NS)]. Olmesartan treatment did not affect the weight gain in controls and mice infused at a low dose (mice treated with 400 ng·kg−1·min−1 plus olmesartan weight was 22.6 ± 0.4 vs. 24 ± 0.6 g after treatment, n = 5, P < 0.05). Furthermore, when given to mice infused at 1,000 ng·kg−1·min−1, olmesartan restored normal weight gain in this group (22.8 ± 0.6 before vs. 24.4 ± 0.6 g after treatment, n = 5, P < 0.05).
ANG II infusions caused a significant reduction of kidney weight (KW) only when infused at the high dose (139.6 ± 3.5 mg, n = 11, P < 0.05) compared with controls (156.6 ± 4.7 mg, n = 7). This reduction was prevented by cotreatment with olmesartan (ANG II 1,000 ng·kg−1·min−1+olmesartan, 160.6 ± 4.9 mg, n = 5, NS). ANG II infusions at the low dose either alone (150 ± 3.0 mg, n = 6) or in combination with olmesartan (ANG II 400 ng·kg−1·min−1+olmesartan, n = 5, 163.6 ± 6.4 mg, NS) did not change KW significantly. Finally, olmesartan treatment did not change KW in controls (161.5 ± 7.5 mg, n = 4, NS).
SBP
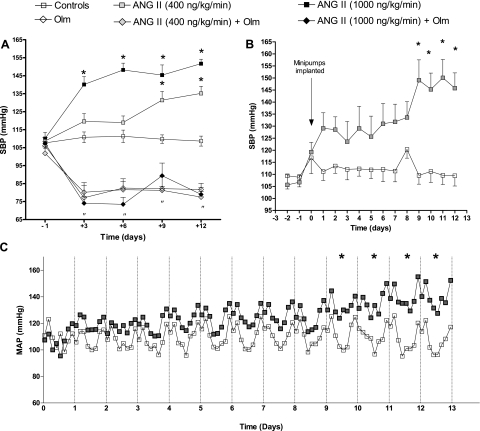
All groups displayed the same level of BP (107–110 mmHg) at the beginning of the study (Fig. 1). SBP determined by tail-cuff plethysmography (Fig. 1A) remained stable in controls during the study, with SBP averaging 109 ± 2.8 mmHg by day 12 (n = 13). ANG II infused at the low dose caused a slowly progressive increase in BP that reached significance (as determined by 2-way ANOVA) after 9 days of infusion; by day 12, the average SBP of this group was 135 ± 4.0 mmHg (n = 16). In contrast, ANG II at the high dose caused a rapid and sustained increase in BP that reached significance by day 3 and remained elevated throughout the experiment; by day 12 the SBP was 152 ± 2.5 mmHg (n = 11).
Fig. 1.
Effects of chronic ANG II and olmesartan infusions on systolic arterial pressure (SAP) as determined by tail-cuff plethysmography (A) and telemetry (B) and mean arterial pressure (MAP; C). Telemetry recordings (B) obtained at the same hours as the tail-cuff measurements (A) (between 1 and 5 P.M.) were averaged to allow comparison between these methods. C: 3-h MAP averages separated in 24-h intervals. Olm, olmesartan (3 mg·kg−1·day−1). *P < 0.05 for daily averages vs. controls. “P < 0.05 for daily averages vs. controls in olmesartan-treated animals.
Olmesartan treatment markedly reduced SBP in control mice and prevented the development of hypertension in mice treated with both doses of ANG II [ANG II 400 ng·kg−1·min−1+olmesartan (n = 5), 77 ± 2.9 mmHg; ANG II 1,000 ng·kg−1·min−1+olmesartan (n = 5), 78 ± 3.4 mmHg]. These changes in SBP were similar to those observed in controls treated only with olmesartan [82 ± 3.4 mmHg (n = 5)].
