Abstract
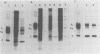
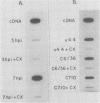
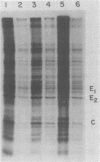
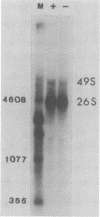
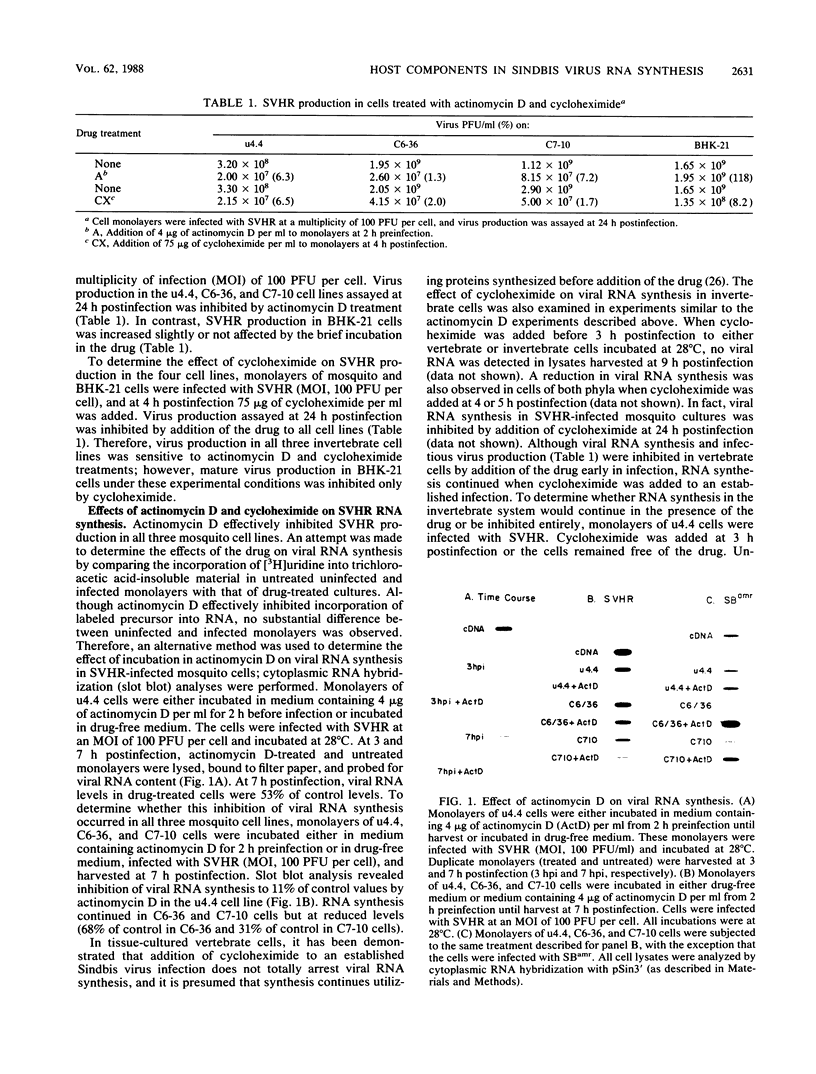
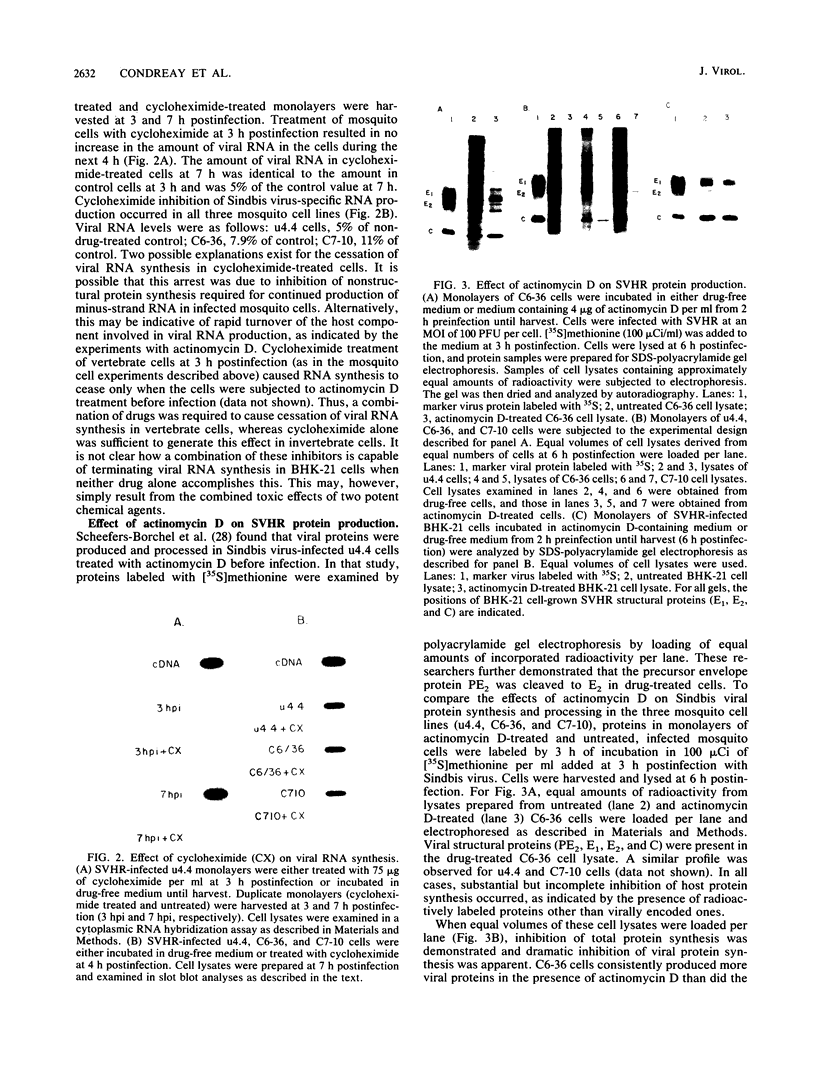
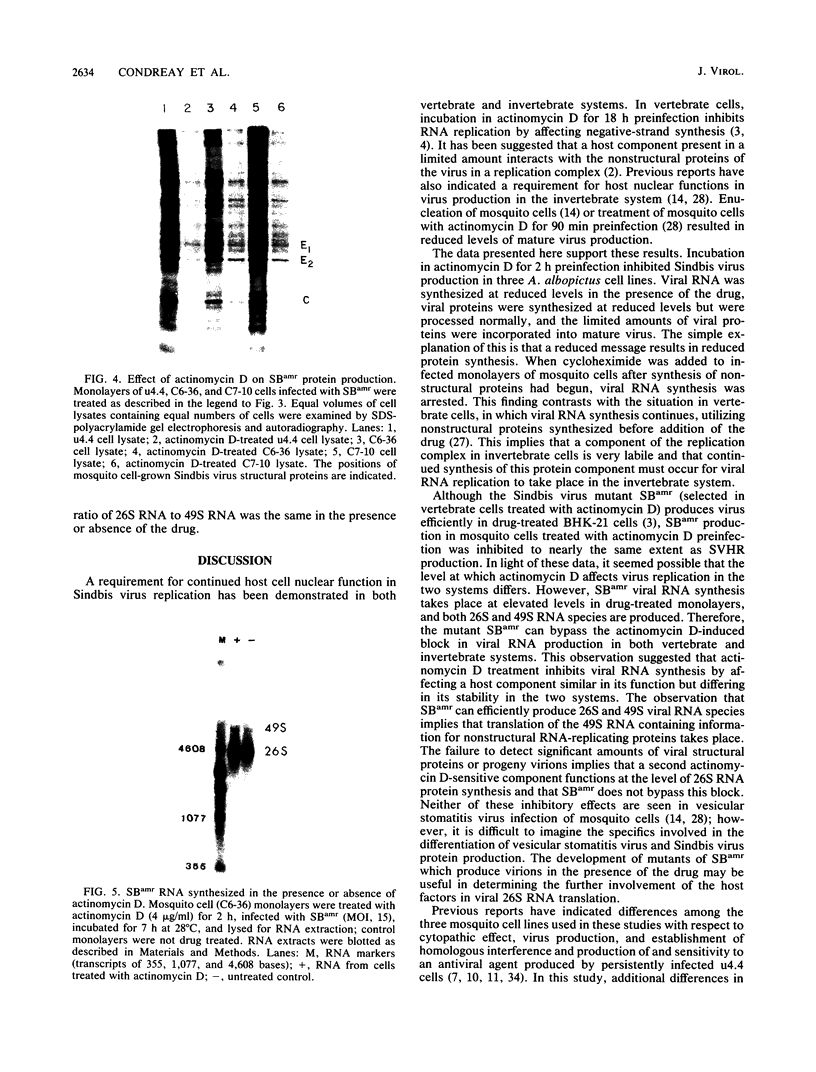
Production of Sindbis virus in the presence of transcription and translation inhibitors was examined in three Aedes albopictus cell lines. Addition of cycloheximide to heat-resistant Sindbis virus (SVHR)-infected mosquito cells arrested viral RNA synthesis completely, in contrast to the effects of this drug on virus-infected vertebrate cells. Production of mature virus by both SVHR (a variant commonly used as a wild-type virus) and SBamr (a mutant which is resistant to the effects of 18 h of pretreatment of vertebrate cells with actinomycin D) in mosquito u4.4, C6-36, and C7-10 cells was inhibited by 2 h of pretreatment with actinomycin D. Pretreatment with this drug for 2 h slightly enhances virus production in vertebrate cells. Treatment of mosquito cells with actinomycin D resulted in shutoff of SVHR RNA synthesis. The mutant SBamr was able to overcome the effects of actinomycin D on viral RNA synthesis and produced both 26S and 49S RNAs, even though no viral structural proteins or mature particles were produced in the presence of the drug. This result suggests that, in the presence of actinomycin, the nonstructural genes of SBamr are translated sufficiently to allow for RNA synthesis but that 26S RNA may not be translated to an extent that allows significant virus production. These data demonstrate that host components are involved in at least two distinct steps in the production of Sindbis virus in mosquito cells: (i) production of viral RNA and (ii) synthesis of viral structural polypeptides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Adams R. H., Brown D. T. BHK cells expressing Sindbis virus-induced homologous interference allow the translation of nonstructural genes of superinfecting virus. J Virol. 1985 May;54(2):351–357. doi: 10.1128/jvi.54.2.351-357.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baric R. S., Carlin L. J., Johnston R. E. Requirement for host transcription in the replication of Sindbis virus. J Virol. 