Because mice infused with 400 ng·kg−1·min−1 of ANG II displayed a SBP profile by tail-cuff plethysmography that resembled the slow pressure response observed in other models of ANG II-dependent hypertension (2, 13, 39), the effects of this dose of ANG II on BP were further assessed by telemetry (Fig. 1, B and C). Systolic arterial pressure telemetric recordings between 1 and 5 P.M. (the same time of the day when tail-cuff measurements were taken) (Fig. 1B) showed similar values for control animals that also remained stable during the experiment (109 ± 4.3 mmHg by day 12, n = 6). Meanwhile, ANG II-infused mice exhibited progressive increases in systolic arterial pressure recordings that reached higher values than those recorded by the tail-cuff method (146 ± 6.4 mmHg by day 12, n = 10). Hence, a higher difference was found between these two groups by telemetry (36 mmHg) than by plethysmography (26 mmHg).
MAP
Both groups, controls and ANG II-infused mice, displayed a circadian pattern in MAP with higher values during night hours (Fig. 1C) than during day hours as previously described (34). However, there was a slow response pattern toward higher BP in ANG II-infused mice that reached significance by day 9 as determined by two-way ANOVA, and these mice displayed higher BP values at all times during the 24-h cycles. By day 12, control animals had a MAP of 107 ± 1.9 mmHg (n = 6) vs. ANG II-infused mice, which had a MAP of 139 ± 4.4 (n = 10) (P < 0.05).
Plasma Levels of ANG II
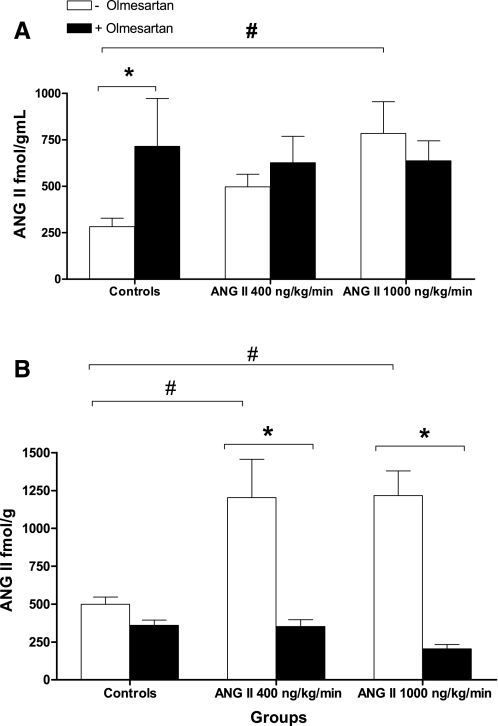
ANG II infusions caused significant changes in plasma ANG II levels (Fig. 2A, P < 0.05 by one-way ANOVA overall). Compared with controls (282 ± 46 fmol/ml, n = 10), plasma ANG II in mice infused with the low dose, although higher, was not statistically different (497 ± 68 fmol/ml, n = 15, NS). In turn, the high dose induced a threefold increase in plasma ANG II that was significant (784 ± 271 fmol/ml, n = 5, P < 0.05).
Fig. 2.
Effects of chronic infusions of ANG II either alone or with olmesartan on ANG II plasma (A) and intrarenal levels (B). #P < 0.05 vs. controls. *P < 0.05 within group by 1-way ANOVA and Bonferroni's posttest.
Mice treated with olmesartan displayed significant increases in ANG II plasma levels compared with controls (sham-operated) as ANG II values in the olmesartan-only group were 715 ± 257 fmol/ml (n = 5, P < 0.05). In mice infused with the low dose of ANG II plus olmesartan, plasma ANG II values were 627 ± 141 fmol/ml (n = 5), and those mice infused with the high dose plus olmesartan showed values of 637 ± 107 fmol/ml (n = 5). These changes were not significant compared with mice infused only with ANG II at the same doses.
Intrarenal Levels of ANG II
ANG II infusions caused significant increases in intrarenal ANG II at low dose (1,203 ± 253 fmol/g, n = 7) and high dose (1,258 ± 173 fmol/g, n = 11). These changes represent a 2.5-fold increase compared with controls (499 ± 40 fmol/g, n = 8) (Fig. 2B). In all groups, ANG II intrarenal levels were higher than plasma values.
Olmesartan treatment prevented the increases in intrarenal ANG II observed during chronic ANG II infusions at both doses (ANG II 400 ng·kg−1·min−1 plus olmesartan, 352 ± 44 fmol/g, n = 5, ANG II 1,000 ng·kg−1·min−1 plus olmesartan 205 ± 28 fmol/g, n = 5).
PRC and KRC
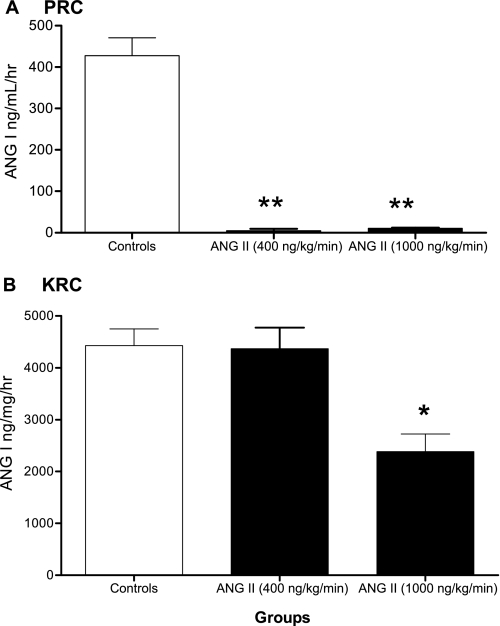
Figure 3A shows that chronic ANG II infusions caused a marked suppression of PRC as control animals displayed a PRC of 427.80 ± 43.14 ng ANG I·ml−1·h−1 (n = 9) vs. ANG II-infused animals that had a PRC of 4.90 ± 4.62 ng ANG I·ml−1·h−1 when infused with the low dose (n = 3) and 10.81 ± 0.87 ng ANG I·ml−1·h−1 when infused with the high dose (n = 9). In contrast, compared with controls KRC (Fig. 3B) (4,428 ± 323.40 ng ANG I·mg−1·h−1, n = 9), no reduction was observed in ANG II-infused mice with the low dose (4,367 ± 408.40 ng ANG I·mg−1·h−1, n = 4) and only a 50% reduction in KRC was achieved during ANG II infusion with the high dose (2,385 ± 337.80 ng ANG I·mg−1·h−1, n = 9).
Fig. 3.
Effects of ANG II infusions on plasma renin concentration (PRC) and kidney renin content (KRC). *P < 0.05 and **P < 0.001 vs. controls.
Expression of Intrarenal Angiotensinogen
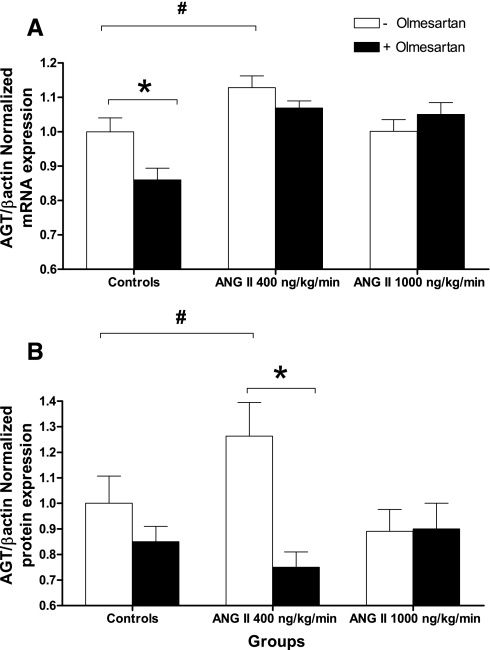
Figure 4 displays the effects of ANG II infusions on angiotensinogen mRNA expression (Fig. 4A) and protein (Fig. 4B) in the renal cortex as determined by qRT-PCR and Western blotting from tissue homogenates. Compared with angiotensinogen mRNA in controls (normalized expression of angiotensinogen/β-actin as 1.00 ± 0.04, n = 11), the low dose of ANG II infusion caused a significant increase in angiotensinogen mRNA levels (1.13 ± 0.03, n = 13). In contrast, the high dose failed to cause significant changes (1.00 ± 0.03, n = 12).