1983 Jan;45(1):200–205. doi: 10.1128/jvi.45.1.200-205.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baric R. S., Lineberger D. W., Johnston R. E. Reduced synthesis of Sindbis virus negative strand RNA in cultures treated with host transcription inhibitors. J Virol. 1983 Jul;47(1):46–54. doi: 10.1128/jvi.47.1.46-54.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Cohen R. S., Meselson M. Separate regulatory elements for the heat-inducible and ovarian expression of the Drosophila hsp26 gene. Cell. 1985 Dec;43(3 Pt 2):737–746. doi: 10.1016/0092-8674(85)90247-8. [DOI] [PubMed] [Google Scholar]
- Condreay L. D., Brown D. T. Exclusion of superinfecting homologous virus by Sindbis virus-infected Aedes albopictus (mosquito) cells. J Virol. 1986 Apr;58(1):81–86. doi: 10.1128/jvi.58.1.81-86.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condreay L. D., Brown D. T. Suppression of RNA synthesis by a specific antiviral activity in Sindbis virus-infected Aedes albopictus cells. J Virol. 1988 Jan;62(1):346–348. doi: 10.1128/jvi.62.1.346-348.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs K., Brown B., Brown D. T. Evidence for a change in capsid morphology during Sindbis virus envelopment. Virus Res. 1984;1(4):297–302. doi: 10.1016/0168-1702(84)90018-2. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Erwin C., Brown D. T. Requirement of cell nucleus for Sindbis virus replication in cultured Aedes albopictus cells. J Virol. 1983 Feb;45(2):792–799. doi: 10.1128/jvi.45.2.792-799.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gliedman J. B., Smith J. F., Brown D. T. Morphogenesis of Sindbis virus in cultured Aedes albopictus cells. J Virol. 1975 Oct;16(4):913–926. doi: 10.1128/jvi.16.4.913-926.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes W. J., Burge B. W. Modification of Sindbis virus glycoprotein by host-specified glycosyl transferases. J Virol. 1971 Mar;7(3):309–313. doi: 10.1128/jvi.7.3.309-313.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973 Jun 5;12(12):2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Igarashi A. Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978 Sep;40(3):531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Raghow R. S., Grace T. D., Filshie B. K., Bartley W., Dalgarno L. Ross River virus replication in cultured mosquito and mammalian cells: virus growth and correlated ultrastructural changes. J Gen Virol. 1973 Oct;21:109–122. doi: 10.1099/0022-1317-21-1-109. [DOI] [PubMed] [Google Scholar]