Fig. 4.
Effects of chronic infusions of ANG II either alone or with olmesartan on angiotensinogen (AGT) mRNA (A) and protein expression (B) in kidney cortices as determined by quantitative real-time-PCR and Western blotting. #P < 0.05 vs. controls. *P < 0.05 within group by 1-way ANOVA and Bonferroni's posttest.
When angiotensinogen protein expression was compared (Fig. 4B), only mice infused at the low dose of ANG II showed a significant increase in angiotensinogen protein expression (1.26 ± 0.13, n = 11, P < 0.05) vs. controls (1.00 ± 0.11, n = 11). The high dose did not cause any significant changes in angiotensinogen protein expression (0.89 ± 0.09, n = 12). Olmesartan treatment prevented the effects of ANG II to stimulate angiotensinogen mRNA and protein expression. Angiotensinogen mRNA expression in the 400 ng·kg−1·min−1 ANG II plus olmesartan group was 1.07 ± 0.02, (n = 5, NS vs. controls). In the same group, angiotensinogen protein expression was 0.75 ± 0.06. In controls, olmesartan treatment also reduced the expression of angiotensinogen mRNA (0.86 ± 0.03, n = 5) but not protein. No significant changes were observed in the group treated with ANG II at 1,000 ng·kg−1·min−1 plus olmesartan in mRNA or protein expression (n = 4).
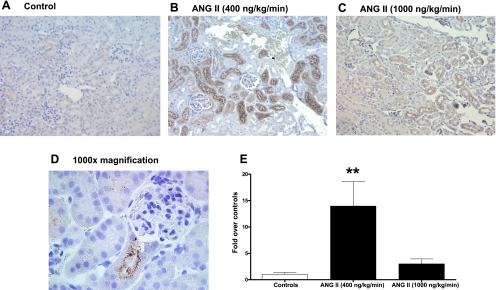
The immunohistochemical analysis (Fig. 5) demonstrates that angiotensinogen in the mouse kidney is expressed mainly in proximal tubules and that this expression was enhanced by ANG II infusions only at low doses, as the semiquantitative analysis revealed a marked induction of tubular angiotensinogen protein expression in infused mice (Fig. 5B, 13.97 ± 4.64, n = 4, P < 0.001) over controls (Fig. 5A, normalized as 1.00 ± 0.40, n = 10); in contrast, the high-dose infusion did not elicit any significant increases (Fig. 5C) (3.00 ± 0.94, n = 5). A higher magnification (×1,000) also revealed the angiotensinogen staining tends to locate to the apical side of the proximal tubule cells (Fig. 5D).
Fig. 5.
Immunohistochemistry analysis of the effect of chronic ANG II infusions on angiotensinogen expression. Top: representative for controls (A) and ANG II-infused animals at low dose (400 ng·kg−1·min−1; B) and at high dose (1,000 ng·kg−1·min−1; C). In every case, immunoreactive areas were circumscribed to mostly cortical brush border-rich tubules (proximal tubules). D: magnification (×1,000) of a positive-stained proximal tubule emerging from the corresponding glomerulus of a kidney from an ANG II-infused animal. Positive areas for angiotensinogen are observed as brown dots in the apical side of proximal tubule cells. Semiquantification of angiotensinogen immunostaining (E) is also shown. **P < 0.001 vs. controls.
DISCUSSION
The present data demonstrate that an infusion of ANG II at a dose of 400 ng·kg−1·min−1 in mice causes a progressive increase in BP accompanied by significant augmentation in intrarenal angiotensinogen mRNA and protein and that this response is dependent on the activation of AT1R. In contrast, a high dose of ANG II (1,000 ng·kg−1·min−1) failed to produce significant increases in intrarenal angiotensinogen despite eliciting a greater increase in BP. The increase in angiotensinogen expression during chronic ANG II infusions at low doses was demonstrated at the mRNA level by qRT-PCR and at the protein level by Western blotting and supported by immunohistochemistry. The difference in magnitude of the observed changes in angiotensinogen protein expression by these two methods might be explained by their different nature as Western blotting is a quantitative method and immunohistochemistry is semiquantitative. Nevertheless, these observations indicate that intrarenal angiotensinogen augmentation is not directly dependent on the ANG II dose or the extent of the increase in BP. Thus other factors participate in proximal tubule angiotensinogen regulation during chronic ANG II infusions.
The results also show that different ANG II doses elicit different phenotypes in mice. The retarded growth shown by the group infused with the high dose is similar to what has been reported in the past by Song et al. (33). In their report, it was demonstrated that mice infused chronically with ANG II (500 ng·kg−1·min−1) develop muscle wasting that is mediated by ANG II-induced downregulation of IGF-1 in skeletal muscles. In turn, mice infused with the low dose of ANG II displayed a normal progression of weight gain. Mice infused with the high ANG II dose displayed a different BP profile characterized by a rapid and pronounced increase in BP compared with the slowly progressive increase observed with the low dose. These responses to the high dose may reflect a malignant form of hypertension and potential target organ damage and might help to explain why this dose failed to induce increases in angiotensinogen expression. However, no evidence specifically demonstrating renal injury in this model is presented. Wesseling et al. (37) reported that C57BL female mice infused with ANG II for 4 wk at different doses, including some higher than those used in the present report, were resistant to kidney damage despite the development of hypertension. It is possible that female mice may be more resistant to hypertension-induced injury. Using a transgene mouse model overexpressing human angiotensinogen in proximal tubule cells and human renin, Kobori et al. (15) reported the development of initial signs of renal injury, including afferent arteriolar wall thickening, increased interstitial macrophage/monocyte infiltration, and increased collagen deposition.
The rapid increase in BP observed during infusion with the high dose also indicates a direct vasoconstrictor effect of ANG II. Furthermore, this dose of ANG II infusion elicits substantial oxidative stress and other extrarenal ANG II effects that might influence renal angiotensinogen expression (12). It is also possible that high doses of ANG II facilitate interactions with other receptors, like AT2R and megalin, that may counteract AT1R actions. However, in the case of the AT2R, findings by Cervenka et al. (4) and Wesseling et al. (37) point out that it is unlikely that AT2R activation contributes significantly to the ANG II-mediated increases in arterial pressure during ANG II-dependent hypertension.
This study demonstrates that ANG II-induced hypertension in mice is characterized by elevated plasma and intrarenal ANG II levels similar to what has been observed in other animal models (8, 23, 39). However, when ANG II levels are compared with those in rats, the best characterized species in ANG II-dependent hypertension, mice display plasma and kidney values that are higher even for the control animals (28). These findings indicate the existence of interspecies differences that need to be considered when the intrarenal RAS is studied and when such results are extrapolated between species, including humans. It has been shown that the development of hypertension elicited by chronic ANG II infusions is AT1R dependent as it is prevented by treatment with angiotensin receptor blockers (16, 18). Also, ANG II infusions into mice lacking AT1Rs in the kidney fail to develop high BP (5). Results from this work further support this notion as olmesartan treatment prevents the development of hypertension, as well as the increases in intrarenal ANG II content or angiotensinogen expression.