- Renz D., Brown D. T. Characteristics of Sindbis virus temperature-sensitive mutants in cultured BHK-21 and Aedes albopictus (Mosquito) cells. J Virol. 1976 Sep;19(3):775–781. doi: 10.1128/jvi.19.3.775-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel B., Brown D. T. Role of extracellular virus on the maintenance of the persistent infection induced in Aedes albopictus (mosquito) cells by Sindbis virus. J Virol. 1977 Sep;23(3):554–561. doi: 10.1128/jvi.23.3.554-561.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sarver N., Stollar V. Sindbis virus-induced cytopathic effect in clones of Aedes albopictus (Singh) cells. Virology. 1977 Jul 15;80(2):390–400. doi: 10.1016/s0042-6822(77)80014-7. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G. Short-lived minus-strand polymerase for Semliki Forest virus. J Virol. 1980 Apr;34(1):108–118. doi: 10.1128/jvi.34.1.108-118.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheefers-Borchel U., Scheefers H., Edwards J., Brown D. T. Sindbis virus maturation in cultured mosquito cells is sensitive to actinomycin D. Virology. 1981 Apr 30;110(2):292–301. doi: 10.1016/0042-6822(81)90061-1. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Bracha M., Schlesinger M. J. Evidence for covalent attachment of fatty acids to Sindbis virus glycoproteins. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1687–1691. doi: 10.1073/pnas.76.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Burge B. W. Biosynthesis of the Sindbis virus carbohydrates. J Virol. 1973 Dec;12(6):1366–1374. doi: 10.1128/jvi.12.6.1366-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M. Immediate glycosylation of Sindbis virus membrane proteins. Cell. 1977 Apr;10(4):659–668. doi: 10.1016/0092-8674(77)90099-x. [DOI] [PubMed] [Google Scholar]
- Simizu B., Maeda S. Growth patterns of temperature-sensitive mutants of Western equine encephalitis virus in cultured Aedes albopictus (mosquito) cells. J Gen Virol. 1981 Oct;56(Pt 2):349–361. doi: 10.1099/0022-1317-56-2-349. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]