The present study extends and confirms previous results regarding the effect of chronic infusions of ANG II at two different doses (400 and 1,000 ng·kg−1·min−1) on SBP (12) and MAP (36). Furthermore, it has been reported that BP and heart rate in mice show a circadian rhythm with higher blood pressure values with high levels of activity during the night phase (34). Although the circadian pattern was preserved during chronic ANG II infusion, higher BP levels were maintained throughout the 24-h period and did not return to normal levels even during the day phase.
Mice infused at a low dose of ANG II (400 ng·kg−1·min−1) display a BP profile that fits the description of the slow pressor response observed in other models for ANG II-dependent hypertension including rats (18, 39), dogs (2), and humans (1). Several observations support the fact that this response involves an activation of the intrarenal RAS as it is a salt-sensitive form of hypertension accompanied by ANG II-mediated reductions in kidney function and sodium excretion (22, 35), and oxidative stress (12, 18, 24, 36). The activation of the angiotensinogen gene observed in mice infused at the low dose likely provides the substrate for additional local generation of ANG II. Furthermore, circulating ANG II, while being effective at reducing plasma renin concentration, failed to suppress kidney renin content in mice infused at the low dose and caused only partial suppression in mice infused with the high dose. While juxtaglomerular renin is suppressed by ANG II infusions, it has been shown that renin in the distal nephron is actually upregulated (30), suggesting that this might be the source of maintained intrarenal renin content. Collectively, these findings suggest that chronic ANG II infusions at the low dose cause an augmentation of the intrarenal RAS. This augmentation is mediated by ANG II uptake by the AT1R and activation of angiotensinogen expression. Indeed, transgenic mice with induced overactivity of this intrarenal RAS develop hypertension and renal damage in a manner that is independent from the systemic RAS (15, 19, 32).
In summary, mice display plasma and intrarenal ANG II levels that are higher than in other species even under basal conditions. During ANG II-induced hypertension, in particular when low doses of ANG II infusions are used, these levels are further increased and they are associated with augmentation of intrarenal angiotensinogen mRNA and protein via AT1R activation as well as AT1R-mediated uptake. These observations provide the foundation for future studies designed to determine the quantitative contribution of the intrarenal RAS and, specifically, proximal tubule angiotensinogen-derived ANG II to the overall levels of ANG II in the kidney and its regulation.
GRANTS
This project was supported by National Institutes of Health Grants P20RR-017659 from the IDeA program of the National Center for Research Resources, HL-26371 (L. G. Navar), and DK-072408 (H. Kobori); The Louisiana Board of Regents Millenium Trust Health Excellence Fund 2001-06-07, and a Consortium for Southeastern Hypertension Control WarrenTrust Fellowship award (R. A. Gonzalez-Villalobos).
Acknowledgments
The authors thank Daiichi Sankyo Co., Ltd. (Tokyo, Japan) for providing olmesartan.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ames RP, Borkowski AJ, Sicinski AM, Laragh JH. Prolonged infusions of angiotensin II and norepinephrine and blood pressure, electrolyte balance, and aldosterone and cortisol secretion in normal man and in cirrhosis with ascites. J Clin Invest 44: 1171–1186, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caravaggi AM, Bianchi G, Brown JJ, Lever AF, Morton JJ, Powell-Jackson JD, Robertson JI, Semple PF. Blood pressure and plasma angiotensin II concentration after renal artery constriction and angiotensin infusion in the dog. (5-Isoleucine)angiotensin II and its breakdown fragments in dog blood. Circ Res 38: 315–321, 1976. [DOI] [PubMed] [Google Scholar]
- 3.Carlson SH, Wyss JM. Long-term telemetric recording of arterial pressure and heart rate in mice fed basal and high NaCl diets. Hypertension 35: E1–5, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Cervenka L, Horacek V, Vaneckova I, Hubacek JA, Oliverio MI, Coffman TM, Navar LG. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension 40: 735–741, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Villalobos R, Klassen RB, Allen PL, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physiol 288: F420–F427, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Graciano ML, Mouton CR, Patterson ME, Seth DM, Mullins JJ, Mitchell KD. Renal vascular and tubulointerstitial inflammation and proliferation in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol 292: F1858–F1866, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Guan S, Fox J, Mitchell KD, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension 20: 763–767, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol 282: F19–F25, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imig JD, Navar GL, Zou LX, O'Reilly KC, Allen PL, Kaysen JH, Hammond TG, Navar LG. Renal endosomes contain angiotensin peptides, converting enzyme, and AT1A receptors. Am J Physiol Renal Physiol 277: F303–F311, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Ingelfinger JR, Jung F, Diamant D, Haveran L, Lee E, Brem A, Tang SS. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol Renal Physiol 276: F218–F227, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol 13: 2860–2868, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension 37: 1329–1335, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol 12: 431–439, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol 293: F938–F945, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension 43: 1126–1132, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koike G, Krieger JE, Jacob HJ, Mukoyama M, Pratt RE, Dzau VJ. Angiotensin converting enzyme and genetic hypertension: cloning of rat cDNAs and characterization of the enzyme. Biochem Biophys Res Commun 198: 380–386, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Kopkan L, Castillo A, Navar LG, Majid DS. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol 290: F80–F86, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am J Physiol Renal Physiol 286: F965–F971, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Li XC, Navar LG, Shao Y, Zhuo JL. Genetic deletion of AT1a receptors attenuates intracellular accumulation of ANG II in the kidney of AT1a receptor-deficient mice. Am J Physiol Renal Physiol 293: F586–F593, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills PA, Huetteman DA, Brockway BP, Zwiers LM, Gelsema AJ, Schwartz RS, Kramer K. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J Appl Physiol 88: 1537–1544, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell KD, Braam B, Navar LG. Hypertensinogenic mechanisms mediated by renal actions of renin-angiotensin system. Hypertension 19: I18–I27, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol Renal Physiol 273: F246–F253, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension 47: 238–244, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest 91: 774–779, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 39: 316–322, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navar LG, Kobori H, Prieto-Carrasquero M. Intrarenal angiotensin II and hypertension. Curr Hypertens Rep 5: 135–143, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navar LG, Nishiyama A. Why are angiotensin concentrations so high in the kidney? Curr Opin Nephrol Hypertens 13: 107–115, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Ploth DW Influences of angiotensin on renal function in renal vascular hypertension. Kidney Int Suppl 30: S97–S101, 1990. [PubMed] [Google Scholar]
- 30.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension 44: 223–229, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz-Ortega M, Esteban V, Ruperez M, Sanchez-Lopez E, Rodriguez-Vita J, Carvajal G, Egido J. Renal and vascular hypertension-induced inflammation: role of angiotensin II. Curr Opin Nephrol Hypertens 15: 159–166, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Sachetelli S, Liu Q, Zhang SL, Liu F, Hsieh TJ, Brezniceanu ML, Guo DF, Filep JG, Ingelfinger JR, Sigmund CD, Hamet P, Chan JS. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int 69: 1016–1023, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest 115: 451–458, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol 549: 313–325, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol 279: F319–F325, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill PS, Aslam S, Wang X, Ji H, Sandberg K, Jose P, Wilcox CS. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension 48: 934–941, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Wesseling S, Ishola DA Jr, Joles JA, Bluyssen HA, Koomans HA, Braam B. Resistance to oxidative stress by chronic infusion of angiotensin II in mouse kidney is not mediated by the AT2 receptor. Am J Physiol Renal Physiol 288: F1191–F1200, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT1 receptor. Hypertension 39: 116–121, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Zou LX, Imig JD, von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal angiotensin II augmentation in angiotensin II-infused rats. Hypertension 28: 669–677, 1996. [DOI] [PubMed] [Google Scholar]